Abstract
Background
Atrial septal defects (ASD) account for 10% of all congenital heart lesions and represent the third most congenital cardiac defect seen in adults.
Objectives
Using strain and strain rate imaging (SRI) to assess right ventricular (RV) function in patients with ASD and correlate the results with the level of N-terminal pro-brain natriuretic peptide (NT-proBNP) before and after transcatheter closure.
Methods
At the Hungarian Institute of Cardiology, 27 females and 18 males (mean age 21.53 years) were diagnosed with ASD and admitted for percutaneous closure. Echocardiography was done to assess theleft ventricular (LV), RV and left atrial (LA) diameters. For assessment of systolic RV function, we measured Tricuspid annular plane systolic excursion (TAPSE), strain, and SRI. Amplatzer ASD closure was done under general anesthesia. NT-proBNP levels were measured before and three months after closure.
Results
ASD closure was achieved in all patients. The mean ASD diameter was 15.15 mm. The size of the occluder ranged from 10 to 24 mm. The mean LA diameter in the pre-closure group was significantly higher than the control; mean left ventricular end diastolic diameter (LVEDD) showed a non-significant difference from either the control group or the post-closure group, while the mean right ventricular end diastolic diameter (RVEDD) markedly reduced post-closure, and it was significantly higher than the control group. Global RV strain and peak systolic strain rate (PSSR) were significantly higher in ASD group than in the control. The NT-proBNP levels were found to be correlated with pulmonary arterial pressure (PAP), TAPSE, global RV strain and PSSR.
Conclusion
Volume overload induced by ASD is associated with increased strain values, which return to normal after closure. NT-proBNP is a parameter which correlates to RV pressure, PAP and the amount of shunt volume caused by an ASD.
Keywords: Atrial septal defect, Strain and SRI, Right ventricular function, NT-proBNP
Background
Atrial septal defect (ASD) is the third most common congenital cardiac defect encountered in adults [1]. It accounts for almost 10% of all congenital heart lesions. The most common complications recorded in association are right ventricular volume overload, congestive heart failure, atrial arrhythmias and latter, the development of pulmonary arterial hypertension (PAH) [1].
Quantitative assessment of right ventricular (RV) function is still challenging due to its complex anatomy and thin wall structure, and therefore is not incorporated into daily clinical practice. However, its assessment is becoming of increasing interest in certain cardiac diseases that affect the right ventricle (pulmonary hypertension, pulmonary stenosis, atrial, or ventricular septal defects [2]). There are several limitations in assessing RV ejection fraction (EF) using conventional echocardiography methods. Tissue Doppler techniques can allow the quantification of myocardial function [3]. Myocardial strain is a dimensionless index of tissue deformation expressed as a fraction or per cent change. Myocardial lengthening gives a positive and myocardial shortening a negative strain value. Strain rate (SR) measures the local rate of deformation per time unit [4,5].
Two-dimensional (2D) strain and SR analyses are novel Doppler-independent techniques to obtain these measurements of myocardial movement and deformation [6]. They have been frequently used to assess left ventricular (LV) function, but rarely been used to examine RV function [7].
In response to an increase in right atrial (RA) pressure and/or volume, atrial natriuretic peptide (ANP) level “a cardiac neurohormone” will increase in RA [8,9]. It is an important regulator of the volume homeostasis [10]. It may be elevated in patients with congenital and acquired heart disease [11]. In left heart failure, high brain natriuretic peptide (BNP) levels are associated with impaired exercise capacity and a poor prognosis [12,13], but the data is limited In right heart failure (there are no data relating serum BNP levels to the severity of RV failure and pulmonary pressure in patients with ASD [14,15]).
Objective of the present study was to use strain and strain rate imaging to assess right ventricular function in patients with right ventricular volume overload due to atrial septal defect and correlate the results with the level of N-terminal pro-brain natriuretic peptide (NT-proBNP) before and after transcatheter closure.
Subjects & methods
At the Hungarian Institute of Cardiology Budapest, Hungary, 45 patients (27 females and 18 males) with a mean age of 21.53 ± 18.09 years (range 5–67 years) were diagnosed with ASD and admitted to the centre for percutaneous closure between April 2008 and March 2009 after giving their informed consent. Decision for ASD closure was based upon haemodynamically significant left-to-right shunt (>40% of pulmonary blood flow) or echocardiographic signs of right heart dilation or shunt related symptoms. None of them had a previous stroke, coronary artery disease, pulmonary disease or a significant valvular lesion (more than mild). Patients with ASD who had other forms of congenital heart disease and/or life-threatening arrhythmias were excluded from the study. Patients with large ASD > 3 cm were excluded and sent for surgical correction.
