Introduction
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease that typically starts in early childhood and can persist into adulthood in some cases. AD is characterized by highly pruritic outbreaks, xerosis, and cutaneous inflammation manifesting as acute, subacute, or chronic eczematous dermatitis.1 Although multiple treatment algorithms for AD have been suggested, they have failed to simplify decision making sufficiently to encourage their routine incorporation into patient management. The primary objectives of this expert roundtable are to document current treatment approaches in an algorithm that is both therapeutically comprehensive and practical for patients and parents (where applicable), with utility in everyday practice.
The prevalence of AD has been increasing worldwide, with rates in the United States (US) ranging from 10 to 25 percent in children and approximately 3 to 5 percent in adults, depending on the population. The annual prevalence of new AD cases among children less than 17 years of age in the US is reported to be approximately 11 percent.2,3 However, accurate diagnosis may be complicated by the wide variability in clinical presentation and the lack of consensus around diagnostic criteria, especially in adults.4 Eighty-five percent of AD cases start before the age of five years.5 Some patients outgrow the disease around puberty, while others continue to suffer throughout their lives. Various reports set the prevalence of adult-onset AD at between 13 and 47 percent.6
Published criteria for diagnosing AD are as varied as the clinical presentation of AD itself. AD is typically diagnosed based on clinical manifestations and patient history. Itchy skin is the cornerstone of the diagnosis, followed by several other criteria, such as age of onset, sites of predilection, family or personal history of atopy, distribution, xerotic skin changes, tendency to relapse, and appearance of lesions.3,4 Patients are often classified as having mild, moderate, or severe disease, which is then used as a guide for treatment selection. Several instruments are used to assess the clinical status of patients and establish severity, but the two most common are the SCORing Atopic Dermatitis (SCORAD) tool7 and the Eczema Area and Severity Index (EASI), which were recently found to be comparable (Table 1).8 There is a paucity of data on the distribution of AD severity, with mild, moderate, and severe AD reported to affect 84, 14, and 2 percent of cases, respectively, in the pediatric population, with much higher percentages of patients with moderate-to-severe AD in the adult population.9
 |
The etiology of AD is not well understood. Evidence suggests that AD is a multifactorial disease with complex interplay between genetic and environmental factors. Clinical phenotypes include xerotic skin changes, eczematous plaques in various stages, lichenification, marked pruritus, associated sleep disturbance, increased Staphylococcus aureus colonization, and predisposition to skin infection, with laboratory assessments showing increased immunoglobulin E (IgE) and eosinophil counts in most cases.10
It is abundantly clear that there is a genetic predisposition to AD. A child has a twofold increased risk of developing AD if one parent has the disease and a threefold increased risk if both parents are affected.10 Evidence overwhelmingly suggests a pathological link between AD, allergic rhinitis, and asthma, with AD being the skin manifestation of a systemic disorder. Data have suggested that AD is often the first step in the emergence of an allergic triad that is often referred to as the “atopic march.” This refers to a progression of atopic disease that is believed to start with atopic dermatitis and subsequently progresses to asthma and allergic rhinitis.5
Pathogenesis of Atopic Dermatitis
With the incidence of AD increasing worldwide, interest in the pathophysiology of AD has increased substantially. There are two competing hypotheses on the pathogenesis of AD. The first is the “outside-in” hypothesis, which posits that there is an inherent barrier disruption with epiphenomenal immunological sequelae. The opposing hypothesis is the “inside-out,” suggesting a primary immune defect with secondary barrier disruption. According to the “outside-in” hypothesis, a defective stratum corneum (SC) permeability barrier may trigger the release of proinflammatory cytokines (e.g., interleukin [IL]-1, IL-6, tumor necrosis factor [TNF]-α), abnormal keratinocyte hyperplasia, and secondary inflammation.11,12 Elias et al11 substantiate this hypothesis with several lines of evidence. The magnitude of SC barrier permeability impairment, characterized by increased transepidermal water loss (TEWL) and decreased SC water content, corresponds with greater AD severity. While normal-appearing skin of AD patients does exhibit SC barrier dysfunction, the increase in TEWL and drop in SC water content are less than in lesional AD skin. The importance of barrier integrity is highlighted by the fact that topical barrier repair therapies are able to reduce the need for topical corticosteroid (TCS) use,11 potentially maintaining the structural and functional integrity of the SC.
