Abstract
Congenital abnormalities of the kidney and urinary tract are some of the most common defects detected in the unborn child. Kidney growth is controlled by the GDNF/RET signalling pathway, but the molecular events required for the activation of RET downstream targets are still poorly understood. Here we show that SOX9, a gene involved in campomelic dysplasia (CD) in humans, together with its close homologue SOX8, plays an essential role in RET signalling. Expression of SOX9 can be found from the earliest stages of renal development within the ureteric tip, the ureter mesenchyme and in a segment-specific manner during nephrogenesis. Using a tissue-specific knockout approach, we show that, in the ureteric tip, SOX8 and SOX9 are required for ureter branching, and double-knockout mutants exhibit severe kidney defects ranging from hypoplastic kidneys to renal agenesis. Further genetic analysis shows that SOX8/9 are required downstream of GDNF signalling for the activation of RET effector genes such as Sprouty1 and Etv5. At later stages of development, SOX9 is required to maintain ureteric tip identity and SOX9 ablation induces ectopic nephron formation. Taken together, our study shows that SOX9 acts at multiple steps during kidney organogenesis and identifies SOX8 and SOX9 as key factors within the RET signalling pathway. Our results also explain the aetiology of kidney hypoplasia found in a proportion of CD patients.
INTRODUCTION
Development of the metanephros commences with a molecular signal involving the glial-derived neurotrophic factor GDNF, which is released from the metanephric blastema, a mesenchymal tissue located at the caudal end of the nephrogenic cord. Binding of GDNF to its receptors GFRα1 and RET, both expressed in the mesonephric (Wolffian) duct, triggers tyrosine kinase signalling, which induces the outgrowth of the ureteric bud (reviewed in 1). Once the ureter has invaded the metanephric mesenchyme, continuous GDNF signalling leads to repetitive branching of the ureter to form the ureteric tree that eventually will give rise to the collecting duct system. The importance of the GDNF/RET signalling pathway is underlined by mutation analysis in mice: loss of Gdnf (2–4), Ret (5,6) or Gfrα1 (7) causes severe kidney defects ranging from renal dysplasia to complete agenesis. On the molecular level, GDNF binding induces a range of intracellular signalling cascades including the ERK/MAPK and the PI3K pathway. Recent evidence suggests that, for ureter branching, PI3K activation is more important and pharmaceutical inhibition of this pathway interferes with the activation of downstream target genes (8). How these targets are activated on the transcriptional level and which factors are involved in this process, however, remain elusive.
Important insights into the transcriptional programme activated by GDNF/RET signalling have recently been made (8). Two key genes in this pathway appear to be the Ets-related transcription factors Etv4 and Etv5. Indeed, reduction of Etv4/Etv5 gene dosage in compound mutant animals leads to reduced ureter branching, and many Etv4−/−Etv5−/+ mutants display renal agenesis.
Although mesenchymal signalling is important for ureter growth, signals released from the ureter are required for the survival of mesenchymal cells and the induction of the nephrogenic programme. Since the ureter is in direct contact with uninduced mesenchymal cells, it is believed that an active mechanism involving the transcription factor SIX2 suppresses premature nephron formation in the outermost kidney precursor cells to maintain a self-renewing pool of nephron progenitors (9). Indeed, deletion of Six2 results in ectopic nephron formation and hypoplastic kidneys as a consequence of renal progenitor depletion (10). An important factor in nephron induction appears to be WNT9b, which is believed to activate the canonical Wnt/β-catenin signalling pathway (11). Genetic analysis demonstrated that Wnt9b is required for mesenchyme-to-epithelial transition (MET), and mutant mice show a complete absence of nephrons (12).
Sox genes are developmental regulators that can be identified by the presence of a DNA-binding domain that shares high homology with the HMG box of the sex-determining gene Sry. Sox genes can be classified into different groups according to their structure and evolutionary conservation (13). In mammals, the SoxE group consists of the three members Sox8, Sox9 and Sox10, all of which appear to act as potent transcriptional regulators. Studies in mice have demonstrated that deletion of Sox8 does not cause severe developmental defects (14), although male mice display lipodystrophy, mild osteopenia and fertility problems at older ages at least on some genetic backgrounds (15–17).
In contrast to Sox8, Sox9 is crucial for normal development, and heterozygous mutations in human cause campomelic dysplasia (CD), a syndrome characterized by severe bone malformations, XY sex reversal and perinatal death. Interestingly, a high proportion of patients also suffer from renal defects, including hydroureter, hydronephrosis, renal hypoplasia and, in rare cases, renal cysts (18). These phenotypes strongly suggest that SOX9 has also an important function during kidney formation.
We have previously generated a conditional knockout allele for Sox9 (19), which allows us to analyse the function of this gene in a tissue-specific manner. Here we show that SOX9 together with SOX8 regulates epithelial branching morphogenesis of the ureter, where they are required for the activation of RET downstream targets.
RESULTS
Sox8 and Sox9 show a dynamic expression pattern during kidney development
The global expression patterns for Sox8 and Sox9 have been described previously (14,20,21), and very recently a more detailed evaluation of Sox9 expression during ureter development has been published (22). Our own analysis extends the presently described expression domains for SOX9. In situ hybridization (ISH) and antibody staining against SOX9 demonstrated low levels of expression within the uninduced Wolffian duct (Fig. 1A). Upon ureter induction, epithelial expression of SOX9 became restricted to the tip of the ureter, where it was maintained throughout kidney development (Fig. 1B–D). Interestingly, the amount of SOX9 protein per cell—as judged by immunofluorescent analysis—was not homogenous throughout the ureteric tip. Apart from the ureteric tip, strong staining was also seen from E11.5 in the mesenchyme surrounding the ureter (Fig. 1B), a tissue that later on will give rise to the smooth muscle cells of the ureter (23). In addition, expression could be detected in the epithelium of the forming ureter from E11.5 until E16.5 (Fig. 1C, E14.5). Finally, SOX9 was expressed in a regionalized pattern during nephrogenesis: immediately after MET, SOX9-expressing cells were detected in the renal vesicle in a subdomain adjacent to the ureteric tip (Fig. 1F). As nephron development proceeded, SOX9 expression became localized to the intermediate and distal domains of the S-shaped body (Fig. 1G). In contrast to SOX9, SOX8 expression was absent from the developing nephrons and restricted to the epithelial tips of the growing ureter (Fig. 1H–J).
