Summary
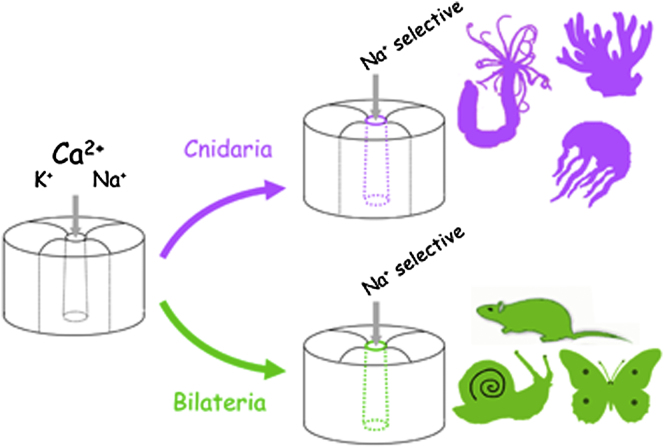
Ion selectivity of metazoan voltage-gated Na+ channels is critical for neuronal signaling and has long been attributed to a ring of four conserved amino acids that constitute the ion selectivity filter (SF) at the channel pore. Yet, in addition to channels with a preference for Ca2+ ions, the expression and characterization of Na+ channel homologs from the sea anemone Nematostella vectensis, a member of the early-branching metazoan phylum Cnidaria, revealed a sodium-selective channel bearing a noncanonical SF. Mutagenesis and physiological assays suggest that pore elements additional to the SF determine the preference for Na+ in this channel. Phylogenetic analysis assigns the Nematostella Na+-selective channel to a channel group unique to Cnidaria, which diverged >540 million years ago from Ca2+-conducting Na+ channel homologs. The identification of Cnidarian Na+-selective ion channels distinct from the channels of bilaterian animals indicates that selectivity for Na+ in neuronal signaling emerged independently in these two animal lineages.
Graphical Abstract
Highlights
► Na+ channel homologs that conduct Ca2+ appeared more than a billion years ago ► Na+ selectivity evolved separately in Cnidaria and Bilateria ► Na+ selectivity is conferred by structural differences at the channel pore
Moran and colleagues have now characterized sodium channel homologs from a sea anemone, revealing four members with a preference for calcium ion conductance, as well as a sodium-selective channel. Sodium selectivity in this channel is conferred by a selectivity filter and pore structure that differ from those of higher animals (Bilateria). A phylogenetic analysis indicates that sodium selectivity in Cnidaria (sea anemones, corals, and jellyfish) evolved independently from that in Bilateria >540 million years ago, reflecting requirements for improved neuronal signaling in a changing environment.
Introduction
The emergence of nervous systems that enable the integration of external stimuli and coordinated responses was a key event in the evolution of animal body plans. Signaling in these systems is based on fast and accurate propagation and conductance of action potentials involving voltage-gated sodium channels (Navs) (Hille, 2001; Meech and Mackie, 2007). Navs are membrane-spanning protein complexes that are composed of pore-forming α-subunits and auxiliary subunits, and conduct Na+ ions in response to changes in the membrane potential (Catterall, 2000; Hille, 2001). The α-subunit, which belongs to a protein superfamily of voltage-gated Na+, K+, and Ca2+ channels (Navs, Kvs, and Cavs, respectively), consists of four domains (DI–DIV) arranged around a central ion-conducting pore. Each domain is comprised of six transmembrane segments (S1–S6), of which the positively charged S4 (voltage sensor) moves outward in response to membrane depolarization, leading to opening of the channel pore and ion conductance. Whereas the α-subunit of Kvs is a single domain tetramer, the α-subunits of Cavs and Navs are large monomers of four homologous, nonidentical domains (Hille, 2001).
Ion selectivity is crucial for fast and accurate signaling, and the emergence of Na+-selectivity likely addressed a need to distinguish neuronal stimuli from intracellular signaling driven by Ca2+ (Hille, 2001; Meech and Mackie, 2007). The selectivity of Navs for Na+ ions is attributed to the ion selectivity filter (SF), a ring of four amino acids (Asp, Glu, Lys, and Ala [DEKA]) that are contributed by the pore-lining loops (p-loop) of the four domains (Catterall, 2000). The Lys at the third position of the DEKA SF is critical for ion selectivity, as indicated by the increase in Ca2+ and K+ conductance when it is substituted in mammalian Navs (Heinemann et al., 1992; Schlief et al., 1996). Although the DEKA SF is conserved in all vertebrate and many invertebrate Navs (Widmark et al., 2011), novel Nav-like channels with a DEEA SF have been observed in many invertebrates, including arthropods, mollusks and tunicates (Zhou et al., 2004; Cui et al., 2012; Sato and Matsumoto, 1992; Nagahora et al., 2000). Still, only two of these Nav-like channels, BSC1 of the cockroach Blattella germanica and DSC1 of Drosophila melanogaster, have been functionally expressed and shown to preferably conduct Ca2+ (Zhang et al., 2011; Zhou et al., 2004).
Understanding the evolutionary relationship between Nav and Nav-like channels may shed light on the development of ion selectivity, and requires a broad data set for phylogenetic analysis. Previous analyses either focused on vertebrate channels or mostly used fragmented and nonverified gene models from invertebrates (Goldin, 2002; Widmark et al., 2011; Liebeskind et al., 2011). These analyses showed that Nav-like channels existed in the common ancestor of animals and their unicellular relatives, choanoflagellates, and are present in the apusozoan Thecamonas trahens (Liebeskind et al., 2011; Cai, 2012). Because apusozoans diverged before the fungal-metazoan split occurred (Derelle and Lang, 2012), Nav-like channels emerged before nervous systems or multicellularity evolved. Because Nav-like channels from early branching phyla of animals or their protist relatives have not been studied directly, their impact on the evolutionary history of neuronal signaling has remained unclear.
To address this critical gap in knowledge, we focused on obtaining a phylogenetic and functional characterization of Nav-like channels from the phylum Cnidaria (sea anemones, corals, hydroids, and jellyfish). This basal animal clade is a sister group to all higher animals (Bilateria) and was among the first lineages to develop a nervous system (Watanabe et al., 2009), and is therefore highly suitable for the study of Nav evolution in animals.