Studies have shown that mutations in the filaggrin gene (FLG), which regulates epidermal terminal differentiation and creates a template for assembly of the cornified envelope, occur in a subset of AD patients.3,13 However, the role of this FLG mutation (FLGm) in creating the AD phenotype remains unclear; the mutation is absent in 50 to 90 percent of the AD population, some of those patients who carry the mutation outgrow the disease, and AD patients are shown to have a broad range of terminal differentiation abnormalities, such as in loricrin (LOR) and involucrin (IVL), which are also important proteins in the formation of the epidermal barrier.14,15
The “inside-out” or immune-driven hypothesis suggests that the abnormal epidermal phenotype found in AD skin is driven by increased expression of cytokines produced by distinct T-cell subsets.16 This hypothesis, and the notion of AD as an immune-driven disease like psoriasis, is supported by the effect of broad T-cell-targeting therapeutics in AD, such as cyclosporine A, and is now gaining wide acceptance after recently published Phase 1b studies showed that dupilumab, an anti-IL-4 receptor alpha (IL-4Rα) monoclonal antibody that blocks IL-4 and IL-13 signaling, potently inhibits the T helper type 2 (Th2) pathway.17,18 Based on recent data, the new paradigm of AD pathogenesis holds that T cells and their related cytokines and chemokines are primarily responsible for the inflammatory responses, with contributions from other factors, such as the epidermal barrier and IgE. The role of IgE in AD remains unclear, but IgE levels have been shown to correlate with disease severity in extrinsic AD, which comprises the majority of cases.19 Th2 immune polarity in AD is well documented. The Th2-related molecules (e.g., IL-4, IL-13, chemokine [C-C motif] ligand 17 [CCL17]) dominate the immune infiltrate in AD.16 Importantly, linking the immune and barrier hypotheses, the Th2-dominant cytokines in addition to the Th22 cytokine IL-22 were shown to downregulate terminal differentiation proteins as well as the production of antimicrobial peptides (AMPs) in keratinocytes, such as cathelicidin (LL-37) and human beta-defensins.20-22 The inhibition of the terminal differentiation proteins and AMPs ultimately contributes to the barrier deficiency in AD and increased skin infections.20,23 The strongest support for the pathogenic role played by the Th2 cytokines IL-4 and IL-13 in AD will now be provided by the clinical efficacy and epidermal changes obtained with drugs targeting the Th2 pathway. What remains to be determined by long-term studies is whether Th2 antagonism will lead to restoration of the terminal differentiation proteins.
IL-31, another important Th2 cytokine that was recently implicated in the induction of pruritus in murine studies, has been shown to play a major role in AD. It, too, was shown to inhibit terminal differentiation proteins and AMPs in human keratinocytes, and its expression was markedly increased with the onset of acute AD.24 Levels of IL-31 are increased in AD lesional skin and increase with the severity of AD. Interestingly, staphylococcal endotoxins can induce IL-31 in macrophages and monocytes, which in turn escalates scratching, further increasing the potential for bacterial colonization or infection with S. aureus.16,26,26 S. aureus colonization contributes to the pathophysiology of AD by serving to induce a flare or prolong an active eczematous flare through production of specific toxins called superantigens.
