An autopropagating wave of reactive oxygen species (the ROS wave) rapidly spreads from a local tissue exposed to stress to the entire plant. In coordination with other systemic signals and abscisic acid, it activates systemic acclimation mechanisms in the entire plant and enhances tolerance to abiotic stress. The enhanced tolerance is specific to the original stress that induces it.
Abstract
Being sessile organisms, plants evolved sophisticated acclimation mechanisms to cope with abiotic challenges in their environment. These are activated at the initial site of exposure to stress, as well as in systemic tissues that have not been subjected to stress (termed systemic acquired acclimation [SAA]). Although SAA is thought to play a key role in plant survival during stress, little is known about the signaling mechanisms underlying it. Here, we report that SAA in plants requires at least two different signals: an autopropagating wave of reactive oxygen species (ROS) that rapidly spreads from the initial site of exposure to the entire plant and a stress-specific signal that conveys abiotic stress specificity. We further demonstrate that SAA is stress specific and that a temporal–spatial interaction between ROS and abscisic acid regulates rapid SAA to heat stress in plants. In addition, we demonstrate that the rapid ROS signal is associated with the propagation of electric signals in Arabidopsis thaliana. Our findings unravel some of the basic signaling mechanisms underlying SAA in plants and reveal that signaling events and transcriptome and metabolome reprogramming of systemic tissues in response to abiotic stress occur at a much faster rate than previously envisioned.
INTRODUCTION
Plants play a principal role in sustaining life on Earth, converting solar energy into bioavailable resources. Being sessile organisms, plants evolved sophisticated acclimation and defense mechanisms to cope with different challenges in their environment (Bray et al., 2000; Boyko and Kovalchuk, 2011; Reddy et al., 2011). These can be activated in the initial tissue(s) exposed to stress as well as in systemic tissues that have not yet encountered stress. The activation of defense or acclimation mechanisms in systemic nonchallenged tissues is often termed systemic acquired resistance (SAR) or systemic acquired acclimation (SAA), respectively, and serves an important role in preventing further infection or damage to the entire plant (Karpiński et al., 1999; Rossel et al., 2007; Carr et al., 2010; Szechyńska-Hebda et al., 2010; Dempsey and Klessig, 2012; Spoel and Dong, 2012; Shah and Zeier, 2013).
Recent studies identified a number of different chemicals and compounds involved in pathogen-induced SAR in plants. These include methyl salicylate (Park et al., 2007), a glycerol-3-phosphate derivative (Chanda et al., 2011), a lipid-transfer protein (DIR1) (Maldonado et al., 2002), azelaic acid (Jung et al., 2009), dehydroabietinal (Chaturvedi et al., 2012), jasmonic acid (Truman et al., 2007), and pipecolic acid (Dempsey and Klessig, 2012; Shah and Zeier, 2013). Some of these signals were proposed to function in a coordinated manner, generating a signaling network that regulates the accumulation of salicylic acid (SA) and the activation of defense mechanisms in systemic tissues. In contrast with the many studies focusing on SAR in plants, little is known about the different signals that control SAA in plants in response to different abiotic stresses. Some of the mechanisms and signals proposed to regulate SAA include alterations in redox and reactive oxygen levels, electric signals, and SA (Szechyńska-Hebda et al., 2010). Recently, SAA and SAR were proposed to be linked via a yet unknown genetic program (Karpiński et al., 2013).
Reactive oxygen species (ROS) function as important signaling molecules in bacteria, plants, animals, and humans (Bae et al., 2011; Halliwell, 2012; Ray et al., 2012). In plant tissues, a burst of ROS production, often occurring as two distinct peaks, accompanies the onset of several different abiotic stresses (Nishimura and Dangl, 2010; Mittler et al., 2011). Examples of localized alterations in ROS levels, as well as ROS oscillation patterns, were also reported for root hairs, guard cells, and pollen–stigma interactions (McInnis et al., 2006; Monshausen et al., 2007; Jammes et al., 2009; Nishimura and Dangl, 2010). We recently uncovered a new type of systemic signal in plants that travels at a rate of 8.4 cm min−1, is self-propagating, dependent on the presence of the respiratory burst oxidase homolog D (RBOHD) protein, but does not require RBOHF, results in rapid ROS accumulation in the apoplast, and responds to a surprisingly broad array of stressors, including wounding, cold, heat, high light (HL), and salinity. (Miller et al., 2009). The identification of this rapid systemic signal, termed the ROS wave (Mittler et al., 2011), demonstrated that the initial abiotic stress–induced burst of ROS generated by a local group of plant cells triggers a cascade of cell-to-cell communication events that results in the formation of a wave of ROS production that propagates throughout the different tissues of the plant and carries a systemic signal over long distances. However, the relevance of this signal to plant acclimation, its specificity and mode of action, and its association with other signals, such as different hormones or electric signals remained unknown (Mittler et al., 2011).
Here, we report that the ROS wave is required for the SAA of plants to heat or HL stresses, demonstrating an important biological function for this signal in the acclimation of plants to abiotic stresses. We further show that the ROS wave functions as a general priming mechanism in plants, alerting systemic tissues to the occurrence of a localized abiotic stress stimuli, and that a temporal–spatial interaction of the ROS wave with abscisic acid (ABA) accumulation in systemic tissues mediates the SAA of plants to heat stress (HS). We also show that SAA in plants is stress specific and that the ROS wave is associated with the production of systemic variation potentials, specifically linking these electric signals with RBOHD function in Arabidopsis thaliana. Our findings underline a role for ROS in plant acclimation to abiotic stresses and reveal that signaling events and transcriptome and metabolome reprogramming of systemic tissues in response to abiotic stress occur at a much faster rate than previously envisioned.
RESULTS
Biological Function and Regulation of the Rapid Autopropagating ROS Signal
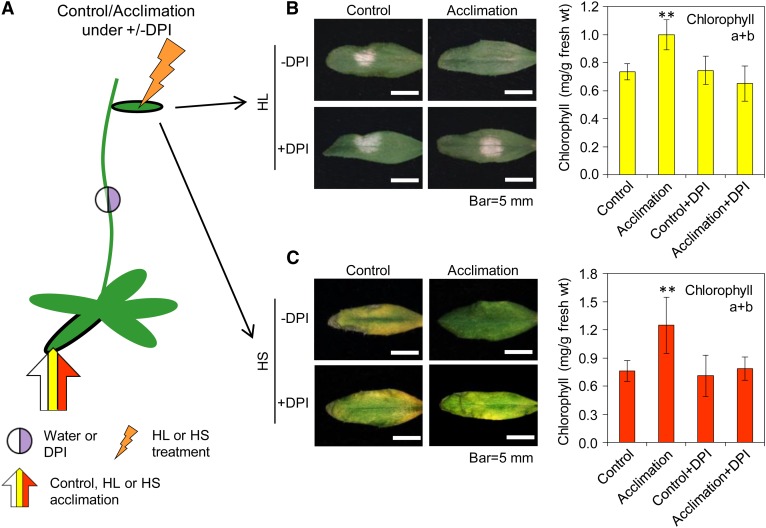
To examine the biological significance of the ROS wave to the SAA of plants, we subjected a single rosette leaf of Arabidopsis to a short period of HL or HS and then exposed a remote systemic leaf to an extended period of the same stress. As shown in Figure 1, systemic leaves of plants that were subjected to a prior local treatment were protected against the subsequent stress, whereas those of control-treated plants were not. These findings demonstrated that SAA was triggered in systemic tissues of HL- or HS-treated plants. To examine whether the ROS wave is required for this SAA response, we applied a drop of diphenylene iodonium (DPI), which inhibits RBOHD and blocks the ROS wave (Miller et al., 2009; Mittler et al., 2011), or a drop of catalase, which scavenges hydrogen peroxide (H2O2) and blocks the ROS wave (Miller et al., 2009), to the middle of the stem of plants 30 min prior to the local HL or HS treatment. As shown in Figure 1 and Supplemental Figure 1 online, the application of a drop of DPI or catalase at the midpoint between local and systemic tissues prevented systemic tissues from acclimating, demonstrating that the rapid ROS signal is required for the SAA response of plants to HL or HS.
Figure 1.
Biological Function of the ROS Wave.
(A) Experimental design showing the application of stress to local leaves (arrow), the placement of water or DPI between the local and systemic tissue (circle), and the subsequent application of stress to systemic leaves (jagged arrow).
(B) Images of systemic leaves (left) and graphs of chlorophyll content (right; n = 15) showing the protection of systemic leaves from HL in acclimated plants and its inhibition by DPI.
(C) Same as (B), but for HS (n = 15).
In (B) and (C), error bars = sd. **Student’s t test significant at P < 0.01. See also Supplemental Figure 1 online.
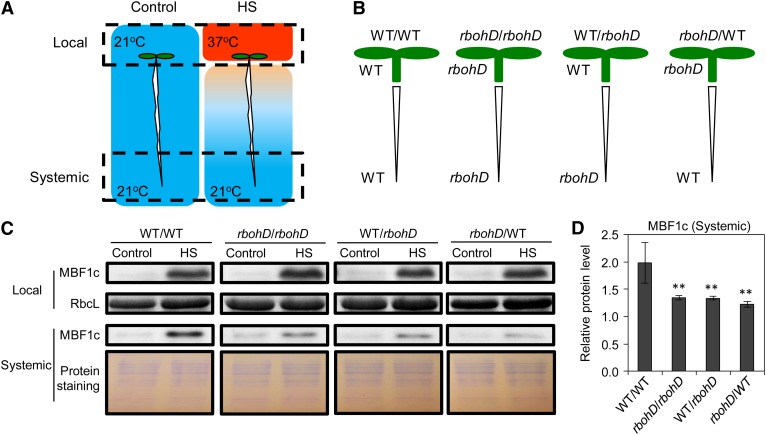
Because DPI has a broad function affecting not only RBOHD, but also different peroxidases (Frahry and Schopfer, 1998), we performed grafting experiments between wild-type and rbohD seedlings and followed, as a marker for HS-induced SAA, the expression of the key HS response transcriptional regulator multiprotein-bridging factor1c (MBF1c; Suzuki et al., 2008, 2011a) in systemic tissues in response to HS applied to local tissues. As shown in Figure 2, local application of HS to the cotyledons (local tissues) of control grafted seedlings resulted in the enhanced expression of MBF1c in root tips (systemic tissues). By contrast, the systemic expression of MBF1c was attenuated in all of the grafting experiments that involved a deficiency in RBOHD in the local or systemic tissues (Figure 2). The presence of RBOHD in systemic (root tips) or local (cotyledons) tissues was therefore required for enhanced MBF1c expression in systemic tissues in response to a local HS treatment. As a control to test whether H2O2 produced in the local cotyledon tissue can diffuse to the root tips, we applied 1 mM of H2O2 (which does not activate the ROS wave; Miller et al., 2009) to the cotyledons and imaged H2O2 in the root tips. As shown in Supplemental Figure 2 online, enhanced levels of H2O2 were not detected in root tips of plants treated with 1 mM H2O2 in their cotyledons. Taken together, our results suggest that ROS produced by RBOHD are responsible for propagating the ROS wave throughout the path of the systemic signal. The finding that MBF1c accumulation was not completely abolished in root tips of rbohD grafts in response to the local HS treatment (Figure 2) further suggests that additional signals that do not involve the ROS wave might be involved in SAA to HS.