The control group consisted of 40 healthy sex and age-matched volunteers after obtaining their informed consent as well. Echocardiography was performed on the day before and three months after percutaneous closure of the ASD.
Echocardiographic examination
Echocardiographic examination was performed with the patient in the supine position or in left lateral semi-recumbence according to the ASE guidelines [16] using an ultrasound system (SONOS 5500, Hewlett-Packard Company and Philips Invisor C) equipped. Standard echocardiography included parasternal long, parasternal short, apical four-chamber, apical three-chamber, and apical two-chamber views. LV, RV and left atrial (LA) diameters were registered by M-mode in the parasternal long-axis view.
RV systolic function was measured using tricuspid annular plane systolic excursion (TAPSE) by conventional M-mode, peak systolic strain and strain rate.
SRI was acquired from the tissue velocity imaging (TVI) mode, frame rates varied from 99 to 134 frames per second, with a mean value of 116. Analysis was performed offline using the built-in Q analysis software (echo pack) to measure peak systolic strain rate (PSSR) as the maximal negative strain rate within 350 ms after the QRS complex.
A region of interest (ROI) was placed in the basal part of the selected segment halfway between the endocardium and epicardium. Auto-correction of ROI location during systolic contraction accounted for the inward motion of the ventricular wall to keep the ROI halfway between the endocardium and epicardium. Three consecutive beats were analyzed and the mean was calculated. The spectral pulsed Doppler signal was adjusted to obtain a Nyquist limit of 40 cm/s, with low-wall filter settings and minimum optimal gain. No angle correction was made. Electrocardiograms were simultaneously recorded in all cases.
Trans-catheter closure of atrial septal defect
Transcatheter ASD closure was conducted under general anesthesia, with fluoroscopic and transesophageal echocardiographic guidance in all patients. Procedures were performed using previously described techniques, including ASD sizing with a compliant balloon (AGA Medical) and closure with an Amplatzer Atrial Septal Occluder (AGA Medical Corp., USA) [17]. Procedural success was defined as the successful deployment of an atrial septal occluder at the end of the procedure.
NT-pro BNP analysis
Blood samples were collected from the antecubital vein before and three months after trans-catheter closure of ASD. Plasma levels of NT-proBNP concentrations were measured on the Elecsys–2010 system (Roche Diagnostics) [18]. This assay is an electrochemiluminescent sandwich immunoassay using two polyclonal antibodies directed at the NT-proBNP molecule.
Statistics
Data are analyzed using Microsoft Excel sheets. The data are expressed as mean ± standard deviation (SD), variables in patient and control group or in patient group pre and post ASD closure were compared using Student t test and correlations between NT-proBNP, tissue Doppler variables or PAP were obtained by using multiple regression analysis. P < 0.05 is considered statistically significant.
Results
Transcatheter closure of ASD was achieved in all patients with an Amplatzer ASD occluder device, and no residual shunt was noted in the entire study group. No major complications occurred after the procedure. All patients had a single device implanted. The clinical characteristics and haemodynamic data of patients with ASD are listed in Table 1 and Fig. 1. The mean ASD diameter was 15.2 ± 3.16 mm and the stretched diameter was 18.7 ± 3.39 mm. The sizes of occluder ranged from 10 to 24 mm, mean 17.35 ± 3.29, using sizing balloon 24 mm of AGA Medical Corp, USA, AEW 0.035″ and Amplatzer sheath 9–10 French.
Table 1.
The clinical characteristics and haemodynamic data of patients with ASD.
| Parameter | Patient group |
|---|---|
| Sex (male/female) | 18/27 |
| Age (years) | 21.53 |
| Height (cm) | 156.22 |
| Weight (kg) | 62.02 |
| ASD diameter by TEE (mm) | 15.15 |
| ASD balloon sizing (mm) | 18.72 |
| Device size (mm) | 17.35 |
| Mean PAP (mmHg) | 35.91 |
| Mean RAP (mmHg) | 10.13 |
| Mean RVSP (mmHg) | 33.31 |
| Qp/Qs | 2.25 |
Figure 1.
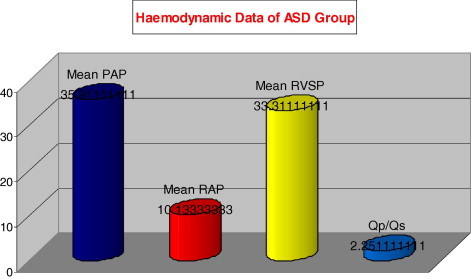
The haemodynamic data of the patients group.
The mean pulmonary artery pressure (PAP) was 35.9 ± 7.86 mmHg; mean right atrial pressure (RAP) was; 10.1 ± 2.04 mmHg, while mean right ventricular systolic pressure (RVSP) was; 33.3 ± 6.63 mmHg. The mean shunt volume was; Qp/Qs 2.25 ± 0.4.
At three months follow up, complete occlusion of the ASD was demonstrated by transesophageal echo (TEE) in all patients, and no significant residual shunting through the device using color Doppler flow.
The echocardiographic measurements of the patients and the controls are given in Table 2. The mean left atrial (LA) diameter 39.7 ± 2.02 mm in the pre-ASD closure group that was significantly higher than the control, P < 0.05; with a non-significant difference after closure; mean left ventricular end diastolic dimension (LVEDD) was 49.1 ± 3.23 mm, that showed non-significant difference from either control group or post ASD closure group. While mean right ventricular end diastolic dimension (RVEDD) 35.41 ± 2.04 mm, that markedly reduced post ASD closure, and it was significantly higher than control group, P < 0.05.
Table 2.
The echocardiography variables pre, post ASD closure & in controls.
| Control group | ASD |
P-value pre and post | ||
|---|---|---|---|---|
| Pre | Post | |||
| LA (mm) | 35.5 | 39.7 | 39.9 | N.S. |
| LVEDD (mm) | 53.2 | 49.1 | 50.7 | N.S. |
| RVEDD (mm) | 26.4 | 35.4 | 28.8 | <0.05 |
| TAPSE (mm) | 17.3 | 22.6 | 19.2 | <0.05 |
| Global strain | −24.1 ± 0.5 | −22.2 ± 0.7 | −23.9 ± 0.4 | <0.05 |
| PSSR | −1.4 ± 0.2 | −1.2 ± 0.5 | −1.4 ± 0.6 | <0.05 |
Global right ventricular strain and PSSR were significantly lower in control group than ASD pre-closure group (−24.1 ± 0.5 vs. −22.2 ± 0.7, P < 0.05 for global strain; and −1.4 ± 0.2 vs. −1.2 ± 0.5, P < 0.05 for PSSR respectively). In the post-device closure group, there was significant reduction of the global RV strain and the PSSR to be −23.9 ± 0.4 and −1.4 ± 0.6 respectively; there was a non-significant difference between this group and control one. There was also a significant reduction of the TAPSE that decreased from 22.6 ± 0.06 mm in pre-ASD closure group to 19.2 ± 3.4 mm in post-ASD closure group, P < 0.05. There was a positive correlation between NT-pro BNP levels in patients with ASD and PAP (r = 0.74, P < 0.05); RA pressure (r = 0.77, P < 0.05); RVSP (r = 0.82, P < 0.01), Qp/Qs (r = 0.71, P < 0.05). It also correlated with TAPSE (r = 0.80, P < 0.01), global RV strain (r = 0.72, P < 0.05) and PSSR (r = 0.79, P < 0.05).
At three months after closure, there was a significant reduction of mean peptide level (Fig. 2) from 194 ± 87 pg/ml to 133 ± 35 pg/ml (P < 0.01).
Figure 2.
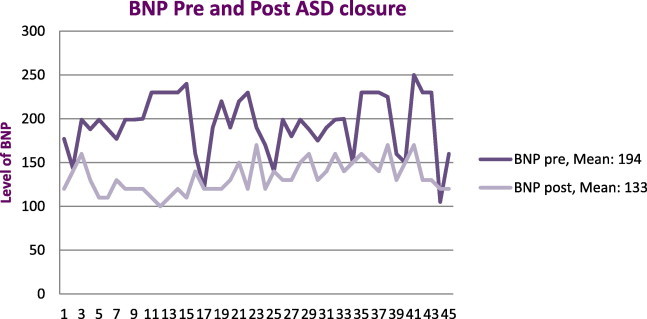
Showed a significant reduction of mean peptide level (BNP) after atrial septal defect closure (P < 0.01).
Discussion
In this study transcatheter closure of ASD was achieved in 45 patients using an Amplatzer device with no residual shunt in the entire patient group. The mean ASD diameter was 15.2 ± 3.16 mm and the stretched diameter was 18.72 ± 3.39 mm. The size of the occluder ranged from 10 to 24 mm, mean 17.35 ± 3.29. A sizing balloon of 24 mm of AGA, AEW 0.035″ and an Amplatzer sheath 9–10 French was used. Our study group had average or mild increased pulmonary artery pressure and mean shunt volume was 2.25 ± 0.4. The mean left atrial diameter in pre-ASD closure group was significantly higher than the control (P < 0.05), with a non-significant difference after closure; mean left ventricular end diastolic dimension showed a non-significant difference from either the control group or post-ASD closure group. While the right ventricular diameter decreased significantly after ASD closure, it was significantly higher than control group (P < 0.05).
Few studies used 2D strain and SR analysis for the assessment of the systolic RV function in patients with RV volume overload secondary to ASD as Smita et al. [19], who concluded that two-dimensional strain appears to be helpful for the assessment of RV function and its response to correction of volume overload. Our study demonstrates that patients with RV volume overload due to an ASD had increased strain values when compared with age-matched healthy adults (P < 0.05). The increased myocardial strain values return to normal three months after trans-catheter closure. Accordingly, we observed a significant reduction of the TAPSE as well (P < 0.05). These results correlated with that of Smita et al. [19].
We discovered that the Plasma NT-pro BNP concentration correlated well with TAPSE, strain and PSSR as well as pulmonary artery pressure, right atrial pressure and right ventricular pressure. Moreover, NT-pro BNP concentration before closure of the defect showed good correlation with the amount of inter-atrial left-to-right shunt. The majority of data from previous studies showed that plasma BNP levels and its co-released peptide NT-pro BNP are linked to haemodynamic indices of left ventricular function [20,21]. Only a few reports deal with BNP levels in the context of RV dysfunction [22]. The findings of the present study are in agreement with the data of Schoen et al. [23] who reported that Plasma NT-pro BNP concentration measured in parallel to MRI scans decreased with good correlation to the restitution of RV dilatation. Muta et al. [24] who described elevated BNP levels in pediatric patients with ASD came with results similar to ours. Additionally, Trojnarska et al. [25] reported on increased BNP concentrations in adult patients with ASD. Recently Weber et al. [26] postulated that NT-pro BNP is not increased in patients with ASD.
Conclusion
Transcatheter ASD closure techniques have become an alternative to surgical procedure using cardiopulmonary bypass. Two-dimensional strain and SR measurements are a novel approach used in assessment of global and regional myocardial function. Right ventricular volume overload is associated with increased strain values, which return to normal after abolishment of the volume overload. SR seems to be less dependent on load of the right ventricle. NT-pro BNP correlates to right ventricular strain, pulmonary pressure and left-to-right shunt in volume load of the right heart caused by an underlying ASD.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Veldtman G.R., Razack V., Siu S. Right ventricular form and function after percutaneous atrial septal defect device closure. J Am Coll Cardiol. 2001;37:2108–2113. doi: 10.1016/s0735-1097(01)01305-5. [DOI] [PubMed] [Google Scholar]
- 2.Dambrauskaite V., Delcroix M., Claus P. Regional right ventricular dysfunction in chronic pulmonary hypertension. J Am Soc Echocardiogr. 2007;20:1172–1180. doi: 10.1016/j.echo.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Weyman A.E., Jiang L. Right ventricle. In: Weyman A.E., editor. Baltimore. Lippincott Williams & Wilkins; MD: 1994. pp. 901–921. [Google Scholar]
- 4.Heimdal A., Stoylen A., Torp H. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr. 1998;11:1013–1019. doi: 10.1016/s0894-7317(98)70151-8. [DOI] [PubMed] [Google Scholar]