The role of staphylococcal superantigens in AD is supported by the correlation of AD severity with the presence of IgE antibodies to superantigens, superantigen-induced activation of infiltrating mononuclear cells augmenting allergen-induced inflammation, induction of mast cell degranulation, and activation of Th2 cells by superantigens.27
Recently, novel T-cell subsets have also been implicated as potential players in AD. These include Th17 and Th22 cells and their respective IL-17 and IL-22 cytokines. Data suggest that IL-17 and IL-22 cytokines regulate the expression of AMPs in keratinocytes, resulting in inflammation.28
IL-17 is expressed in acute skin lesions,29 and Th17 cells are increased in peripheral blood mononuclear cells from patients with acute AD, further supporting the possible role of this axis in acute disease.24,28 Additionally, there may be interplay between IL-17 and IL-22 in inducing the terminal differentiation genes, specifically the S100 proteins.24 However, while psoriasis is a disease that is heavily centered on Th17, IL-17 does not play an equal role in AD.22,30
Environmental Factors
There is a paucity of rigorous data regarding environmental factors that cause or exacerbate AD. Much of the data is generalized and then complicated by individual differences within AD. Nevertheless, management of patients with AD should include questions about their environment in an effort to improve quality of life. Langan et al31 conducted a study of 60 children with AD, with a “bother” score as the primary outcome. Results showed that an increase in severity was associated with nylon clothing, dust, exposure to unfamiliar pets, shampoo, and sweating; these factors were enhanced by cold temperatures. Body site-specific flares were associated with nylon clothing (trunk and limbs), wool clothing (trunk), and unfamiliar pets (hands). Moreover, a combination of any 3 of the 7 variables increased AD severity.31
House dust mites have been documented as a possible risk factor for the development of allergic diseases. A study of 19 children with AD and 21 healthy-skin controls was conducted over a two-year period. Samples were collected from bedding, clothing, and skin. Results showed that the children with AD had significantly more positive skin samples of dust mites than the control subjects (84 vs. 14%). In addition, there was no correlation with bedding or clothing. The researchers concluded that a higher prevalence of dust mites on the skin of AD patients compared with controls suggested a sensitizing or disease-exacerbating effect of dust mites in these patients.32
Industrialization has been implicated as a primary reason for the increased incidence of AD over the last 30 years. Air pollution from motor vehicles, energy production, and factories have been suggested as factors in the development or aggravation of AD.33 A direct correlation exists between the increase in risk of atopic diseases and allergic sensitization and exposure to traffic-related air pollution. The closer a child lives to a busy street, the higher that child’s risk for AD due primarily to nitrogen dioxide.34 On the other hand, a farming environment appears to protect against the development of allergic diseases. In a cross-sectional survey study, exposure before or within the first year of life to stables, farm milk, or both was associated with lower frequencies of allergic disease relative to study participants who were not so exposed. The children who had the lowest frequency of allergic disease were those who were continually exposed from birth through five years of age.35
Allergic contact dermatitis as a result of topical treatment for AD is a factor to consider if treatment is not improving symptoms. In a study of 641 children with AD under the age of 16 years, six percent had a positive patch test to one of seven agents tested: chlorhexidine, hexamidine, budesonide, tixocortol pivalate, bufexamac, sodium fusidate, and the current emollient used by the child. The agents with the highest percentage of sensitization were the emollients (47.5%) and chlorhexidine (42.5%). Risk factors associated with increased sensitivity were AD severity, early AD onset, and IgE-mediated sensitization.36 In recalcitrant AD patients, a patch test may be warranted. A study of 79 children resulted in 51 percent having one or more positive allergic patch test reactions, of whom 55 percent had AD. Nickel was the most common contact allergen.37 Oat sensitization is a surprising potential culprit for AD, considering the plethora of oat products on the market specifically for skin conditions; nevertheless, Boussault et al38 showed a higher-than-expected rate of reaction to oat-containing products.
