Abstract
Purpose
To identify local retinal abnormalities and evaluate the nature and extent of retinal dysfunction in diabetics using full field electroretinogram (ERG) and multifocal ERG (MF-ERG) and to determine the correlation between features of optical coherence tomography (OCT) and MF-ERG.
Methods
Twenty-eight normal subjects (Control Group; 56 eyes) and 37 patients (72 eyes) with diabetes mellitus (DM Group) were evaluated. In the DM Group, 17 eyes had no retinopathy (grade 1), 18 eyes had early non proliferative diabetic retinopathy (NPDR) (grade 3), 16 eyes had late NPDR (grade 4), 21 eyes had proliferative diabetic retinopathy (PDR) (grade 5). Full field ERG and MF-ERG, were used to assess the effects of diabetic retinopathy on retinal function. OCT and fluorescein angiography were used to assess and compare morphological changes with functional changes in diabetes mellitus.
Results
In diabetic patients without retinopathy (17 eyes), the amplitudes of the second order component of MF-ERG were reduced and implicit times were delayed, while only implicit times of first order component of MF-ERG were delayed but the amplitudes of first order component were normal. In diabetic patients with retinopathy (55 eyes), the overall amplitudes were reduced and peak implicit time increased in the first order component and second order component.
OCT of the DM Group showed the fovea of eyes with edema were thicker than the Normal Group. The fovea of eyes with cystoid macular edema (CME) were significantly thicker than the fovea of eyes with diffuse swelling. The implicit times of MF-ERG were directly correlated with foveal thickness.
Conclusion
MF-ERG reveals local retinal dysfunction in diabetic patients. MF-ERG offers the advantage of topographic mapping of retinal dysfunction. The magnitude of delay of MF-ERG implicit time reflects the degree of local clinical abnormalities in eyes with retinopathy. Local response delays found in eyes without retinopathy detects subclinical local retinal dysfunction in diabetics. The combination of OCT and MF-ERG may provide objective criteria for evaluation and assessment of diabetic retinopathy.
Keywords: Electroretinogram (ERG), Diabetes mellitus (DM), Optical coherence tomography (OCT)
Introduction
Diabetic retinopathy is a leading cause of blindness among individuals in the working age group in the United States.1 Visual loss is generally irreversible once non perfusion regions, neovascularization or both are clearly identified by ophthalmoscopy and fluorescein angiography.2
The evaluation of the functional properties of the diabetic retina with objective methods such as electroretinogram (ERG) is an important aspect of the diagnostic and therapeutic approach to diabetic retinopathy.3 The full field ERG has been used as an objective tool to detect alterations of retinal function during early stages of diabetes mellitus,4 to predict progression of diabetic retinopathy,5 and to monitor treatment effects.6 However the sensitivity of full-field ERG is limited, because it reflects the activity of the entire retina. Even advanced disease, if confined to small discrete patches can remain undetected by full field ERG.7
In diabetes mellitus, the earliest clinical changes are typically confined to the posterior pole.8 Therefore, the ability to measure local ERG in diabetes mellitus would improve objective detection of early functional alterations and assessment of local changes over time. Focal ERG has been used to evaluate retinal function within the macula (central 3–10°) in diabetic patients.9 However, the technique is too time consuming to allow testing of even a few retinal areas during one session. In contrast, MF-ERG was developed by Sutter and Tran10 enables assessment of up to hundreds of distinct retinal regions within approximately 8 min per eye.11 MF-ERG is an objective technique for mapping retinal function.
Functional abnormalities preceding retinal disease and changes associated with retinal disease have been studied with MF-ERG, an objective technique for mapping retinal function.11,12
The purpose of this study was to explore the efficacy of MF-ERG in detecting and localizing dysfunctional retinal areas in diabetes and to compare the features of optical coherence tomography (OCT) and MF-ERG outcomes.
Methods
Subjects for this study were enrolled from patients presenting to the outpatient clinic of Mansoura Ophthalmic Center from January 2009 to May 2010. Subjects were enrolled into one of two groups: 28 normal subjects (Control Group) and; 37 diabetic patients (DM Group). All eyes had 20/40 or better corrected visual acuity with refractive error between −1.00 D and +1.00 D. Patients with visible media opacities or a history of other ocular disease or surgery were excluded from the study.
The level of diabetic retinopathy and degree of macular edema were diagnosed by dilated eye examination, slit lamp biomicroscopy, direct and indirect ophthalmoscopy, and photography and fluorescein angiography).
All subjects underwent full ophthalmic examination, full field ERG, MF-ERG and OCT. All control subjects had no known abnormalities of the visual system, normal findings in ophthalmic examination, normal full field ERG, normal MF-ERG and normal OCT features.
This study was approved by the Human Subjects Committee of the University of Mansoura, and adhered to the Declaration of Helsinki. The purpose and potential risks of the study were explained to the subjects and written informed consent was obtained from all participants.
ERG
Full field ERG and MF-ERG were recorded using Roland Consult (Roland Consult, Elektrophysiologische Diagnostik Systeme, Brandenburg, Germany). Pupils were fully dilated (⩾7 mm) using 1% tropicamide and 2.5% phenylephrine. Before placing electrodes, the skin was cleansed. Topical corneal anesthesia (Benoxinate hydrochloride 4%) was instilled and a Dawson, Trick and Litzkow (DTL) electrode (positive electrode) was placed contacting corneal limbus, a ground electrode was placed on the forehead and a negative electrode was placed near the orbital rim. The electrodes were placed under dim red light and after dark adaption for 20 min. The recording was monocular and the contralateral eye was occluded with light pressure to suppress blinking.
Full field ERG
Prior to beginning the patients had to in a comfortable body position, the head was still before stimulation and the eyes fixated on red light within the Ganzfeld globe. Subsequently the test was started and recorded in 5 steps: scotopic rod response, scotopic combined response, oscillatory potential then light adaptation for 10 min then photopic cone response and flicker response recording.