Figure 1.
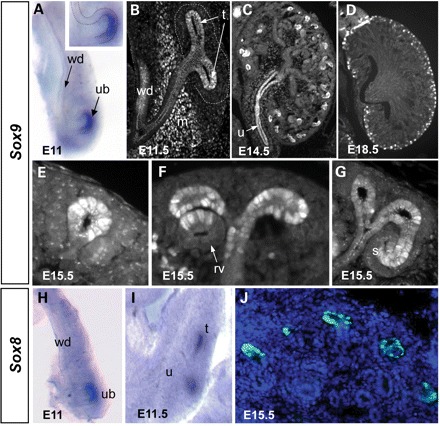
Sox8 and Sox9 are expressed in a highly dynamic pattern during kidney development. (A) Whole-mount ISH at E11 reveals Sox9 expression at the tip of the emerging ureteric bud (ub) and, to a lower level, along the entire length of the Wolffian duct (wd). (B–G) Sox9 antibody staining reveals a complex expression pattern including the urothelium (u), mesenchyme surrounding the developing ureter (m), ureteric tips (t), renal vesicle (rv) and within the intermediate segment of the S-shaped body (s). ISH (H and I) and immunostaining (J) demonstrate that Sox8 shows an overlapping expression with Sox9 at the ureteric tips. Note that other cell types are negative for Sox8.
Before discussing the role of Sox8 and Sox9 in ureter development in detail, we would like to point out that a proportion of Pax2:Cre;Sox9fl/fl animals showed defects in nephron patterning. A detailed analysis of this phenotype will however require further studies and will be reported separately.
Sox8/Sox9 are required in a functionally redundant manner for ureter branching
The early expression of Sox8 and Sox9 within the Wolffian duct and the ureteric tips suggested that these genes play a role during metanephric kidney induction. Deletion of Sox8 alone does not result in an overt renal phenotype, and mutant mice appear to be normal (14; Supplementary Material, Fig. S1). Complete deletion of Sox9 using germline-specific Cre lines (male Sox9fl/fl;Prm1:Cre× female Sox9fl/fl;Zp3:Cre from now on called Sox9co/co) results in embryonic lethality (24). To address the function of Sox8 and Sox9 in kidney induction and ureter branching, we first analysed Sox8−/−;Sox9co/co mutants at E11.5, the latest stage at which mutant embryos could be recovered alive. In wild-type control or Sox8−/−;Sox9+/co embryos, the ureter had invaded the metanephric mesenchyme and undergone the first dichotomous branching event (Fig. 2A and data not shown). In contrast, in the majority of double-knockout embryos, induction of the ureteric bud occurred, but it failed to initiate branching (Fig. 2B). Since the metanephric mesenchyme was normally condensed, the branching defect is likely to have a ureteric bud origin.
Figure 2.
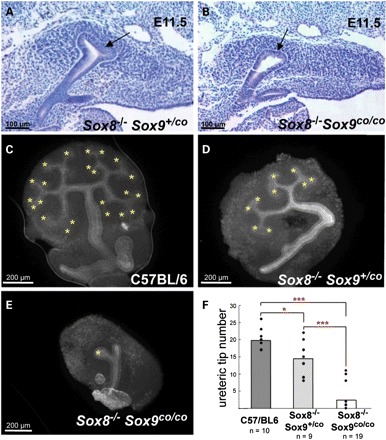
Sox8 and Sox9 are required for ureter branching. (A and B) Histological analysis showing a blind ending ureter in double-knockout animals (generated by crossing male Prm1:Cre;Sox9fl/fl;Sox8−/− with female ZP3:Cre;Sox9fl/fl;Sox8−/−). (C–E) In vitro analysis of ureter branching demonstrates a mild reduction after the removal of both copies of Sox8 and one copy of Sox9 and an almost complete absence of ureter branching in double-mutants. The ureter is visualized using an anti-cytokeratin antibody. (F) Quantification of data from (C) to (E). The y-axis represents the number of ureteric tips. The number of explants analysed for each genotype is given below the diagram.
To assess the role of Sox8 and Sox9 in ureter branching in greater detail, we placed kidneys dissected from wild-type and mutant E11.5 embryos in culture and counted the number of ureteric tips after 48 h of growth in vitro (Fig. 2C–E). Deletion of Sox8 alone had no effect on ureter branching when compared with wild-type animals (data not shown). In contrast, removal of one additional copy of Sox9 revealed a significant reduction of ureteric tips in Sox8−/−;Sox9+/co tissues (Fig. 2D and F). Strikingly, complete deletion of Sox8 and Sox9 (Sox8−/−;Sox9co/co) resulted in severely reduced ureter branching (>6×, Fig. 2E and F), and a complete absence of the ureter was observed in many cases.
Sox8 activation in the developing testis depends on the expression of Sox9 (25) and we wondered whether a similar dependency also exists for the kidney. Expression of Sox8 within the early branching ureter persisted in the absence of Sox9 at E11.5 (Supplementary Material, Fig. S2A and B). Similarly, Sox9 expression was maintained in E16.5 Sox8−/− kidneys (Supplementary Material, Fig. S2C). We conclude that neither Sox8 nor Sox9 expression in the developing kidney is dependent on the activity of its paralogue.