Figure 2.
The Role of RBOHD in Mediating Rapid Systemic Signaling.
(A) Experimental design showing the application of stress to local cotyledon tissue (top dashed box) and the sampling of systemic root tissue (bottom dashed box).
(B) Experimental design showing the different grafting combinations used between wild-type (WT) and rbohD seedlings.
(C) Images of protein blot analysis showing the accumulation of MBF1c in local and systemic tissues of grafted seedlings (B) in response to HS (A).
(D) Quantification of protein expression in (C) (n = 3). Error bars = sd. **Student’s t test significant at P < 0.01.
See also Supplemental Figure 2 online.
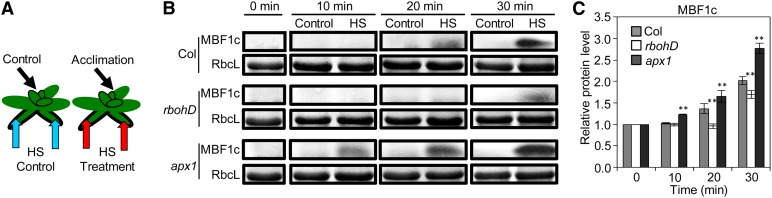
To further study how different ROS production and ROS scavenging mechanisms regulate the ROS wave in plants, we measured the accumulation of MBF1c in systemic tissues of the wild type, the rbohD mutant (deficient in ROS production), and a mutant deficient in the key H2O2-scavenging enzyme cytosolic ascorbate peroxidase1 (apx1) (Davletova et al., 2005a; Vanderauwera et al., 2011) in response to local application of HS. As shown in Figure 3, the accumulation of MBF1c was delayed in the ROS-production rbohD mutant, but accelerated in the ROS-scavenging apx1 mutant, demonstrating that interplay between ROS production and ROS scavenging by these two enzymes (RBOHD and APX1, respectively) functions to modulate the ROS wave in response to HS.
Figure 3.
Interplay between ROS Production and ROS Scavenging Modulates the ROS Wave in Response to HS.
(A) Experimental design showing the application of HS to local leaves (blue or red arrows) and the subsequent sampling of systemic tissue (black arrows).
(B) Time-course protein blot analysis showing the accumulation of MBF1c in systemic tissue of wild-type, rbohD, and apx1 mutants in response to a local HS stimulus.
(C) Quantification of protein expression in (B) (n = 3). Error bars = sd. **Student’s t test significant at P < 0.01.
Specificity in Rapid Systemic Acclimation
The ROS wave is triggered by different abiotic stimuli, such as HL, HS, salinity, cold, and wounding (Miller et al., 2009), and is required for SAA to HL or HS (Figure 1). However, the degree of specificity it confers is unknown (Mittler et al., 2011). One possibility is that the ROS wave activates a general acclimation response, regardless of the specific abiotic stimuli that is locally applied. Such a general response will trigger a broad and general reprogramming of the transcriptome and metabolome of systemic tissues and render them tolerant to a variety of different stresses. Alternatively, the ROS wave could act as a triggering signal, required but not sufficient, for SAA. In such a case, the function of the ROS wave would be coordinated with abiotic stress–specific signals and the reprogramming of the transcriptome and metabolome in systemic tissues, as well as the induced SAA tolerance, would be stress specific. Another possibility is that different abiotic stresses result in different oscillation patterns of the ROS wave that convey specificity in SAA (Mittler et al., 2011). However, so far, we were unable to detect such a phenomenon in mature Arabidopsis plants.
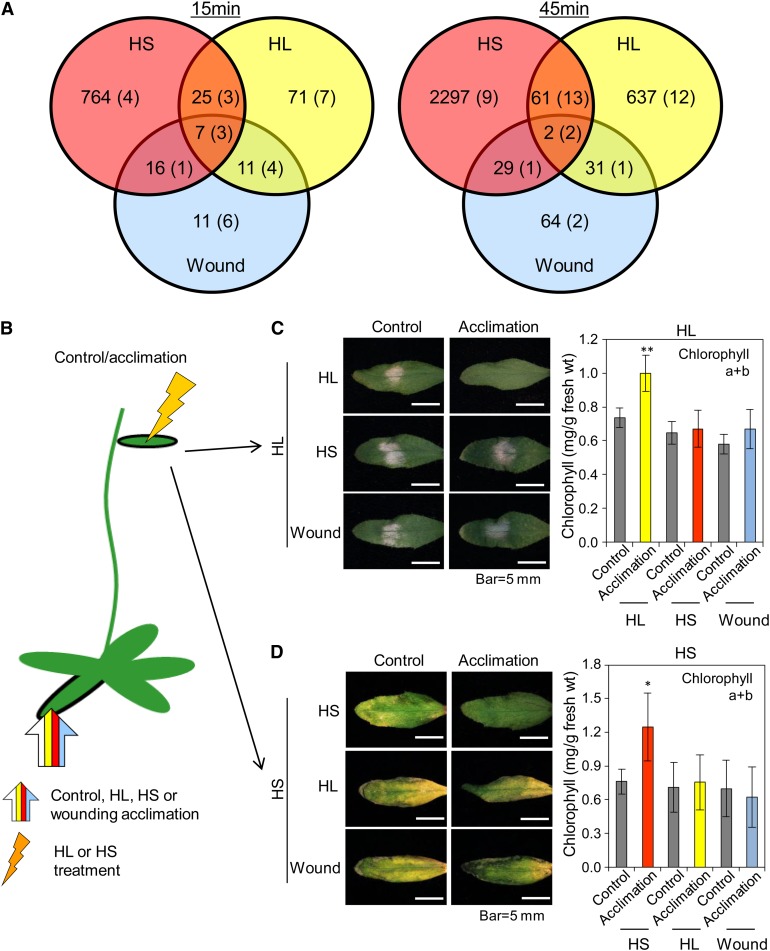
To address the ROS wave specificity question, we examined the degree of overlap between changes in the transcriptome and metabolome in systemic tissues of plants 15 and 45 min following the application of HL, HS, or wounding to local leaves (Figure 4A). In addition, we tested the potential of these three different systemic responses to induce cross-protection against each other (Figures 4B to 4D). Considerable specificity was found in the expression of transcripts and the accumulation of metabolites between the different systemic responses at 15 and 45 min following a local abiotic stress treatment (Figure 4A; see Supplemental Figures 3 to 5 and Supplemental Data Sets 1 to 22 online). In addition, as shown in Figures 4B to 4D, no cross-protection of systemic tissues was found between the different treatments. Local HL treatment was therefore able to only induce protection against HL, and local treatment of HS was able to only induce protection against HS (Figures 4B to 4D). The activation of the ROS wave by all three treatments (Miller et al., 2009) was evident by the high proportion of H2O2 response transcripts elevated at 15 min in all tissues (Table 1; see Supplemental Figure 4 and Supplemental Data Set 21 online). Because HL, HS, or wounding triggered the ROS wave (Miller et al., 2009; Table 1; see Supplemental Figure 4 and Supplemental Data Set 21 online), and acclimation to HL or HS required it (Figure 1), our findings that each of the different abiotic treatments induced a unique response that resulted in a stress-specific SAA (Figure 4) indicate that the ROS wave could function as a general priming signal required, but not sufficient, for rapid SAA in plants. Specificity in SAA is therefore likely to involve stress-specific signals that function in coordination with the ROS wave.
Figure 4.
SAA in Plants Is Abiotic Stress Specific.
(A) Venn diagrams showing the accumulation of stress-specific transcripts and metabolites (in parentheses) in systemic tissues of wild-type plants subjected to a local stimulus of HS, HL, or wounding (wound) for 15 or 45 min. Experimental design for stress application is similar to Figure 3A. See also Supplemental Figures 3 to 5 and Supplemental Data Sets 1 to 22 online.
(B) Experimental design showing the application of HL, HS, or wounding to local leaves (wide arrow) and the subsequent application of HL or HS to systemic leaves (jagged arrow).
(C) Images of systemic leaves (left) and graphs of chlorophyll content (right; n = 15) showing that local HL stimulus can only protect systemic leaves against HL.
(D) Images of systemic leaves (left) and graphs of chlorophyll content (right; n = 15) showing that local HS stimulus can only protect systemic leaves against HS.
In (C) and (D), error bars = sd. Student’s t test significant at *P < 0.05 or **P < 0.01.
Table 1. Representation of Hormone or ROS Response Transcripts among the Transcripts Elevated in Systemic Tissues of Plants in Response to Local HS, HL, or Wounding Stimuli at 15 and 45 min.
| 15 min |
45 min |
|||||
|---|---|---|---|---|---|---|
| Hormone/ROS | HS | HL | Wound | HS | HL | Wound |
| Total | 764 | 71 | 11 | 2297 | 637 | 64 |
| ABA | 141 (18.45) | 6 (8.45) | 1 (9.09) | 325 (14.15) | 82 (12.87) | 10 (15.63) |
| ACC | 10 (1.31) | 2 (2.82) | 0 (0.00) | 34 (1.48) | 5 (0.78) | 2 (3.13) |
| Brassinolide | 32 (4.19) | 3 (4.23) | 0 (0.00) | 46 (2.00) | 28 (4.40) | 2 (3.13) |
| Cytokinin | 6 (0.79) | 5 (7.04) | 0 (0.00) | 24 (1.04) | 40 (6.28) | 1 (1.56) |
| Gibberellin | 3 (0.39) | 1 (1.41) | 1 (9.09) | 8 (0.35) | 3 (0.47) | 2 (3.13) |
| Indole-3-acetic acid | 43 (5.63) | 5 (7.04) | 2 (18.18) | 112 (4.88) | 28 (4.40) | 0 (0.00) |
| Methyl jasmonate | 50 (6.45) | 7 (9.86) | 7 (63.64) | 124 (5.40) | 75 (11.77) | 49 (75.56) |
| SA | 13 (1.70) | 0 (0.00) | 0 (0.00) | 27 (1.18) | 9 (1.41) | 2 (3.13) |
| H2O2 | 143 (18.72) | 8 (11.27) | 4 (36.36) | 171 (7.44) | 50 (7.85) | 8 (12.50) |
| O2− | 68 (8.90) | 2 (2.82) | 0 (0.00) | 91 (3.96) | 25 (3.92) | 5 (7.81) |
| 1O2 | 50 (6.54) | 4 (5.63) | 2 (18.18) | 67 (2.92) | 20 (3.14) | 13 (20.31) |
Numbers in parentheses indicate percentage relative to the total. Bold indicates transcript representation >10%.