One reasonable concept to explain the increase in prevalence of AD is the “hygiene hypothesis.” Epidemiological studies have associated higher socioeconomic status with reduced exposure to bacteria. Data support a relationship between low levels of bacterial exposure and reduced activation of Th1 cell-mediated immunity and a subsequent increase in Th2 cells leading to diseases, such as AD.14,33 However, challenges to this theory are emerging. Data from cohort studies bring into question the validity of the hypothesis, concluding that the effects of environmental factors are disease specific.39 Alternatively, AD prevalence may be dependent on environmental stressors that interfere with the epidermal barrier. Irritants or chemicals may account for this breach in the skin leading to or exacerbating AD. The results of a recent cross-sectional study indicated that exposure to detergents or salts in hard water and chlorine-based oxidants in swimming pool water increased the prevalence of childhood eczema.40 This contradicts an earlier prospective cohort study that found no detrimental effect of babies’ exposure to chlorinated swimming pools on the development of atopic disease.41
Dysbiosis, the imbalance of micro-organisms on the skin, can occur with the application of topical irritants or the use of systemic antibiotics. The homeostasis maintained by AMPs that reside in the SC antimicrobial barrier is reduced in AD, which may explain the increased incidence of flares and S. aureus infections in these patients. In addition, normally occurring Staphylococcus epidermidis acts as a barrier to potentially pathogenic microbes by enhancing AMPs. However, when AMPs are reduced and an immune imbalance results, S. epidermidis becomes potentially pathogenic.42
Cohabitation of patients with allergic diseases with pet dogs or cats has long been a topic of discussion. Previous reports have endorsed the avoidance of exposure to dogs or cats due to their purported promotion of allergic responses. In contrast, more recent reports have refuted these claims and suggest that living with pets might actually confer immunity.43,44
Food has been raised as a factor in AD and represents an area of continuing conflict. Food allergies are classified as either IgE-mediated or non-IgE-mediated.45 A large international study tested the association between AD and IgE-mediated food allergies, specifically to milk, eggs, and peanuts. Children with the most severe AD and at the youngest age of onset had the highest frequency of IgE-mediated food sensitivity (64%).46 Late atopic reaction to food is considered a non-IgE-mediated reaction. Noh et al45 found that patients with a milk allergy have an allergen-specific response of decreased regulatory B cells compared with an increase in controls. However, both milk-allergic and milk-tolerant subjects, when stimulated with an allergen, demonstrated increases in regulatory T cells. Thus, regulatory B cells seem to influence non-IgE-mediated immune responses to food allergens.45 The delay of the dietary introduction of cow’s milk and other foods is still a common recommendation. However, a study showed that delaying foods—particularly cow’s milk—in the first two years of life did not prevent development of atopy. In fact, delaying their introduction was associated with a higher risk for AD. In addition, the elimination of milk in the diets of children with AD may lead to an unnecessary dietary deficiency.47 This was also true for other food products tested.48 The American Academy of Pediatrics has changed their guidelines on food restrictions in children, including children with AD, citing a lack of evidence that diet manipulation among pregnant or breastfeeding mothers can either cause or prevent allergy in a child. The new guidelines for children at high risk for AD and those who already have AD indicate that breastfeeding for the first four months of life (or substituting extensively hydrolyzed formula) may be beneficial. The guidelines also call into question the benefit of delaying the introduction of solid foods beyond 4 to 6 months of age, as there is insufficient evidence of a protective effect of delaying food beyond this time frame on the development of AD.49 A review of epidemiological studies failed to show that breastfeeding directly protects infants from AD; however, breastfeeding remains a recommendation due to its many other benefits to the infant.50,51
It has been proposed that probiotic bacteria may be protective against AD due to their stimulation of Th1 cytokines and suppression of Th2 cytokines. This theory would also support the hygiene hypothesis previously discussed. Wickens et al52 showed a 50-percent decrease in AD prevalence at two years of age (with a persistent effect up to 4 years) in children whose mothers ingested Lactobacillus rhamnosus from 35 weeks’ gestation until birth, following which L. rhamnosus was given to infants from birth to two years.52 L. rhamnosus GG, a specific genotype, has also demonstrated potential in the treatment and primary prevention of AD, but intervention trials have been mixed. A clinical trial with seven-year follow-up shows that L. rhamnosus GG is useful in the prevention of AD in children at high risk of allergy.53 Contradictory results were reported in another trial, in which supplementation with L. rhamnosus GG during pregnancy and early infancy neither reduced AD nor altered its severity.54