MF-ERG
Patients were positioned 30 cm from the stimulus monitor. Stimulus clarity was optimized by overrefraction and the final adjustment of the test distance was made to maintain constant stimulus magnification. The stimulus was presented on a 32 cm × 22 cm monitor driven at a 75 Hz frame rate and consisted of an array of 61 hexagonal elements across a field subtending 44° horizontally and 40° vertically. White hexagons had a luminance of 185–200 cd/m2 and dark frames were 1–2 cd/m2 resulting in local contrast of 98–99%. Each hexagon was temporally modulated between light and dark according to a binary m-squence.10 At any time, approximately 50% of the stimulus elements displayed were white and 50% were black. Observers fixated on a small gray spot in the center of the stimulus during 8-min recording sessions. To improve fixation stability, the sessions were divided into 30 s segments with brief rest periods between each segment. Signals were amplified (gain, 106), band pass filtered (10–300 Hz) and recorded at 16 samples per display frame. Two recordings were obtained from each eye per stimulus condition. Recording segments contain two or more amplifier saturating artifacts were discarded.
The results of two 8-min recordings were averaged to improve the signal to noise ratio. An artifact elimination technique described by Sutter and Tran was applied twice.10 For m-sequence stimulation, local first and second order components can be derived by means of a signal cross correlation between m-sequence stimulation and the response.
The first and second order components were examined. For each stimulus area, the first order component is the difference between the mean response to all white frames in the sequence and the mean response to all black frames (Fig. 1). It can be described as the mean local response to all flashes occurring in a stimulus cycle.
Figure 1.

(A) Patient with ERG connection; (B) patient on Ganzfeld stimulator; and (C) patient in front of MF-ERG.
The second order component response represents the temporal interaction between two focal flashes (white-frames) separated by an integral number of stimulus base intervals. The amplitudes and implicit times of first and second orders were analyzed using a computer program similar to that described by Hood and Li.13
Amplitude was calculated as the voltage difference between first trough and the first peak of the scaled template wave. Implicit time was measured to the first prominent response peak of the scaled template wave. This method of determining response amplitude and implicit time is reliable.13–15
Grading system for local characteristics of retinopathy
Stereoscopic 30° color fundus photographs were taken in each eye. The severity of diabetic retinopathy was classified according to the Diabetic Retinopathy Study (DRS)16 as follows: Grade 1, indicates absence of diabetic retinopathy; Grade 2, only microaneurysms (MAs) present (MAs definitely other characteristics absent); Grade 3, mild to moderate non-proliferative diabetic retinopathy (NPDR) (MAs definitely present and any one or more of the following: venous loops, hemorrhage, hard exudates or soft exudates definitely present or soft exudates, intra-retinal micro-vascular abnormalities (IRMA) or venous beading questionably present); Grade 4, moderate to severe NPDR (moderate to severe hemorrhages, MAs or IRMA definitely present); Grade 5 proliferative diabetic retinopathy (PDR). Fluorescein angiography was used to evaluate the presence and extent of leakage.
Optical coherence tomography
OCT was performed with the 3-dimensional OCT-1000 (Topcon Corp., Tokyo, Japan). OCT measurements were performed using a fiberoptic optically integrated Michelson interferometer with short coherence length superluminescent diode.
Subjects underwent (6) linear scans spaced (30) over the macula for measurements total retinal thickness. Internal fixation (subject fixated with the eye being tested) was chosen due to better reproducibility in our experience.
The macular thickness map was divided into nine sections and it was displayed as three concentric circles including a central circle, an inner ring and outer ring, with each ring divided into four quadrants: superior, inferior, nasal, and temporal. The central 1 mm circular region represents the foveal region. The location of the vitreo–retinal interface and the retinal pigment epithelium defined the inner and outer boundaries respectively of the retina. These two boundaries were associated with the sharpest edge in each OCT scan because of the high contrast in optical reflectively between the relative non-reflective vitreous and reflective neurosensory retina and between the minimally reflective outer segments of the photoreceptors and the highly reflective retinal pigment-choriocapillaris complex layer. A good quality scan was defined as one with signal to noise ratio of more than (50 dB). Subjects with scans that did not meet the criteria of good quality scan after three attempts were excluded.
Statistical analysis
Data were analyzed using SPSS version 10 (IBM Corp., NY, USA). Quantitative data are presented as mean ± standard deviation (SD). Chi square and test of significance were used for comparison between groups. Spearman’s correlation variables, R ⩾ 0.5 indicate good correlation, P ⩽ 0.01 indicated statistical significance.
Results
A total of 128 eyes from 65 subjects were included in the study. On ophthalmoscopic examination, 9 diabetic patients showed no clinical signs of retinopathy whereas the remaining 28 had diabetic retinopathy. Both eyes of each patient were examined except two diabetic patient in whom the other eye had to be excluded because of cloudy media (1 eye) and because of previous surgery (1 eye). Demographics are included in Table 1.
Table 1.
Demographic features among groups.
| Number | Age (years) | Sex |
||
|---|---|---|---|---|
| Female | Male | |||
| Normal control group | 28 patients (56 eyes) | 30–60 (45 ± 7) | 10 | 18 |
| Diabetic group | 37 patients (72 eyes) | 30–65 (44 ± 10) | 18 | 19 |
| Grade 1 | 17 eyes | 32–50 | 4 | 5 |
| Grade 2 | 0 | – | – | – |
| Grade 3 | 18 eyes | 30–55 | 5 | 4 |
| Grade 4 | 16 eyes | 40–60 | 4 | 4 |
| Grade 5 | 21 eyes | 35–65 | 5 | 6 |
First order component of MF-ERG
In normal arrays, the responses were equal in height. To compare, the wave forms between control and diabetic patients, the responses were average across all 61 local responses. Peak implicit times varied more among diabetic subjects than among control subjects. In control subjects the mean implicit times were (44 ± 1.0 ms) for the first positive peak (p. wave) and the mean amplitude was (48 ± 10 nv) per degree squared. In diabetic patients without retinopathy, mean latency of the first positive peak (49 ± 2.0 ms) was significantly increased (P ⩽ 0.01), the mean amplitude (mean 47 ± 11 nv) per degree squared did not differ significantly from the Control Group (P > 0.01). In patients with clinically apparent diabetic retinopathy, the latency of first positive peak was significantly increased (P ⩽ 0.01). The amplitudes of first order component were also significantly reduced per degree square (Table 2, Fig. 2).