Involvement of ABA in the SAA Response of Plants to HS
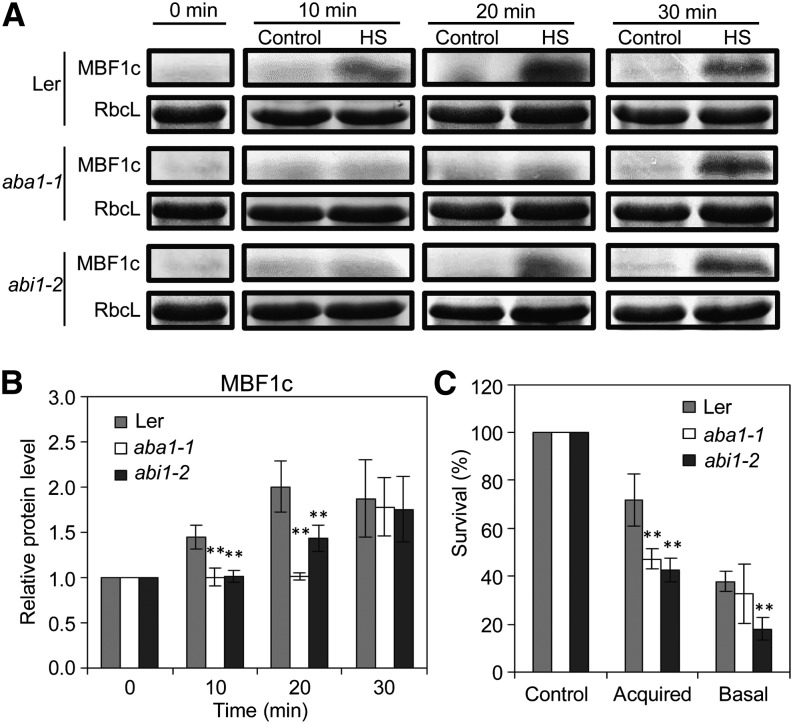
Meta-analysis of transcriptome reprogramming in systemic tissues during SAA to different abiotic stimuli revealed that >18% of the systemic transcripts elevated within 15 min of a local HS stimulus are classified as ABA responsive (Table 1). A high representation of ABA response transcripts (>12%) was also found among transcripts elevated in systemic tissues of plants subjected to all three stresses at 45 min (Table 1). The high representation of ABA response transcripts in systemic tissues of HS-treated plants at 15 and 45 min suggests that ABA could play a role in HS-induced SAA. We therefore tested the expression of the HS-induced SAA marker MBF1c protein in systemic tissues of mutants impaired in ABA signaling in response to local HS treatment. As shown in Figures 5A and 5B, the HS-induced expression of MBF1c in systemic tissues of locally treated plants was attenuated in mutants deficient in ABA biosynthesis (aba1-1) or ABA sensing (abi1-2), indicating that ABA signaling could be required for HS-induced SAA. Accordingly, and in agreement with previous studies (Larkindale et al., 2005), mutants impaired in ABA signaling were found to have a decreased survival rate following HS (Figure 5C).
Figure 5.
Involvement of ABA Signaling in the SAA Response of Plants to HS.
(A) Time-course protein blot analysis showing the accumulation of MBF1c in systemic tissue of wild-type, aba1-1, and abi1-2 mutants in response to a local HS stimulus. Ler, Landsberg erecta.
(B) Quantification of protein expression in (A) (n = 3).
(C) Survival of wild-type, aba1-1, and abi1-2 seedlings in response to HS applied with (acquired) or without (basal) a HS pretreatment (n = 5).
In (B) and (C), error bars = sd. **Student’s t test significant at P < 0.01.
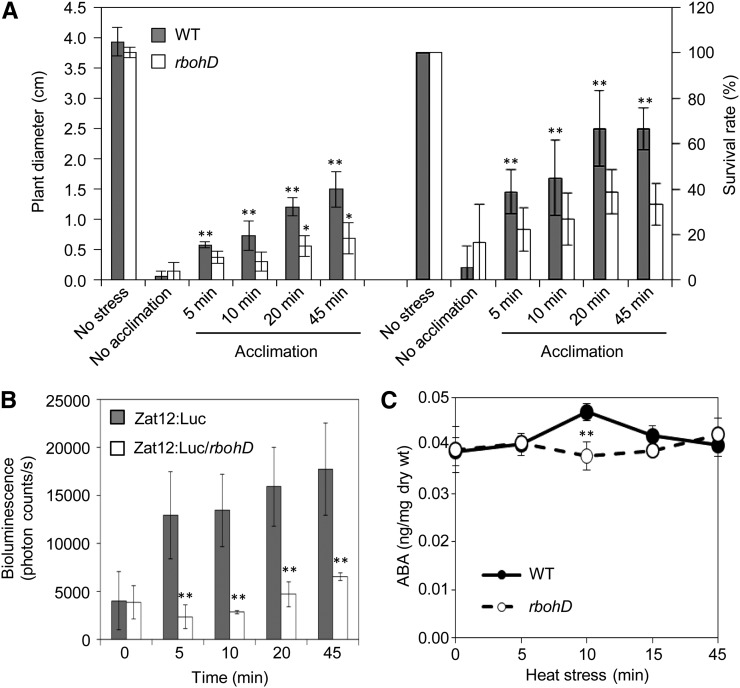
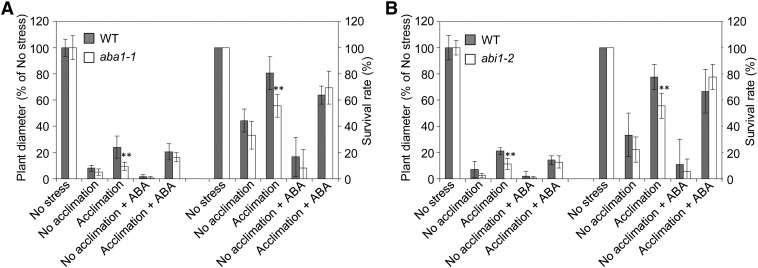
To further test the link between HS-induced SAA and ABA, we performed a time-course analysis comparing HS acclimation time, activation of rapid ROS signaling (imaged indirectly with the Zat12 promoter fused to luciferase; Miller et al., 2009), and ABA accumulation in wild-type and rbohD plants subjected to a local HS treatment for different time durations. As shown in Figures 6A and 6B, enhanced tolerance of plants to HS was correlated with the activation of the ROS wave and occurred as early as 5 to 10 min following HS application. Interestingly, detectable levels of free ABA transiently accumulated in systemic tissues 10 min following HS application (Figure 6C). Moreover, ABA accumulation in systemic tissues, rapid ROS signaling, and plant survival were suppressed in rbohD mutants (Figures 6A to 6C) that did not accumulate ROS in their systemic tissues (Miller et al., 2009). Although elevated levels of free ABA were only found 10 min following the local HS treatment (Figure 6C), that is 5 min after SAA to HS was induced (Figure 6A), we could not rule out the possibility that conjugated ABA plays a role during the early stages of the SAA to HS. To further test the involvement of ABA in the SAA of plants to heat, we subjected wild-type, aba1-1, and abi1-2 plants to a lethal HS with or without prior acclimation of a local leaf. As shown in Figure 7, the two ABA mutants failed to induce a SAA in response to a local HS treatment. Interestingly, application of ABA to systemic tissues of aba1-1 or abi1-2 prior to HS was able to recover SAA only in plants subjected to the local HS acclimation. This finding indicated that ABA as well as the ROS wave and/or other systemic signals are required to induce SAA in the ABA mutants. The findings that MBF1c accumulation and plant survival in response to HS require ABA signaling (Figures 5 and 7), coupled with the findings that ABA transiently accumulates in systemic tissues of plants subjected to a local HS in an RBOHD-dependent manner (Figure 6C), indicate that systemic acclimation to HS requires the coordinated function of ABA as well as ROS signaling and that ABA accumulation in systemic tissues is dependent on ROS production by RBOHD during HS. The finding that ABA application to systemic tissues (in the absence of acclimation) caused a reduction in plant growth and survival (Figure 7) is not surprising because ABA application would result in closure of stomata that would in turn result in higher leaf temperature during HS, causing a decrease in growth and survival (Rizhsky et al., 2004b).
Figure 6.
Accumulation of ABA, Survival, and ROS Signaling in Systemic Tissues of Plants Subjected to a Local HS Treatment for Different Durations.
(A) Measurements of plant diameter and survival of wild-type (WT) or rbohD plants subjected to a lethal HS treatment with or without a local HS stimulus for different durations.
(B) Quantification of the rapid ROS signal using the Zat12:luc reporter in wild-type or rbohD plants subjected to a local HS stimulus for different durations (n = 3).
(C) Measurements of ABA in systemic tissues of wild-type or rbohD plants subjected to a local HS stimulus for different durations (n = 5).
In (A) and (B), error bars = sd. In (C), error bars = se. Student’s t test significant at *P < 0.05 or **P < 0.01.
Figure 7.
Impaired SAA Response to HS in Mutants Deficient in ABA Signaling and Its Recovery by ABA Application.
(A) Measurements of plant diameter and survival of wild-type (WT) and aba1-1 plants subjected to a lethal HS treatment with or without a local HS stimulus and/or ABA application.
(B) Measurements of plant diameter and survival of wild-type and abi1-2 plants subjected to a lethal HS treatment with or without a local HS stimulus and/or ABA application.
Acclimation time in (A) and (B) was 45 min. Plant diameter is indicated in percentage relative to control (No stress) because plant diameter was different between wild-type and the ABA mutants under controlled conditions. In (A) and (B), error bars = sd. **Student’s t test significant at P < 0.01.
Subcellular Localization of MBF1c during Rapid Systemic Acclimation
The function of certain regulatory proteins elevated in systemic tissues in response to a local stress stimulus is dependent on their ability to localize to their correct cellular compartment. The HS-induced transcriptional regulator MBF1c was previously reported to be transported from the cytosol to nuclei during HS (Suzuki et al., 2008). The finding that the level of this key HS response protein is elevated in systemic nonstressed tissues in response to a local HS treatment (Figures 2 and 3) and that these tissues are more tolerant to a subsequent HS treatment (Figures 1C, 4D, and 6A) suggest that MBF1c could be localized to the nuclei in systemic tissues in the absence of an apparent HS treatment applied to this tissue. To test this possibility, we used transgenic Arabidopsis plants constitutively expressing an MBF1c-GFP (for green fluorescent protein) fusion protein under the control of the cauliflower mosaic virus 35S promoter (Suzuki et al., 2008).
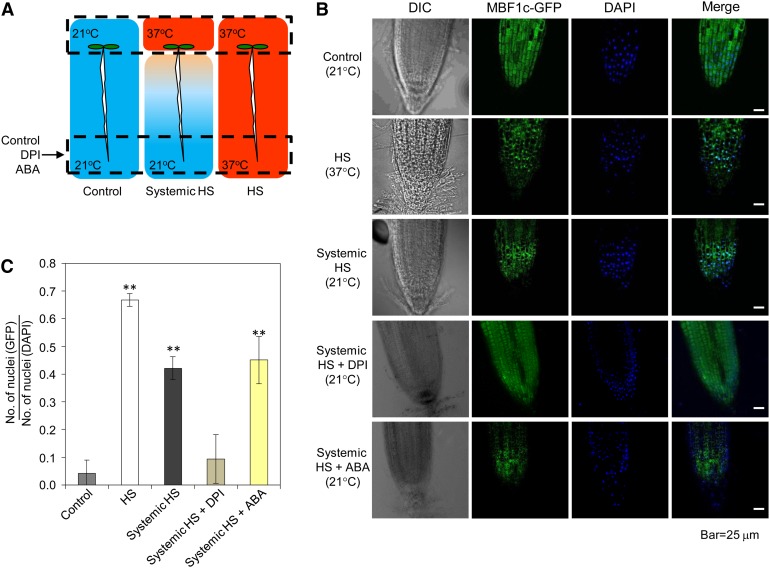
As shown in Figure 8, the constitutively expressed MBF1c-GFP protein was localized to the cytosol in control untreated seedlings. Upon a HS treatment of transgenic seedlings, the MBF1c-GFP protein was localized to the nuclei of cells. Interestingly, a local HS treatment of the cotyledons caused rapid nuclear localization of the MBF1c-GFP protein in the systemic nonstressed root tip tissue (Figure 8). The systemic nuclear localization of MBF1c could be suppressed by the application of DPI or catalase (see Supplemental Figure 6 online), but ABA had no apparent positive or negative effect on this process (Figure 8). These findings demonstrated that proper protein localization is an important component of rapid systemic signaling and that this ROS wave–dependent process could be induced in systemic tissues in the absence of stress.
Figure 8.
Nuclear Localization of MBF1c Is Induced in Systemic Tissues during SAA to HS.
(A) Experimental design showing the application of stress to local tissue (top dashed box) and the imaging of systemic tissue (bottom dashed box) of transgenic seedlings expressing the MBF1c-GFP fusion protein under the control of the cauliflower mosaic virus 35S promoter.
(B) Images of root tips from control and HS-treated tissues showing that nuclear localization of the MBF1c-GFP protein can be induced in systemic tissues of plants subjected to a local HS stimulus and that this process is inhibited by DPI. DIC, differential interference contrast; DAPI, 4′,6-diamidino-2-phenylindole.
(C) Quantification of MBF1c-GFP nuclear localization in control, local and systemic tissues of seedlings subjected to a local HS stimulus (n = 100) with or without ABA or DPI application. Error bars = sd. **Student’s t test significant at P < 0.01.
See also Supplemental Figure 6 online.
A Possible Role for the ROS Wave in Propagating Electric Signals
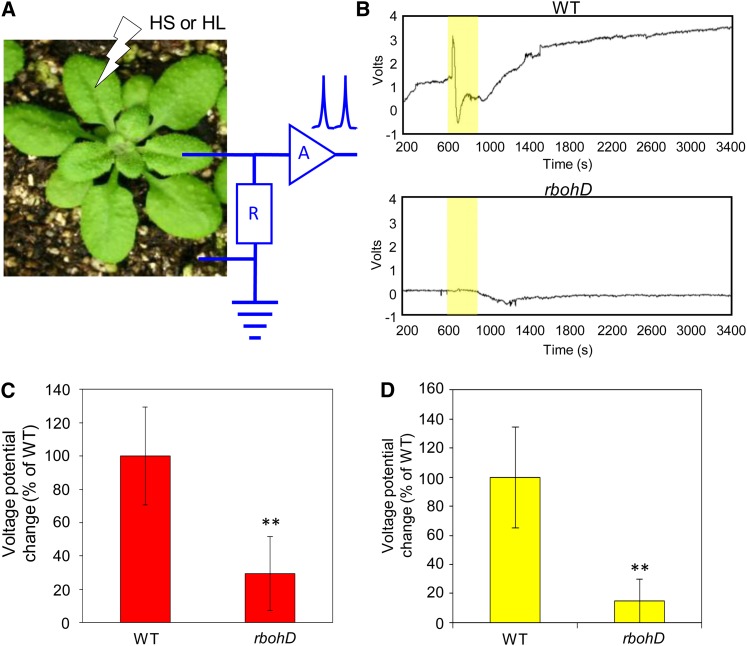
The accumulation of ROS at the outer surface of the plasma membrane (PM) during the progression of the ROS wave along its systemic path could cause membrane depolarization and the generation and/or propagation of electric signals (DeCoursey, 2003; Matoba and Shimokawa, 2003; Miller et al., 2010; Mittler et al., 2011). The ROS wave may therefore directly promote electric signals at the PM along its path (Mittler et al., 2011). In support of this possibility, the velocity of at least one type of electric signals (system potentials) (Zimmermann et al., 2009) is very similar to the rate previously recorded for the ROS wave in Arabidopsis (Miller et al., 2009). To test this possibility, we measured extracellular potential variations in systemic leaves (Fromm and Lautner, 2007; Volkov et al., 2010) of wild-type and rbohD mutants in response to local application of HS or HL. Compared with wild-type plants, a significant reduction was found in the amplitude of HS- or HL-induced extracellular systemic potential variations in the rbohD mutant (Figure 9), supporting a possible link between the ROS wave and electric signals.
Figure 9.
Possible Association between the ROS Wave and Systemic Potential Variations during SAA to HS or HL.
(A) Experimental design used to measure extracellular systemic potentials in wild-type and rbohD plants subjected to a local HS or HL stimulus.
(B) Representative extracellular systemic potential variation plots from wild-type (WT) and rbohD plants subjected to a local HL stimulus. The period of HL stress is indicated in yellow.
(C) Quantification of changes in extracellular systemic variations in wild-type and rbohD plants subjected to a local HS stimulus (n = 30).
(D) Same as (C) but for HL.
In (C) and (D), error bars = sd. **Student’s t test significant at P < 0.01.
See also Supplemental Figure 7 online.
To further examine the possibility that the ROS wave is associated with electric signals, we used a different measuring method. Instead of using an extracellular method to measure systemic potential variations, we used feeding aphids as a measuring probe (see Supplemental Figure 7 online; Tjallingii, 2006; Louis et al., 2010). Aphid feeding from sieve elements generates a unique waveform (periodic potential drops and increases; type E2 waveform; Tjallingii, 2006; Louis et al., 2010) that appears as a background noise in Supplemental Figure 7B online. Upon HL treatment of a lower leaf (see Supplemental Figure 7B online, top panels, yellow highlight), a systemic HL-induced potential variation signal was recorded superimposed on this insect feeding waveform (see Supplemental Figure 7B online, bottom panels, gray highlight). Compared with the wild type, and in agreement with the extracellular potential variations measurements (Figure 9), the HL-stimulated superimposed systemic signal was suppressed in the rbohD mutant. The results presented in Figure 9 and Supplemental Figure 7 online could be very important to our understanding of electric signals in plants and their relationship to other signaling pathways (Fromm and Lautner, 2007) because they demonstrate that ROS accumulation at the outer surface of the PM, regulated by the calcium binding and/or phosphorylation of the RBOHD protein (Kobayashi et al., 2007; Suzuki et al., 2011b; Dubiella et al., 2013), during the progression of the ROS wave, could be required for systemic potential signals.
Metabolic Responses Associated with Rapid SAA
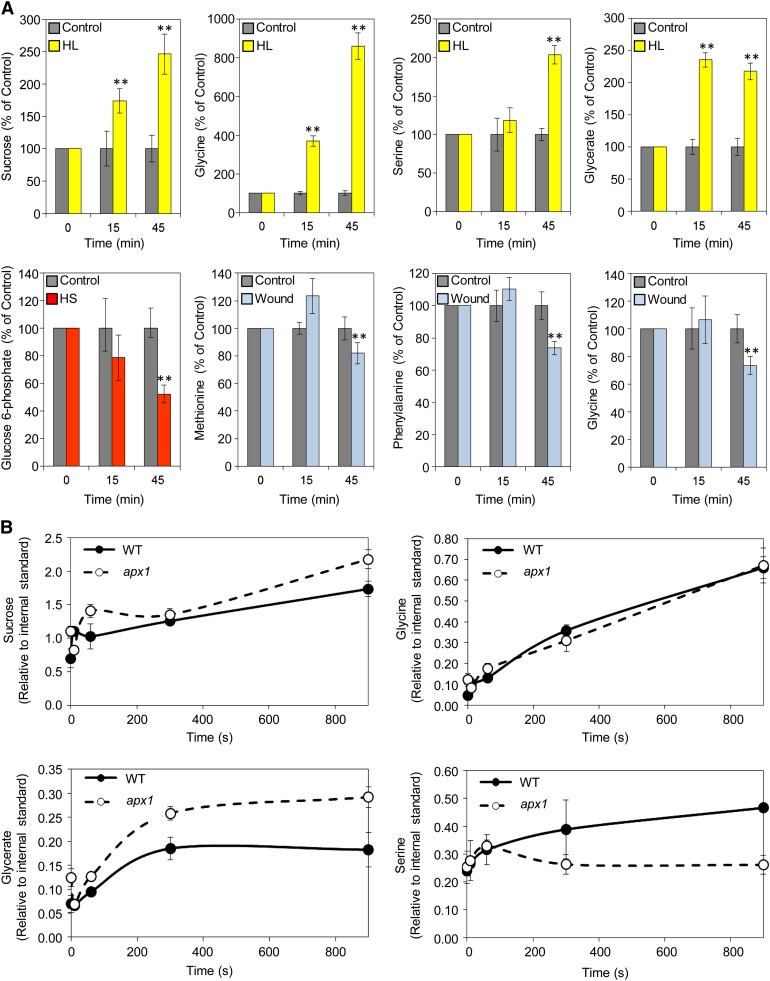
A derivative of glycerol 3-phosphate was recently found to be involved in SAR (Chanda et al., 2011), suggesting that specific primary metabolites could play an important function in SAA. Our metabolome analysis identified Gly, glycerate, Ser, and Suc as putative compounds involved in rapid systemic acclimation to HL (Figure 10A; see Supplemental Data Sets 15 to 20 online). The rapid accumulation of Gly and glycerate in systemic tissues of plants at 15 and 45 min following local HL treatment was followed by accumulation of Ser at 45 min (Figure 10A), suggesting that at least part of the photorespiratory pathway could be activated in systemic nonstressed leaves of plants subjected to a local treatment of HL. This finding could suggest a novel mechanism for systemic HL acclimation.
Figure 10.
Systemic and Local Metabolic Responses of Plants Subjected to Abiotic Stimuli.
(A) Top panels: Accumulation of Suc, Gly, Ser, and glycerate in systemic tissues of wild-type plants subjected to a local HL stimulus for 15 or 45 min. Bottom panels: Suppression of Glc 6-phosphate or several amino acids (Met, Phe, and Gly) in systemic tissues of wild-type plants subjected to a local HS or wound stimulus, respectively, for 15 or 45 min (n = 5). **Student’s t test significant at P < 0.01. See also Supplemental Figure 5 online.
(B) Accumulation of Suc, Gly, Ser, and glycerate in local tissues of wild-type (WT) or apx1 plants exposed to short-term HL stimuli (0, 10, 60, 300, and 600 s). Results are relative to internal control (ribitol) (n = 5).
In (A) and (B), error bars = se.
In contrast with the accumulation of Gly, glycerate, and Ser during HL stress, the level of Glc 6-phosphate and several amino acids was found to be suppressed in systemic tissues in response to HS or wounding, respectively (Figure 10A; see Supplemental Figure 5 and Supplemental Data Sets 15 to 20 online). These findings could implicate additional primary metabolites in systemic signaling in plants as well as uncover a type of systemic response that decreases the availability of free amino acids in systemic tissues in response to wounding.
The accumulation of Gly, glycerate, and Suc in systemic tissues of plants subjected to a local HL treatment occurred as early as 15 min following the application of stress (Figure 10A). This finding prompted us to test how fast these responses would occur in the local leaves that were directly subjected to the HL treatment. As shown in Figure 10B, the accumulation of Gly, glycerate, Ser, and Suc initiated within 60 s of HL application to local leaves. This finding demonstrated that metabolome reprogramming of local tissues could be at least partially similar to that of systemic tissues and that these responses occur at a very rapid rate. Interestingly, some of the local responses to HL were altered in the apx1 mutant, demonstrating that they could be associated with H2O2 scavenging (Figure 10B).
DISCUSSION
Because not all plant tissues are likely to sense an external threat simultaneously, mechanisms that rapidly transmit cellular signals across long distances in plants could be viewed as an evolutionary advantage enabling the plant to better prepare for, and survive, different environmental stimuli or stresses. Triggered by a wide range of abiotic stimuli (Miller et al., 2009), and functioning as a priming signal required for rapid systemic acclimation (Figures 1 to 7), the ROS wave could be one such mechanism.
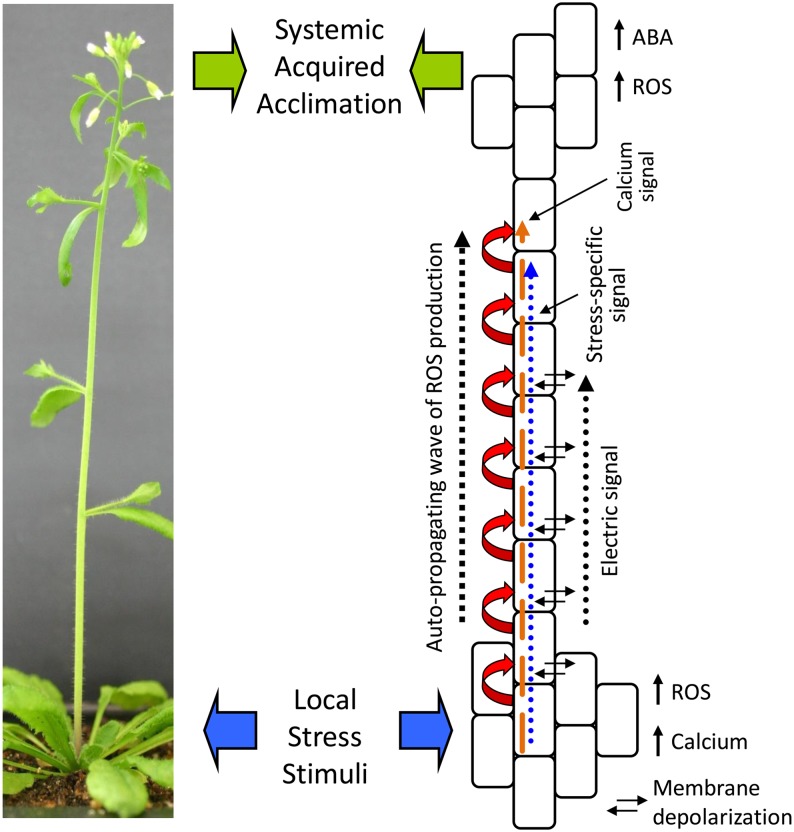
Our study of the ROS wave suggests that sensing of changes in environmental conditions by a group of cells in a particular tissue of the plant could generate at least three different systemic signals: a general priming signal in the form of the ROS wave, a stress-specific signal that could be a plant hormone, such as ABA, a basic plant metabolite, such as Gly, or any of the previously proposed signals and chemicals involved in SAA or SAR (Shah and Zeier, 2013) and electric signals (Szechyńska-Hebda et al., 2010). The recent finding that the activation of RBOHD along the path of the rapid systemic signal requires calcium-dependent protein kinase5 and that this process is H2O2 dependent (Dubiella et al., 2013) strongly suggests that a process of calcium-regulated ROS-induced ROS production is involved in the propagation of the ROS wave. Calcium signaling and the potential existence of a calcium ROS wave could therefore add an additional dimension to this process (Figure 11).
Figure 11.
Suggested Model of the SAA Response of Plants to Abiotic Stimuli.
Local stress treatment (wide blue arrows) is shown to result in local calcium and ROS accumulation and the activation of a calcium-dependent autopropagating wave of ROS production (red and gold arrows). The ROS wave spreads to the entire plant and is required for SAA (wide green arrows). It could be carrying with it a stress-specific signal (blue arrow) that conveys abiotic stress specificity to the signal as well as a change in membrane depolarization (electric signal).
The autopropagating nature of the ROS wave signal indicates that each cell along its path independently activates its own RBOHD enzyme and generates ROS (Figure 2; Miller et al., 2009; Mittler et al., 2011; Dubiella et al., 2013). This observation opens the door to a very interesting possibility: In addition to activating its own RBOHD, each cell along the systemic path of the ROS wave could also activate different pathways that generate and accumulate the stress-specific signal. The finding that ABA accumulation in systemic tissues of plants subjected to a local HS treatment is RBOHD dependent (Figure 6C) supports such a possibility. The function of the ROS wave may therefore be to carry with it a stress-specific signal or metabolic response all the way from its initiation site to the systemic tissues, potentially explaining why it is required for, but not sufficient, to induce a stress-specific SAA (Figures 1 to 7 and 11).
Our findings that the ROS wave is associated with the generation and/or propagation of systemic potential variations (Figure 9) may demonstrate a link between electric signals in plants and the highly regulated process of ROS production. Under certain conditions, activating ROS production by protein phosphorylation or calcium signaling via RBOHD could therefore amplify or attenuate electric signals. Although our findings suggest a link between the ROS wave and a certain type of electric signals, it should be noted that the velocity of many electric signals recorded in plants is faster than that of the ROS wave (Zimmermann et al., 2009; Oyarce and Gurovich, 2011). Furthermore, the electric signals we recorded did not seem to be specific to a particular stress and were induced by HS or HL (Figure 9). Additional studies are required to address the involvement of electric signals in SAA.
Our metabolome analysis of SAA in plants uncovered a number of possible acclimation strategies (Figures 4A and 10). The rapid accumulation of Gly and glycerate that was followed by accumulation of Ser during HL SAA (Figure 10) could suggest that a portion of the photorespiratory machinery is activated as part of the HL SAA process in systemic nonstressed tissues. Such activation could facilitate Gly-to-Ser respiration in mitochondria of systemic leaves, increasing the level of CO2 and priming cells to resist the HL stress via a faster activation of photorespiration upon HL stress of the entire plant. The increased levels of Suc also observed during HL SAA (Figure 10) could provide further energy to drive mitochondrial respiration. The net outcome of these processes would be an increase in NAD(P)H levels that could be used to scavenge excess ROS upon HL, a partially activated photorespiratory machinery primed for HL stress, and a higher cellular level of CO2 to provide a photosynthetic sink for the high photon flux induced by the HL stress. In the case of mechanical wounding, frequently associated with insect feeding in plants, our observation that wound-induced SAA results in a decrease in the free level of certain amino acids (Figures 10A; see Supplemental Figure 5 online), could indicate a metabolic response that would make plants less nutritious for the insect and thus deter further feeding and infestation. Our findings that rbohD mutants are more susceptible to aphid feeding (Miller et al., 2009) could support this possibility, especially if these systemic alterations are blocked in the rbohD mutant. Further studies are required to address some of these tantalizing mechanisms potentially involved in SAA to HL, HS, or wounding.
Compared with previous work on systemic signaling in plants that described signaling and acclimation within 15 to 45 min (SAA) or 6 to 48 h (SAR) (Karpiński et al., 1999; Rossel et al., 2007; Carr et al., 2010; Szechyńska-Hebda et al., 2010; Dempsey and Klessig, 2012; Spoel and Dong, 2012; Shah and Zeier, 2013), our findings uncover a much faster systemic and local response and acclimation times in plants (e.g., a propagation rate of 8.4 cm min−1 for the ROS wave [Figure 6B; Miller et al., 2009], a 5-min SAA time for HS [Figure 6A], and a rapid reprogramming of metabolome that occurs within seconds in local or minutes in systemic tissues in response to HL [Figure 10]). Transcriptome, proteome, and metabolome reprogramming of cells in local and systemic tissues of stress- or pathogen-challenged cells may therefore occur faster than was previously thought, suggesting that a gap in short-term biotic or abiotic stress studies could exist in many of the currently available -omics databases. Filling this gap could reveal early events in signaling and acclimation that are unknown at present.
METHODS
Plant Material and Growth Conditions
Arabidopsis thaliana cv Columbia-0, cv Wassilewskija-0, rbohD knockout (Torres et al., 2002), Arabidopsis deficient in cytosolic APX1 (apx1; SALK_000249 and KO-APX1; Davletova et al., 2005a), cv Landsberg erecta-0, and aba1-1, abi1-1 (Assmann et al., 2000), and abi1-2 (Saez et al., 2006) were grown on peat pellets (Jiffy-7) under controlled conditions: 21°C, 16-h light cycle, 100 μmol m−2 s−1, and relative humidity of 70% (E-30 AR-66; Percival Scientific) as described by Rizhsky et al. (2004a).
SAA Assays
Three different setups were used to study SAA in plants. Bolting plants were used to study the role of the ROS wave in SAA because they could be treated with a drop of DPI or catalase along the systemic path of the signal (e.g., Figure 1). Rosette leaves were used to study the role of SAA in whole-plant protection and signaling because the entire plant could be treated following local application of a single leaf and the ROS signal could be imaged (e.g., Figures 5 to 7). Seedlings were used to study SAA because they could be used for grafting (Figure 2) and because their root tips could be imaged for GFP localization (Figure 8).
One rosette leaf of each bolting plant with an inflorescence stem of 13 to 17 cm was exposed to abiotic stress. For HL stress, one rosette leaf was exposed to 1500 μmol m−2 s−1 light using a gooseneck light source (ACE I; Schott) for 45 min. For HS, one rosette leaf was dipped into a water bath (40 or 21°C) for 45 min. For wounding, one rosette leaf was pricked 10 times with the tip of a scalpel. Plants were then incubated for 45 min under controlled conditions. Following this recovery period, one cauline leaf located between 9 and 12 cm above the rosette leaves in each plant was exposed to 1500 μmol m−2 s−1 light for 90 min or dipped into a 45 or 21°C water bath for 60 min. Leaves were then photographed and sampled for chlorophyll measurement immediately after HL stress or 7 d following HS. Total chlorophyll concentration was determined according to Yalovsky et al. (1992). To test the effect of DPI or catalase on the SAA response of plants to abiotic stress, a 1-mL drop of 0.1% agarose containing 50 μM DPI or 100 units/μL catalase was placed on the inflorescence stem at the middle between the rosette and the cauline leaf for 30 min prior to the local stress treatment of the rosette leaf.
To compare the effects of different HS acclimation times on SAA, two leaves of 21- to 24-d-old plants were treated in a water bath at 40 or 21°C for 0, 5, 10, 20, and 45 min, and the entire plant was then subjected to a 50°C HS in a growth chamber for 4.5 h. Plants were then allowed to recover under controlled conditions for 5 d and scored for their diameter and survival. To test the effect of ABA on the SAA response of plants to HS, 1 μM ABA was applied to the entire plant 30 min prior to the local stress treatment. Protein blot analysis was performed as previously described (Suzuki et al., 2008), and quantification of protein level was performed using Image J software (rsbweb.nih.gov/ij/). Acquired and basal thermotolerance of seedlings was determined as previously described (Suzuki et al., 2008).
For nuclear localization, seeds of transgenic plants expressing MBF1c-GFP fusion protein (Suzuki et al., 2008) were surface sterilized and placed in a row on 1% agar plates as previously described (Luhua et al., 2008). Leaves of 7-d-old seedlings were subjected to 37 or 21°C using a heat block for 30 min. Root tips were maintained under 21°C during exposure of leaves to HS. Localization of MBF1c-GFP fusion protein was observed in root tips using an Olympus confocal microscope (FV1000) (Suzuki et al., 2008). To test the effect of ABA, DPI, or catalase on nuclear localization, 0.1% agarose containing 1 μM ABA, 50 μM DPI, or 100 units/μL catalase was placed on the root tip 30 min prior to the HS treatment.
Luciferase Imaging
The ROS was imaged indirectly with the Zat12 promoter fused to luciferase as described by Miller et al. (2009). Two leaves of 21- to 24-d-old plants were exposed to 40°C HS as described above. Plants were then sprayed with 1 mM luciferin (GOLD Bio Technology) and imaged using a NightOWL LB983 NC100 (Berthold) imager. Images were captured with 30 s of exposure time. Luminescence intensity was measured with IndiGO v2.0.3.0 (Berthold). Despite repeated attempts, we were unable to record oscillations in the ROS wave signal.
Micrografting Assays
Single-hypocotyl grafts were constructed as previously described (Turnbull et al., 2002; Nalam et al., 2012) with minor modifications. Columbia-0 and rbohD were grown on 2% agar plates for 4 d as previously described (Luhua et al., 2008). Prior to grafting, 4-d-old seedlings were moved to 27°C for 1 d. Scion and rootstock were then cut transversely and aligned precisely under sterile conditions. Following grafting, the seedlings were incubated at 27°C for two more days to promote graft union, transferred to 21°C, and grown for an additional 7 d. The grafted seedlings were monitored every day, and any adventitious roots were crushed with forceps. Leaves of grafted seedlings were then subjected to 37 or 21°C for 30 min. Local and systemic tissues were sampled in parallel and analyzed by protein blots.
Imaging of ROS in Root Tips of Seedlings Treated with H2O2
A 0.5-mL drop of water containing 1 mM H2O2 was placed on the cotyledons of 7-d-old seedlings grown on 1% agar plates as described above and seedlings were incubated for 30 min at room temperature. Following this incubation, H2O2 was removed by pipetting, and cotyledons were washed with distilled water. Seedlings were then sprayed with 2 μM Amplex Red (Life Technologies) and incubated for 20 min at room temperature under dark. H2O2 was observed under an E-VOS-fl microscope (Advanced Microscopy Group).
Stress Treatments for Microarray and Metabolome Analyses
Two fully expanded leaves of nonbolting 21- to 24-d-old plants were subjected to HS, HL, or wounding, and systemic tissues were sampled at 15 and 45 min. Samples were also collected in parallel from plants that were exposed to control treatments corresponding to each stress. For HS, two leaves were dipped into 40°C water. Two leaves of similar sizes and developmental stages were also dipped into 21°C water as control. For wounding, two leaves were pricked 10 times as described above. Unwounded plants were used as control. For HL, plants were covered with foil, exposing only two leaves to 1500 μmol m−2 s−1 light. Two leaves of plants covered with foil were also exposed to 100 μmol m−2 s−1 light as control. For microarray analysis, three independent biological replicates, each composed of leaves pooled from at least 28 different plants, were used per experimental condition. For metabolome analysis, five independent biological replicates, each containing at least 100 mg of fresh weight from a pool of at least 15 different plants were used for each experimental condition.
For short-term HL exposure, 15 to 20 wild-type or apx1- plants were grown in 9 × 9 × 6-cm3 pots covered with a fiberglass screen net at 23°C under constant low light conditions (50 µmol m−2 s−1) for 3 weeks. HL stress was applied in a growth chamber at a light intensity of 1000 µmol m−2 s−1 at 20°C for periods of 0, 0.25, 1, 5, and 15 min. Samples were collected by immediately dipping the pots in liquid nitrogen. Frozen shoots were then cut onto aluminum foil, ground, and transferred into 1.5-mL tubes (∼300 to 350 mg/tube). Samples were kept frozen during the entire collecting process and stored at −80°C. For ABA measurement, two fully expanded leaves of nonbolting 21- to 24-d-old plants were subjected to HS as described above, and meristems were sampled at 0, 5, 10, 15, and 45 min.
Microarray, Quantitative RT-PCR, and Meta-Analyses
RNA samples for microarray analysis were processed at the Nevada Genomics Center of the University of Nevada, Reno as previously described (Davletova et al., 2005a, 2005b; Miller et al., 2009; Suzuki et al., 2011a). Array data were analyzed as previously described (Irizarry et al., 2003; Gautier et al., 2004; Davletova et al., 2005a, 2005b; Miller et al., 2009; Suzuki et al., 2011a) and deposited in Array Express (E-MEXP-3754).
The expression of several transcripts detected by the array analysis was confirmed by quantitative real-time PCR (Miller et al., 2009; see Supplemental Figure 1 online) using the StepOnePlus real-time PCR system (Applied Biosystems). The quantitative PCR data were analyzed with StepOnePlus software v2.0.1 (Applied Biosystems). Threshold cycle values for glutaredoxin, HSFA2, CRF2, GPT2, JAS1, JAZ6, AOC3, and PBP1, were calculated with the cycle threshold of EF1-a as an internal control. Primer pairs used for amplifications are shown in Supplemental Table 1 online.
The overlap between transcripts upregulated in systemic tissues in response to each of the different stresses and transcripts upregulated in response to ABA, ethylene (1-aminocyclopropane-1-carboxylic acid - ACC), brassinolide, cytokinin, gibberellin, auxin (indole-3-acetic acid), methyl jasmonate (Nemhauser et al., 2006), SA (Blanco et al., 2009), H2O2 (Davletova et al., 2005b), O2− (Scarpeci et al., 2008), or 1O2 (Gadjev et al., 2006) was determined as described by Miller et al. (2009). Venn diagrams were generated according to Davletova et al. (2005b) and Rizhsky et al. (2004b).
Metabolome Analysis
To examine the accumulation of stress-associated metabolites in systemic and local leaves of Arabidopsis, we performed gas chromatography–mass spectrometry (Fiehn et al., 2000; Roessner et al., 2000; Shuman et al., 2011) and liquid chromatography–mass spectrometry (Armenta et al., 2010; Salazar et al., 2012) analyses and expressed the level of individual compounds as relative to an internal control (e.g., ribitol; Rizhsky et al., 2004b). For gas chromatography–mass spectrometry analysis, extraction and derivatization were performed as described previously (Fiehn et al., 2000; Roessner et al., 2000; Shuman et al., 2011). Amino acids were extracted and quantified by liquid chromatography–mass spectrometry analysis as previously described (Armenta et al., 2010; Salazar et al., 2012). ABA was extracted as previously described (Pan et al., 2010). Leaf material (15 mg) in a 1.5-mL microcentrifuge tube was flash frozen in liquid nitrogen and ground in a reciprocating ball mill (Mixer Mill MM 300 and Mixer Mill adapter set from Qiagen) at 30 cycles/s using 2.3-mm stainless steel balls. The sample was soaked with 50 μL of the internal standard solution (1 ng/μL ABA-d6 in methanol) and 300 μL of extraction solvent (2:1:0.002 isopropanol/water/hydrochloric acid [v/v/v]). After 24 h extraction at 4°C with 200 rpm using an orbital shaker (Multi Purpose Rotator Model 2314; Thermo Scientific), the sample was spun at 13,000g for 10 min in a bench top refrigerated (4°C) microcentrifuge (Beckman Coulter) and the supernatant was transferred to a clean tube. Dichloromethane (0.6 mL) was added to the supernatant followed by centrifugation for 5 min at 4°C. The lower phase was transferred into a clean screw-cap glass vial using a Pasteur pipette. The extract was concentrated (but not completely dry) in a nitrogen evaporator (Organomation Associates) and then redissolved in 100 μL of 100% methanol. Complete dissolution was ensured by vortexing and sonicating the extract, which was transferred to a reduced volume liquid chromatography vial.
ABA was analyzed by ultraperformance liquid chromatography–electrospray ionization–tandem mass spectrometry using a Waters Acquity UPLC system interfaced to a Waters Xevo TQ mass spectrometer. The chromatographic separation of ABA and its internal standard from the plant extracts was performed on an Acquity UPLC BEH C18 column (2.1 mm i.d. × 50 mm, 1.7-μm particles) at 40°C using a binary solvent system comprising 0.1% formic acid in water (Solvent A) and 0.1% formic acid in methanol (Solvent B) at a flow rate of 0.5 mL/min. The solvent gradient used was as follows: 0 min (99.9% A, held for 0.5 min), 1.0 min (80.0% A), 1.5 min (40.0% A, held for 0.5 min), 2.5 min (35.0% A, held for 0.5 min), and 4 min (99.9% A, held for 1.8 min). Analysis of the compounds was based on multiple reaction monitoring using their most sensitive parent-daughter ion transitions (ABA, mass-to-charge ratio 263.16 > 153.05; d6-ABA, mass-to-charge ratio 269.20 > 159.10) at the optimal collision energy (CE) and cone voltage (CV) values determined with IntelliStart software (ABA, CV = 22 V, CE = 12 eV; ABA-d6, CV = 22 V, CE = 10 eV). The response of the mass spectrometer was calibrated by triplicate analysis of standard solutions of known concentration of ABA (ranging from 0.24 to 31.25 ng/mL) with a constant concentration of 50 ng/mL internal standard. The mass spectrometer was operated in the negative mode using the following source settings: capillary voltage, 3.5 kV (ESI); desolvation temperature, 600°C; desolvation gas flow rate, 1000 liters/h; and source temperature, 150°C. Argon was used as the collision gas at a flow rate of 0.15 mL/min. The autosampler was kept at 4°C during the analysis. The ultraperformance liquid chromatography–electrospray ionization–tandem mass spectrometry system control and data acquisition were performed with Waters MassLynx software. Data analysis was conducted with TargetLynx software (Waters).
Measurements of Systemic Potential Variations
Systemic potential variations were recorded extracellularly in 21- to 24-d-old plants as previously described (Tjallingii, 2006; Pegadaraju et al., 2007; Louis et al., 2010; Volkov et al., 2010) with the following modifications. A 10-μm gold wire was directly attached to the conductive surface of the leaf using water-based colloidal silver glue (Ted Pella). An output wire from the monitor was inserted into the soil in which the plant was rooted. A fully expanded leaf older than the leaf connected to the electrode was exposed to 1500 μmol m−2 s−1 light with a gooseneck light source for 5 min or 40°C HS by placing a heat block at close proximity to the leaf for 10 min. Leaf temperature was monitored with an infrared thermometer as described (Miller et al., 2009). Systemic extracellular potential differences were continuously recorded with a Giga-4/8 EPG system for 10 min prior to stress treatment, for 5 or 10 min during the HL or HS treatment, respectively, and for an additional 45 min following the HL or HS treatments. Plants were held inside a Faraday cage during the stress and recording periods at an ambient temperature of 22°C. Thirty replications were performed, and waveform recordings obtained were analyzed using stylet +a software (W.F. Tjallingii, Wageningen University, Wageningen, The Netherlands). The EPG system (Tjallingii, 2006) was also used to record systemic potential variations using feeding green peach aphids (Myzus persicae) on a systemic leaf of wild-type and rbohD plants subjected to a local HL stimulus. An aphid attached to a 10-μm gold wire using water-based colloidal silver glue was released on a leaf, and its behavior was electrically monitored as previously described (Tjallingii, 2006; Pegadaraju et al., 2007; Louis et al., 2010). Once phloem feeding in the form of type E2 waveform (Tjallingii, 2006) was initiated and maintained for several minutes, HL stress was applied to a fully expanded leaf older than the one used for recording, and electric signals were recorded for 10 min.
Statistical Analysis
Analysis of variance was performed on microarray data as previously described (Davletova et al., 2005a, 2005b; Miller et al., 2009; Suzuki et al., 2011a). Other statistical analyses were performed by one-tailed Student’s t test as described by Davletova et al. (2005a). Results are presented as mean ± sd or se (*P < 0.05; **P < 0.01).
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for genes mentioned in this article are as follows: RbohD (At5g47910), APX1 (At1g07890), MBF1c (At3g24500), glutaredoxin (At4g15680), HSFA2 (At2g26150), CRF2 (At4g23750), GPT2 (At1g61800), JAS1 (At5g13220), JAZ6 (At1g72450), AOC3 (At3g25780), PBP1 (At5g54490), and EF1-a (At5g60390). Microarray data were deposited in Array Express (E-MEXP-3754).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Inhibition of SAA to HL or HS by Catalase.
Supplemental Figure 2. Detection of H2O2 in Local (Cotyledons) and Systemic (Root Tip) Tissues in Wild-Type and rbohD Seedlings in Response to Local Application of 1 mM H2O2.
Supplemental Figure 3. Expression of Stress Response Transcripts in Systemic Tissues.
Supplemental Figure 4. Categorization of Expression Patterns of the Transcripts Elevated by Heat Stress, High Light Stress, or Wounding in Systemic Tissues.
Supplemental Figure 5. Suppression of Metabolites in Systemic Tissues of Plants Subjected to a Local Wound Stimulus.
Supplemental Figure 6. Nuclear Localization of MBF1c in Systemic Tissues during SAA to HS Is Attenuated by Catalase.
Supplemental Figure 7. Association between the ROS Wave and Systemic Potential Variations during the SAA Response of Plants to HL Stress.
Supplemental Table 1. Primer Pairs Used for qRT-PCR.
Supplemental Data Set 1. Transcripts Specifically Upregulated in Systemic Tissues 15 min Following Local Heat Stress Application.
Supplemental Data Set 2. Transcripts Specifically Upregulated in Systemic Tissues 15 min Following Local High Light Stress Application.
Supplemental Data Set 3. Transcripts Specifically Upregulated in Systemic Tissues 15 min Following Wounding of Local Tissue.
Supplemental Data Set 4. Transcripts Specifically Upregulated in Systemic Tissues 45 min Following Local Heat Stress Application.
Supplemental Data Set 5. Transcripts Specifically Upregulated in Systemic Tissues 45 min Following Local High Light Stress Application.
Supplemental Data Set 6. Transcripts Specifically Upregulated in Systemic Tissues 45 min Following Wounding of Local Tissue.
Supplemental Data Set 7. Transcripts Upregulated in Systemic Tissues 15 min Following Local Heat Stress or High Light Stress Application.
Supplemental Data Set 8. Transcripts Upregulated in Systemic Tissues 15 min Following Local Heat Stress or Wounding Application.
Supplemental Data Set 9. Transcripts Upregulated in Systemic Tissues 15 min Following High Light Stress or Wounding Application.
Supplemental Data Set 10. Transcripts Upregulated in Systemic Tissues 15 min Following Local Heat Stress, High Light Stress, or Wounding Application.
Supplemental Data Set 11. Transcripts Upregulated in Systemic Tissues 45 min Following Local Heat Stress or High Light Stress Application.
Supplemental Data Set 12. Transcripts Upregulated in Systemic Tissues 45 min Following Local Heat Stress or Wounding Application.
Supplemental Data Set 13. Transcripts Upregulated in Systemic Tissues 45 min Following Local High Light Stress or Wounding Application.
Supplemental Data Set 14. Transcripts Upregulated in Systemic Tissues 45 min Following Local Heat Stress, High Light Stress, or Wounding Application.
Supplemental Data Set 15. Amino Acids Significantly Upregulated in Systemic Tissues 15 or 45 min Following Local Abiotic Stress or Wounding Treatment.
Supplemental Data Set 16. Amino Acids Significantly Downregulated in Systemic Tissues 15 or 45 min Following Local Abiotic Stress or Wounding Treatment.
Supplemental Data Set 17. Sugars Significantly Upregulated in Systemic Tissues 15 or 45 min Following Local Abiotic Stress or Wounding Treatment.
Supplemental Data Set 18. Sugars Significantly Downregulated in Systemic Tissues 15 or 45 min Following Local Abiotic Stress or Wounding Treatment.
Supplemental Data Set 19. Organic Acids Significantly Upregulated in Systemic Tissues 15 or 45 min Following Local Abiotic Stress or Wounding Treatment.
Supplemental Data Set 20. Organic Acids Significantly Downregulated in Systemic Tissues 15 or 45 min Following Local Abiotic Stress or Wounding Treatment.
Supplemental Data Set 21. Proportion of Hormone or ROS Response Transcripts among the Transcripts Elevated in Systemic Tissues in Response to More Than Two Different Stimuli at 15 and 45 min. Supplemental Data Set 22. Putative Pathways Identified among the Different Transcripts Elevated in Systemic Tissues in Response to a Local HS, HL, or Wound Stimuli at 15 and 45 Min.
Acknowledgments
This work was supported by funding from the National Science Foundation (NSF-0431327, IOS-0639964, IOS-0743954, and IOS-0919192) and the University of North Texas College of Arts and Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article. We thank Craig Osborne, Rachel Tam, and Yuting Luo for technical support.
AUTHOR CONTRIBUTIONS
R.M., N.S., V.S., and J.S. designed and supervised the research. N.S., G.M., C.S., H.A.M., E.S., D.F.C., J.L.S., and X.L. performed the research. K.S. analyzed transcriptome data. R.M. and N.S. wrote the article.