Results and Discussion
Characterization of Nav-like Homologs from N. vectensis
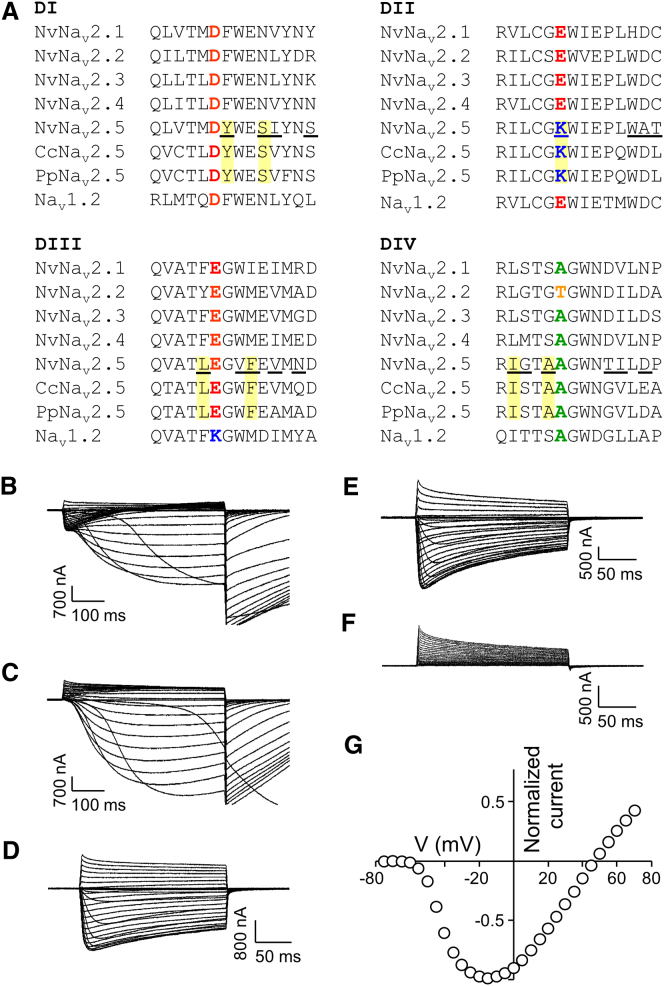
To study cnidarian Nav-like channels, we used the starlet sea anemone Nematostella vectensis (Cnidaria, Anthozoa), whose genome has been fully sequenced (Putnam et al., 2007). A homology search of the genome retrieved five putative Nav genes. cDNA cloning and sequencing revealed SFs resembling those found in Nav-like channels. Channels with DEKA SF are termed Nav1; therefore, we named the Nav-like channels Nav2, and accordingly refer to the five Nematostella channels as NvNav2.1–2.5. To functionally characterize these channels, we expressed them in Xenopus oocytes and examined their ion selectivity.
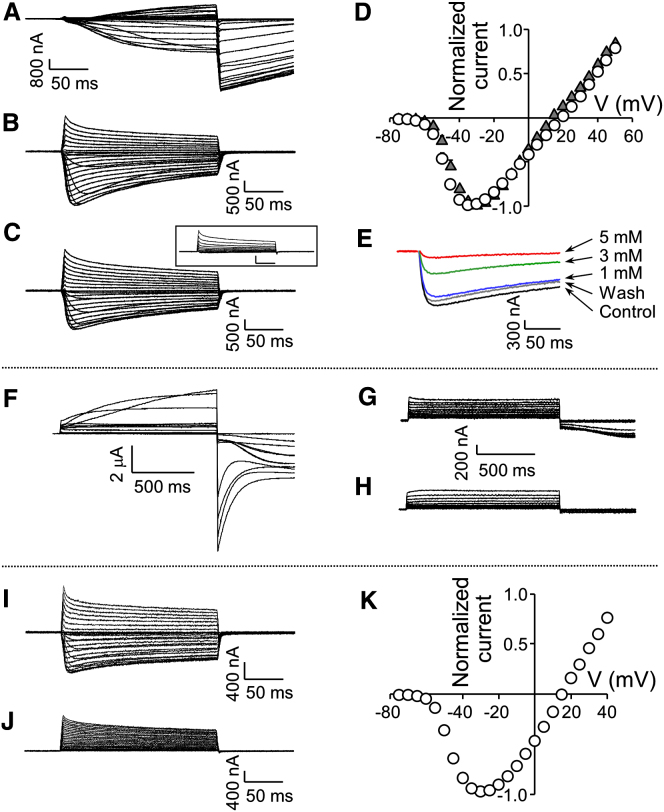
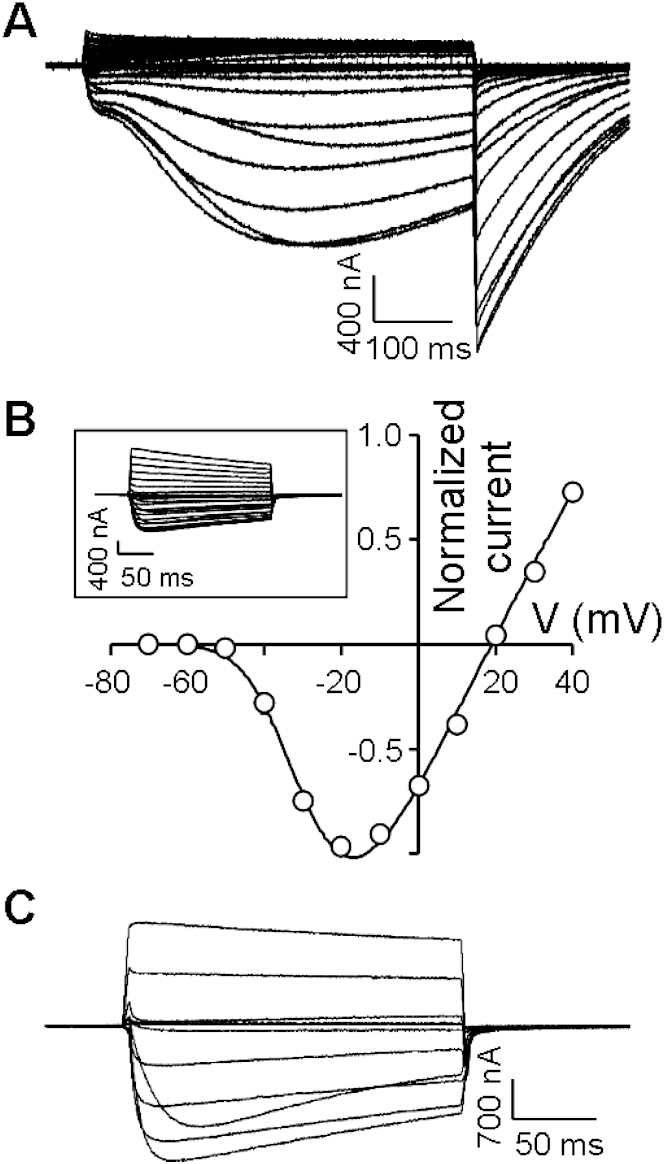
NvNav2.1, bearing a DEEA SF, exhibited slowly developing activating and inactivating currents in response to depolarizing voltage pulses, and substantial tail currents when returned to a holding potential (Figure 1A). Such currents with a reversal potential (Erev) of −13 ± 1.2 mV (n = 8; Figure S1A) are reminiscent of Cl− ion conductance through Ca2+-activated Cl− channels endogenous to Xenopus oocytes (Barish, 1983). Because these currents were reduced by the Nav1 blocker lidocaine, the Cl− currents were apparently triggered by Ca2+ influx through NvNav2.1 (Figure S1B). We removed the Cl− currents by injecting the Ca2+ chelator BAPTA into the oocytes, which uncovered voltage-dependent inward currents characterized by fast activation and slow inactivation kinetics, and Erev = 21.6 ± 2.2 mV (n = 7; Figure S1C). Further, we eliminated the Ca2+-induced Cl− currents by injecting either BAPTA or EGTA, and substituted CaCl2 in the ND96 bath solution with BaCl2, which is less likely to activate Ca2+-activated Cl− channels (Barish, 1983). Under these conditions, the inward currents measured were similar to those obtained with CaCl2 (Erev = 16.2 ± 0.8 mV, n = 14; Figures 1B and 1D). Whereas 100 μM tetrodotoxin (TTX), a Nav1 blocker, had no effect (Figure S1D), lidocaine inhibited these currents in a dose-dependent manner (Figure 1E), suggesting a structural similarity between Nav2 and Nav1 channels at the lidocaine-binding site (Cestèle and Catterall, 2000). Moreover, in the presence of >10 mM lidocaine in the medium, N. vectensis adult polyps were paralyzed within 20 min. The lidocaine effects observed in vitro and in vivo suggest that the Nav2 channels in N. vectensis have a crucial physiological role.