Table 2.
First order component responses of MF-ERG among groups.
| P wave latency in millisecond (ms) | P wave amplitude in nanovolt (nv) | |
|---|---|---|
| Control group | 44 ± 1.0 | 48 ± 10 |
| P = 0.001 | P = 0.002 | |
| Diabetic group | P = 0.006 | P = 004 |
| Grade 1 | 49 ± 2.0 | 47 ± 11 |
| Grade 3 | 51 ± 1.5 | 25 ± 5.0 |
| Grade 4 | 53 ± 2.0 | 24 ± 7.0 |
| Grade 5 | 60 ± 3.50 | 20 ± 10.0 |
Figure 2.
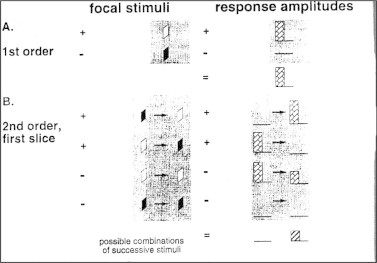
Stimulus of first and second order of MF-ERG.
First order wave forms are known to vary mainly as a function of eccentricity. Hence, responses were averaged over concentric rings around the fovea for more accurate comparison of these parameters. The results in Table 2 held true for all eccentricities and also when first order responses were averaged over retinal quadrants (Figs. 3–5).
Figure 3.
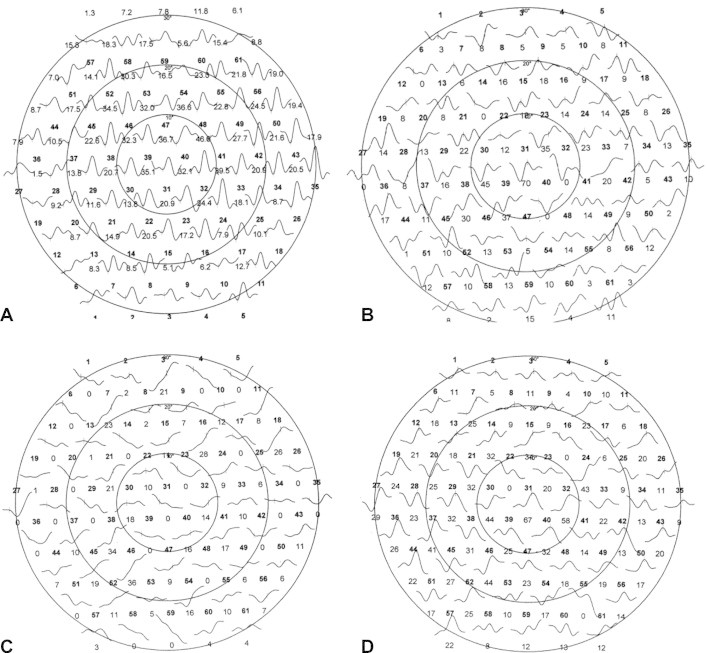
First order component of MF-ERG among groups. (A) MF-ERG in control group; (B) MF-ERG in diabetic eyes with mild retinopathy in which there is mild reduction of amplitude & delay in latency; (C) MF-ERG in late retinopathy in which no apparent peak and through; and (D) MF-ERG in diabetic subject without retinopathy (just slight delay).
Figure 4.
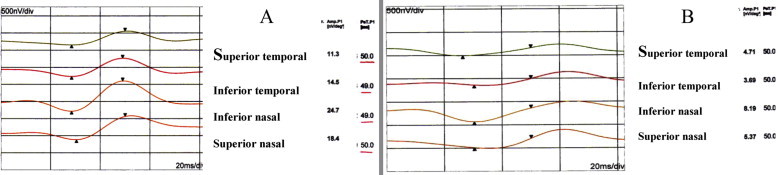
First order component of MF-ERG in diabetes. (A) Without retinopathy; and (B) with retinopathy.
Figure 5.
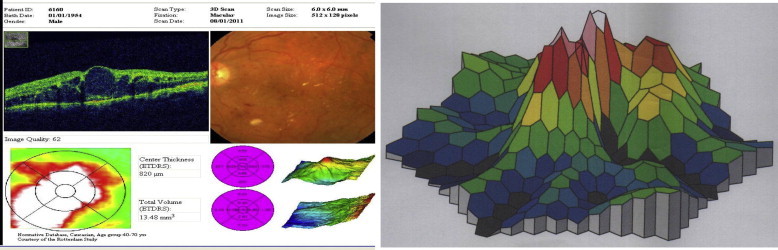
OCT in case with cystoid macula edema in which focal areas of hypo reflectivity corresponding to CMO and; three dimensional MF-ERG CMO showing no foveal peak.
There was very low inter-individual variability of implicit time. Also, the intra-individual variability of local response implicit time was small. Implicit time varied slightly with eccentricity. In contrast, local response amplitudes varied to a much greater extent both between and within normal eyes. It is important to note the hexagonal stimuli are scaled with retinal eccentricity to produce an approximately equal amplitude response throughout the array.
The amplitudes of the MF-ERG in eyes with diabetic retinopathy were reduced relative to normal across most of the fundus. Both ophthamloscopically normal and abnormal regions within eyes with diabetic retinopathy produced MF-ERG that were delayed relative to normal. The increased local severity of retinopathy was associated with increased delay of implicit time. No association between local MF-ERG amplitude and retinopathy grade was apparent.