Glossary
- SAR
systemic acquired resistance
- SAA
systemic acquired acclimation
- SA
salicylic acid
- ROS
reactive oxygen species
- ABA
abscisic acid
- HL
high light
- HS
heat stress
- DPI
diphenylene iodonium
- H2O2
hydrogen peroxide
- PM
plasma membrane
- CE
collision energy
- CV
cone voltage
References
- Armenta J.M., Cortes D.F., Pisciotta J.M., Shuman J.L., Blakeslee K., Rasoloson D., Ogunbiyi O., Sullivan D.J., Jr., Shulaev V. (2010). Sensitive and rapid method for amino acid quantitation in malaria biological samples using AccQ.Tag ultra performance liquid chromatography-electrospray ionization-MS/MS with multiple reaction monitoring. Anal. Chem. 82: 548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann S.M., Snyder J.A., Lee Y.-R.J. (2000). ABA-deficient (aba1) and ABA-insensitive (abi1-1, abi2-1) mutants of Arabidopsis have a wild-type stomatal response to humidity. Plant Cell Environ. 23: 387–395 [Google Scholar]
- Bae Y.S., Oh H., Rhee S.G., Yoo Y.D. (2011). Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 32: 491–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco F., Salinas P., Cecchini N.M., Jordana X., Van Hummelen P., Alvarez M.E., Holuigue L. (2009). Early genomic responses to salicylic acid in Arabidopsis. Plant Mol. Biol. 70: 79–102 [DOI] [PubMed] [Google Scholar]
- Boyko A., Kovalchuk I. (2011). Genome instability and epigenetic modification—Heritable responses to environmental stress? Curr. Opin. Plant Biol. 14: 260–266 [DOI] [PubMed] [Google Scholar]
- Bray, E.A., Bailey-Serres, J., and Weretilnyk, E. (2000). Responses to abiotic stresses. In Biochemistry and Molecular Biology of Plants, B.B. Buchanan, W. Gruissem, and R.L. Jones, eds (Rockville, MD: American Society of Plant Physiologists), pp. 1158–1249. [Google Scholar]
- Carr J.P., Lewsey M.G., Palukaitis P. (2010). Signaling in induced resistance. Adv. Virus Res. 76: 57–121 [DOI] [PubMed] [Google Scholar]
- Chanda B., Xia Y., Mandal M.K., Yu K., Sekine K.T., Gao Q.M., Selote D., Hu Y., Stromberg A., Navarre D., Kachroo A., Kachroo P. (2011). Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat. Genet. 43: 421–427 [DOI] [PubMed] [Google Scholar]
- Chaturvedi R., Venables B., Petros R.A., Nalam V., Li M., Wang X., Takemoto L.J., Shah J. (2012). An abietane diterpenoid is a potent activator of systemic acquired resistance. Plant J. 71: 161–172 [DOI] [PubMed] [Google Scholar]
- Davletova S., Rizhsky L., Liang H., Shengqiang Z., Oliver D.J., Coutu J., Shulaev V., Schlauch K., Mittler R. (2005a). Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S., Schlauch K., Coutu J., Mittler R. (2005b). The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 139: 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T.E. (2003). Interactions between NADPH oxidase and voltage-gated proton channels: Why electron transport depends on proton transport. FEBS Lett. 555: 57–61 [DOI] [PubMed] [Google Scholar]
- Dempsey D.A., Klessig D.F. (2012). SOS - Too many signals for systemic acquired resistance? Trends Plant Sci. 17: 538–545 [DOI] [PubMed] [Google Scholar]
- Dubiella U., Seybold H., Durian G., Komander E., Lassig R., Witte C.P., Schulze W.X., Romeis T. (2013). Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 110: 8744–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn O., Kopka J., Trethewey R.N., Willmitzer L. (2000). Identification of uncommon plant metabolites based on calculation of elemental compositions using gas chromatography and quadrupole mass spectrometry. Anal. Chem. 72: 3573–3580 [DOI] [PubMed] [Google Scholar]
- Frahry G., Schopfer P. (1998). Inhibition of O2-reducing activity of horseradish peroxidase by diphenyleneiodonium. Phytochemistry 48: 223–227 [DOI] [PubMed] [Google Scholar]
- Fromm J., Lautner S. (2007). Electrical signals and their physiological significance in plants. Plant Cell Environ. 30: 249–257 [DOI] [PubMed] [Google Scholar]
- Gadjev I., Vanderauwera S., Gechev T.S., Laloi C., Minkov I.N., Shulaev V., Apel K., Inzé D., Mittler R., Van Breusegem F. (2006). Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 141: 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier L., Cope L., Bolstad B.M., Irizarry R.A. (2004). affy—Analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20: 307–315 [DOI] [PubMed] [Google Scholar]
- Halliwell B. (2012). Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 70: 257–265 [DOI] [PubMed] [Google Scholar]
- Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U., Speed T.P. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264 [DOI] [PubMed] [Google Scholar]
- Jammes F., et al. (2009). MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. USA 106: 20520–20525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H.W., Tschaplinski T.J., Wang L., Glazebrook J., Greenberg J.T. (2009). Priming in systemic plant immunity. Science 324: 89–91 [DOI] [PubMed] [Google Scholar]
- Karpiński S., Reynolds H., Karpińska B., Wingsle G., Creissen G., Mullineaux P. (1999). Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284: 654–657 [DOI] [PubMed] [Google Scholar]
- Karpiński S., Szechyńska-Hebda M., Wituszyńska W., Burdiak P. (2013). Light acclimation, retrograde signalling, cell death and immune defences in plants. Plant Cell Environ. 36: 736–744 [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Ohura I., Kawakita K., Yokota N., Fujiwara M., Shimamoto K., Doke N., Yoshioka H. (2007). Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19: 1065–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J., Hall J.D., Knight M.R., Vierling E. (2005). Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 138: 882–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis J., Lorenc-Kukula K., Singh V., Reese J., Jander G., Shah J. (2010). Antibiosis against the green peach aphid requires the Arabidopsis thaliana MYZUS PERSICAE-INDUCED LIPASE1 gene. Plant J. 64: 800–811 [DOI] [PubMed] [Google Scholar]
- Luhua S., Ciftci-Yilmaz S., Harper J., Cushman J., Mittler R. (2008). Enhanced tolerance to oxidative stress in transgenic Arabidopsis plants expressing proteins of unknown function. Plant Physiol. 148: 280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado A.M., Doerner P., Dixon R.A., Lamb C.J., Cameron R.K. (2002). A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 419: 399–403 [DOI] [PubMed] [Google Scholar]
- Matoba T., Shimokawa H. (2003). Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J. Pharmacol. Sci. 92: 1–6 [DOI] [PubMed] [Google Scholar]
- McInnis S.M., Desikan R., Hancock J.T., Hiscock S.J. (2006). Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: Potential signalling crosstalk? New Phytol. 172: 221–228 [DOI] [PubMed] [Google Scholar]
- Miller E.W., Dickinson B.C., Chang C.J. (2010). Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 107: 15681–15686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Schlauch K., Tam R., Cortes D., Torres M.A., Shulaev V., Dangl J.L., Mittler R. (2009). The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2: ra45. [DOI] [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V., Van Breusegem F. (2011). ROS signaling: The new wave? Trends Plant Sci. 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Monshausen G.B., Bibikova T.N., Messerli M.A., Shi C., Gilroy S. (2007). Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc. Natl. Acad. Sci. USA 104: 20996–21001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalam V.J., Keeretaweep J., Sarowar S., Shah J. (2012). Root-derived oxylipins promote green peach aphid performance on Arabidopsis foliage. Plant Cell 24: 1643–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser J.L., Hong F., Chory J. (2006). Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126: 467–475 [DOI] [PubMed] [Google Scholar]
- Nishimura M.T., Dangl J.L. (2010). Arabidopsis and the plant immune system. Plant J. 61: 1053–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarce P., Gurovich L. (2011). Evidence for the transmission of information through electric potentials in injured avocado trees. J. Plant Physiol. 168: 103–108 [DOI] [PubMed] [Google Scholar]
- Pan X., Welti R., Wang X. (2010). Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 5: 986–992 [DOI] [PubMed] [Google Scholar]
- Park S.W., Kaimoyo E., Kumar D., Mosher S., Klessig D.F. (2007). Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318: 113–116 [DOI] [PubMed] [Google Scholar]
- Pegadaraju V., Louis J., Singh V., Reese J.C., Bautor J., Feys B.J., Cook G., Parker J.E., Shah J. (2007). Phloem-based resistance to green peach aphid is controlled by Arabidopsis PHYTOALEXIN DEFICIENT4 without its signaling partner ENHANCED DISEASE SUSCEPTIBILITY1. Plant J. 52: 332–341 [DOI] [PubMed] [Google Scholar]
- Ray P.D., Huang B.W., Tsuji Y. (2012). Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24: 981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A.S., Ali G.S., Celesnik H., Day I.S. (2011). Coping with stresses: Roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 23: 2010–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L., Davletova S., Liang H., Mittler R. (2004a). The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J. Biol. Chem. 279: 11736–11743 [DOI] [PubMed] [Google Scholar]
- Rizhsky L., Liang H., Shuman J., Shulaev V., Davletova S., Mittler R. (2004b). When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 134: 1683–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U., Wagner C., Kopka J., Trethewey R.N., Willmitzer L. (2000). Technical advance: Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J. 23: 131–142 [DOI] [PubMed] [Google Scholar]
- Rossel J.B., Wilson P.B., Hussain D., Woo N.S., Gordon M.J., Mewett O.P., Howell K.A., Whelan J., Kazan K., Pogson B.J. (2007). Systemic and intracellular responses to photooxidative stress in Arabidopsis. Plant Cell 19: 4091–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez A., Robert N., Maktabi M.H., Schroeder J.I., Serrano R., Rodriguez P.L. (2006). Enhancement of abscisic acid sensitivity and reduction of water consumption in Arabidopsis by combined inactivation of the protein phosphatases type 2C ABI1 and HAB1. Plant Physiol. 141: 1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar C., Armenta J.M., Cortés D.F., Shulaev V. (2012). Combination of an AccQ·Tag-ultra performance liquid chromatographic method with tandem mass spectrometry for the analysis of amino acids. Methods Mol. Biol. 828: 13–28 [DOI] [PubMed] [Google Scholar]
- Scarpeci T.E., Zanor M.I., Carrillo N., Mueller-Roeber B., Valle E.M. (2008). Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: A focus on rapidly induced genes. Plant Mol. Biol. 66: 361–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J., Zeier J. (2013). Long-distance communication and signal amplification in systemic acquired resistance. Front. Plant Sci. 4: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman J.L., Cortes D.F., Armenta J.M., Pokrzywa R.M., Mendes P., Shulaev V. (2011). Plant metabolomics by GC-MS and differential analysis. Methods Mol. Biol. 678: 229–246 [DOI] [PubMed] [Google Scholar]
- Spoel S.H., Dong X. (2012). How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 12: 89–100 [DOI] [PubMed] [Google Scholar]
- Suzuki N., Bajad S., Shuman J., Shulaev V., Mittler R. (2008). The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. J. Biol. Chem. 283: 9269–9275 [DOI] [PubMed] [Google Scholar]
- Suzuki N., Miller G., Morales J., Shulaev V., Torres M.A., Mittler R. (2011b). Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 14: 691–699 [DOI] [PubMed] [Google Scholar]
- Suzuki N., Sejima H., Tam R., Schlauch K., Mittler R. (2011a). Identification of the MBF1 heat-response regulon of Arabidopsis thaliana. Plant J. 66: 844–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechyńska-Hebda M., Kruk J., Górecka M., Karpińska B., Karpiński S. (2010). Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell 22: 2201–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjallingii W.F. (2006). Salivary secretions by aphids interacting with proteins of phloem wound responses. J. Exp. Bot. 57: 739–745 [DOI] [PubMed] [Google Scholar]
- Torres M.A., Dangl J.L., Jones J.D. (2002). Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman W., Bennett M.H., Kubigsteltig I., Turnbull C., Grant M. (2007). Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proc. Natl. Acad. Sci. USA 104: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull C.G., Booker J.P., Leyser H.M. (2002). Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J. 32: 255–262 [DOI] [PubMed] [Google Scholar]
- Vanderauwera S., Suzuki N., Miller G., van de Cotte B., Morsa S., Ravanat J.L., Hegie A., Triantaphylidès C., Shulaev V., Van Montagu M.C., Van Breusegem F., Mittler R. (2011). Extranuclear protection of chromosomal DNA from oxidative stress. Proc. Natl. Acad. Sci. USA 108: 1711–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov A.G., Foster J.C., Ashby T.A., Walker R.K., Johnson J.A., Markin V.S. (2010). Mimosa pudica: Electrical and mechanical stimulation of plant movements. Plant Cell Environ. 33: 163–173 [DOI] [PubMed] [Google Scholar]
- Yalovsky S., Ne’eman E., Schuster G., Paulsen H., Harel E., Nechushtai R. (1992). Accumulation of a light-harvesting chlorophyll a/b protein in the chloroplast grana lamellae. The lateral migration of the membrane protein precursor is independent of its processing. J. Biol. Chem. 267: 20689–20693 [PubMed] [Google Scholar]
- Zimmermann M.R., Maischak H., Mithöfer A., Boland W., Felle H.H. (2009). System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol. 149: 1593–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]