Abstract
Transcatheter closure of secondum atrial septal defect (ASD) is an alternative option to open heart surgery with good short and long-term outcomes. For this purpose, the Amplatzer septal occluder (ASO) device is widely used. Arrhythmias are known complications of ASD device closure including atrial ectopy and heart block.
We report a seven-year-old female patient who developed second degree atrioventricular block (AVB) within few hours after ASD device closure using ASO device. At the seventh post-procedure day; while under close observation; patient regained sinus rhythm which was maintained thereafter. A 3-day course of prednisolone was given.
Keywords: Amplatzer Septal Occluder, Secondum atrial septal defect, Heart block, PSCC-Qassim
Case report
A seven-year-old girl presented to the outpatient department with a history of palpitations on exertion for two months. It was not associated with syncope or dizziness. She was not on any medication. Examination revealed a thin, young girl weighing 13 kg (below the 5th percentile). Her height was 109 cm (below the 5th percentile). Cardiovascular examination showed a regular pulse, fixed splitting of the second heart sound, and an ejection systolic murmur grade 3/6 at the upper left sternal border. Pre-intervention 12-lead electrocardiogram (ECG) showed a normal sinus rhythm at a rate of 100 per minute with a normal PR interval. There was an incomplete right bundle branch block with QRS duration of 90 ms (Fig. 1). The echocardiogram was diagnostic of a 20-mm atrial septal defect (ASD) secondum type with moderate dilatation of the right atrium and right ventricle. The ASD rims were adequate with a 15 mm rim to the superior vena cava (SVC), 6 mm rim to the inferior vena cava (IVC), 4 mm rim to the aorta, and 6 mm rim to the atrioventrivcular (AV) valves.
Figure 1.
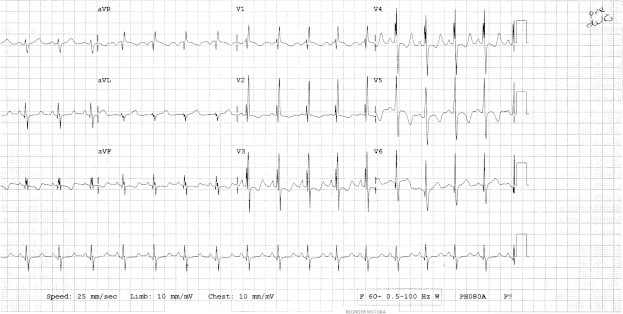
ECG prior to ASD device closure. Sinus rhythm, with normal PR interval and rSR in V1 (incomplete RBBB).
In the cardiac catheterization laboratory, under general anesthesia, transesophageal echocardiography (TEE) confirmed the diagnosis and showed the presence of a deficient rim to the aorta and a small rim to the AV valves.
The patient had transcatheter ASD device closure using a 22 mm AMPLATZER® Septal Occluder (ASO) device (AGA Medical Corporation), under fluoroscopy and TEE guidance. The device/patient height ratio was 0.20. The procedure was uncomplicated and the patient was transferred to the recovery area in stable condition. Eight hours after the procedure she had an irregular rhythm. A 12-lead ECG showed second degree atrioventricular block (Mobitz I/Wenckebach periodicity) (Fig. 2).
Figure 2.
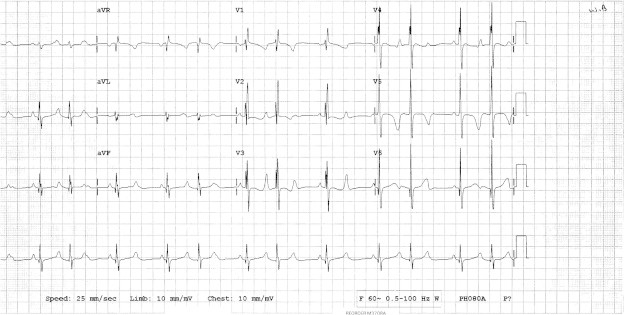
ECG after ASD device. Second degree heart block type 1 (progressive prolongation of the PR interval).
A transthoracic echocardiogram showed that the ASD device was in good position with no residual shunt and no compromisation to nearby structures (SVC, IVC, Pulmonary veins, and AV valves). There was no pericardial effusion (see Fig. 3).
Figure 3.
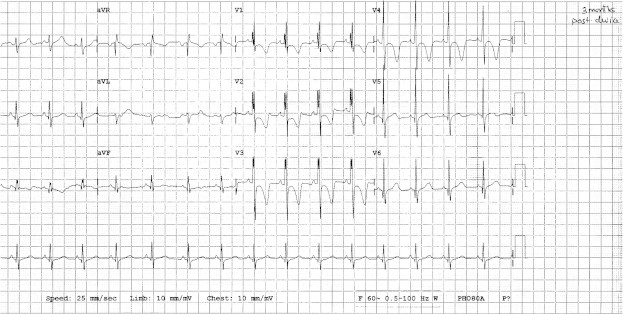
Follow up ECG post ASD device. Resolution of heart block, sinus rhythm, normal PR interval and signs of incomplete RBBB.
