Abstract
HIV seroconversion outcomes in preexposure prophylaxis (PrEP) trials of oral tenofovir (TFV)-containing regimens are highly sensitive to drug concentration, yet less-than-daily dosing regimens are under study. Description of TFV and its active moiety, TFV diphosphate (TFV-DP), in blood, vaginal tissue, and colon tissue may guide the design and interpretation of PrEP clinical trials. Six healthy women were administered a single oral dose of 300 mg tenofovir disoproxil fumarate (TDF) and 4.3 mg (12.31 MBq, 333 μCi) 14C-TDF slurry. Blood was collected every 4 h for the first 24 h, then at 4, 8, 11, and 15 days postdosing. Colonic and vaginal samples (tissue, total and CD4+ cells, luminal fluid and cells) were collected 1, 8 and 15 days postdose. Samples were analyzed for TFV and TFV-DP. Plasma TFV demonstrated triphasic decay with terminal elimination half-life median [interquartile range (IQR)] 69 h (58–77). Peripheral blood mononuclear cell (PBMC) TFV-DP demonstrated biphasic peaks (median 12 h and 96 h) followed by a terminal 48 h (38–76) half-life; Cmax was 20 fmol/million cells (2–63). One day postdose, the TFV-DP paired colon:vaginal tissue concentration ratio was 1 or greater in all subjects' tissue homogenates, median 124 (range 1–281), but was not sustained. The ratio was lower and more variable in cells extracted from tissue. Among all sample types, TFV and TFV-DP half-life ranged from 23 to 139 h. PBMC TFV-DP rose slowly in the hours after dosing indicating that success with exposure-driven dosing regimens may be sensitive to timing of the dose prior to exposure. Colonic tissue homogenate TFV-DP concentrations were greater than in vaginal homogenate at 24 h, but not in cells extracted from tissue. These and the other pharmacokinetic findings will guide the interpretation and design of future PrEP trials.
Introduction
Randomized clinical trials of HIV preexposure prophylaxis (PrEP), using tenofovir (TFV) disoproxil fumarate (TDF)-containing oral dosing regimens, have yielded mixed, and conflicting, results. Relative risk reduction has ranged from 65% to 75% in heterosexual men and women on daily TDF or Truvada (Partners, TDF2),1,2 44% in men who have sex with men (MSM) on oral Truvada (iPrEx),3 and no statistically significant benefit in women on daily TDF or Truvada (FEM-PrEP, VOICE).4,5 In spite of modest or no benefit in some of these trial populations with daily dosing, studies are underway to assess less frequent TDF dosing. In CAPRISA 004, coitally dependent dosing around the time of sex with vaginal 1% TFV gel yielded a modest 39% risk reduction6 and daily dosing in VOICE was ineffective. Both within and among these trials, drug concentration is a powerful explanatory variable for the seroconversion outcomes.7 The relative contribution of adherence and pharmacokinetics, however, has not been rigorously assessed.
Understanding the pharmacokinetics (PK) of TFV and its active moiety, TFV diphosphate (TFV-DP), in blood, colon, and vaginal tissue concentrations could be used to interpret PrEP trials of TDF that involve populations of women and men with different anatomic sites of predominant HIV risk. Outcome sensitivity to adherence would also be better informed by a more complete PK picture. While drug concentration in blood is easily accessible and was measured in all of these trials, concentrations in vaginal and colonic tissue were not measured on study in any of them. Small bridging studies with simultaneous sampling of blood and tissues associated with sexual HIV exposure are needed to better understand concentration–response relationships at the active site. Furthermore, a quantitative description of the distribution and time course of TFV-DP among potentially HIV-exposed tissue sites following oral dosing, viewed critically in light of the outcomes of prior double-blind randomized controlled trials (RCTs), would provide a means of assessing the protective plausibility of alternate, less frequent dosing regimens. Through PK/PD modeling and simulation, concentration–time profiles with different dosing regimens can be compared to drug concentrations achieved in RCTs that were associated with protective effects.
We performed a PK study using a single oral dose of TDF followed by sampling of the blood, colon, and female genital tract for 2 weeks to assess time-dependent changes in drug concentration among these anatomic sites. Near simultaneous sampling of vagina and colon in each woman over 2 weeks enabled a paired comparison within each woman. We estimated a single TDF dose would result in TFV and TFV-DP concentrations below the detection limits of our existing UPLC-MS/MS assays, especially 2 weeks after dosing and in tissue samples with small numbers of extracted cells. For this reason, we used 14C-TDF with detection by accelerator mass spectrometry (AMS), commonly used in 14C dating, to detect a very small radioisotope mass in samples.8 AMS detection of 14C, instead of a fragment of a chemical entity as in traditional mass spectrometry, enhances the sensitivity by reducing the sample background to the 14C present in nature, which results in a detection limit of 0.1 Modern.