To further assess the role of Sox9 in kidney development, we specifically deleted this gene within the mesonephric duct and ureter using a HoxB7:Cre line (26) in combination with our conditional Sox9 knockout allele (19). Alternatively, a Pax2:Cre line was used, which also mediated deletion within the cap mesenchyme (27). Macroscopic analysis of embryos and newborn mice demonstrated that a proportion of mice carrying a kidney-specific deletion of Sox9 displayed either uni- or bilateral agenesis (Fig. 3A–D). The low penetrance of the renal agenesis phenotype could be due to inefficient deletion of Sox9 and/or delayed expression of the Pax2:Cre transgene. Analysis by immunofluorescence staining, however, indicated that excision of the Sox9fl allele within the ureter was complete even at early time points (Fig. 3E–H). We therefore speculated that persistent expression of Sox8 at the tips of the ureter compensated for the loss of Sox9. Indeed, mice carrying deletions for both Sox8 and Sox9 (Pax2:Cre;Sox9fl/fl;Sox8−/−) showed renal agenesis with >90% penetrance (45% bilateral; 45% unilateral, Fig. 3I). In those cases where kidneys did form, they were always hypoplastic, reaching sometimes only 50% of their normal size (Fig. 4). Taken together, these results demonstrate that SOX8 and SOX9 play crucial but partially redundant roles in kidney development.
Figure 3.
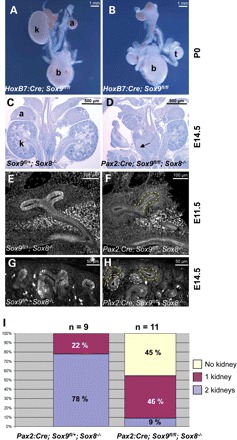
Tissue-specific ablation of Sox8 and Sox9 leads to renal agenesis. (A and B) Macroscopic analysis at E18.5 shows unilateral (A) or bilateral (B) kidney agenesis in HoxB7:Cre;Sox9fl/fl knockout animals (a, adrenal; b, bladder; k, kidney; t, testis). (C and D) PAS staining depicting unilateral agenesis in a Sox8/9 double-knockout mouse. Note the presence of a blind ending ureter in (D) (arrow). (E–H) Immunofluorescent analysis with antibodies against SOX9 confirms complete deletion of Sox9 within the ureteric bud tips and the developing nephron (compare E and G with F and H), whereas staining within the ureter mesenchyme persists (arrowhead in F). Ureteric bud tips are outlined by a dashed yellow line, and developing nephrons by a dashed white line. (I) Quantification of renal agenesis in mice lacking one or two copies of Sox9 in the absence of Sox8. The number of embryos analysed for each genotype is given above the diagram.
Figure 4.
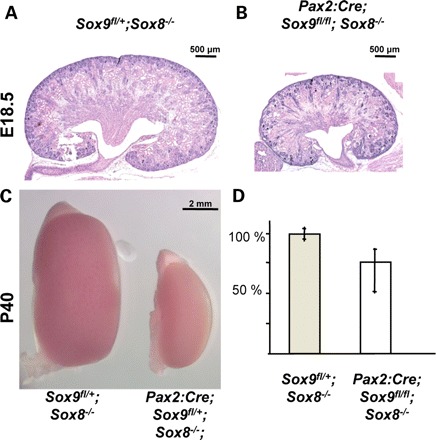
Tissue-specific deletion of Sox9 in the absence of Sox8 results in a hypoplastic kidney phentoype. (A and B) PAS staining of E18.5 kidneys from wild-type and Pax2:Cre;Sox9fl/fl;Sox8−/− mutant animals. (C) Macroscopic view of the hypoplasia in Pax2:Cre;Sox9fl/+;Sox8−/− at post-natal day 40. Note that the majority of these mice die at birth. (D) Measurement of renal size at E15.5 shows a reduction of up to 50% in double-mutant kidneys. Error bars represent the range of kidney sizes observed.
GDNF/RET signalling is impaired in Sox8/Sox9 double-mutant mice
Ureter branching is regulated by GDNF/RET signalling, and mutations in these genes result in a kidney phenotype similar to that found in Sox8/Sox9 double-mutants (2–5). Given the overlapping expression patterns at the tip of the ureter, we speculated that SOX8/SOX9 might be directly involved in the transcriptional regulation of the Ret gene. ISH analysis and quantitative PCR assays did not reveal dramatic changes in Ret expression in E14.5 Sox8/9 double-mutants (Fig. 5A and B and data not shown).
Figure 5.
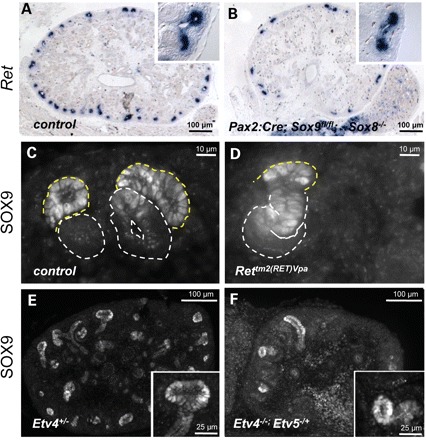
SOX9 expression does not depend on RET signalling. (A and B) Ret expression remains unaffected in Sox8/9 mutant mice. (C and D) Nuclear SOX9 expression persists in the Ret hypomorph Rettm2(RET)Vpa at levels comparable with those found in control mice. Similarly, SOX9 remains expressed in the ureteric tips of Etv4+/− (E) and Etv4−/−;Etv5−/+ (F) compound knockout kidneys. Note the reduction of ureteric tips in Etv4−/−;Etv5−/+ kidneys. All samples were dissected from E14.5 embryos. Ureteric bud tips are outlined by a dashed yellow line, and developing nephrons by a dashed white line.
Since Ret expression seemed independent of the presence or absence of SOX8/SOX9, we next asked whether these two genes might be downstream targets of the RET signalling pathway. To test this hypothesis, we analysed tissues from Rettm2(RET)Vpa mutant mice (28), in which kidneys are hypoplastic due to a severely reduced RET signalling. Although ureteric tips were sparse in these mutants, those that formed showed strong nuclear expression of SOX9 comparable with that found in wild-type kidneys (Fig. 5C and D). SOX9 expression within the forming nephrons also persisted in these mutants.