She was kept in the hospital for close observation and a twice daily ECG. Holter telemetry recording for 24 h confirmed the presence of second degree heart block with 2:1 conduction as well as Wenckebach periodicity. In the third post procedure day she was started on Prednisolone 2 mg/kg/day divided every eight hours for three days. At the seventh post cath day, her rhythm returned to sinus with normal AV conduction. The 24 h telemetry recording confirmed the normal conduction with no evidence of AV block. She was discharged home on Aspirin 5 mg/kg/day orally for six months. She was seen three weeks later and then at three and six months post intervention. ECG revealed that she is still in sinus rhythm with normal PR interval. Echo revealed that the device is in position, with no impingement on nearby structures and no pericardial effusion.
Discussion
ASDs are one of the most common congenital heart diseases. It is well known that ASDs may be associated with subclinical ECG abnormalities, including sinus node dysfunction, conduction delay, and AV block [1,2].
The ECG of patients with ASD often shows right-axis deviation, right atrial enlargement, incomplete right bundle-branch block (secundum ASD), superior left-axis deviation (primum ASD), or an abnormal P-wave axis (superiorly located sinus venosus ASD). Complete heart block may be present in association with familial ASD [3]. Familial ASD with AV conduction defect is an autosomal dominant trait, with mutations in the cardiac homeobox transcription factor gene NKX2–5 [4,5].
Closure of an ASD either percutaneously or surgically is indicated for right atrial and RV enlargement with or without symptoms. (Level of Evidence: B)
Surgical closure has been the “gold standard” form of treatment, with excellent late outcome. The development of percutaneous transcatheter closure techniques has provided an alternative method of closur for uncomplicated secundum ASDs with appropriate morphology [6,7].
ASO can be used for ASD closure in infants and young children provided the diameter of the disk is smaller than the diameter of the atrial septum [8,9].
Follow-up for patients after device closure requires clinical assessment of symptoms of arrhythmia, chest pain, or embolic events and echocardiographic surveillance for device position, residual shunting, and complications such as thrombus formation or pericardial effusion.
Reported complications after ASD catheter intervention include residual shunt, device misalignment, embolization, erosion of atrial wall or aorta, impingement on adjacent structures (AV valves, Coronary sinus, SVC, pulmonary veins, Aorta) and device thrombosis. Endocarditis for the first six months is also a possible complication [10].
Heart block can occur during or after ASD closure using the ASO device with a frequency of 6.1% [11,12].
Johnson et al. reported a low risk of clinically significant post-procedure arrhythmias after device placement with an incidence of 5.2% of arrhythmias in the four months following device placement, including atrial tachyarrhythmias, junctional tachycardia, and heart block. Clinically significant heart block occurred in only two of their patients (0.3%) [13].
Cardiac arrhythmias and heart block were not of the causes for cardiac surgery after ASD device closure in some reported complications [14,15].
The size of the device can be a predisposing factor for AVB after ASD closure. Device size equal to or more than 18 mm, and a device/height ratio equal to or more than 0.18 was reported as a predisposing factor for AVB after ASD closure [11,12].
Patients who receive 18 mm or larger device, or a device/height ratio >0.18 need closer hemodynamic and ECG monitoring during and after the procedure. Twelve-lead ECG and 24-h Holter recordings are necessary later on, whereas exercise testing may be required for selected patients.
AVB during ASO device closure may necessitate the immediate removal of the device during the procedure if the AVB did not improve within 30 min.
Patients with AVB that appears after the ASD device closure need to be observed in the hospital until hemodynamically stable and regression of AVB is observed. Subsequent ambulatory follow-up with Holter monitoring is required.
The mechanism of AVB secondary to ASO device could be due to continuous pressure or friction of atrial discs on the AV node, leading to edema and AVB. This could be due to a deficient rim to the atrioventricular valves and a small distance between the right atrial disc and the tricuspid valve [10].
AVB after ASD device may resolve within two weeks after the procedure, which may or may not be related to steroid administration [11,12].
In some series, AVB after ASO device closure of ASDs resolves or improves spontaneously, with no recurrence at mid-term follow-up [16].
Once it is decided to remove the device in patients with AVB after ASD device closure it is important not to delay this to avoid ischemia and fibrosis resulting in permanent injury of the AV node. Leaving the device in place and inserting a permanent pacemaker is also an option but this carry the morbidity associated with pacemaker placement, such as equipment malfunction, certain sports restrictions, infection, and the requirement of life-long battery changes. Therefore prompt surgical removal of the device and ASD surgical closure could be the best option in such situation.