Materials and Methods
Clinical study plan
The study protocol was approved by the Johns Hopkins Medicine Institutional Review Board. Research participants provided informed consent prior to study screening. Eligible subjects were healthy HIV-negative, premenopausal women with a negative pregnancy test. Six research participants were orally coadministered a slurry of 300 mg tenofovir disoproxil fumarate (TDF; Viread, Gilead Sciences, Inc., Foster City, CA) and 12.31 MBq (333 μCi, 4.3 mg) adenine-8-14C-tenofovir disoproxil fumarate in 1:1 ethanol/water (14C-TDF; Moravek Biochemicals, Brea, CA) followed by a high-fat meal and apple juice. Blood was drawn into cell preparation tubes (CPT) once every 4 h for 24 h after dosing, then at 4, 8, 11, and 15 days postdose. Colonic biopsies and luminal brushings, vaginal biopsies, cervicovaginal lavage (CVL), and cervical cytobrush cells were sampled 24 h after the dose and then 8 and 15 days after dosing.
Sample collection and processing
Blood
Peripheral blood mononuclear cells (PBMCs) were isolated via centrifugation from the CPTs at 1,800×g for 25 min at 28°C. Plasma was isolated (1 ml) from the CPT tube, and then the PBMCs were collected from the buffy coat and washed twice with phosphate-buffered saline (PBS). Cells were resuspended in 1 ml PBS for cell counting. Cell pellets were lysed with 70% methanol and stored at −80°C until analysis.
Colon samples
Colon samples were collected through a flexible sigmoidoscope (Evis Exera, Olympus America Corp., Center Valley, PA). Endoscopic brushes (Kimberly Clark, Roswell, GA) were used to collect superficial colonic fluid and cells.9 Brushes were weighed and luminal fluid was eluted using 1 ml PBS and centrifugation at 800×g, 10 min, 4°C. Samples were immediately stored at −80°C. Brushes were later dried and weighed to determine the original brush, and hence sample, weight.
Thirty biopsies were collected using 3.7-mm pinch biopsy forceps (Microvasive no. 1599; Boston Scientific Corp., Natick, MA) 10–20 cm from the anus, and placed in RPMI medium with 10% fetal bovine serum (R10 media) until processing.10 Four colonic biopsies were weighed and homogenized in 500 μl ice-cold 70% methanol; samples were immediately frozen at −80°C. The remaining biopsies were used for enzymatic extraction of mucosal cells.
Vaginal sampling
Samples were scheduled not to coincide with menses. CVL was performed with 10 ml of Normosol-R (Hospira, Lake Forest, IL) repeatedly applied against the cervix and vaginal walls for 30 s and collected into a 10-ml syringe with Luer-Lok tip. CVL was transferred into a 15-ml conical tube and centrifuged at 450×g, 10 min, 4°C to pellet CVL cells.11 Supernatant was aliquoted into 1-ml fractions for analysis. The CVL pellet was washed twice and lysed in 70% methanol. For vaginal biopsies, a speculum was inserted into the vagina and five vaginal biopsies were taken with 2.3×4.2-mm Tischler gold-plated gynecological forceps. One biopsy was homogenized and flash frozen similar to the colon biopsies. The remaining biopsies were used for cell extraction.
Tissue cell extraction
To release cells for intracellular analysis from colonic and vaginal tissue, biopsies were incubated with a dissociative enzyme cocktail consisting of collagenase (0.5 mg/ml, Sigma-Aldrich, St. Louis, MO), DNase I (0.083 U/ml, Roche, Indianapolis, IN), elastase (0.07 U/ml, Worthington Biochemicals, Lakewood, NJ), and hyaluronidase (0.4 U/ml, Worthington Biochemicals, Lakewood, NJ). The digestions were carried out in RPMI with 7.5% FBS in 50 ml-conical tubes at 37°C with agitation (Invitrogen, Carlsbad, CA) essentially as described.11 Cells were counted using Guava/Millipore EasyCyte Plus (Millipore, Billerica, MA).
Cell separation with MACS
CD4 cells were isolated via positive selection with CD4 microbeads using magnetic affinity column separation (MACS) according to the manufacturer's recommended protocol (Miltenyi Biotec, Auburn, CA). CD4-positive and CD4-negative fractions were collected for cell counting and intracellular drug analysis. To maximize cell yield, we did not take aliquots to test CD4 cell purity. In our prior studies, PBMC CD4 purity is 95% and tissue cell extraction CD4 purity is 75–85%.
Drug concentration analysis
Accelerator mass spectrometry (AMS): Sample preparation:
14C analysis of neat plasma, tissue homogenate, colonic fluid, and CVL samples for TFV concentration was performed at the Massachusetts Institute of Technology (MIT) Biological Engineering Accelerator Mass Spectrometry (BEAMS) laboratory.12,13
For TFV-DP analysis, the isolation procedure used for LC-MS/MS detection of TFV-DP (described below) was used prior to AMS detection. Samples were reconstituted in 50 μl 0.5% acetic acid in water and analyzed using AMS. No 13C-TFV internal standard was added to these samples, as there was concern that 13C in samples would increase the background in each sample, thereby lowering the sensitivity of detection of 14C.