Figure 1.
Current Recordings from NvNav2.1, NvNav2.2, and NvNav2.1DEKA Channels Expressed in Xenopus Oocytes
Oocytes were clamped at −80 mV holding potential, and currents were elicited by 200 ms depolarizations from −75 mV to 50 mV.
(A) Ca2+-activated Cl− currents recorded in ND96 bath solution from an oocyte expressing NvNav2.1.
(B–E) NvNav2.1 currents recorded in bath solution with Ba2+ substituting for Ca2+, and in addition with choline substituting for Na+ (C) and also without Ba2+ as control (see inset). See Figure S1 for further characterization of NvNav2.1. (D) Current-voltage relations of NvNav2.1 (circles: Erev = 16.2 ± 0.8 mV; n = 14) and with choline substituting for Na+ (triangles: Erev = 13.3 ± 0.9 mV; n = 7). (E) Inward currents elicited by 200 ms depolarizing pulse to −30 mV in the presence of increasing concentrations of lidocaine. The inhibitory effect of lidocaine was removable by washes with bath solution (gray).
(F) Outward and tail currents elicited by 1 s depolarizations from −75 mV to 50 mV, measured for an oocyte expressing NvNav2.2 in ND96 bath solution.
(G and H) Currents decreased in the presence of 5 mM lidocaine (G) and were eliminated when Ca2+ was substituted with Ba2+ ions in the bath solution (H).
(I and J) NvNav2.1DEKA currents in ND96 bath solution (I) and with choline substituting for Na+ (J).
(K) Current-voltage relations of NvNav2.1DEKA in ND96 bath solution (Erev = 16.7 ± 1.1 mV; n = 14). Each point represents the mean ± SEM of n cells.
See also Figure S1.
Figure S1.
Current Recordings from NvNav2.1 Expressed in Xenopus Oocytes, Related to Figure 1
Oocytes were clamped at −100 or −80 mV holding potential in ND96 bath solution, and currents were elicited by depolarizing pulses from −75 mV to 50 mV for 200 ms.
(A) Current-voltage relations of calcium-activated chloride channels from a representative oocyte, which were activated by Ca2+ influx through NvNav2.1 (Erev = −15.9 mV).
(B) Ca2+-activated Cl− currents recorded from an oocyte expressing NvNav2.1. Addition of 5 mM lidocaine reduced the Ca2+ inward current through NvNav2.1, which decreased the chloride currents.
(C) Current-voltage relations of NvNav2.1 expressed in oocytes injected with BAPTA prior to the measurement (Erev = 21.6 ± 2.2 mV; n = 7). Each point represents mean ± SEM of n cells. Injection of BAPTA to oocytes eliminated the Ca2+-activated Cl− currents (see inset).
(D) NvNav2.1 inward current elicited by 200 ms depolarizing pulse to −30 mV with BaCl2 substituting for CaCl2 in the ND96 bath solution. The current was not affected by 100 μM TTX (red).
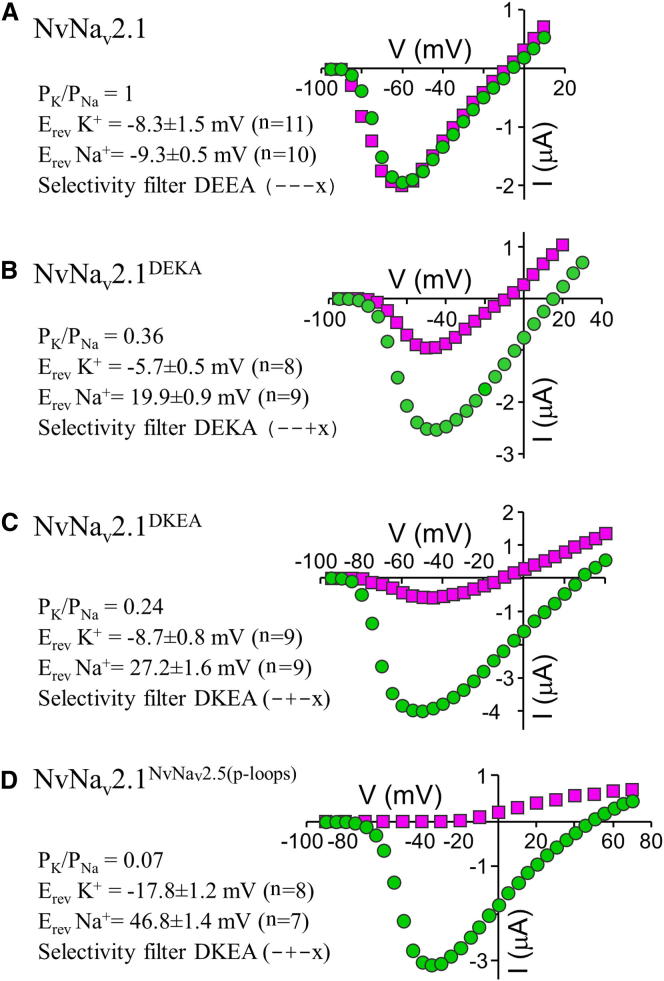
We next examined the NvNav2.1 ion conductance by substituting Na+ in the bath solution with impermeable choline ions. Because the reversal potentials with and without Na+ in the bath solution were similar (Erev Na+ = 16.2 ± 0.8 mV, n = 14; Erev choline = 13.3 ± 0.9 mV, n = 12; Figures 1C and 1D), Ba2+ ions were evidently responsible for most of the current measured, implying that under physiological conditions, Ca2+ ions are the main charge carrier conducted by these channels. Using single ion solutions of identical concentrations, we found that the channel was also permeable to Na+ and K+ ions (permeability ratio PK/PNa = 1; Figure 2A).