Prebiotics, nondigestible carbohydrates that encourage the growth of healthy bacteria in the colon, may reduce the risk of developing AD. Pooled results of four studies showed that prebiotic supplementation of infants reduced AD risk by a ratio of 0.68. There was no reduction in risk for the development of asthma or urticaria.55
The confounding issue is whether AD can develop or be exacerbated due to food or supplement sensitization, or whether AD causes food sensitization. A study by Lowe et al56 found that, in some infants, sensitization preceded the development of AD (hazard ratio [HR]: 1.63), while AD in the first six months was associated with increased risk of allergen sensitization at one year (HR: 2.34) and two years (HR: 3.47).56 The guidelines of the National Institute of Allergy and Infectious Diseases recommend that children less than five years of age with moderate-to-severe AD be considered for a food allergy evaluation.57 Nevertheless, food allergy testing is controversial. A study assessing the value of serological testing for food allergies in AD children concluded that “physician and patient misinterpretation of the relevance and reliability of allergy testing may misdirect proper prevention and therapy of AD.”58
Quality of life-related factors may also increase the risk of developing AD or exacerbate the disease. Stress has been shown to increase flares and disease risk; divorce or separation increased the risk by an odds ratio (OR) of 3.59.59 Nonrestorative sleep due to sleep problems has also been associated with various medical conditions, including AD (OR: 2.18).60
Pruritus
Pruritus is the hallmark of AD. This symptom is arguably present in 100 percent of patients and has the most profound adverse impact on quality of life. Pruritus results from a complex interchange of chemical and peripheral mediators. Alterations in the epidermis of AD patients are associated with itch. Increased TEWL occurs due to the damaged epidermal barrier and causes xerosis and often pruritus. Irritants, both chemical and mechanical, can more easily permeate the suboptimal physical barrier of the SC in AD patients and exacerbate the condition.61
Several mediators of pruritus are directly involved in AD. Neuronal protease-activated receptor 2 (PAR2) is involved in the pain pathway, activating both somatic and visceral afferent nerve fibers.62 Data now suggest PAR2 involvement in pruritus in AD patients. Mast cell tryptase is a PAR2 agonist that is also enhanced in AD patients. The SC chymotryptic enzyme, kallikrein 7, involved in cell turnover and desquamation, activates PAR2 in nerve fibers, and such signaling is increased in AD.61
Histamine-sensitive afferent nerve fibers have been identified that may cause a histamine-induced itch via the peripheral nerves. However, recent data indicate that histamine and the H1 receptor may have limited involvement in AD. This has been demonstrated pharmacologically in that high doses of antihistamines do not usually alleviate itch in AD. We now know that H3 receptors pacify itch and H4 receptors foster itch.61
IL-2, like IL-31, may have a role in AD pruritus. IL-2 is a product of T-cell activation, and its inhibition is the rationale for topical and systemic immunosuppressant therapies, such as topical calcineurin inhibitors and cyclosporin A. As mentioned previously, IL-31 is expressed by Th2 and therefore overexpressed in AD.61
Keratinocytes and free nerve endings in the skin secrete substance P (SP), a peptide in the tachykinin family. It can elicit itch by two different mechanisms, one dependent on histamine and the other independent of histamine. High levels of SP exist in AD, and SP plasma levels have been suggested as a marker for AD severity.61
Prostaglandin E2 (PGE2) is a prostanoid with a direct, low-level, histamine-independent pruritogenic effect in AD. Another prostaglandin, PGD2, inhibits itch. Interestingly, scratching reduces levels of PGD2, suggesting that low levels of PGD2 are associated with the itch-scratch cycle. Thromboxane A2, another prostanoid, induces itch-associated responses through the thromboxane prostanoid receptors located in keratinocytes and skin nerve fibers.61
Recently, the itch-specific neuron MrgprA3+ has been isolated. These neurons transmit only itch, exclusive of pain, and are associated with both acute and chronic pruritic conditions. This discovery could potentially lead to new antipruritic therapies in the future.63
Treatment of Atopic Dermatitis
An individualized approach to the treatment of AD is warranted based on age, severity, distribution of lesions, family history, medication history, and disruption of the patient’s and their family’s quality of life. While there is no cure for AD, the disease can be effectively managed. The focus of treatment of an acute flare of AD is symptomatic relief with control of pruritus and rapid control of cutaneous inflammation using agents to clear eczematous dermatitis and reverse xerotic skin changes. The focus of treatment between flares of AD is to promote the maintenance of SC (epidermal) barrier integrity and function. This approach implies the importance of regular incorporation of proper skin care and rational product selection, which serve to prevent at least some of the exogenous triggers that can induce flares of AD.