Accelerator mass spectrometry (AMS): Sample analysis
Aliquots of cell lysates or luminal fluid samples (1–2.75 μl) were used for AMS analysis. Samples were adsorbed onto CuO powder pellets prepared by exposure to an O2 atmosphere in a vacuum oven. Pellets were transferred to a laser-induced combustion interface for AMS analysis. Five samples were run after a quantitation standard of 0.0030 dpm/μl. The combustion interface produces CO2, which is then delivered to the ion source and used to determine the total 14C in the sample. The TFV-DP lower limit of quantitation was 1.32 fmol/million cells based on a 1.5-μl sample. The TFV lower limit of quantitation was 0.09 ng/ml based on a 1.5-μl plasma sample. The TFV data showed a subject-dependent (analytical run) difference between the AMS and LC-MS/MS concentrations, ranging from 1- to 6-fold. To adjust for this, we scaled the AMS TFV results using individual LC-MS/MS results on the same sample as a reference.
LC-MS/MS
Plasma samples from several subjects were also assayed for TFV and TFV-DP for comparison with AMS results. TFV was assayed in multiple biological matrices as previously described.14 Briefly, thawed aliquots of plasma and tissue homogenate with 13C5-TFV internal standard were protein precipitated with methanol. CVL and rectal fluid aliquots, also with 13C5-TFV internal standard, underwent solid phase extraction using HLB oasis cartridges. The supernatants and eluants were collected, dried, and reconstituted in 0.5% acetic acid for analysis. For chromatographic separation of samples we employed gradient elution with a Zorbax Eclipse XDB-C18 column, with positive electrospray ionization (ESI), and detection via multiple reaction monitoring using an LC-MS/MS system (Waters Acquity UPLC and Agilent 1100 HPLC Applied Biosystem API4000 mass spectrometer). Calibration standards for the TFV assay ranged from 0.31 to 1,280 ng/ml (0.25–50 ng/sample for tissue).
For intracellular TFV-DP analysis, cell lysates were analyzed using an indirect assay, essentially as described by King, et al.15 TFV-DP was isolated from cell lysates on a Waters QMA cartridge (Waters Corporation, Milford, MA) over a salt (KCl) gradient. TFV and tenofovir monophosphate (TFV-MP) were separated from the cartridge under lower salt concentrations followed by elution of TFV-DP with application of 1 M KCl to the cartridge. Isolated TFV-DP was then enzymatically dephosphorylated to TFV via phosphatase digestion with incubation with phosphatase and 13C-TFV internal standard. TFV was isolated from the KCl solution using trifluoroacetic acid and eluted in methanol. TFV with 13C-TFV internal standard was analyzed via UPLC-MS/MS mass spectrometer.14
Pharmacokinetic analysis
TFV and TFV-DP PK parameter estimates were determined by manual curve stripping using Microsoft Excel and noncompartmental analysis using WinNonlin (Version 5.0, Pharsight, Inc., Cary, NC). Given the small number of sample times for tissue matrices, zero AMS values preceded by measurable value were imputed as one-half the lowest observed value for the matrix. The data were summarized using median and interquartile range. The Wilcoxon rank sum test was used to test the statistical significance of values between matrices (IBM SPSS Statistics v.19.0.0, Armonk, NY). Testing was performed only when the number of pairs exceeded 3; otherwise, statistical significance could not be achieved.
Results
AMS vs. UPLC-MS/MS comparison
AMS detection of 14C-TFV in plasma was compared to our established LC-MS/MS method using plasma and PBMC lysate samples. The linear regression slopes for AMS vs. LC-MS/MS for TFV and TFV-DP were 0.96 (r2=0.92, p<0.0001) and 0.96 (r2=0.61, p<0.0001), respectively.
Overall trends
The PK parameters for all matrices are summarized in Table 1. While the peak concentration (Cmax) and time to peak concentration (Tmax) are indicated in the table, these estimates have little precision as they are largely influenced by the sampling scheme—blood was sampled only every 4 h during the first day and tissue was sampled only weekly. With this sampling scheme, Tmax is useful to indicate matrices that peak beyond the 24-h sample time, specifically, TFV-DP in both vaginal and colon CD4 cells (but not total cells), CVL cell TFV-DP, and colon brush TFV. Most subjects had measurable TFV and TFV-DP in all matrices (except CVL cells) 1 week after dosing, but nearly half of the matrices were below detection for most subjects by 2 weeks.
Table 1.