GDNF/RET signalling results in the activation of a series of downstream target genes, with Etv4(Pea3) and Etv5(Erm) seemingly acting as key effectors of this signalling pathway (8). Complete ablation of Etv5 results in embryonic lethality before kidneys form and thus does not allow us to test for SOX9 expression in this mutant. However, Etv4−/−;Etv5−/+ compound mutants can be generated, which develop severely hypoplastic kidneys. Downstream target genes of ETV4/5, such as Cxcr4, are dramatically reduced in these mutants (8). Immunofluorescent analysis demonstrated that SOX9 expression was maintained in these compound mutants (Fig. 5E and F). Thus, SOX9 expression appears to be independent of functional RET signalling and does not depend on the expression of ETV4/5.
Since SOX9 neither regulates Ret expression nor is controlled by RET signalling, we speculated that it may act as a mediator of RET activation. We therefore tested whether the expression of RET effector genes may be affected in our Sox8/9 mutants. Indeed, ISH analysis demonstrated that while Etv5 expression was maintained in the condensing mesenchyme, ISH signal was specifically lost within the ureteric tips of double-mutant kidneys (Fig. 6A and B). Similarly, the RET downstream targets Etv4, Cxcr4, Met, Spry1 and Dusp6 were severely reduced or absent in the ureteric tips of mutant mice (Fig. 6 and data not shown).
Figure 6.
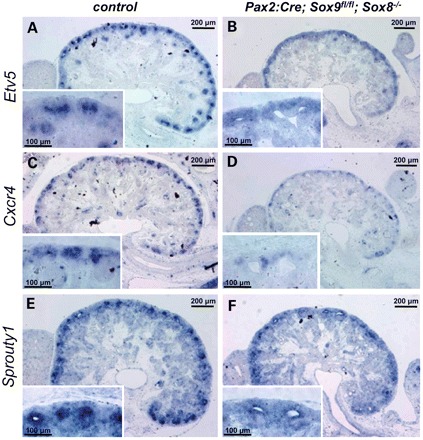
SOX8/9 regulate downstream targets of RET signalling. Expression of Etv5 (A and B), Cxcr4 (C and D) and Sprouty1 (E and F) within the ureteric tips is severely reduced or completely absent in mutant mice. Note the persistence of Etv5 expression within the metanephric mesenchyme (inset in B).
To test whether SOX9 may be sufficient to activate RET downstream targets in vivo, we next cloned a Sox9 cDNA downstream of HoxB7 regulatory elements (HoxB7:Sox9). Analysis of transient HoxB7:Sox9 transgenic animals revealed the expression of SOX9 along the collecting ducts of the ureter in addition to the endogenous expression at the ureteric tips (Supplementary Material, Fig. S4). No major changes in renal architecture were observed upon histological examination. Importantly, ISH analysis failed to reveal the activation of RET downstream target genes in the ureteric stalk expressing SOX9 ectopically. We conclude that SOX8/9 are required, but not sufficient, for GDNF/RET signalling by controlling the expression of its major effector genes.
Abnormal nephron induction at the end of embryonic development
Nephrogenesis during kidney formation occurs in a repetitive manner, with new nephrons being born throughout development at the outer cortex of the kidney. Precursor cells residing in this area proliferate to replenish a pool of mesenchymal cells that are then transformed into nascent nephrons. This process is highly regulated, involving both inductive and repressive signals of nephrogenesis. Six2 appears to fulfil a key role in this process by actively suppressing nephrogenesis in uninduced mesenchymal precursor cells (10).
Although nephron induction at early stages of metanephric kidney development appeared normal in Sox8/Sox9 mutant mice, histological analysis from E16.5 onwards demonstrated the presence of highly dilated ureteric tips accompanied by abnormal cellular aggregates in the outermost layer of the cortex of all Sox8/Sox9 double-mutant kidneys (Fig. 7A–D). Similar histology was also found in 100% of the Pax2:Cre;Sox9fl/fl, but not in Sox8−/− animals. ISH analysis demonstrated the expression of early nephron markers such as Lim1, Fgf8 and Wnt4 (Fig. 7E and F and data not shown), suggesting that these ectopic structures represent pretubular aggregates that are located at the peripheral side of the ureter tips. Expression of Six2 remained unchanged in the mutant kidneys but, as expected, expression was excluded from ectopic pretubular aggregates (Fig. 7G and H).
Figure 7.
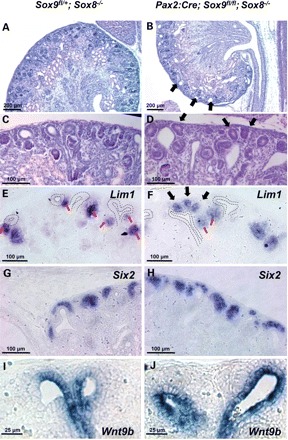
Abnormal induction of nephrogenesis at late stages of development in Sox8/Sox9 knockout kidneys. (A and B) Overview of E18.5 kidneys reveals the presence of highly dilated ureteric tips (black arrows, B). (C and D) Higher magnification shows dilated ureteric tips and abnormal epithelialization at the outermost layer of the kidney (black arrows, D). (E and F) ISH for Lim1 confirms the presence of both normal (red arrows) and abnormal renal vesicles (black arrows). Dotted lines represent the outline of the ureteric tips. (G and H) ISH analysis for Six2 shows no change of expression pattern in double-mutant animals. (I and J) ISH for Wnt9b showing extended expression throughout the entire epithelial structure in knockout tissue.