Figure 2.
Current-Voltage Relations for NvNav2.1 Mutants in Na+ and K+ Single Ion Solutions and Relative Permeabilities
Currents were elicited for 200 ms from −95 up to 70 mV from a holding potential of −100 mV.
(A–D) Current-voltage relations of representative oocytes expressing NvNav2.1 (A), NvNav2.1DEKA (B), NvNav2.1DKEA (C), or NvNav2.1NvNav2.5(p−loops) (D). Circles: Na+ single ion solution; squares: K+ single ion solution. The relative ion permeability was calculated from the difference in reversal potential between K+ and Na+ single ion solutions of identical concentrations (see Experimental Procedures). The values provided are the mean ± SEM of n cells. The protonation state of each SF residue is indicated in parentheses, with x designating an uncharged residue.
See also Figure S2.
In contrast to NvNav2.1, NvNav2.2, bearing a DEET SF, did not exhibit inward currents under various voltage protocols, but lidocaine-sensitive outward and tail currents in ND96 bath solution (with CaCl2) were observed (Figures 1F and 1G). Because no inward currents were detected with Ba2+ substitution for Ca2+ (Figure 1H), the tail currents observed were of Ca2+-activated Cl− channels, indicating that NvNav2.2 conducts Ca2+ ions.
We further examined the ion selectivity by introducing the DEKA SF, which is conserved in all Nav1 channels, in the background of NvNav2.1 (substitution E1239K, mutant NvNav2.1DEKA). No inward current was detectable when Na+ in the bath solution was substituted with choline, indicating that the channel mutant was Ca2+ impermeable (Erev = 16.7 ± 1.1 mV, n = 14; Figures 1I–1K). However, whereas Nav1 channels are Na+ selective (PK/PNa ≤ 0.1; Schlief et al., 1996), NvNav2.1DEKA conducted both Na+ and K+ ions (PK/PNa = 0.36; Figure 2B). This finding substantiates the structural difference between Nav1 and Nav2 at the channel pore.
NvNav2.5 Is a Na+-Selective Channel
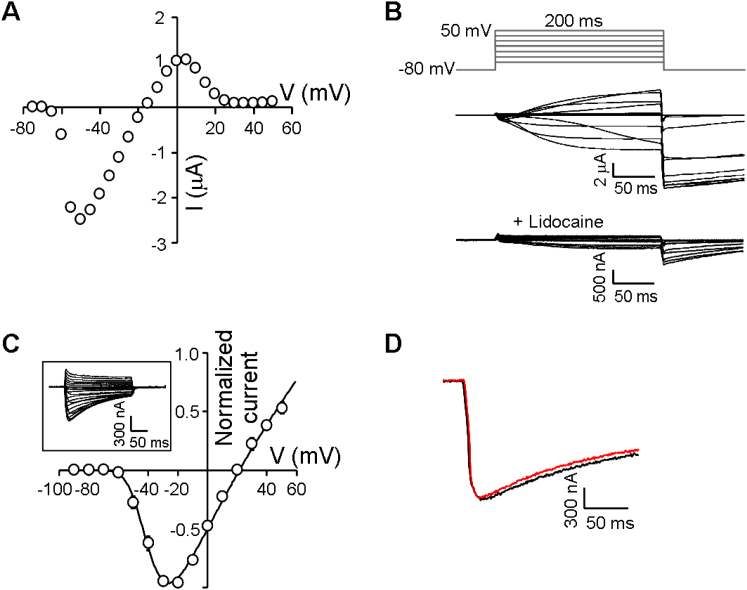
The unique DKEA SF (Figure 3A) raised questions regarding the ion preference of NvNav2.5. Because NvNav2.5 did not express in oocytes, we used NvNav2.1 as a platform for analysis of NvNav2.5 ion selectivity. Substitution E800K at DII resulted in mutant NvNav2.1DKEA, which exhibited biphasic inward currents with a first peak that appeared within milliseconds and a second, slowly developing peak with tail currents (Figure 3B). Substituting Na+ with choline or chelating the Ca+ in the bath solution with EGTA (Figures 3C and 3D) indicated that the first peak corresponded to Na+ currents and the second peak corresponded to Cl− currents induced by Ca2+ conducted by NvNav2.1DKEA. Furthermore, inward currents were observed in K+ single ion solution, indicating that NvNav2.1DKEA is nonselective (PK/PNa = 0.24; Figure 2C). However, in comparison with NvNav2.1, which conducts mainly Ca2+, considerable Na+ currents were measured through NvNav2.1DKEA.
Figure 3.
Sequence Alignment of NvNav2 Channels and Current Recordings from NvNav2.1DKEA and NvNav2.1NvNav2.5(p−loops) Channels Expressed in Xenopus Oocytes
(A) Alignment of the pore-loop regions of the five N. vectensis (Nv) channels (see also Figure S3 for spatiotemporal expression of Nav2 cnidarian channels), as well as a channel from the medusae P. penicillatus (Pp) and C. capillata (Cc) and the mammalian brain channel Nav1.2. Substitutions of NvNav2.1NvNav2.5(p−loops) are underlined and substitutions unique to the Nav2.5 channel subfamily are in yellow boxes. For current recordings the oocytes were clamped at −80 mV holding potential and currents were elicited by 200 or 500 ms depolarizing voltage pulses from −75 mV to either 50 or 70 mV.
(B) NvNav2.1DKEA in ND96 bath solution.
(C) NvNav2.1DKEA with choline substituting for Na+ in the bath solution.
(D) NvNav2.1DKEA with Ca2+ in the bath solution chelated by EGTA.
(E and F) NvNav2.1NvNav2.5(p−loops) in ND96 bath solution (E) and with choline substituting for Na+ (F).
(G) Current-voltage relations of NvNav2.1NvNav2.5(p−loops) (Erev = 46.5 ± 1.2 mV; n = 19). Each point represents mean ± SEM of n cells (see Figure S2 for analysis of the SF in NvNav2.1NvNav2.5(p−loops)).
The p-loops of NvNav2.5 differ in sequence from those of the other four NvNav2 channels (Figure 3A). Moreover, transcripts encoding Nav2.5 homologs with DKEA SF were previously identified in motor neurons of the hydrozoan Polyorchis penicillatus and the scyphozoan Cyanea capillata, and whole-cell recording in P. penicillatus showed Na+-selective voltage-gated ion currents (Anderson et al., 1993; Spafford et al., 1996). Therefore, we constructed the NvNav2.5 p-loops of all four domains in the background of NvNav2.1 and examined the ion selectivity. The resulting channel chimera, NvNav2.1NvNav2.5(p−loops), was Ca2+ impermeable (Figures 3E and 3F), with an Erev value of 46.5 ± 1.2 mV (n = 19; Figure 3G) and PK/PNa ratio of 0.07 (Figure 2D) resembling those of Nav1 channels (insect channel DmNav1 Erev = 47.8 ± 1.1 mV, n = 17; mammalian brain channel rNav1.2 Erev = 48.9 ± 2.3 mV, n = 14; data not shown). These characteristics suggest that NvNav2.1NvNav2.5(p−loops) is Na+ selective. Of note, the Na+ selectivity was lost when the DKEA SF was substituted with DEEA or DEKA (Figure S2).