Pharmacokinetic Parameters for All Matrices
| Anatomic location | Moiety | Units | Tmax Median (IQR) | Cmax Median (IQR) | C24 (day 1) Median (IQR) | Terminal half-life Median (IQR) |
|---|---|---|---|---|---|---|
| Plasma | TFV | ng/mL | 4 (4, 4) | 175 (136, 232) | 40 (24, 51) | 69 (58, 77)a |
| PBMC | TFV-DP | fmol/M | 12 (8, 96) | 20 (20, 63) | 10 (9, 12) | 48 (38, 76) |
| Blood CD4+ Cells | TFV-DP | fmol/M | 12 (12, 15) | 10 (7, 16) | 5 (5, 7) | 112 (100, 118) |
| VT homogenate | TFV | ng/gm | 24 (24, 24) | 6 (1, 10) | 4 (1, 9) | 47 (38, 53) |
| VT homogenate | TFV-DP | fmol/gm | 24 (24, 150) | 1,022 (572, 1,684) | 526 (437, 1,216) | 53 (45, 68) |
| VT Total Cells | TFV-DP | fmol/M | 24 (24, 192) | 60 (56, 101) | 56 (39, 101) | 66 (43, 202) |
| VT CD4+ Cells | TFV-DP | fmol/M | 192 (108, 192) | 13 (BLQ, 54) | 23 (17, 39) | 139 (121, 167) |
| CVLb | TFV | ng/mL | 24 (24, 24) | 7 (4, 8) | 7 (4, 8) | 40 (38, 43) |
| CVL Cells | TFV-DP | fmol/M | 108 (66, 150) | BLQ (BLQ, 6) | BLQ (BLQ, BLQ) | - |
| CT homogenate | TFV | ng/gm | 108 (24, 192) | 25 (6, 164) | 33 (2, 208) | 31 (24, 36) |
| CT homogenate | TFV-DP | fmol/gm | 24 (24, 150) | 36,651 (5,598, 63,609) | 7,463 (3,517, 60,910) | 34 (21, 40) |
| CT Total Cells | TFV-DP | fmol/M | 24 (24, 192) | 76 (25, 88) | 25 (15, 88) | 82 (43, 89) |
| CT CD4+ Cells | TFV-DP | fmol/M | 192 (24, 192) | 17 (4, 17) | 1 (BLQ, 4) | 60 (52, 72) |
| Colon Brush | TFV | ng/mL | 192 (24, 192) | 57 (5, 565) | BLQ (BLQ, 565) | 20 (20, 21) |
Terminal gamma half-life based on 4 subjects; median (IQR) for alpha half-life 4 h (4-5h), beta half-life 18 h (16-20).
CVL is not corrected for dilutional volume of the lavage solution.
IQR, interquartile range; TFV, tenofovir; PBMC, peripheral blood mononuclear cells; TFV-DP, tenofovir diphosphate; M, million cells; VT, vaginal tissue; BQL, below limits of AMS assay quantitation; CVL, cervicovaginal lavage; CT, colon tissue.
Blood samples
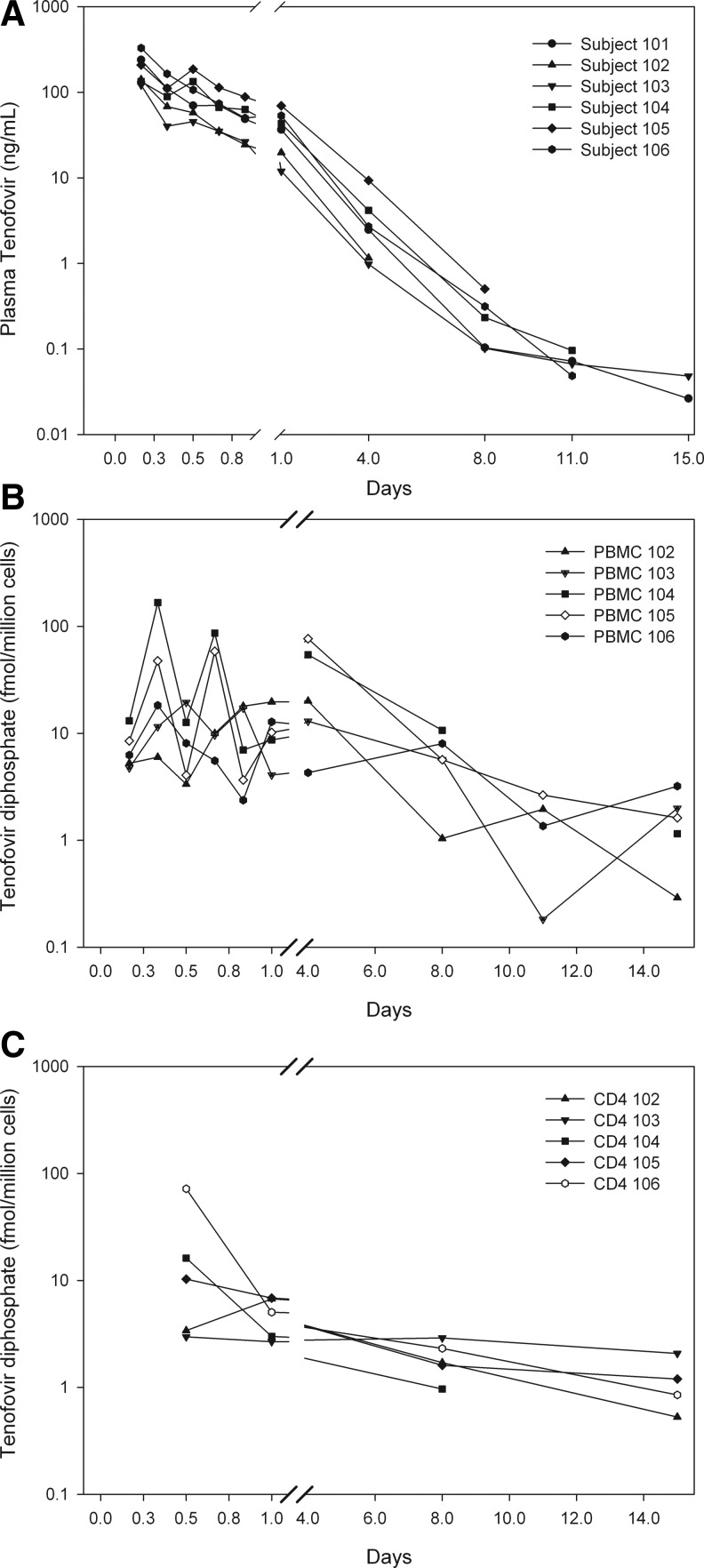
The plasma TFV concentration–time course showed triphasic decay in most subjects and only two of the subject's samples were still quantifiable at 360 h (day 15) (Fig. 1A and Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid). The median (IQR) estimate for the terminal gamma elimination half-life, measurable in four of six subjects, was 69 h (58–77) (Table 1). The beta elimination half-life (the terminal phase in two subjects) was 18 h (16–20).