The ureteric tip and stalk have two distinct cell identities that are characterized by the expression of different sets of marker genes. Although the ureteric tip has an important function for the survival and growth of the surrounding metanephric mesenchyme, signals from the stalk, notably WNT9b, have been proposed to induce nephron formation (12). We therefore wondered whether the formation of ectopic nephrons may be caused by changes of tip identity. ISH demonstrated that the tip-specific markers Ret and Wnt11 and also the more general markers Emx2, Gata3 and calbindin were dramatically reduced in E18.5 tips of Pax2:Cre;Sox9fl/fl;Sox8−/− kidneys (Supplementary Material, Fig. S3). Furthermore, expression of Wnt9b, an inducer of β-catenin signalling in the developing nephron, which is usually excluded from the extremity of the tips, extended throughout the entire epithelial structure in mutant embryos at E18.5 (Fig. 7I and J). Thus, ablation of Sox9 leads to a loss of ureteric tip identity at late stages of kidney development and redistribution of Wnt9b expression in the entire ureteric tip area.
DISCUSSION
Sox9 encodes a transcriptional regulator that fulfils essential roles during vertebrate embryogenesis. In this study, we have discovered a new function of Sox9 and its close paralogue Sox8 in kidney development and demonstrate a key role of these two genes in ureter branching.
The early ureter phenotype observed in our Sox8/Sox9 double-mutants is reminiscent of defects seen in RET knockout animals and we speculated that this signalling pathway could be disrupted in mutant kidneys. RET expression was maintained at least initially in Sox8/Sox9 mutants, suggesting that they are not required for the activation of this gene. Similarly, Sox9 expression was maintained in a Ret hypomorph, demonstrating that activation of the RET pathway is not essential for Sox9 expression. These findings are also supported by microarray experiments performed on ureteric bud cultures, where activation of RET signalling by GDNF did not induce SOX9 expression (8).
In contrast, RET downstream targets that are specifically activated within the ureteric tip were severely affected in Sox8/9 mutant mice. In particular, expression of Etv4 and Etv5, two key players in ureter growth and branching (8), is lost from the epithelial tips in double-mutant animals. Analysis by Lu et al. (8) suggested at least two independent classes of genes downstream of Ret signalling. Whereas genes such as Cxcr4, Met and Myb depend on the expression of ETV4/5, others such as Spry1, Crlf1 and Dusp6 seem to be regulated in an independent manner. Our data suggest that both classes of genes require the presence of SOX8/9 for their full activation. Although Sox8/9 are required for the activation of RET downstream targets, it does not seem to be sufficient to induce their expression. Indeed, expression of SOX9 along the ureter stalk in HoxB7:Sox9 transgenic animals did not result in ectopic activation of Etv5 (Supplementary Material, Fig. S4). These results are in agreement with a model where a combination of RET signalling and SOX8/SOX9 is required for the activation of RET downstream effector genes.
If SOX8/9 are required for RET signalling, how do they act at a molecular level? Several potential modes of action can be envisaged: first, RET activation might induce posttranslational modification of SOX8/9, possibly through phosphorylation or sumoylation, both of which have been reported to influence the biochemical properties of SOX9 (29,30). Modified SOX9 alone or potentially in conjunction with an adaptor molecule may then lead to the activation of Etv5 and other downstream target genes. Although this is a possibility, we have so far been unable to demonstrate direct binding of SOX9 to Etv5 regulatory regions using ChIP technology, which may however be due to technical difficulties. Alternatively, SOX8/9 might act as a competence factor, by regulating the expression of a presently unknown mediator of RET signalling within the ureteric tip. Finally, it is conceivable that SOX9 is required to suppress genes that otherwise interfere with normal ureter branching. A potential candidate for such a gene could be Wnt9b, which is expressed along the ureteric stalk but excluded from the tips. Ectopic expression of Wnt9b along the entire ureter has recently been shown to interfere with branching events (31). However, although we observed ectopic expression of Wnt9b in the tips of E18.5 Sox8/9 mutant animals (Fig. 7), molecular analysis at earlier time points (E14.5/E15.5) showed an absence of Wnt9b from the ureteric tips (data not shown). Changes in Wnt9b expression are therefore unlikely to be responsible for the observed ureter branching defects. Further experiments using biochemical approaches will be required to precisely determine the cellular action of Sox8/9 during GDNF/RET signalling.
Since ETV4/5 are essential for ureter branching, the loss of activation of these genes explains the dramatic branching defects observed in most of the embryos of the Sox8/9 double-mutants. However, if Sox8/9 are essential for GDNF/RET signalling, why do a proportion of mutants develop kidneys? Apart from the absence of Etv4/5 in the ureteric tip, deletion of SOX8/9 also leads to a dramatic downregulation of the FGF inhibitor SPRY1. Recent analysis in mice has demonstrated that in the absence of Spry1, kidney development does not require GDNF/RET signalling (32). We can therefore speculate that lack of SPRY1 in Sox8/9 mutant kidneys may lead to increased FGF signalling, which in turn may support ureter outgrowth and branching. An alternative explanation for the observed incomplete penetrance could be persistence of small amounts of other SOX proteins within the ureteric tip that may be able to partially compensate for the loss of SOX8/9 function.
Apart from the GDNF/RET pathway, ureter branching is also stimulated by FGF signalling (32). Similar to the GDNF RET pathway, FGF signalling also involves a tyrosine kinase receptor (FGFR) that can activate intracellular signalling cascades. To induce ureter branching, both cascades must converge on the expression of the canonical RET targets, such as Etv4/5. Since SOX8/9 are required for the activation of Etv4/5, it is likely that the absence of SOX8/9 could also interfere (at least to some extent) with FGF signalling and thus lead to reduced ureter branching and, in many cases, renal agenesis.