Figure S2.
Currents Mediated by Channel Mutants NvNav2.1NvNav2.5(p−loops DEEA) and NvNav2.1NvNav2.5(p−loops DEKA) Expressed in Oocytes, Related to Figure 3
(A) Currents elicited by 500 ms depolarizing pulses from −75 to 50 mV mediated by NvNav2.1NvNav2.5(p−loops DEEA) in ND96 bath solution. Note the large tail currents upon returning to the −80 mV holding potential.
(B) Inward currents elicited by 200 ms depolarizing pulses from −75 to 50 mV mediated by NvNav2.1NvNav2.5(p−loops DEKA) in ND96 bath solution. Note the absence of tail currents (see inset). The Erev value obtained for the current-voltage relations is 19.1 ± 1.6 (n = 6). Each point represents mean ± SEM of n cells.
(C) Currents elicited by 200 ms depolarizing pulses from −90 to 50 mV mediated by NvNav2.1NvNav2.5(p−loops DEKA) in K+ single ion bath solution.
Based on mutagenesis of the Nav1 DEKA SF, DKEA in the Nav2.5 channel was considered an intermediate between DEKA and DEEA SFs (Schlief et al., 1996; Liebeskind et al., 2011). However, here we show not only that NvNav2.5 is as Na+ selective as Nav1, but also that this selectivity cannot be conferred by the DKEA SF alone. Because Na+ selectivity in NvNav2.1 was achieved only when the entire p-loop region was exchanged by that of NvNav2.5, other residues in addition to those of the SF are involved in determining the Na+ selectivity of NvNav2.5.
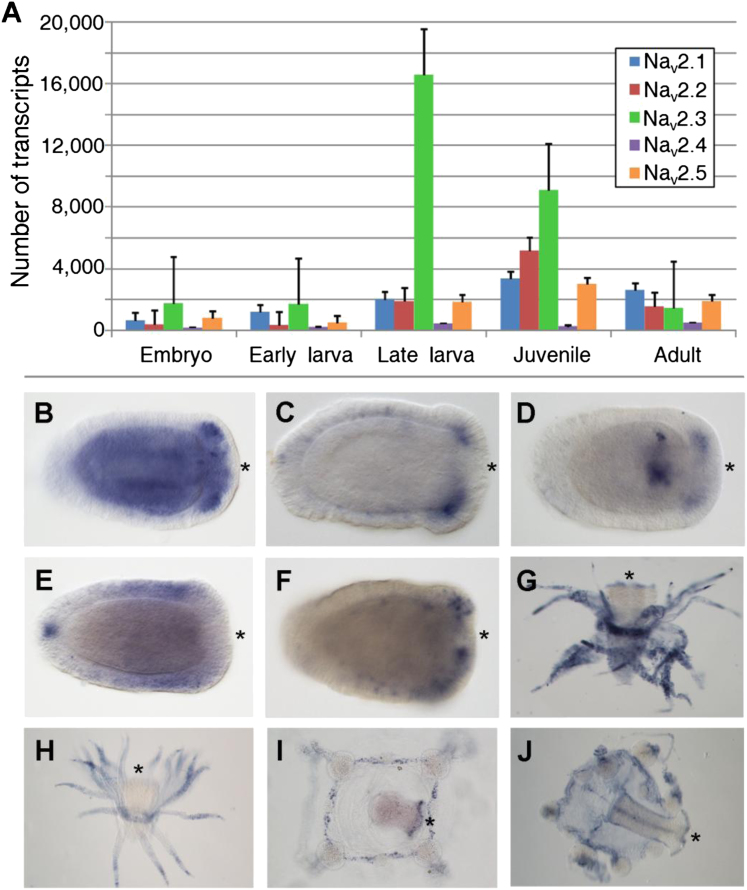
The multiple Nav2 genes in N. vectensis suggested variable spatiotemporal expression, and indeed this was demonstrated by quantitative PCR (qPCR) analysis and distinct in situ hybridization expression patterns (Figure S3). Using two Clytia hemisphaerica sodium channel cDNA clones homologous to NvNav2.1 and NvNav2.5 (ChNav2.1 and ChNav2.5), and in situ hybridization, we observed differential channel expression in two distinct developmental stages of this hydrozoan (Figures S3G–S3J). Thus, similarly to bilaterian animals, cnidarians possess several channel subtypes distributed in a spatiotemporal manner, possibly reflecting specialized physiological roles. Given the complex expression of other neuronal genes in N. vectensis (Marlow et al., 2009) and the correlation between the increase in the number of Nav genes and neuronal complexity in bilaterians (Widmark et al., 2011), cnidarian excitability is probably more complex than was initially hypothesized.
Figure S3.
Spatiotemporal Expression of Cnidarian Navs, Related to Figure 3
(A) Developmental time series of expression for the five Nav2 channel genes from N. vectensis. The data were generated from qPCR for five developmental stages. Expression is presented in molecules per μl cDNA. Bars represent mean + SE of n = 3 replicates.
(B–F) In situ hybridization with probes for NvNav2.1 (B), NvNav2.2 (C), NvNav2.3 (D), NvNav2.4 (E), and NvNav2.5 (F) revealed the spatial expression pattern of the different channel subtypes in 5-day-old N. vectensis larvae. A similar assay on C. hemisphaerica revealed the spatial expression patterns of two channel homologs.
(G–J) Probes for ChNav2.1 (G) and ChNav2.5 (H) in the gastrozoid developmental stage, and for ChNav2.1 (I) and ChNav2.5 (J) in the medusa stage. The oral end is indicated by an asterisk.