Second order component of MF-ERG
The beginning of each trace of second order response component is time locked to the beginning of the second of the two stimuli defining the second order response component. The mean implicit times of the control group was (38 ± 0.9 ms) for first peak. The mean amplitude was (34 ± 4 nv). In diabetic patients without retinopathy the mean implicit times (40 ± 3.02 ms) were significantly delayed (P ⩽ 0.01). The mean amplitude were (28 ± 6 nv) significantly decreased (P ⩽ 0.01). In diabetic patients with retinopathy, the mean implicit times were significantly markedly delayed (P ⩽ 0.01). The amplitude was significantly decreased (P ⩽ 0.01) (Fig. 6, Table 3).
Figure 6.
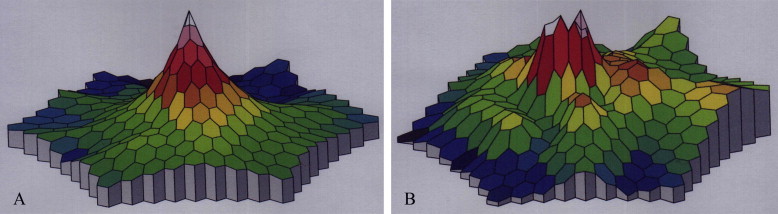
Three dimensional MF-ERG in diabetes. (A) Without retinopathy (in which there is foveal peak with dark areas at periphery corresponding to affected areas); and (B) with retinopathy (in which no foveal peak with areas of decreased response corresponding to affected areas).
Table 3.
Second order responses component of MF-ERG (P = 0.001).
| P wave latency (ms) | P wave amplitude nanovolt (nv) | |
|---|---|---|
| Control subjects | 38 ± 0.9 | 34 ± 4 |
| Diabetic patients | ||
| Grade 1 | 40 ± 2.0 | 28 ± 6 |
| Grade 3 | 46 ± 2.5 | 20 ± 4.5 |
| Grade 4 | 50 ± 2.8 | 19.9 ± 5.7 |
| Grade 5 | 55 ± 3.5 | 18.0 ± 9.00 |
Full field ERG
According to current ISCEV standard, five major ERG responses are defined: rod response, maximal combined response and, oscillatory potential (OPs), cone response and 30-Hz flicker response. The b-wave is a large positive ERG potential present in rod response, the maximal combined response and photopic response.
The amplitude of the b-wave is measured from trough to peak. Implicit time is measured from flash onset to the peak of the b-wave.
In the Control Group, the scotopic b-wave amplitude was 75 ± 13 nv, the combined response b-wave amplitude was 200 ± 30 nv and photopic b-wave amplitude was 45 ± 9 nv. The implicit time of the Ops waves were 23 ± 5 ms and amplitude of Ops waves were 27 ± 3 nv. The implicit time of flicker response were 50 ± 5 ms and the amplitude were 55 ± 6 nv (Table 4, Fig. 7).
Table 4.
Full field ERG parameters among groups.
| Control | Diabetic without retinopathy | Diabetic with retinopathy | |
|---|---|---|---|
| Scotopic b-wave amplitude | 75 ± 13 nv | 72 ± 10 nv | 45 ± 20 nv |
| Combined b-wave amplitude | 200 ± 30 nv | 177 ± 35 nv | 100 ± 50 nv |
| Ops | |||
| Implicit time | 23 ± 5 ms | 27 ± 7 ms | 35 ± 10 ms |
| Amplitude | 27 ± 3 nv | 18 ± 2 nv | 8 ± 5 nv |
| Photopic b-wave amplitude | 45 ± 9 nv | 43 ± 5 nv | 26 ± 12 nv |
| 30 Hz flicker | |||
| Implicit time | 50 ± 5 ms | 51 ± 7 ms | 70 ± 10 ms |
| Amplitude | 55 ± 6 nv | 50 ± 10 nv | 30 ± 15 nv |
Figure 7.
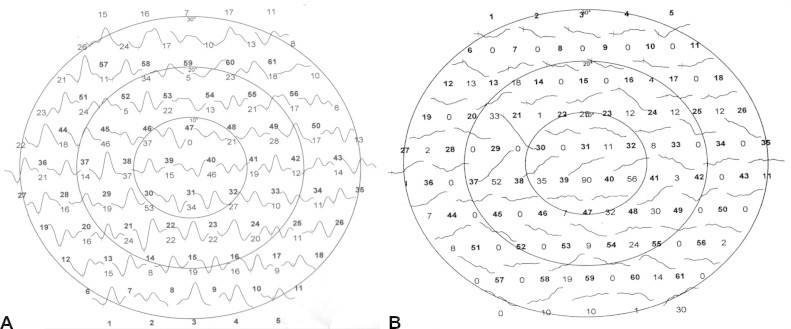
Second order component of MF-ERG in diabetes. (A) Without retinopathy; and (B) with late retinopathy (just irregular line with no apparent peak through).
In subjects with diabetic retinopathy, there were statistically significant delays in Ops implicit time and reduction Ops amplitude in all cases (P ⩽ 0.001, all tests). The severity of diabetic retinopathy correlate with the Ops amplitude (R = 0.66, P = 0.001). A significant reduction of scotopic and photopic b-wave amplitude, 30 Hz flicker amplitude were found in these patients (P ⩽ 0.001, all tests). In diabetic patients without retinopathy, there was a significant reduction in Ops amplitude (P ⩽ 0.001) and a significant increase (P ⩽ 0.001) in Ops implicit time while other parameters (photopic –b-wave, scotopic b-wave, 30 Hz flicker amplitude and implicit times) were normal (see Fig. 8).
Figure 8.