Moisturizers and Barrier Repair Agents. Maintaining skin hydration and preventing increased TEWL is an essential component of AD treatment. Research shows that maintaining epidermal hydration can improve the management of AD by reducing the overall need for topical corticosteroid therapy.64-66 Ultimately, epidermal enzymes involved in maintaining normal skin function and desqua-mation are dependent upon an adequate concentration gradient of water across the SC.67 The physiological water content of skin ranges between 10 and 30 percent.68 When the water content of the SC decreases, cascades of self-repair of the SC permeability barrier are immediately initiated, some with an immediate impact on barrier repair and others with more delayed and substantive reparative responses.67,69 The most immediate event is the release of stored precursor lipids from the granular layer into the SC to assist in repair of the intercellular lipid bilayer, which functions to reduce TEWL. Another important process in barrier self-repair is the increased formation and enzymatic breakdown of filaggrin into several components of natural moisturizing factor (NMF), mostly free amino acids, which serve as “nature’s humectant.” Much of the humectancy within the SC is due to the hygroscopic properties of NMF, which attracts and retains water where it is needed rather than letting it proceed further upward in the SC and be lost to evaporation. These filaggrin-generated free amino acids, along with pyrrolidone carboxylic acid (PCA), lactate, urea, sugars, and small concentrations of other compounds (mostly electrolytes), provide the necessary humectancy to sustain SC hydration. Additionally, the content, flux, and gradient of water within the SC is actively controlled and modulated by the intercellular lipid bilayer between corneocytes.67,70,71 Impairments of any of the components needed to maintain proper epidermal water content can lead to increased TEWL and the development of xerotic changes, skin inflammation, pruritus, and eczematous dermatitis. Furthermore, atopic skin is inherently compromised by deficiencies in SC ceramides, and some patients exhibit various patterns of FLGm that decrease the production of NMF.72-74 As a result, patients with atopic skin are inherently further compromised by increased levels of TEWL even when their skin appears normal or xerotic (without flare of eczema) and are less capable of self-repair of the SC barrier due to innate deficiencies associated with AD.67,72
Exposure to irritants can compromise the SC permeability barrier with an increase in TEWL and lessened protection against environmental factors. True soaps, poorly formulated skin cleansers, and overwashing are common sources of SC barrier compromise as they increase TEWL, initiate inflammation, and over time reduce the water-holding capacity of the skin.75