Na+-Channel Phylogeny Reveals Convergent Evolution of Sodium Selectivity
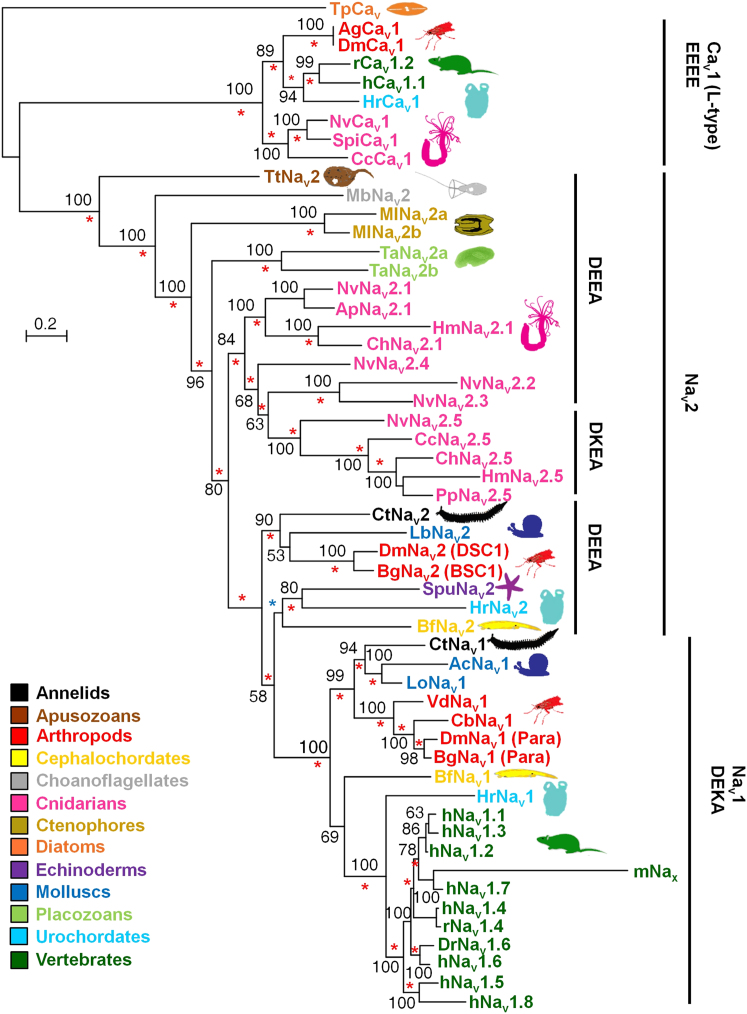
The characterization of sea anemone Nav2 channels, especially the Na+-selective NvNav2.5, prompted us to reanalyze the evolutionary history of sodium channels. We extended the data set of Nav sequences with full-length cDNAs encoding Nav2 homologs, which we cloned from basal metazoans and their relatives (Figure 4 and Table S1). We also identified and cloned Nav2.1 and Nav2.5 homologs from the hydroid cnidarians Hydra magnipapillata (Chapman et al., 2010) and Clytia hemisphaerica (J.E.M.K., unpublished data). Our phylogenetic analysis with the extended data set using the putative channel of the diatom Thalassiosira pseudonana as an outgroup (Derelle and Lang, 2012) positioned the Nav2 channels within a single cluster containing all metazoan Navs and a putative homolog from T. trahens (Figure 4). This phylogeny confirms that Nav2 channels appeared prior to the metazoan-fungal split (Cai, 2012), and that they were retained in most extant nonbilaterian animals but not in fungi or the unicellular opisthokont Capsaspora owczarzaki. Nav2 channels are also found in many bilaterians, but were lost in vertebrates. Nav1 channels, however, are found only in bilaterians (Figure 4; Liebeskind et al., 2011). Although Nav2 channels mediate Ca2+, they cluster with Nav1 channels rather than Cavs. Because Nav2 channels coexisted with Cavs in the ancestor of T. trahens and opisthokonts (Cai, 2012), the separation of Navs from Cavs occurred more than a billion years ago.
Figure 4.
Phylogeny of Voltage-Gated Sodium Channels
A maximum-likelihood tree was constructed using the LG (+F +G +I) model. The bootstrap support out of 100 is indicated at the branches. A Bayesian analysis using the WAG model resulted in identical topology. Posterior probabilities of 1.0 are indicated by a red asterisk, and those of 0.95 < X < 1.0 are indicated by a blue asterisk. All sequences are from cloned cDNA unless otherwise mentioned. Accession numbers and species names are available in Table S1. Animal clades are indicated by colors.
The N. vectensis channels (NvNav2.1–NvNav2.5) are clustered with channels of other cnidarians, indicating a monophyletic origin (Figure 4). NvNav2.5, however, forms a small subcluster together with hydrozoan and scyphozoan Navs bearing a unique DKEA SF, suggesting that the Nav2.1 and Nav2.5 subtypes resulted from a gene duplication event in the common ancestor of all extant cnidarians >540 million years ago (Park et al., 2012). The selective Na+ conductance of the Nav2.5 subfamily explains the sodium-based action potentials measured in isolated motor neurons of the medusozoans Cyanea and Polyorchis (Anderson, 1987; Spafford et al., 1996).
Our results indicate that selectivity for Na+ evolved separately in the cnidarian and bilaterian lineages. Moreover, a single substitution at the third position in the SF of NvNav2.1, to resemble the DEKA SF of Nav1 channels (NvNav2.1DEKA), abolished Ca2+ but not K+ conductance. Because K+ ion conductance through Kvs generates the falling phase of the action potential, whereas Na+ ion conductance through Navs is responsible for its rising phase, a clear functional advantage would be gained by separating these two fluxes and increasing the selectivity of Nav2 channels to Na+ ions. We therefore propose that the pore regions in both the urbilaterian Nav1 and the primordial Nav2.5 cnidarian channel evolved under selective pressure to cease K+ and Ca2+ ion conductance, and that this required additional substitutions at the p-loops other than those at the SF. The selectivity for Na+ was achieved in the two channel types in different ways, as is evident from the lack of selectivity for Na+ ions in channel mutants NvNav2.1NvNav2.5(p−loops DEKA) bearing the SF of Nav1, and Nav1.2DKEA (a mammalian brain channel mutant) bearing the SF of NvNav2.5 (Figure S2; Schlief et al., 1996). This conclusion argues for a structural difference between the pore regions of Nav2.5 and Nav1, which is conceivable given that Na+ selectivity in cnidarian Nav2.5 channels and bilaterian Nav1 channels has evolved in a convergent manner.
Bacterial homotetrameric Navs bear an EEEE SF similar to that of metazoan Cavs, but they are still selective for Na+ ions (Payandeh et al., 2011). Thus, Na+ selectivity in voltage-gated ion channels evolved independently in three lineages on the tree of life. This highlights the plasticity of molecular evolution and the importance of Na+ selectivity in biological systems ranging from prokaryotes to advanced eukaryotes.
Intriguingly, despite the advantages of Na+ selectivity, several animal lineages with simple nervous systems (e.g., nematodes and echinoderms) appear to have independently lost the Nav1 channels (Figure 4; Widmark et al., 2011; Jegla et al., 2009). Moreover, Nav2 channels with a Ca2+ preference were retained in parallel to Na+ selective channels in many animal groups, such as ascidians, insects, and cnidarians (Figure 4; Nagahora et al., 2000; Cui et al., 2012). Thus, it seems that Ca2+-based action potentials are not merely an evolutionary relic but may be advantageous in simple neuronal circuits.