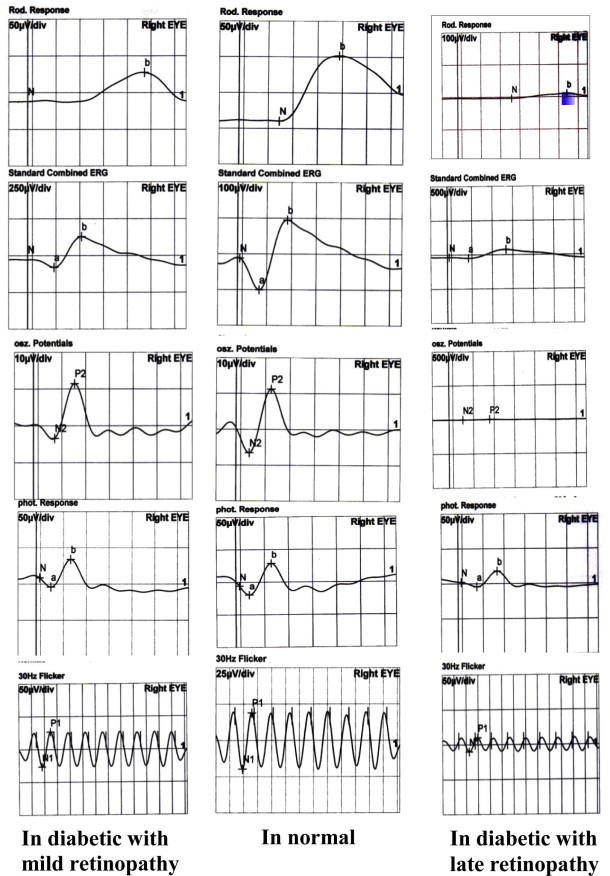
Full field ERG among groups.
Optical coherence tomography images showed cystoid macular edema in 10 eyes and diffuse edema in 30 eyes. OCT showed an increase in foveal thickness concomitant with a clinical increase in macular edema. The implicit times of MF-ERG were directly correlated with foveal thickness (R = 0.55, P = 0.002) while MF-ERG amplitude and foveal thickness were inversely correlated (R = −0.5, P = 0.001) in cases of diabetic edema. In patients without edema there was no correlation between foveal thickness and MF-ERG parameters (R = 0.3, P = 0.1 for implicit time, R = 0.22, P = 0.01 for amplitude).
Discussion
Ocular complications due to diabetes mellitus are a major cause of irreversible vision loss in adults in the United States.2 An objective test that can detect retinal function prior the appearance of diabetic retinopathy, thereby identifying individuals and retinal locations at risk for development of retinopathy can reduce visual morbidity.
MF–ERG technique can measure and map retinal functions at more than 60 locations within 8 min and can distinguish separate responses of inner and outer retina. MF-ERG has been used to examine a large number of eye diseases including diabetes related retinal function.17
Several reports have provided information regarding retinal dysfunction associated with DM. Investigators who have analyzed MF-ERG have reported abnormally reduced amplitudes and/or delayed implicit times in diabetic subjects18 and in diabetic subjects without signs of retinopathy.19,20 The outcomes of the current study concurs with previous reports. For example, there were reductions in the amplitudes of first order and second order component responses and delays in implicit times in patients with diabetic retinopathy and macular edema. Farahvash and Mohammed Zadoh21 reported that local ERG responses were significantly delayed and decreased in amplitude in patients with significant diabetic edema. Similarly, Fortune et al.22 reported that implicit times were increased and amplitudes were mildly reduced.
Greenstein et al.23 found that implicit times were significantly increased. Weiner et al.24 have reported that mean amplitude of ERG was lower in eyes without diabetic edema compared to normal eyes and was even lower in eyes with edema.
The magnitude of MF-ERG implicit time delays was correlated with the severity of retinopathy. By contrast, response amplitude although reduced in eyes with retinopathy had no such correlation with the degree of retinopathy in this study. The increased implicit time of local ERG responses were associated with increased severity of the local retinopathy signs.22 Bearse et al.25 and Han et al.26 found no correlation between amplitude reduction and retinal abnormalities.
ERG abnormalities can be present at a very early stage of disease while there are no visible changes in the fundus and before the onset of clinical symptoms. In this study, there were delays in implicit times without reduction of amplitude in the first order component, however the second order component response was reduced in amplitude and there was an increase in implicit times in diabetic patients without retinopathy. Fortune et al.22 reported that the delay in implicit times were not only in retinal regions manifesting abnormalities but also significantly increased in regions that appeared to be free of fundus abnormalities on fluorescein angiography. Weiner et al.24 found that the mean implicit time was significantly delayed in eyes with edema while in eyes without edema, implicit times were the same as normal eyes. Fortune et al.22 found that implicit times are often significantly delayed in retinal location without retinopathy and in diabetic eyes without retinopathy. Han et al.26 reported similar results of abnormal implicit times in diabetic patients without retinopathy.