FIG. 1.
Individual concentration versus time curves for plasma tenofovir (TFV) (A), peripheral blood mononuclear cell (PBMC) total cell tenofovir diphosphate (TFV-DP) (B), and PBMC CD4+ cell subset TFV-DP (C). The y-axis scale for PBMC total and CD4+ cell subsets are the same. Unique symbols are used to display each subject and the same symbol is used for both (A) and (B).
The PBMC TFV-DP Tmax of 12 (8–96) h misses the fact that four of five subjects with PBMC data had two distinct peaks (Fig. 1B and C). The initial peak occurred 12 (8–16) h after the dose with concentration of 20 (20–59) fmol/million cells. A second peak occurred in four of five measured subjects, all at 96 h with a concentration of 20 (13–54) fmol/million cells. The terminal half-life phase of TFV-DP, median (IQR) 48 h (38–76), largely overlapped the plasma half-life distribution, 69 (58–77) h.
Vaginal and colon samples
Both vaginal and colon tissue matrices had Tmax values beyond 24 h (median 108 h and 192 h, respectively). However, sampling occurs only at weekly intervals, so this pattern may not be very different from the more frequently sampled PBMC TFV-DP, which appears to peak on day 4 (96 h) when there are no corresponding vaginal or colon samples. As noted above, vaginal tissue CD4 cell TFV-DP half-life is markedly longer than blood and, especially, colon CD4 cells. The half-life of vaginal tissue homogenates for both TFV and TFV-DP are also longer than values in the colon.
Colon homogenate TFV and TFV-DP concentrations were greater than paired vaginal matrices (Table 2, p<0.05). The ratio of colon to vaginal tissue homogenates (C:V ratio) was 33.8 (6.8–37.8) and 123.7 (8.4–155.4) for TFV and TFV-DP, respectively, 24 h postdosing. However, this difference did not persist quantitatively or statistically at the 8 day observation. For total cells extracted from tissue, TFV-DP tended to be less in colon than vaginal tissue and this was statistically significant at week 2. Incomplete data pairs (due to small vaginal tissue mass, low cell yields, and low concentrations) prevent any statistical testing of extracted CD4 cells at any time or homogenates at 2 weeks. Homogenates and cells were not correlated between colon and vaginal sources (r<0.01, p>0.05).
Table 2.
Colon-to-Vaginal Tissue Concentration Ratio
| |
Homogenate TFV |
Homogenate TFV-DP |
Total Cell TFV-DP |
CD4+ Cells TFV-DP |
||||
|---|---|---|---|---|---|---|---|---|
| Days | N>0 | Median (IQR) | N>0 | Median (IQR) | N>0 | Median (IQR) | N>0 | Median (IQR) |
| 1 | 5 | 33.8 (6.8, 37.8)* | 5 | 123.7 (8.4, 155.4)* | 5 | 0.3 (0.1, 0.4) | 2 | 19.2 (9.6, 28.8) |
| 8 | 6 | 4.5 (0.9, 31.3) | 5 | 1.7 (0.3, 2.8) | 4 | 1.1 (0.8, 1.4) | 2 | 0.2 (0.2, 0.2) |
| 15 | 1 | 0.3 | 2 | 2.6 (2.5, 2.6) | 4 | 0.3 (0.1, 0.5)* | 1 | 0.2 |
Values are median (interquartile range [IQR]) except where N = 2 where minimum and maximum are shown, and where N = 1 where IQR is not shown; TFV, tenofovir; N>0, number of participants with complete non-zero value (above limits of assay quantitation) for both colon and vaginal drug concentration; TFV-DP, tenofovir diphosphate; *p < 0.05.
Cell subset comparisons
PBMCs had greater TFV-DP content compared to CD4+ subsets by 24 h after dosing (p=0.025), but this difference was not statistically significant at later times (Table 3). A similar pattern was seen for total and CD4+ cells extracted from colon and vaginal tissue, but the number of complete total:CD4 pairs was inadequate for statistical testing. Pairwise correlations among colon tissue homogenates, total extracted cells, and CD4+ cell subsets were well correlated (all Pearson correlation coefficients >0.98, p<0.01). Vaginal tissue total cells correlated poorly with vaginal tissue homogenate and CD4+ cell subsets, but vaginal homogenate and CD4 cells were reasonably well correlated (r=0.74, p<0.01).
Table 3.