Conclusion
AVB is one of the important complications after ASD device closure especially when using large devices (18 mm or more or the device/height ratio is more than 0.18). Close observation of the ECG during and after the procedure is very important. AVB after ASD device may resolve spontaneously with or without steroids administration or may require removal of the device.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Clark E.B., Kugler J.D. Preoperative secundum atrial septal defect with coexisting sinus node and atrioventricular dysfunction. Circulation. 1982;65:976–980. doi: 10.1161/01.cir.65.5.976. [DOI] [PubMed] [Google Scholar]
- 2.Bink-Boelkens M.T.E., Bergstra A., Landsman M.L.J. Functional abnormalities of the conduction system in children with an atrial septal defect. Int J Cardiol. 1988;20:263–272. doi: 10.1016/0167-5273(88)90271-9. [DOI] [PubMed] [Google Scholar]
- 3.Bizarro R.O., Callahan J.A., Feldt R.H., Kurland L.T., Gordon H., Brandenburg R.O. Familial atrial septal defect with prolonged atrioventricular conduction. A syndrome showing the autosomal dominant pattern of inheritance. Circulation. 1970;41:677–683. doi: 10.1161/01.cir.41.4.677. [DOI] [PubMed] [Google Scholar]
- 4.Schott J.J., Benson D.W., Basson C.T. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281:108–111. doi: 10.1126/science.281.5373.108. [DOI] [PubMed] [Google Scholar]
- 5.Pease W.E., Nordenberg A., Ladda R.L. Familial atrial septal defect with prolonged atrioventricular conduction. Circulation. 1976;53:759–762. doi: 10.1161/01.cir.53.5.759. [DOI] [PubMed] [Google Scholar]
- 6.Fischer G., Stieh J., Uebing A., Hoffmann U., Morf G., Kramer H.H. Experience with transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder: a single centre study in 236 consecutive patients. Heart. 2003;89:199–204. doi: 10.1136/heart.89.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhillon R., Thanopoulos B., Tsaousis G., Triposkiadis F., Kyriakidis M., Redington A. Transcatheter closure of atrial septal defects in adults with the Amplatzer septal occluder. Heart. 1999;82:559–562. doi: 10.1136/hrt.82.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diab K.A., Cao Q.L., Bacha E.A., Hijazi Z.M. Device closure of atrial septal defects with the Amplatzer septal occluder: safety and outcome in infants. J Thorac Cardiovasc Surg. 2007 Oct;134(4):960–966. doi: 10.1016/j.jtcvs.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Du Z.D., Hijazi Z.M., Kleinman C.S., Silverman N.H., Larntz K. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39:1836–1844. doi: 10.1016/s0735-1097(02)01862-4. [DOI] [PubMed] [Google Scholar]
- 10.Hill S.L., Berul C.I., Patel H.T., Rhodes J., Supran S.E., Cao Q.L. Early ECG abnormalities associated with transcatheter closure of atrial septal defects using the Amplatzer septal occluder. J Interv Card Electrophysiol. 2000 Oct;4(3):469–474. doi: 10.1023/a:1009852312907. [DOI] [PubMed] [Google Scholar]
- 11.Suda Kenji, Raboisson Marie-José, Piette Eric. Reversible atrioventricular block associated with closure of atrial septal defects using the Amplatzer device. J Am Coll Cardiol. 2004;43:1677–1682. doi: 10.1016/j.jacc.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Al-Anani S.J., Weber H., Hijazi Z.M. Atrioventricular block after transcatheter ASD closure using the Amplatzer septal occluder: risk factors and recommendations. Catheter Cardiovasc Interv. 2010 Apr 1;75(5):767–772. doi: 10.1002/ccd.22359. [DOI] [PubMed] [Google Scholar]
- 13.Johnson J.N., Marquardt M.L., Ackerman M.J. Electrocardiographic changes and arrhythmias following percutaneous atrial septal defect and patent foramen ovale device closure. Catheter Cardiovasc Interv. 2011;78(2):254–261. doi: 10.1002/ccd.23028. [Epub 2011 May 11] [DOI] [PubMed] [Google Scholar]
- 14.Sarris G.E., Kirvassilis G., Zavaropoulos P. Surgery for complications of trans-catheter closure of atrial septal defects: a multi-institutional study from the European Congenital Heart Surgeons Association. Eur J Cardiothorac Surg. 2010;37(6):1285–1290. doi: 10.1016/j.ejcts.2009.12.021. [Epub 2010 Mar 28] [DOI] [PubMed] [Google Scholar]
- 15.Chessa Massimo, Carminati Mario, Butera Gianfranco. Early and Late Complications Associated With Transcatheter Occlusion of Secundum Atrial Septal Defect. J Am Coll Cardiol. 2002;39:1061–1065. doi: 10.1016/s0735-1097(02)01711-4. [DOI] [PubMed] [Google Scholar]
- 16.Suda K., Raboisson M.J., Piette E. Reversible atrioventricular block associated with closure of atrial septal defects using the Amplatzer device. J Am Coll Cardiol. 2004;43(9):1677–1682. doi: 10.1016/j.jacc.2003.12.042. [DOI] [PubMed] [Google Scholar]