During kidney organogenesis, nephrons are continuously induced in the cortical zone of the growing kidney, where a pool of nephron precursor cells is maintained (9,10). This process is tightly regulated, and nephron induction occurs always at the medullary side of the T-shaped ureter. After E17.5, Sox9 mutant mice consistently showed the formation of ectopic nephrons at the outermost area of the developing kidney. The expression of Six2, which suppresses ectopic nephron formation and thus maintains a pool of mesenchymal precursor cells, was not changed in double-mutant kidneys. This suggests that an additional signal is required to suppress MET in the nephron precursor compartment. Such a signal could be released from the ureteric tip that is in constant contact with these mesenchymal precursor cells. The fact that abnormal nephron induction coincided with the loss of tip-specific markers, such as Ret and Wnt11 (Supplementary Material, Fig. S3), is in agreement with this notion. Alternatively, ectopic nephron induction could be caused by increased or ectopic expression of an inducer within the mutant tips. An excellent candidate for such a molecule is WNT9b, a member of the Wnt signalling family that is required for nephron induction by activating canonical β-catenin signalling in mesenchymal cells (12). In wild-type embryos, Wnt9b is excluded from the most proximal part of the ureter. In contrast, Sox8/9 double-mutants at E18.5 showed Wnt9b expression along the entire ureter including the tips. Since Wnt9b expression was excluded from the tip at earlier time points (E14.5 and E15.5), a direct repression of this gene by SOX8/9 seems unlikely. Instead, the expansion of Wnt9b expression may reflect a general loss of ureteric tip identity, which is further supported by the loss of tip-specific markers such as Wnt11 and Ret. Since the expansion of the Wnt9b expression domain, coincided with the appearance of ectopic nephrons, it is conceivable that induced Wnt9b signalling in late-stage mutant animals is responsible for the observed ectopic nephron formation.
A premature cessation of nephrogenesis could be an important factor contributing to the hypoplastic phenotype observed in our mutant animals. Although this may be the case, the fact that even at early time points (E14.5) mutant kidneys are smaller than wild-type controls suggests that hypoplasia is not exclusively caused by premature exhaustion of the nephrogenic precursor compartment. This point of view is further corroborated by the fact that kidneys from mutant embryos (even carrying a single-mutant Sox9 allele) showed a clear reduction in the number of ureteric branches after 48 h in culture (Fig. 2).
A proportion of CD patients carrying heterozygous mutations in SOX9 present with renal abnormalities that range from hydronephrosis to kidney dysplasia and, in rare cases, renal cysts (18). The tissue-specific knockout mice described in the present study also develop a hypodysplasia phenotype and may therefore be considered a model for some aspects of the human disorder. In contrast, we have never observed hydronephrosis in mutant animals, which is the most common renal defect in CD patients. Although this may seem surprising at a first glance, Hoxb7:Cre- or Pax2:Cre-mediated deletion did not remove the strong SOX9 expression within the developing ureter mesenchyme (Fig. 3). Mesenchymal cells surrounding the ureter have been shown to be essential for both the normal differentiation of the ureteric epithelium and the development of the smooth muscle cell layer, as exemplified by recent studies describing the analysis of Tbx18- and Tshz3-deficient mouse mutants (23,33). Indeed, a recent study using the mesenchyme-specific Cre lines Pax3:Cre and Tbx18:Cre to ablate Sox9 demonstrated an essential function for this gene in smooth muscle cell differentiation of the ureter (22).
In conclusion, our study has identified SOX9 as a major regulator of kidney development. It acts as a central controller of epithelial branching by allowing GDNF/RET signalling to occur and maintains tip-specific cell identity, thus suppressing the formation of ectopic nephrogenesis in the outer cortex of the kidney.
MATERIALS AND METHODS
Mouse strains and genetic background
All animal work has been conducted according to national and international guidelines. All mouse lines were kept on a mixed 129/C57Bl6 background. The generation of the Sox9fl allele (19), HoxB7:Cre (26) and the Pax2:Cre lines (27) was reported previously. Mice with a homozygous deletion of Sox9 were generated using the Prm1:Cre (34) and Zp3:Cre (35) strain as described (25).
Genotyping of embryos and mice
Wild-type and Sox9fl alleles were identified using the primers 5′-GGGGCTTGTCTCCTTCAGAG-3′, or 5′-ACACAGCATAGGCTACCTG-3′ and 5′-TGGTAATGAGTCATACACAGTAC-3′, respectively. The Sox9 knockout allele was identified using the primers 5′-GTCAAGCGACCCATG-3′ and 5′-TGGTAATGAGTCATACACAGTAC-3′. Genotyping for the Sox8 knockout allele was performed as described in Sock et al. (14). The presence of the Pax2:Cre transgene was determined using primers 5′-TCAAATGGCTCTCCTCAAGC-3′ and 5′-AGCTGGCCCAAATGTTGCTG-3′.
Histological and immunological analyses
Embryonic samples from timed matings (day of vaginal plug = E0) were collected, fixed with 4% paraformadehyde overnight at 4°C and then embedded in paraffin. Microtome sections of 7 μm thickness were stained with PAS according to standard procedures. Immunofluorescent analysis was performed after antigene retrieval in 10 mm Na citrate (pH 6) at 2 min in a pressure cooker. Sections were incubated for 45 min in blocking solution (3% BSA, 10% donkey serum, 0.1% Triton) at room temperature. Blocking solution was replaced by a solution of primary antibodies prepared in dilutent (3% BSA, 3% donkey serum, 0.1% Triton) at the following concentrations: SOX9, SOX8 (1:1000) (36,37), WT1 (C-19: sc-192, Santa-Cruz, 1:100). Relevant Cy3- or Cy2-conjugated anti-rabbit or anti-rat antibodies (1:150, Jackson Laboratories) were used for the detection of primary antibodies. Slides were mounted using Vectashield, with DAPI as a mounting reagent (Vector Labs). Fluorescent studies were performed with a Leica microscope DMLB, and pictures were taken with a spot RT-slider camera (Diagnostic Instruments) and processed with Adobe Photoshop.
ISH analysis
Embryos were fixed in 4% paraformaldehyde in PBS overnight at 4°C. Further processing of the embryos and ISH were carried out as described (38). Riboprobes for Sox8 (14), Sox9 (39), Ret (40), Emx2, Gata3 (kind gift from M. Bouchard), Six2 (10), Lim1 (41), Wnt11 (42) were synthesized as described previously.