The cause of the reduction of amplitude of second order while the amplitude of first order was normal in early diabetes is likely because the first order response components originate predominantly in the outer (cone photoreceptors) retina and/or middle retina (cone bipolar cells, Muller cells). Sutter and Tran10 showed that under photopic conditions, the decreased first order response component with eccentricity follows approximately that of retinal cone density. In addition, when all the focal responses of the multifocal cone ERG are averaged together, the response bears a strong similarity to the full field flash ERG. Flash ERG responses originate predominantly in the outer 70% of the retina.10 The reduced overall amplitudes and the delayed latencies in the first order component observed in diabetic patients with retinopathy may indicate same impairment of outer retinal function in diabetes, although the second order component of the MF-ERG contains outer retinal contributions, it appears to have substantial contributions from sources in the inner retina and also from the optic nerve head. A response originating from nerve fibers near the location of the optic nerve head can be extracted from the second order component of MF-ERG. The reduction of amplitude of second order component of MF-ERG in diabetic eyes without retinopathy are consistent with the previous suggestion that early diabetes causes a dysfunction within the inner retina before photoreceptors are affected.27,28
The cause of MF-ERG delays associated with and even occurring before the diabetic lesion provide some insights of the pathophysiology of the effects of diabetes on the retina. Diabetic retinopathy is a disease of small retinal vessels, prior to the appearance of visible fundus lesions and early characteristic changes in the retinal vasculature of diabetic eyes there is pericyte apoptosis and basement membrane thickening resulting in acellular capillaries.29
Three major theories have been proposed to explain how chronic hyperglycemia and subsequent retinal hypoxia might lead to these anatomic changes: increased formation of advanced glycosylation end products;30 abnormal bypass of growth metabolism through the sorbitol pathway and; activation of growth factors (such as vascular endothelial growth factor. Oxidative stress and free radical generation also promotes the development of diabetic lesions.31
Compromised local metabolism may affect the function of MF-ERG generators leading to delayed neural conduction and prolonged implicit time. Moreover, in the diabetic eye, early or undetected perfusion defects associated with degeneration of choriocapillaries may also result in implicit time delays that occur before the anatomic signs of abnormal vasculature within the inner retina.32
In contrast to the findings with implicit times, MF-ERG amplitudes changes of first order component response were not associated with early retinopathy. One possible reason for the insensitivity of amplitude to diabetic dysfunction is that this measure has larger inter-subject variability than implicit time in normal subjects.
This large inter-individual variability of amplitude diminishes the usefulness of this parameter for detection of local retinal abnormalities. In contrast, the variability in MF-ERG implicit times was very small, consistent with the findings of other MF-ERG studies.
Another consideration is that amplitude measures reflect the strength of the summed responses generated by retinal cells and may be significantly affected only at a later stage when the generators are severely damaged or cell loss occurs.
Additionally it has been demonstrated that decreased stimuli contrast or luminance affect MF-ERG amplitude to much greater extent than implicit time. Thus it is possible that decreased effective stimulus contrast and/or luminance within patches of retinal abnormalities may be responsible for alteration of local MF-ERG amplitude in diabetic retinopathy (that is absent in diabetic patients without retinopathy).33
Full field Ganzfeld ERG in the current study showed reductions in scotopic and photopic responses in diabetic patients with retinopathy. Reduction in oscillatory potential amplitude and flicker response were also observed in those patients with delayed implicit times. Under scotopic conditions, the wave is directly generated by bipolar cells.34 Under photopic conditions, several types of neurons contribute to the generation of the response.35 Ops are four to six low amplitude, high frequency wavelets superimposed on the ascending limb of ERG b-wave. The Ops are thought to result from feedback between the amacrine cells and the bipolar cell and/or feedback from ganglion cells to amacrine cells.36
Other ERG Studies of diabetic patients have reported inconsistent results. Arden et al.37 found reduced ERG amplitudes of diabetic patients only in the presence of cotton wool spots and angiographic evidence of capillary non-perfusion whereas others, reported normal or even supernormal amplitudes in the flash ERG of diabetic patients with retinopathy. Wanger and Persson38 could not find any flash ERG changes that could distinguish between the presence or absence of retinopathy in diabetic patients.
Reduction in amplitudes of oscillatory potentials have also been reported in diabetic retinopathy.39 However, others did not find any such changes.40
Bresnick and Palta41 and Hood and Birch42 reported that Ops amplitudes correlate well with the severity of diabetic retinopathy. Whereas Chung et al.43 and Satoh et al.5 observed that photopic b-wave implicit times correlate well with severity of diabetic retinopathy. In the current study, there was a statistically significant correlation between oscillatory potential amplitudes and severity of diabetic retinopathy (R = 0.55, P = 0.006) while there was no correlation between severity of diabetic retinopathy and any other parameters of full field ERG (R = 0.2, P = 0.5).
In diabetic patients without retinopathy, there were only changes in oscillatory potentials (reduction in amplitudes and delay in implicit times) while other full field ERG parameters were normal in this study. Similarly, Holopigian et al.4 and Shirao and Ohta44 found that the most consistent alteration in diabetic eyes without retinopathy was a significant increase in the implicit time of the oscillatory potential. Juen and Kieselbach45 and Papakostopulos et al.46 reported a significant reduction in scotopic amplitude in diabetics without retinopathy. Cone amplitude was also reduced in similar these cases.45,46
In the current study, there was correlation between tomographic features of OCT and MF-ERG in cases of diabetic retinopathy. The implicit times of MF-ERG were positively correlated with foveal thickness measured with OCT. The increased foveal thickness was associated with increased implicit times of ERG, while in cases without retinopathy there was no correlation between OCT and MF-ERG parameters. Similarly, Yamamoto et al.47 found that best corrected visual acuity and macular response density of MF-ERG were inversely correlated and implicit times were directly correlated with foveal thickness.
MF-ERG is well suited to the study of diabetic retinopathy for several reasons: Firstly, diabetic retinopathy is a retinal disease with local lesions typically confined to the posterior pole where the MF-ERG techniques test local retinal function (the central 45)5; Secondly, diabetic retinopathy is largely caused by defects of retinal capillaries in the inner nuclear layer where the cell bodies of the bipolar cells, the primary generators of MF-ERG are located. Thus there is an anatomic basis for the detection of MF-ERG abnormality in diabetes.
MF-ERG provide very sensitive objective assessment of local retinal health in diabetes mellitus. MF-ERG implicit time is a sensitive measure of retinal function that can be used to monitor the progression of diabetic retinopathy. It is evident that, MF-ERG is abnormal very early in diabetic retinopathy. MF-ERG can demonstrate local abnormalities while the abnormalities in flash OPs and b-wave demonstrate the widespread nature of retinopathy. ERG has several uses in diabetes including: the ability to discriminate between patients who are more likely to develop retinopathy and patients with a lesser chance of doing so and; use as non-invasive objective measure of the progression during follow up. The combination of MF-ERG and OCT may provide objective criteria for the evaluation and assessment of diabetic edema.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Diabetes Research working Group. Conquering diabetes. A strategic plan for the 21st century. National Institutes of Health; NIH, Publication; 1999. p. 99–4398.