Experimental Procedures
Identification of Nav Homologs
Putative Nav homologs were detected in GenBank (nr), Broad Institute, and Joint Genome Institute databases via BLAST. The voltage sensors, SF, and inactivation loop were assigned manually. Because some of these regions were missing or contained noncanonical substitutions, we cloned and sequenced overlapping cDNA fragments encoding the putative Navs from several basal metazoans and a choanoflagellate (see Table S1).
Functional Expression of Navs in Oocytes and Two-Electrode Voltage Clamp Measurements
Nav-like transcripts were cloned into a modified pAlter expression vector (Promega). Constructs encoding the Nav α-subunits were linearized, transcribed in vitro, and injected into Xenopus oocytes as described previously (Shichor et al., 2002). Currents were measured 1–3 days later using a two-electrode voltage clamp and a Gene Clamp 500 amplifier (Axon Instruments). Data were sampled at 10 kHz and filtered at 5 kHz. Unless otherwise stated, the ND96 bath solution contained (in mM) 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, 5 HEPES, pH 7.5. For G-V analysis, the mean conductance (G) was calculated from the peak current-voltage relations using the equation G = I/(V − Erev), where I is the peak current, V is the membrane potential, and Erev is the reversal potential. The normalized conductance-voltage relations were fit with either a one- or two-component Boltzmann distribution according to the equation:
where V11/2 and V21/2 are the respective membrane potentials for two populations of channels for which the mean conductance is half maximal, k1 and k2 are their respective slopes, and A defines the proportion of the second population (amplitude) over the total. When only one population of channels was apparent, A was set to zero. To avoid the Ca2+-activated chloride currents in oocytes expressing NvNav2.1, CaCl2 in the ND96 bath solution was substituted with BaCl2, and the oocytes were injected 30 min or 2 hr prior to the measurements with 25 nL of either 50 mM BAPTA or EGTA, respectively. The relative ion permeability PK/PNa was determined by measuring the difference in reversal potential between the test solution (K+ single ion solution) and the reference solution (Na+ single ion solution; see Extended Experimental Procedures). In the case of equal concentrations, the following equation was used (Hille, 2001):
where R, T, and F are the gas constant, absolute temperature, and Faraday’s constant, respectively.
Extended Experimental Procedures.
Composition of Single Ion Solutions Used in the Analysis of Relative Ion Permeability
The K+ single ion bath solutions used in this study were made of the following salts (in mM): 96 KCl, 5 HEPES, 1 MgCl2, and 0.5 EGTA, or alternatively of 96 KCl, 5 HEPES, and 1.8 EGTA. Both solutions were adjusted to pH 7.5 with KOH. The reversal potential values obtained in both solutions were similar. MgCl2 was included in order to inhibit Ca2+-inactivated chloride currents (Weber et al., 1995) and EGTA was included to chelate residual Ca2+.
The Na+ single ion bath solutions contained (in mM) 96 NaCl, 5 HEPES, 1 MgCl2, and 0.5 EGTA, or alternatively 96 NaCl, 5 HEPES, and 1.8 EGTA. Both solutions were adjusted to pH 7.5 with NaOH and gave similar reversal potential values.
qPCR
The main parameters of the qPCR analysis followed an established protocol (Reitzel and Tarrant, 2009). In brief, 19-to 21-nt-long primers with a 45%–55% guanine-cytosine content were designed to span one or more introns and produce amplicons of 72–120 bp with minimal predicted secondary structure (m-fold). A standard curve was constructed from serially diluted plasmids containing a larger portion of each transcript, and 18S ribosomal RNA was used as control. Gene expression was assayed in five developmental stages (embryo: 12 hr to 1 day; early larva: 3–5 days; late larva: 5–8 days; juvenile: 9–13 days; adult: months). RNA extraction and cDNA synthesis were conducted as previously described (Reitzel and Tarrant, 2009). qPCR was performed using iQ SYBR Green Supermix (Bio-Rad) in a MyCycler real-time PCR detection system (Bio-Rad). For each gene, standards and experimental samples were run in duplicates (technical replicates of a single plate). PCR products were subjected to melt curve analysis to ensure amplification of only a single product. The number of molecules per microliter for each gene was calculated by comparing the threshold cycle (Ct) from the sample with the standard curve.
Characterization of Spatiotemporal Expression Patterns
The main parameters of the qPCR analysis followed an established protocol (Reitzel and Tarrant, 2009; see Extended Experimental Procedures). In situ hybridization in N. vectensis was carried out according to an established method (Genikhovich and Technau, 2009). C. hemisphaerica young medusae and gastrozoids were fixed in 3.7% formaldehyde and 0.2% glutaraldehyde in seawater for 1 hr at 4°C. In situ hybridization in Clytia was performed according to the N. vectensis protocol, but specimens were digested in a higher concentration of Proteinase K (0.02 mg/ml) to improve permeability.
Phylogenetic Analysis
Channel protein sequences were aligned with the use of MUSCLE (Edgar, 2004), and low-quality alignment regions were removed by the TrimAl program (Capella-Gutiérrez et al., 2009). ProtTest was used to determine the most suitable model for phylogenetic reconstruction of Navs (Abascal et al., 2005). A maximum-likelihood phylogenetic tree was constructed using PhyML with the LG Model (+I +G +F; Guindon et al., 2010). Support values were calculated using 100 bootstrap replicates. Bayesian phylogenetic reconstruction was carried out using MrBayes 3.1 and the WAG model. A total of 5,000,000 generations were calculated and every 100th generation was sampled.
Acknowledgments
We thank N. King and M.J. Westbrook (University of California, Berkeley), L.Z. Holland (University of California, San Diego), P.A.V. Anderson (University of Florida), and B. Schierwater and M. Eitel (Institut für Tierökologie und Zellbiologie, Hannover) for providing RNA and tissue samples; D. Fredman (University of Vienna) for sharing data; and T.J. Jegla (Penn State University) and N. Dascal (Tel Aviv University) for critical comments. Y.M. was supported by an EMBO long-term fellowship (ALTF 1096-2009). A.M.R. was supported by award F32HD062178 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. This study was supported by a research grant from the Austrian National Science Foundation (FWF P 21108-B17) to U.T., and by a United States-Israel Binational Agricultural Research and Development Grant (IS-4313-10) and an Israeli Science Foundation grant (107/08) to M.G.
Published online: July 26, 2012
Footnotes
Supplemental Information includes Extended Experimental Procedures, three figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2012.06.016.
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 Unported License (CC-BY-NC-ND; http://creativecommons.org/licenses/by-nc-nd/3.0/legalcode).
Accession Numbers
All novel sequences have been deposited in GenBank under accession numbers HQ877452–HQ877461 and JQ066819–JQ066822.