Moisturizers and barrier repair products are used to decrease TEWL and improve SC hydration, which leads to reversal of xerotic skin changes and inflammation associated with permeability barrier compromise.67,76,77 Additionally, there is some evidence that use of a barrier repair product alone may improve function of both the SC permeability barrier function and the antimicrobial barrier function. In one study, a ceramide-containing barrier repair product demonstrated permeability barrier and antimicrobial barrier restoration and a reduction in cytokines associated with AD equivalent to topical tacrolimus.78,79
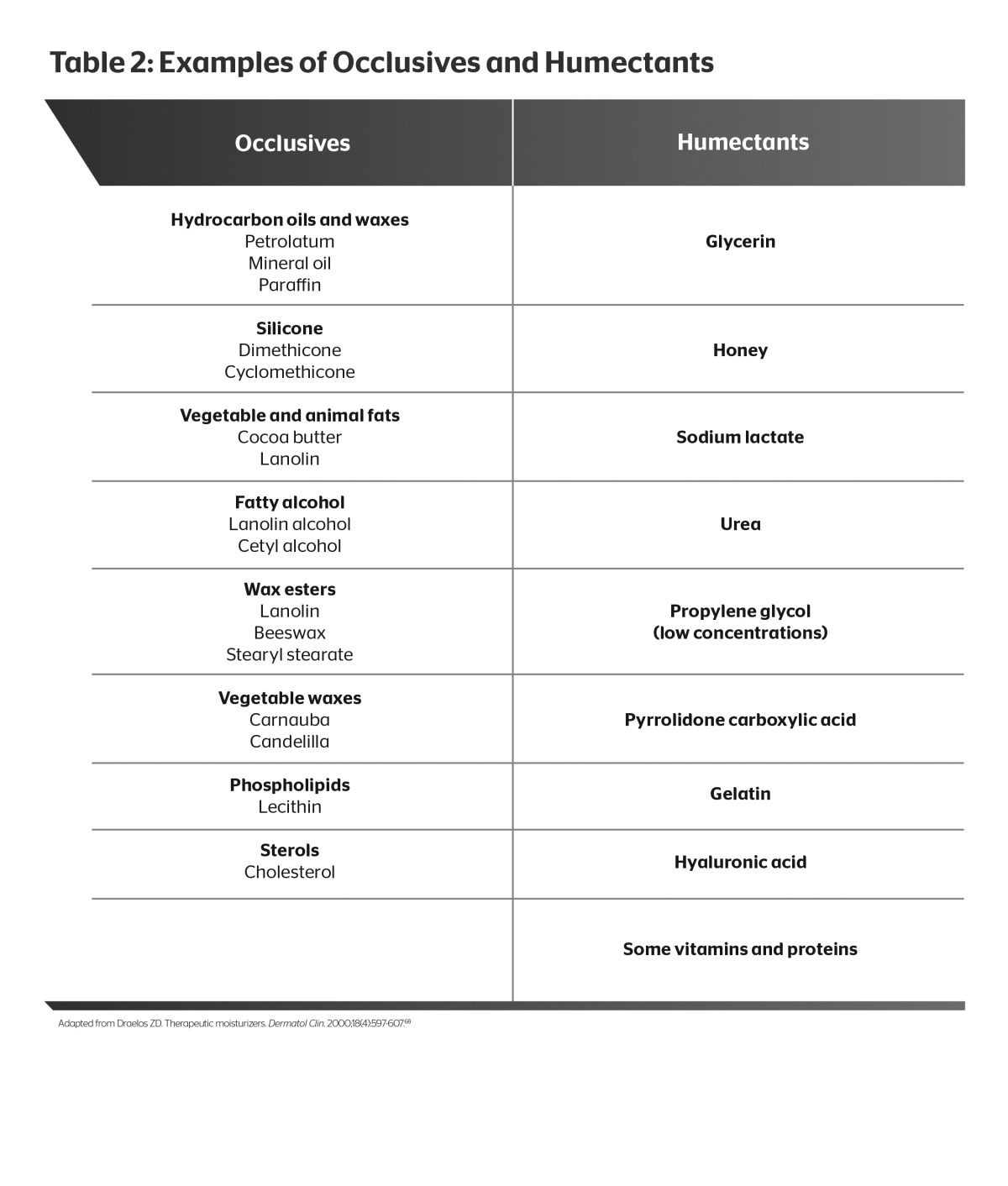
There are two fundamental components incorporated into barrier repair products to promote SC hydration: Occlusives to retard TEWL and humectants to attract and retain water. Well-formulated barrier repair products contain both. Petrolatum is the most efficacious occlusive, producing a near-immediate reduction in TEWL. However, cosmetic acceptability of petrolatum is poor due to its greasy and messy characteristics, which inhibit adherence. Silicates provide some occlusivity along with favorable emolliency and cosmetic acceptability. Hyaluronic acid is an endogenous humectant that is used in some barrier repair products and moisturizers. Other commonly used humectants include glycerin, urea, propylene glycol, and sodium lactate (Table 2). Moisturizers comprise substances that penetrate between desquamating corneocytes and provide lubrication to the skin.68 Such products are typically available over the counter, without a prescription, and represent a wide array of different formulations.
 |
Barrier Repair Medical Devices. Barrier repair medical devices (BRMDs) are different from over-the-counter moisturizers in that they have been approved by the US Food and Drug Administration as a 510(k) medical device. BRMDs are a unique class of products that incorporate ingredients intended to augment barrier repair, such as physiological lipids, niacinamide, and hyaluronic acid. These products provide barrier occlusivity and humectancy and are formulated to replenish physiological lipids, promote substantive barrier repair, and incorporate other agents that may assist in the reduction of inflammation and promote healing.80,81 Primary barrier abnormalities in AD were discussed previously and include an inherent reduction in SC ceramides that correlates with TEWL impairment. Thus, ceramides are commonly incorporated into products designed to restore SC integrity in patients with AD.