Total Cell-to-CD4+ T Cell Subset Ratio
| |
PBMC |
Colon |
Vagina |
|||
|---|---|---|---|---|---|---|
| Days | N>0 | Median | N>0 | Median | N>0 | Median |
| 0.5 | 5 | 0.8 (0.4, 1.0) | - | - | - | - |
| 1 | 5 | 2.6 (1.5, 2.9)* | 3 | 6.2 (3.5, 36.0) | 2 | 34.0 (17.2, 50.68) |
| 8 | 5 | 3.5 (0.6, 3.5) | 3 | 4.3 (3.0, 4.5) | 2 | 0.5 (0.4, 0.6) |
| 15 | 4 | 1.2 (0.9, 2.0) | 3 | 0.1 (0.1, 1.0) | 1 | 0.3 |
Values are median (interquartile range [IQR]) except where N = 2 where minimum and maximum are shown, and where N = 1 where IQR is not shown; TFV, tenofovir; N > 0, number of participants with complete non-zero value (above limits of assay quantitation) for both total cell and CD4+ cell subset drug concentration; TFV-DP, tenofovir diphosphate; *p < 0.05.
Half-life estimates
The terminal elimination half-life for all matrices ranged from 20 h (colon brush TFV) to 139 h (vaginal CD4 cell TFV-DP), but the medians of most matrices were between 42 h and 82 h with largely overlapping interquartile ranges and less than 2-fold variation between quartiles for all matrices. There were notable deviations from this generalization about overlapping interquartile ranges. Colon brush and colon tissue homogenates had the shortest half-lives. Colon brush TFV half-life was shorter, 23 (21–23) h, than all other evaluable matrices (p<0.05) except for colon tissue homogenate TFV-DP. With values similar to colon brushes, colon tissue homogenates half-lives for TFV, 31 (24–36) h, and TFV-DP, 34 (21–40) h, were shorter than almost all other matrices (p<0.05). The exceptions were vaginal tissue homogenates (too variable and statistically insignificant) and vaginal cells (insufficient complete pairs to be significant). The point estimate for TFV-DP half-life in PBMC CD4+ cells, 112 (100–118) h, is longer than and has a nonoverlapping interquartile range compared to all other matrices. This is statistically significant compared only to CVL, colon homogenates, and colon brushes. The matrix with the longest half-life estimate, vaginal tissue CD4 cells, 139 (121–167) h, had insufficient complete pairs for testing against other matrices.
Discussion
Plasma TFV demonstrated a gamma elimination phase with a median (IQR) half-life of 69 (58–77) h, which largely overlapped with the PBMCs TFV-DP half-life distribution, 48 (38–76) h. A similar plasma TFV gamma phase was reported by Patterson, et al., with a median 47 h half-life, also in a single dose study.16 These triphasic decay estimates may be useful in understanding TFV interrelationship with TFV-DP and in simulation of dosing regimens, but the absolute concentrations in this terminal gamma tail are very low. We speculate that the deep compartment associated with this gamma phase is TFV trapped within cells as TFV-DP that is dephosphorylated either before or after cell turnover and released back into the circulation.
The relatively long 48-h PBMC TFV-DP half-life is far shorter than median estimates of the terminal half-life of PBMC TFV-DP in several prior reports in HIV-infected patients, which range from to 150 h to 180 h.17–19 This may be due to differing intracellular phosphorylation rates or cell turnover between healthy and HIV-infected populations. The biphasic peak and delayed 96 h to peak concentration indicate complicated intracellular phosphorylation. This pattern is, however, consistent with another single-dose study we reported.20 We did not measure TDF, TFV, or TFV-MP concentrations in the cells, but intracellular phosphorylation kinetics and complexities in TFV and TDF cellular uptake may contribute to the complex and variable intracellular kinetics. Careful assessment of these variables would be helpful for physiologically based PK modeling with these data, which is essential for their optimal application to future study planning through simulation.
In terms of absolute concentrations, PBMC TFV-DP concentrations at 24 h (10 fmol/million cells) and at each of two observed peaks (20 fmol/million cells) from this study were well below those measured in HIV-infected patients at steady-state on TDF-containing regimens for which median trough estimates range from 85 fmol/million cells to 223 fmol/million cells among different reports.17–19 We would expect a significantly higher TFV-DP concentration at steady-state due to accumulation of TFV-DP with its long half-life. However, a PrEP study using daily oral tenofovir in healthy women recently reported median peak steady-state PBMC TFV-DP concentrations of only 49–51 fmol/million cells, which is well below values in HIV patients.14 Taken together, these studies in healthy women indicate a shorter half-life and less accumulation of PBMC TFV-DP than in HIV patients. Either poor adherence, individual PK differences between study populations, or saturable TFV-DP kinetics are potential explanations for the difference between treatment and prevention studies. Dose-escalation studies using directly observed dosing will be helpful in teasing out potential explanations.
In the PrEP context, the gradual rise to the initial PBMC TFV-DP peak, occurring between 8 h and 16 h, is highly relevant, though itself insufficient, for choosing the timing of coitally dependent dosing regimens where the dose precedes sex and potential HIV exposure. The lead time needed to prevent HIV infection depends, first, on the critical concentration target needed to prevent HIV replication. This critical concentration may be achieved at some point earlier in the initial rise or, possibly, even the peak concentrations achieved with a single dose could be inadequate for the task. Second, the critical location for this HIV inhibiting concentration is not in the accessible PBMCs, but, more likely, in the tissues. While we did not biopsy tissue as early or frequently as blood, one might anticipate that the initial 24-h time course in a well-perfused colon or vaginal tissue compartment would have a slightly delayed Tmax relative to simultaneous concentrations in blood. However, our data indicate very different TFV-DP concentrations at 24 h, indicative of greatly differing rates of intracellular TFV phosphorylation followed by very different rates of dephosphorylation or cell turnover.
In contrast to coitally dependent dosing, the long rise, sustained peak, and 48-h terminal half-life obtained with daily dosing predict a flat concentration–time course at steady-state for both PBMCs and tissue as has been reported previously in a steady-state oral and vaginal dosing study in healthy women.14 If the frequency of dosing is to be decreased in an attempt to simplify the regimen or reduce costs, then our data should prove useful for performing model-based simulations of potential dosing regimens. However, the critical protective concentration of TFV-DP is still needed to understand the limits of less than daily dosing. PK data from completed PrEP RCTs can guide these decisions.
One significant variable in such PrEP planning is the site of HIV infection risk, whether the rectosigmoid colon or vagina. Our measured TFV and TFV-DP concentrations in colon tissue were 34 times and 124 times higher, respectively, than in vaginal tissue homogenates 24 h after dosing in the same woman. This finding is very similar to the report by Patterson et al., which reported TFV-DP in colon tissue homogenates in men to be a mean 126-fold higher compared to vaginal tissue concentrations in women 24 h after dosing.16 This confirms that the colonic:vaginal tissue gradient reported in Patterson et al. was not due to sexual differences in the men (colon samples) and women (vaginal samples) sampled.16 Importantly, this large colonic:vaginal tissue homogenate gradient was not sustained in our study or in the data presented by Patterson et al.16
Turning from tissue homogenates to intact cells extracted from tissue, we saw lower and less consistent gradients when looking at total cells and CD4 cells extracted from tissue biopsies. In fact, the total cell colonic:vaginal TFV-DP ratio was at or below one at all observation times. Due to small cell yields from only a few vaginal biopsies, we had complete CD4 cell ratios in only two women—too few to draw important conclusions. Both the magnitude and temporal pattern of colon and vaginal TFV-DP concentrations are important for study planning for less than daily dosing regimens. With daily dosing, however, the large colonic:vaginal tissue TFV-DP ratio seen at 24 h in homogenates, consistent with the time of daily concentration troughs, is likely maintained both within and between subjects. The reason for the discordance between homogenate and extracted cell value, however, is not clear. Substantial variability in cellular type and content of vaginal biopsies may introduce significant variability to cell extraction results.
If we assume that similar concentrations are required for HIV protection in colon tissue and vaginal tissue, our findings and those of Patterson et al.16 suggest a PrEP advantage for protection from receptive anal intercourse (RAI) compared to receptive vaginal intercourse (RVI).16 These colonic:vaginal differences (in tissue homogenates and only at one day) may partly explain the contrast between a 44% relative risk reduction in the iPrEx study of daily TDF/FTC in MSM compared to the absence of benefit in the FEM-PREP study of daily TDF/FTC in women.
The other critically influential variable, adherence, was roughly similar in both studies as indicated by similar low plasma TFV concentrations at or below 10 ng/ml.3,4 However, this kind of interpretation must be viewed cautiously as RAI transmission risk per exposure is estimated to be greater than RVI risk based on epidemiological data, which at least partially off-sets a colonic tissue TFV-DP advantage. Furthermore, Garcia-Lerma et al. reported that dATP, the natural substrate with which TFV-DP competes for HIV reverse transcriptase binding, is found at far higher concentrations in macaque colon tissue compared to vaginal tissue, positing another factor that may offset the TFV-DP colon advantage.21
Our study was limited by the number of observations made in the mucosal compartments—only three samples spanning two weeks—so Cmax and Tmax estimates are far less precise than that of plasma and PBMCs, which were sampled, at most, every 4 h during the first day. Due to total blood volume and tissue biopsy limitations, even with AMS sensitivity, TFV and TFV-DP were not measurable in all matrices for all subjects at 2 weeks, thus reducing the ability to confidently estimate terminal half-life (with three declining concentrations) in several vaginal matrices. In sensitivity analyses, the median half-life estimates were little different regardless of whether we used an imputed value to provide the terminal value or not. In correlating TFV-DP AMS with UPLC-MS/MS, the coefficient of determination was not high, indicating substantial between method variability, though the relationship between AMS and LC-MS/MS was linearly proportional with slope near unity. Finally, because we used the same single dose in all subjects to minimize the radiation exposure to 14C-TDF, a dose-ranging study is needed to confirm dose proportionality to rule out saturable TFV-DP kinetics when simulating multiple dose regimens.
In conclusion, this study provides the most intensive observations of the complex PBMC and blood CD4 cell TFV-DP concentration–time course in the first days following dosing and combines them with 2 weeks of continued observation in blood as well as tissue homogenates and cells from paired colon and vaginal mucosal sites. The delayed time-to-peak concentration of TFV-DP in PBMCs and CD4 cells raises concerns about the practicality of coitally dependent dosing strategies for oral PrEP regimens. We provide the first estimates of paired colonic:vaginal tissue and intracellular CD4+ cell TFV-DP concentrations that provide interpretive value in recently reported oral PrEP studies of men and women. This pairing of TFV and TFV-DP concentrations in blood, colon, and vagina, is useful for (1) estimating active drug, TFV-DP, concentrations in blood and tissue within PrEP outcome trials through linkage to plasma TFV concentrations reported in those trials and (2) modeling and simulation of alternative TDF-containing PrEP regimens for future studies.
Supplementary Material
Acknowledgments
We acknowledge the essential contributions of the women who volunteered to participate in this complex study. This study was supported in part by grant UL1 RR 025005 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH.
Author Disclosure Statement
Gilead Sciences partially funded this study through a contract with Johns Hopkins University. Gilead Sciences, Inc., also markets the TDF-containing products Viread, Truvada, and Stribild.
References
- 1.Baeten JM. Donnell D. Ndase P. Mugo NR. Campbell JD, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–401. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thigpen MC. Kebaabetswe PM. Paxton LA. Smith DK. Rose CE, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 3.Grant RM. Lama JR. Anderson PL. McMahan V. Liu AY, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Damme L. Corneli A. Ahmed K. Agot K. Lombaard J, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MTN. Network MT; 2011. MTN Statement on Decision to Discontinue Use of Oral Tenofovir Tablets in VOICE, a Major HIV Prevention Study in Women. Web site article. [Google Scholar]
- 6.Abdool Karim Q. Abdool Karim SS. Frohlich JA. Grobler AC. Baxter C, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendrix CW. The clinical pharmacology of antiretrovirals for HIV prevention. Curr Opin HIV AIDS. 2012;7:498–504. doi: 10.1097/COH.0b013e32835847ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel JS. Turteltaub KW. Accelerator mass spectrometry as a bioanalytical tool for nutritional research. Adv Exp Med Biol. 1998;445:397–410. doi: 10.1007/978-1-4899-1959-5_25. [DOI] [PubMed] [Google Scholar]
- 9.Louissaint NA. Nimmagadda S. Fuchs EJ. Bakshi RP. Cao YJ, et al. Distribution of cell-free and cell-associated HIV surrogates in the colon after simulated receptive anal intercourse in men who have sex with men. J Acquir Immune Defic Syndr. 2012;59:10–17. doi: 10.1097/QAI.0b013e3182373b5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs EJ. Lee LA. Torbenson MS. Parsons TL. Bakshi RP, et al. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: Potential implication for HIV transmission. J Infect Dis. 2007;195:703–710. doi: 10.1086/511279. [DOI] [PubMed] [Google Scholar]
- 11.Louissaint NA. Fuchs EJ. Bakshi RP. Nimmagadda S. Du Y, et al. Distribution of cell-free and cell-associated HIV surrogates in the female genital tract after simulated vaginal intercourse. J Infect Dis. 2012;205:725–732. doi: 10.1093/infdis/jir841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe K. Liberman RG. Skipper PL. Tannenbaum SR. Guengerich FP. Analysis of DNA adducts formed in vivo in rats and mice from 1,2-dibromoethane, 1,2-dichloroethane, dibromomethane, and dichloromethane using HPLC/accelerator mass spectrometry and relevance to risk estimates. Chem Res Toxicol. 2007;20:1594–1600. doi: 10.1021/tx700125p. [DOI] [PubMed] [Google Scholar]
- 13.Hillier SM. Marquis JC. Zayas B. Wishnok JS. Liberman RG, et al. DNA adducts formed by a novel antitumor agent 11beta-dichloro in vitro and in vivo. Mol Cancer Ther. 2006;5:977–984. doi: 10.1158/1535-7163.MCT-05-0464. [DOI] [PubMed] [Google Scholar]
- 14.Hendrix CW. Chen BA. Guddera V. Hoesley C. Justman J, et al. MTN-001: Randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLOS One. 2013;8:e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King T. Bushman L. Kiser J. Anderson PL. Ray M, et al. Liquid chromatography-tandem mass spectrometric determination of tenofovir-diphosphate in human peripheral blood mononuclear cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;843:147–156. doi: 10.1016/j.jchromb.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 16.Patterson KB. Prince HA. Kraft E. Jenkins AJ. Shaheen NJ, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: Implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112re114. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins T. Veikley W. St Claire RL., 3rd Guyer B. Clark N, et al. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J Acquir Immune Defic Syndr. 2005;39:406–411. doi: 10.1097/01.qai.0000167155.44980.e8. [DOI] [PubMed] [Google Scholar]
- 18.Pruvost A. Negredo E. Benech H. Theodoro F. Puig J, et al. Measurement of intracellular didanosine and tenofovir phosphorylated metabolites and possible interaction of the two drugs in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2005;49:1907–1914. doi: 10.1128/AAC.49.5.1907-1914.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pruvost A. Negredo E. Theodoro F. Puig J. Levi M, et al. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): Investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob Agents Chemother. 2009;53:1937–1943. doi: 10.1128/AAC.01064-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J. Flexner C. Liberman RG. Skipper PL. Louissaint NA, et al. Biphasic elimination of tenofovir diphosphate and nonlinear pharmacokinetics of zidovudine triphosphate in a microdosing study. J Acquir Immune Defic Syndr. 2012;61:593–599. doi: 10.1097/QAI.0b013e3182717c98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Lerma JG. Otten RA. Qari SH. Jackson E. Cong ME, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5:e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.