Supplemental Information
References
- Abascal F., Zardoya R., Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics. 2005;21:2104–2105. doi: 10.1093/bioinformatics/bti263. [DOI] [PubMed] [Google Scholar]
- Anderson P. Properties and pharmacology of a TTX insensitive Na+ current in neurones of the jellyfish Cyanea Capillata. J. Exp. Biol. 1987;133:231–248. [Google Scholar]
- Anderson P.A., Holman M.A., Greenberg R.M. Deduced amino acid sequence of a putative sodium channel from the scyphozoan jellyfish Cyanea capillata. Proc. Natl. Acad. Sci. USA. 1993;90:7419–7423. doi: 10.1073/pnas.90.15.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M.E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J. Physiol. 1983;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. Ancient origin of four-domain voltage-gated Na+ channels predates the divergence of animals and fungi. J. Membr. Biol. 2012;245:117–123. doi: 10.1007/s00232-012-9415-9. [DOI] [PubMed] [Google Scholar]
- Capella-Gutiérrez S., Silla-Martínez J.M., Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W.A. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Cestèle S., Catterall W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- Chapman J.A., Kirkness E.F., Simakov O., Hampson S.E., Mitros T., Weinmaier T., Rattei T., Balasubramanian P.G., Borman J., Busam D. The dynamic genome of Hydra. Nature. 2010;464:592–596. doi: 10.1038/nature08830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y.J., Yu L.L., Xu H.J., Dong K., Zhang C.X. Molecular characterization of DSC1 orthologs in invertebrate species. Insect Biochem. Mol. Biol. 2012;42:353–359. doi: 10.1016/j.ibmb.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Derelle R., Lang B.F. Rooting the eukaryotic tree with mitochondrial and bacterial proteins. Mol. Biol. Evol. 2012;29:1277–1289. doi: 10.1093/molbev/msr295. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genikhovich G., Technau U. In situ hybridization of starlet sea anemone (Nematostella vectensis) embryos, larvae, and polyps. Cold Spring Harb. Protoc. 2009;2009(9) doi: 10.1101/pdb.prot5282. pdb.prot5282. [DOI] [PubMed] [Google Scholar]
- Goldin A.L. Evolution of voltage-gated Na(+) channels. J. Exp. Biol. 2002;205:575–584. doi: 10.1242/jeb.205.5.575. [DOI] [PubMed] [Google Scholar]
- Guindon S., Dufayard J.F., Lefort V., Anisimova M., Hordijk W., Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Heinemann S.H., Terlau H., Stühmer W., Imoto K., Numa S. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 1992;356:441–443. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- Hille B. Third Edition. Sinauer Associates Inc.; Sunderland, MA: 2001. Ion Channels of Excitable Membranes. [Google Scholar]
- Jegla T.J., Zmasek C.M., Batalov S., Nayak S.K. Evolution of the human ion channel set. Comb. Chem. High Throughput Screen. 2009;12:2–23. doi: 10.2174/138620709787047957. [DOI] [PubMed] [Google Scholar]
- Liebeskind B.J., Hillis D.M., Zakon H.H. Evolution of sodium channels predates the origin of nervous systems in animals. Proc. Natl. Acad. Sci. USA. 2011;108:9154–9159. doi: 10.1073/pnas.1106363108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow H.Q., Srivastava M., Matus D.Q., Rokhsar D., Martindale M.Q. Anatomy and development of the nervous system of Nematostella vectensis, an anthozoan cnidarian. Dev. Neurobiol. 2009;69:235–254. doi: 10.1002/dneu.20698. [DOI] [PubMed] [Google Scholar]
- Meech R.W., Mackie G.O. Evolution of excitability in lower metazoans. In: North G., Greenspann J., editors. Cold Spring Harbor Laboratory Press; New York: 2007. pp. 581–615. [Google Scholar]
- Nagahora H., Okada T., Yahagi N., Chong J.A., Mandel G., Okamura Y. Diversity of voltage-gated sodium channels in the ascidian larval nervous system. Biochem. Biophys. Res. Commun. 2000;275:558–564. doi: 10.1006/bbrc.2000.3290. [DOI] [PubMed] [Google Scholar]
- Park E., Hwang D.S., Lee J.S., Song J.I., Seo T.K., Won Y.J. Estimation of divergence times in cnidarian evolution based on mitochondrial protein-coding genes and the fossil record. Mol. Phylogenet. Evol. 2012;62:329–345. doi: 10.1016/j.ympev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Payandeh J., Scheuer T., Zheng N., Catterall W.A. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam N.H., Srivastava M., Hellsten U., Dirks B., Chapman J., Salamov A., Terry A., Shapiro H., Lindquist E., Kapitonov V.V. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- Reitzel A.M., Tarrant A.M. Nuclear receptor complement of the cnidarian Nematostella vectensis: phylogenetic relationships and developmental expression patterns. BMC Evol. Biol. 2009;9:230. doi: 10.1186/1471-2148-9-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C., Matsumoto G. Primary structure of squid sodium channel deduced from the complementary DNA sequence. Biochem. Biophys. Res. Commun. 1992;186:61–68. doi: 10.1016/s0006-291x(05)80775-2. [DOI] [PubMed] [Google Scholar]
- Schlief T., Schönherr R., Imoto K., Heinemann S.H. Pore properties of rat brain II sodium channels mutated in the selectivity filter domain. Eur. Biophys. J. 1996;25:75–91. doi: 10.1007/s002490050020. [DOI] [PubMed] [Google Scholar]
- Shichor I., Zlotkin E., Ilan N., Chikashvili D., Stuhmer W., Gordon D., Lotan I. Domain 2 of Drosophila para voltage-gated sodium channel confers insect properties to a rat brain channel. J. Neurosci. 2002;22:4364–4371. doi: 10.1523/JNEUROSCI.22-11-04364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spafford J., Grigoriev N., Spencer A. Pharmacological properties of voltage-gated Na+ currents in motor neurones from a hydrozoan jellyfish Polyorchis penicillatus. J. Exp. Biol. 1996;199:941–948. doi: 10.1242/jeb.199.4.941. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Fujisawa T., Holstein T.W. Cnidarians and the evolutionary origin of the nervous system. Dev. Growth Differ. 2009;51:167–183. doi: 10.1111/j.1440-169X.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Widmark J., Sundström G., Ocampo Daza D., Larhammar D. Differential evolution of voltage-gated sodium channels in tetrapods and teleost fishes. Mol. Biol. Evol. 2011;28:859–871. doi: 10.1093/molbev/msq257. [DOI] [PubMed] [Google Scholar]
- Zhang T., Liu Z., Song W., Du Y., Dong K. Molecular characterization and functional expression of the DSC1 channel. Insect Biochem. Mol. Biol. 2011;41:451–458. doi: 10.1016/j.ibmb.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Chung I., Liu Z., Goldin A.L., Dong K. A voltage-gated calcium-selective channel encoded by a sodium channel-like gene. Neuron. 2004;42:101–112. doi: 10.1016/s0896-6273(04)00148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplemental Reference
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.