- 5.Marwick T. Measurement of strain and strain rate by echocardiography. Ready for prime time? J Am Coll Cardiol. 2006;47:113–127. doi: 10.1016/j.jacc.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 6.Perk G., Tunick P.A., Kronzon I. Non-Doppler two-dimensional strain imaging by echocardiography—from technical considerations to clinical applications. J Am Soc Echocardiogr. 2007;20:234–243. doi: 10.1016/j.echo.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Leitman M., Lysyansky P., Sidenko S. Two-dimensional strain—a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–1029. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Nagaya N., Nishikimi T., Uematsu M. Secretion patterns of brain natriuretic peptide and atrial natriuretic peptide in patients with or without pulmonary hypertension complicating atrial septal defect. Am Heart J. 1998;136:297–301. doi: 10.1053/hj.1998.v136.89729. [DOI] [PubMed] [Google Scholar]
- 9.Suda K., Matsumura M., Matsumoto M. Clinical implication of plasma natriuretic peptides in children with ventricular septal defect. Pediatr Int. 2003;45:249–254. doi: 10.1046/j.1442-200x.2003.01716.x. [DOI] [PubMed] [Google Scholar]
- 10.De Zeeuw D., Janssen W.M.T., de Jong P.E. Atrial natriuretic factor: its (patho) physiological significance in humans. Kidney Int. 1992;41:1115–1133. doi: 10.1038/ki.1992.172. [DOI] [PubMed] [Google Scholar]
- 11.Levin E.R., Gardner D.G., Samson W.K. Natriuretic peptides. N Engl J Med. 1998;339:321–331. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 12.Kruger S., Graf J., Kunz D. Brain natriuretic peptide levels predict functional capacity in patients with chronic heart failure. J Am Coll Cardiol. 2002;40:718–722. doi: 10.1016/s0735-1097(02)02032-6. [DOI] [PubMed] [Google Scholar]
- 13.Koglin J., Pehlivanli S., Schwaiblmair M. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol. 2001;38:1934–1941. doi: 10.1016/s0735-1097(01)01672-2. [DOI] [PubMed] [Google Scholar]
- 14.Nagaya N., Nishikimi T., Okano Y. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol. 1998;31:202–208. doi: 10.1016/s0735-1097(97)00452-x. [DOI] [PubMed] [Google Scholar]
- 15.Nagaya N., Nishikimi T., Uematsu M. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- 16.Cheitlin MD, Armstrong WF, Aurigemma GP, et al.: ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). 2003.
- 17.Du Z., Hijazi Z., Kleinman C. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39:1836–1844. doi: 10.1016/s0735-1097(02)01862-4. [DOI] [PubMed] [Google Scholar]
- 18.Collinson P.O., Barnes S.C., Gaze D.C. Analytical performance of the N-terminal pro B type natriuretic peptide (NT-proBNP) assay on the Elecsys 1010 and 2010 analysers. Eur J Heart Fail. 2004;6(3):365–368. doi: 10.1016/j.ejheart.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Smita R., Werner S., Thomas B. Two-dimensional strain and strain rate imaging of the right ventricle in adult patients before and after percutaneous closure of atrial septal defects. Eur J Echocardiogr. 2009;10(4):499–502. doi: 10.1093/ejechocard/jen315. [DOI] [PubMed] [Google Scholar]
- 20.Galasko G., Lahiri A., Barnes S. What is the normal range for N-terminal pro-brain natriuretic peptide? How well does this normal range screen for cardiovascular disease? Eur Heart J. 2005;26:2269–2276. doi: 10.1093/eurheartj/ehi410. [DOI] [PubMed] [Google Scholar]
- 21.Grantham J., Burnett J. BNP: increasing importance in the pathophysiology and diagnosis of congestive heart failure. Circulation. 1997;96:388–390. [PubMed] [Google Scholar]
- 22.Leuchte H., Holzapfel M., Baumgartner R. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol. 2004;43:764–770. doi: 10.1016/j.jacc.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 23.Schoen S., Zimmermann T., Kittner T. NT-pro BNP correlates with right heart haemodynamic parameters and volumes in patients with atrial septal defects. Eur J Heart Fail. 2007;9(6–7):660–666. doi: 10.1016/j.ejheart.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Muta H., Ishii M., Maeno Y. Quantitative evaluation of the changes in plasma concentrations of cardiac natriuretic peptide before and after transcatheter closure of atrial septal defect. Acta Paediatr. 2002;91(6):649–652. doi: 10.1080/080352502760069043. [DOI] [PubMed] [Google Scholar]
- 25.Trojnarska O., Szyszka A., Gwizdala A. Evaluation of exercise capacity with cardiopulmonary exercise testing and type b natriuretic peptide concentrations in adult patients with patent atrial septal defect. Cardiology. 2006;106(3):154–160. doi: 10.1159/000092770. [DOI] [PubMed] [Google Scholar]
- 26.Weber M., Dill T., Deetjen A. Left ventricular adaptation after atrial septal defect closure assessed by increased concentrations of N-terminal pro-brain natriuretic peptide and cardiac magnetic resonance imaging in adult patients. Heart May. 2006;92(5):671–675. doi: 10.1136/hrt.2005.065607. [DOI] [PMC free article] [PubMed] [Google Scholar]