Data from a study with a triple-lipid emulsion BRMD revealed improved SCORAD values and lower levels of TEWL in children with AD.64 Similarly, a BRMD containing ceramide, hyaluronic acid, and free fatty acid showed improvement in structural barrier repair and reduced TEWL as well as superior patient preference scores; patients chose this product over a triple-lipid emulsion primarily due to the favorable characteristics of the foam of the former.82,83 The high patient acceptance and efficacy of this product may be correlated, as therapeutic adherence improves clinical outcomes. Glycyrrhetinic acid is a mild anti-inflammatory ingredient that is incorporated, along with hyaluronic acid, in another BRMD. A randomized, controlled study has shown this product to be effective in reducing pruritus and to be statistically superior to a placebo vehicle in reducing symptoms in mild-to-moderate AD.84
The benefits of using a BRMD in AD patients include the potential reduction in overall use of topical corticosteroids, and possibly topical calcineurin inhibitors, as well as restoration of the altered SC permeability function inherently associated with AD. This is most apparent for flared sites, but is also applicable to xerotic and normal-appearing skin of atopic patients.67,85 Sugarman et al86 compared a lipid-based barrier repair formulation with fluticasone propionate cream in moderate-to-severe AD patients. Although fluticasone showed greater improvement than the BRMD at 14 days, there was no statistically significant difference at 28 days. The authors concluded that BRMD therapy offers targeted, disease-specific lipid replacement therapy in addition to substantial safety advantages.86 Another study compared the ceramide-hyaluronic acid BRMD foam formulation with pimecrolimus cream, a topical calcineurin inhibitor (TCI). Both products exhibited efficacy after four weeks (82% of BRMD subjects vs. 71% of pimecrolimus subjects); however, patients preferred the BRMD to the pimecrolimus cream 68 percent to 32 percent.87 Patient preference is significant because, as we have noted, adherence to therapy is a significant factor in the treatment of AD. The potential downside of BRMDs relative to moisturizers is the need for a prescription.
Topical Corticosteroids. Topical corticosteroids (TCSs) are the cornerstone of AD treatment and provide a level of efficacy that has been confirmed in multiple studies.88,89 A variety of mechanisms contribute to their effectiveness, including anti-inflammatory, antiproliferative, and immunosuppressive effects. TCSs suppress the quantity and activities of many inflammatory cell types and cytokines including neutrophils, monocytes, lymphocytes, Langerhans cells, IL-1α, IL-1β, IL-2, TNF, and granulocyte-monocyte colony-stimulating factor, and they induce anti-inflammatory proteins (e.g., lipocortins, vasocortin, vasoregulin).3,89 Thus, TCS therapy induces rapid inhibition of multiple mechanisms of cutaneous inflammation, provided the TCS potency is properly matched with the type and severity of disease. The potency of the TCSs is classified by their potential for vasoconstriction, which accounts for not only their clinical efficacy, but also their adverse reaction potential (e.g., skin thinning, telangiectasia, striae, petechiae, and atrophy).3,4
TCSs are available in a range of potencies from ultra-high (Class 1) to low (Class 7). Choice of potency depends on the distribution and surface area of the lesions, their severity, and the age of the patient. General practice has classically been to use a low- to mid-potency TCS on the face and intertriginous areas, and the lowest potency that is effective on the other areas. Caution should also be used when choosing TCSs for infants and children.90 Many corticosteroids are not FDA approved for children; it should be noted, however, that many clinicians utilize TCSs for treatment of pediatric AD in an off-label manner. Products approved for use down to the age of three months include fluticasone 0.05% cream, desonide 0.05% foam and gel, fluocinolone acetonide oil 0.01%, and hydrocortisone butyrate 0.1% lotion and cream (Table 3). A 29-day study was conducted with hydrocortisone butyrate 0.1% lotion versus vehicle in children aged three months to less than 18 years with mild-to-moderate AD. Of relevance to the consideration of age-appropriate therapy selection was the even distribution of ages in the treatment group—3 months to less than 2 years, 2 years to less than 6 years, 6 years to less than 12 years, and 12 years to less than 18 years (n=32, 32, 38, and 37, respectively). Results for the primary endpoint of achieving a Physician Global Assessment score of 0 or 1 were statistically significant at 49 percent for the treatment group and 24 percent for the control group at 29 days (P<0.001). