Abstract
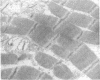
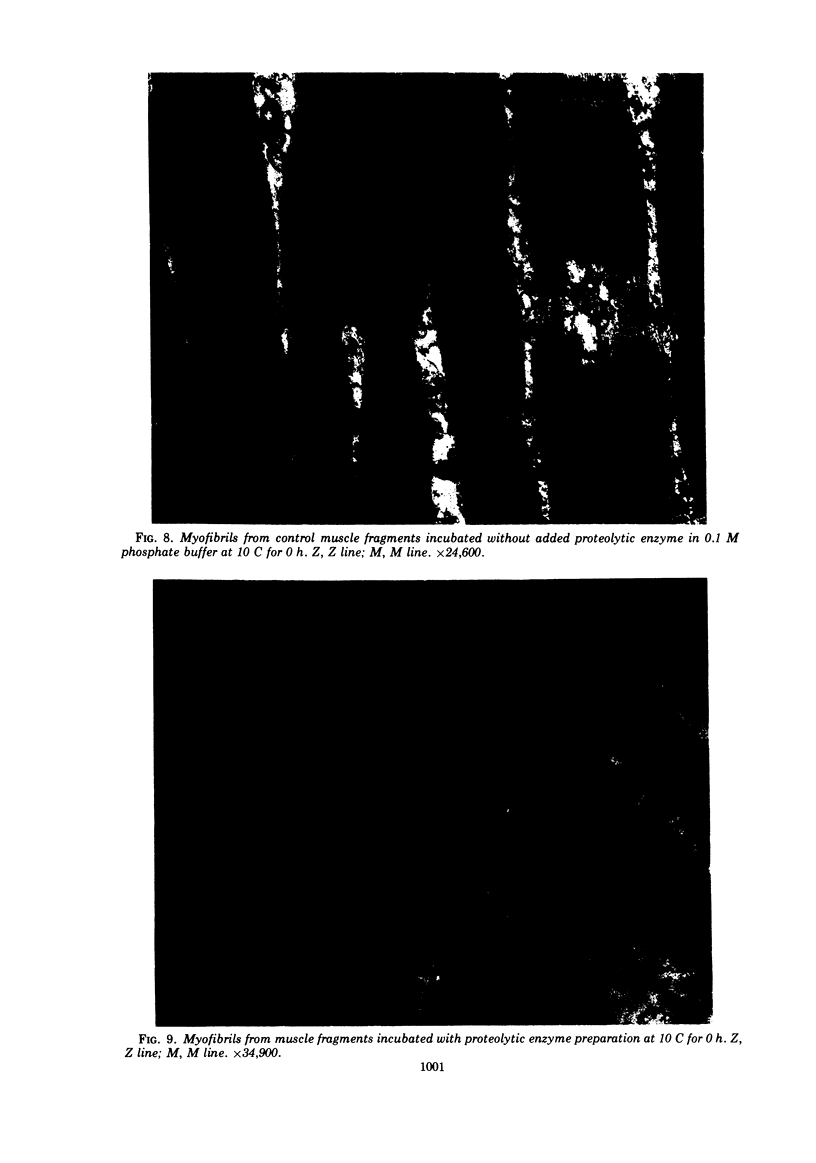
An extracellular preparation from Pseudomonas fragi with proteolytic enzyme activity was isolated, and its action on meat proteins and meat protein ultrastructure was studied. First, a suitable growth medium for proteolytic enzyme production was determined, and a method for partial purification of the proteolytically active fraction was developed. The enzyme preparation displayed optimal proteolytic activity at neutral pH and 35 C. Proteolytic activity was irreversibly lost by mild heat treatment. The enzyme preparation was tested for its ability to hydrolyze isolated pig muscle proteins. Myofibrillar protein was rapidly degraded, G-actin and myosin were broken down at a slower rate, and the sarcoplasmic proteins were least susceptible to hydrolysis. Electron micrographs of pork muscle showed that the proteolytic enzyme preparation caused a complete loss of dense material from the Z line. Similarities are discussed between the action of P. fragi extracellular proteolytic enzyme(s) on meat and normal bacterial spoilage of meat.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dutson T. R., Pearson A. M., Price J. F., Spink G. C., Tarrant P. J. Observations by electron microscopy on pig muscle inoculated and incubated with Pseudomonas fragi. Appl Microbiol. 1971 Dec;22(6):1152–1158. doi: 10.1128/am.22.6.1152-1158.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D. W., Goll D. E., Stromer M. H. A comparison of shortening and Z line degradation in post-mortem bovine, porcine, and rabbit muscle. Am J Anat. 1970 May;128(1):117–135. doi: 10.1002/aja.1001280109. [DOI] [PubMed] [Google Scholar]
- Jay J. M. Nature, characteristics, and proteolytic properties of beef spoilage bacteria at low and high temperatures. Appl Microbiol. 1967 Jul;15(4):943–944. doi: 10.1128/am.15.4.943-944.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER V., SPICER S. S. Experience in the preparation of myosin. Biochim Biophys Acta. 1952 Apr;8(4):474–476. doi: 10.1016/0006-3002(52)90074-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerke P., Farber L., Adams R. Bacteriology of spoilage of fish muscle. IV. Role of protein. Appl Microbiol. 1967 Jul;15(4):770–776. doi: 10.1128/am.15.4.770-776.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOMMAERTS W. F. H. M. The molecular transformations of actin. I. Globular actin. J Biol Chem. 1952 Sep;198(1):445–457. [PubMed] [Google Scholar]
- PERRY S. V. The adenosinetriphosphatase activity of myofibrils isolated from skeletal muscle. Biochem J. 1951 Mar;48(3):257–265. doi: 10.1042/bj0480257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seraydarian K., Briskey E. J., Mommaerts W. F. The modification of actomyosin by alpha- actinin. I. A survey of experimental conditions. Biochim Biophys Acta. 1967 Apr 11;133(3):399–411. doi: 10.1016/0005-2795(67)90544-2. [DOI] [PubMed] [Google Scholar]
- Tarrant P. J., Pearson A. M., Price J. F., Lechowich R. V. Action of Pseudomonas fragi on the proteins of pig muscle. Appl Microbiol. 1971 Aug;22(2):224–228. doi: 10.1128/am.22.2.224-228.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]