- 2.Early Treatment Diabetic Retinopathy Study (ETDRS) Research Group. Fundus photographic risk factors for progression of diabetic retinopathy: report number 12. Ophthalmology 1991;98:823–33 [quoted from Fortune B, Schneck ME, Adams AJ. Multifocal electroretinogram delays neural local retinal dysfunction in early diabetic retinopathy. Invest ophthalmol Vis Sci 1999;40:2638–57]. [PubMed]
- 3.Tzekov R., Arden G.B. The electroretinogram in diabetic retinopathy. Surv Ophthamol. 1999;44:53–60. doi: 10.1016/s0039-6257(99)00063-6. [DOI] [PubMed] [Google Scholar]
- 4.Holopigian K., Seiple W., Lorenzo M. A comparsion of photopic and scotopic electroretinographic changes in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1992;33:2773–2780. [PubMed] [Google Scholar]
- 5.Satoh S., IiJima H., Imai M. Photopic electroretinogram implicit time in diabetic retinopathy. Jpn J Ophthalmol. 1997;38:178–184. [PubMed] [Google Scholar]
- 6.Bresnick G.H., Palta M. Temporal-aspects of electroretinogram in diabetic retinopathy. Arch Ophthalmol. 1987;105:660–664. doi: 10.1001/archopht.1987.01060050078042. [DOI] [PubMed] [Google Scholar]
- 7.Birch DG. Focal electroretingraphy. In: Heckenlively JR, Arden GB, editors. Principles and practice of clinical electrophysiology of vision. St. Louis: Mosby; 1991. p. 334–38.
- 8.Bresnick KGH. Non proliferative diabetic retinopathy. In: Ryan SJ, editor. Retina, vol. 2. St. Louis: Mosby; 1994. p. 1277–318.
- 9.Dileo M.A., Caputo S., Falisin B. Presence and further development of retinal dysfunction after 3-year follow up in IDDM patients without angiograhically documented vasculopathy. Diabetologia. 1994;37:911–916. doi: 10.1007/BF00400947. [DOI] [PubMed] [Google Scholar]
- 10.Sutter E.E., Tran D. The field topography of ERG components in man – I. The photopic luminance response. Vision Res. 1992;32:433–446. doi: 10.1016/0042-6989(92)90235-b. [DOI] [PubMed] [Google Scholar]
- 11.Bearse M.A., Sutter E.E. Imaging localized retinal dysfunction with multifocal electroretinogram. J Opt Soc Am A. 1996;13:634–640. doi: 10.1364/josaa.13.000634. [DOI] [PubMed] [Google Scholar]
- 12.Hood D.C., Odel H.G., Winn B.J. The multifocal electroretingram. J Neuro Ophthalmol. 2003;23:225–235. doi: 10.1097/00041327-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Hood DC, Li J. A technique for measuring individual multifocal ERG records. In: Yages D, editor. Non invasive assessment of the visual system. Trends in optic and photonics, vol. 11. Washington: Optical Society of America; 1997. p. 280–83.
- 14.Seeligar M.W., Kretschmann U.H., Apfelstedt- Sylla E., Zenner E. Implicit time topography of multifocal electroretingram. Invest Ophthalmol Vis Sci. 1998;39:718–723. [PubMed] [Google Scholar]
- 15.Verdan W.A., Haegerstrom-Portnoy G. Topography of the multifocal electroretinogram. Doc Ophthalmol. 1998;95:73–90. doi: 10.1023/a:1001732613424. [DOI] [PubMed] [Google Scholar]
- 16.Diabetic Retinopathy Study (DRS) research group II: a modification of the Airlee House classification of diabetic retinopthy: DRS report number 7. Invest Ophthalmol Vis Sci 1981;21:210–20. [PubMed]
- 17.Fortune B., Schneck M.E., Adams A.J. Multifocal electroretinogram delays neural local retinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1999;40:2638–2657. [PubMed] [Google Scholar]
- 18.Hood D.C., Seiple W., Holopigien K. A comparison of the components of multifocal and full field ERG. Vis Neuro Sci. 1997;14:533–544. doi: 10.1017/s0952523800012190. [DOI] [PubMed] [Google Scholar]
- 19.Shimada Y., Li Y., Bearse M.A. Assessment of early retinal changes in diabetes using a new multifocal ERG protocol. Br J Ophthalmol. 2001;85:414–419. doi: 10.1136/bjo.85.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomura R., Terasaki H., Hirose H. Blue-on yellow perimetry to evaluate S-cone sensitivity in early diabetics. Ophthalmic Res. 2003;32:69–72. doi: 10.1159/000055592. [DOI] [PubMed] [Google Scholar]
- 21.Farahvash M.S., Mohammed Zadoh S. Multifocal electroretinogram in clinically significant diabetic macular oedema. Arch Iran Med. 2006;9(3):261–265. [PubMed] [Google Scholar]
- 22.Fortune B, Adams AJ, Schneck ME. Ophthalmoscopic and angiographic features of diabetic retinopathy are associated with local ERG response delays. Invest Ophthalmol Vis Sci 1999;40(4):5614.