Whole-mount X-Gal staining
Embryos were fixed in 0.2% glutaraldehyde in 0.1 m phosphate buffer, 2 mm MgCl2 and 5 mm EGTA for 35 min and processed as described (43).
Kidney culture
Kidney rudiments were dissected from E11.5 mouse embryos and placed on filters (Millipore, 0,5 µm pore size) that were in direct contact with DMEM medium supplemented with 10% heat-inactivated newborn calf serum and 1% penicillin and penicillin/streptomycin (Sigma). After 48 h (37°C in 5% CO2), kidney rudiments were fixed in cold methanol at 4°C while still attached to their filters, washed in PBS and blocked for 1 h in 2% BSA/PBS at room temperature. Staining was performed using cytokeratin antibodies (C-2562, Sigma; 1:100 dilution in PBS/0.1% BSA/0.1% Triton) followed by detection with a Texas Red-conjugated anti-mouse antibody (1:150, Jackson Laboratories) and kidneys examined using a fluorescence microscope.
AUTHOR CONTRIBUTIONS
A.R. carried out all of the experiments if not stated otherwise. M.C. performed transfection experiments. Y.N. carried out expression analysis. B.L. and F.C. provided in situ probes and Rettm2(RET)Vpa, Etv4 and Etv5 knockout embryos. E.S. and M.W. provided the Sox8 and Sox9 antibodies and the Sox8 knockout mouse, and T.O. and A.K.G., the Pax2:Cre mouse strain. A.R., M.C., M.-C.C. and A.S. designed experiments and interpreted the results.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by grants from the European Commission (EuReGene LSHG-CT-2004-005085) to A.S., the ARC (Association pour la recherche sur le cancer) grant 5198 and the FRM (Fondation de la Recherche médicale) to A.S. A.R. was supported by a fellowship from the FRM.
ACKNOWLEDGEMENTS
We would like to thank the staff of the animal house for their outstanding work and dedication; Rannar Airik and Andreas Kispert (Hannover Medical School, Germany) for characterizing in situ hybridization probes; and Seppo Vainio (University of Oulu, Finland) and Maxime Bouchard (McGill University, Montreal, Quebec, Canada) for their kind gifts of reagents. We are also grateful to Andy McMahon (Harvard University) for providing the HoxB7:Cre mice.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Costantini F., Shakya R. GDNF/Ret signaling and the development of the kidney. Bioessays. 2006;28:117–127. doi: 10.1002/bies.20357. doi:10.1002/bies.20357. [DOI] [PubMed] [Google Scholar]
- 2.Moore M.W., Klein R.D., Farinas I., Sauer H., Armanini M., Phillips H., Reichardt L.F., Ryan A.M., Carver-Moore K., Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. doi:10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 3.Pichel J.G., Shen L., Sheng H.Z., Granholm A.C., Drago J., Grinberg A., Lee E.J., Huang S.P., Saarma M., Hoffer B.J., et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. doi:10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez M.P., Silos-Santiago I., Frisen J., He B., Lira S.A., Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. doi:10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 5.Schuchardt A., D'Agati V., Larsson-Blomberg L., Costantini F., Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret [Comments] Nature. 1994;367:380–383. doi: 10.1038/367380a0. doi:10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 6.Schuchardt A., D'Agati V., Pachnis V., Costantini F. Renal agenesis and hypodysplasia in ret-k- mutant mice result from defects in ureteric bud development. Development. 1996;122:1919–1929. doi: 10.1242/dev.122.6.1919. [DOI] [PubMed] [Google Scholar]
- 7.Cacalano G., Farinas I., Wang L.C., Hagler K., Forgie A., Moore M., Armanini M., Phillips H., Ryan A.M., Reichardt L.F., et al. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. doi:10.1016/S0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu B.C., Cebrian C., Chi X., Kuure S., Kuo R., Bates C.M., Arber S., Hassell J., MacNeil L., Hoshi M., et al. Etv4 and Etv5 are required downstream of GDNF and Ret for kidney branching morphogenesis. Nat. Genet. 2009;41:1295–1302. doi: 10.1038/ng.476. doi:10.1038/ng.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi A., Valerius M.T., Mugford J.W., Carroll T.J., Self M., Oliver G., McMahon A.P. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. doi:10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Self M., Lagutin O.V., Bowling B., Hendrix J., Cai Y., Dressler G.R., Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006;25:5214–28. doi: 10.1038/sj.emboj.7601381. doi:10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J.S., Valerius M.T., McMahon A.P. Wnt/{beta}-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134:2533–2539. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- 12.Carroll T.J., Park J.S., Hayashi S., Majumdar A., McMahon A.P. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. doi:10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Bowles J., Schepers G., Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 2000;227:239–255. doi: 10.1006/dbio.2000.9883. doi:10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- 14.Sock E., Schmidt K., Hermanns-Borgmeyer I., Bosl M.R., Wegner M. Idiopathic weight reduction in mice deficient in the high-mobility-group transcription factor Sox8. Mol. Cell. Biol. 2001;21:6951–6959. doi: 10.1128/MCB.21.20.6951-6959.2001. doi:10.1128/MCB.21.20.6951-6959.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Bryan M.K., Takada S., Kennedy C.L., Scott G., Harada S., Ray M.K., Dai Q., Wilhelm D., de Kretser D.M., Eddy E.M., et al. Sox8 is a critical regulator of adult Sertoli cell function and male fertility. Dev. Biol. 2008;316:359–370. doi: 10.1016/j.ydbio.2008.01.042. doi:10.1016/j.ydbio.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guth S.I., Schmidt K., Hess A., Wegner M. Adult-onset degeneration of adipose tissue in mice deficient for the Sox8 transcription factor. J. Lipid. Res. 2009;50:1269–1280. doi: 10.1194/jlr.M800531-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt K., Schinke T., Haberland M., Priemel M., Schilling A.F., Mueldner C., Rueger J.M., Sock E., Wegner M., Amling M. The high mobility group transcription factor Sox8 is a negative regulator of osteoblast differentiation. J. Cell. Biol. 2005;168:899–910. doi: 10.1083/jcb.200408013. doi:10.1083/jcb.200408013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houston C.S., Opitz J.M., Spranger J.W., Macpherson R.I., Reed M.H., Gilbert E.F., Herrmann J., Schinzel A. The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al. in 1971. Am. J. Med. Genet. 1983;15:3–28. doi: 10.1002/ajmg.1320150103. doi:10.1002/ajmg.1320150103. [DOI] [PubMed] [Google Scholar]