- 23.Greenstein V.C., Holopigian K., Hood D.C. The nature and extent of retinal dysfunction associated with diabetic macular oedema. Invest Ophthalmol Vis Sci. 2000;41:3643–3654. [PubMed] [Google Scholar]
- 24.Weiner A., Christopoulos V.A., Gusseler C.H. Foveal cone function in non proliferative diabetic retinopathy and macular edema. Invest Ophthalmol Vis Sci. 1997;38:1443–1449. [PubMed] [Google Scholar]
- 25.Bearse M.A., Han Y., Schneck M.F. Retinal function in normal and diabetic eyes mapped with the slow flash multifocal electretinogram. Invest Ophthalmol Vis Sci. 2004;45:296–304. doi: 10.1167/iovs.03-0424. [DOI] [PubMed] [Google Scholar]
- 26.Han Y., Bearse M.A., Schneck M.E. Multifocal electroretinogram delay predict sites of subsequent diabetic retinopathy. Invest Ophthalmol. 2004;45:948–954. doi: 10.1167/iovs.03-1101. [DOI] [PubMed] [Google Scholar]
- 27.Heynen H., Van Norren D. Origin of the electroretinogram in the intact macaque eye I. Principal component analysis. Vision Res. 1985;25:697–707. doi: 10.1016/0042-6989(85)90176-2. [DOI] [PubMed] [Google Scholar]
- 28.Hood D.C., Frishman I.J., Saszik S. Retinal origins of the primate multifocal: implications for the human response. Invest Ophthalmol Vis Sci. 2002;43:1673–1685. [PubMed] [Google Scholar]
- 29.Ca J., Boulton M. The pathogenesis of diabetic retinopathy old concepts and new questions. Eye. 2002;16:242–260. doi: 10.1038/sj.eye.6700133. [DOI] [PubMed] [Google Scholar]
- 30.Stitt A.W. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br J Ophthalmol. 2001;85:746–753. doi: 10.1136/bjo.85.6.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witmer A.N., Vrensen G.F., Van Noorden C.J. Vascular endothelial growth factors and angiogensis in eye disease. Prog Retina Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 32.Tsilibary E.C. Microvascular basement membranes in diabetes mellitus. J Pathol. 2003;200:537–546. doi: 10.1002/path.1439. [DOI] [PubMed] [Google Scholar]
- 33.Bearse MA, Suttet EE. Contrast dependence of multifocal ERG components. Vision science and its applications technical gests, series vol. 1. Washington, DC: Optical Society of America; 1998. p. 24–7.
- 34.Hood D., Birch D. B-wave of the scotopic (rod) electroretinogram as a measure of the activity of human on – bipolar cells. J Opt Soc Am. 1996;13:623–633. doi: 10.1364/josaa.13.000623. [DOI] [PubMed] [Google Scholar]
- 35.Robson J.G., Frishman L.J. Photoreceptor and bipolar cell contributions to the cat electroretinogram: a kinetic model for the early part of the flash response. J Opt Soc Am. 1996;13:613–622. doi: 10.1364/josaa.13.000613. [DOI] [PubMed] [Google Scholar]
- 36.Sieving P.A., Murayama K., Naarendorp F. Push-pull model of the primate photopic electroretinograms a role for hyperpolarizing neurons in shoping the b-wave. Vis Neuro Sci. 1994;11:519–532. doi: 10.1017/s0952523800002431. [DOI] [PubMed] [Google Scholar]
- 37.Ardenr T.W., Antonetti D.A., Barber A.J. New insights into the pathophysiology of diabetic retinopathy: potential cell-specific therapeutic targets. Diabetes Technol Ther. 2000;2:601–608. doi: 10.1089/15209150050502023. [DOI] [PubMed] [Google Scholar]
- 38.Wanger P., Persson H.E. Early diagnosis of retinal changes in diabetes: a comparison between electroretinogram and retinal biomicroscopy. Acta Ophthalmol. 1985;63:716–720. doi: 10.1111/j.1755-3768.1985.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 39.Bellini G., Bocin E., Cosenzi A. Oscillatory potentials of the electroretinogram in hypertensive patients. Hypertension. 1995;25:839–841. doi: 10.1161/01.hyp.25.4.839. [DOI] [PubMed] [Google Scholar]
- 40.Currcio C.A., Sloan K.R., Kalina R.E. Human photoreceptors topography. J Comp Neural. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 41.Bresnick G.H., Palta M. Oscillatory potential amplitudes relation to severity of diabetic retinopathy. Arch Ophthalmol. 1987;105:923–933. doi: 10.1001/archopht.1987.01060070065030. [DOI] [PubMed] [Google Scholar]
- 42.Hood D., Birch D. B-wave of the scotopic (rod) electroretinogram as a measure of the activity of human on – bipolar cells. J Opt Soc Am. 1996;13:623–633. doi: 10.1364/josaa.13.000623. [DOI] [PubMed] [Google Scholar]
- 43.Chung N., Kim S., Kwak M. The electroretinogram sensitivity in patients with diabetes. Korean J Ophthalmol. 1993;7:43–47. doi: 10.3341/kjo.1993.7.2.43. [DOI] [PubMed] [Google Scholar]
- 44.Shirao Y., Ohta T. Clinical importance of electroretinographic oscillatory potentials in early detection and objective evaluation for diabetic retinopathy. Clin Vis Sci. 1991;6:445–450. [Google Scholar]
- 45.Juen S., Kieselbach G.F. Electro physiological changes in juvenile diabeties without retinopathy. Arch Ophthalmol. 1990;108:372–375. doi: 10.1001/archopht.1990.01070050070033. [DOI] [PubMed] [Google Scholar]
- 46.Papakostopulos D., Hart J., Corrall R. The scotopic electroretinogram to blue flash and pattern reversal visual evoked potential in insulin dependent diabetes. Int J Psycho-Physiol. 1996;21:33–43. doi: 10.1016/0167-8760(95)00040-2. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto S., Yamamoto T., Hayashi M. Morphological and functional analyses of diabetic macular odema by optical coherence tomography and multifocal electroretinograms. Graefes Arch Clin Exp Ophthalmol. 2001;239(2):96–101. doi: 10.1007/s004170000238. [DOI] [PubMed] [Google Scholar]