- 19.Akiyama H., Chaboissier M.C., Martin J.F., Schedl A., De Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. doi:10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kent J., Wheatley S.C., Andrews J.E., Sinclair A.H., Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- 21.Wright E., Hargrave M.R., Christiansen J., Cooper L., Kun J., Evans T., Gangadharan U., Greenfield A., Koopman P. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat. Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. doi:10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- 22.Airik R., Trowe M.O., Foik A., Farin H.F., Petry M., Schuster-Gossler K., Schweizer M., Scherer G., Kist R., Kispert A. Hydroureternephrosis due to loss of Sox9-regulated smooth muscle cell differentiation of the ureteric mesenchyme. Hum. Mol. Genet. 19:4918–4929. doi: 10.1093/hmg/ddq426. [DOI] [PubMed] [Google Scholar]
- 23.Airik R., Bussen M., Singh M.K., Petry M., Kispert A. Tbx18 regulates the development of the ureteral mesenchyme. J. Clin. Invest. 2006;116:663–674. doi: 10.1172/JCI26027. doi:10.1172/JCI26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akiyama H., Chaboissier M.C., Behringer R.R., Rowitch D.H., Schedl A., Epstein J.A., de Crombrugghe B. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc. Natl Acad. Sci. USA. 2004;101:6502–6507. doi: 10.1073/pnas.0401711101. doi:10.1073/pnas.0401711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaboissier M.C., Kobayashi A., Vidal V.I., Lutzkendorf S., van de Kant H.J., Wegner M., de Rooij D.G., Behringer R.R., Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. doi: 10.1242/dev.01087. doi:10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- 26.Yu J., Carroll T.J., McMahon A.P. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–5312. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- 27.Ohyama T., Groves A.K. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–199. doi: 10.1002/gene.20017. doi:10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- 28.de Graaff E., Srinivas S., Kilkenny C., D'Agati V., Mankoo B.S., Costantini F., Pachnis V. Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 2001;15:2433–2444. doi: 10.1101/gad.205001. doi:10.1101/gad.205001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang W., Zhou X., Lefebvre V., de Crombrugghe B. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9's ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol. Cell. Biol. 2000;20:4149–4158. doi: 10.1128/mcb.20.11.4149-4158.2000. doi:10.1128/MCB.20.11.4149-4158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor K.M., Labonne C. SoxE factors function equivalently during neural crest and inner ear development and their activity is regulated by SUMOylation. Dev. Cell. 2005;9:593–603. doi: 10.1016/j.devcel.2005.09.016. doi:10.1016/j.devcel.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Kiefer S.M., Robbins L., Stumpff K.M., Lin C., Ma L., Rauchman M. Sall1-dependent signals affect Wnt signaling and ureter tip fate to initiate kidney development. Development. 2010;137:3099–3106. doi: 10.1242/dev.037812. doi:10.1242/dev.037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michos O., Cebrian C., Hyink D., Grieshammer U., Williams L., D'Agati V., Licht J.D., Martin G.R., Costantini F. Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet. 2010;6:e1000809. doi: 10.1371/journal.pgen.1000809. doi:10.1371/journal.pgen.1000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caubit X., Lye C.M., Martin E., Core N., Long D.A., Vola C., Jenkins D., Garratt A.N., Skaer H., Woolf A.S., et al. Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development. 2008;135:3301–3310. doi: 10.1242/dev.022442. doi:10.1242/dev.022442. [DOI] [PubMed] [Google Scholar]
- 34.O'Gorman S., Dagenais N.A., Qian M., Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc. Natl Acad. Sci. USA. 1997;94:14602–14607. doi: 10.1073/pnas.94.26.14602. doi:10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Vries W.N., Binns L.T., Fancher K.S., Dean J., Moore R., Kemler R., Knowles B.B. Expression of Cre recombinase in mouse oocytes: a means to study maternal effect genes. Genesis. 2000;26:110–112. doi:10.1002/(SICI)1526-968X(200002)26:2<110::AID-GENE2>3.0.CO;2-8. [PubMed] [Google Scholar]
- 36.Stolt C.C., Lommes P., Sock E., Chaboissier M.C., Schedl A., Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. doi:10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stolt C.C., Schmitt S., Lommes P., Sock E., Wegner M. Impact of transcription factor Sox8 on oligodendrocyte specification in the mouse embryonic spinal cord. Dev. Biol. 2005;281:309–317. doi: 10.1016/j.ydbio.2005.03.010. doi:10.1016/j.ydbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson D.G. Whole mount in situ hybridisation of vertebrate embryos. In: Wilkinson D.G., editor. In Situ Hybridization. Oxford: Oxford University Press; 1992. pp. 75–83. [Google Scholar]
- 39.Morais da Silva S., Hacker A., Harley V., Goodfellow P., Swain A., Lovell Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat. Genet. 1996;14:62–68. doi: 10.1038/ng0996-62. doi:10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- 40.Pachnis V., Mankoo B., Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi A., Kwan K.M., Carroll T.J., McMahon A.P., Mendelsohn C.L., Behringer R.R. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development. 2005;132:2809–2823. doi: 10.1242/dev.01858. doi:10.1242/dev.01858. [DOI] [PubMed] [Google Scholar]
- 42.Kispert A., Vainio S., Shen L., Rowitch D.H., McMahon A.P. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development. 1996;122:3627–3637. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- 43.Hogan B.L., Beddington R.S., Constantini F., Lacy E. Manipulating the Mouse Embryo. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]


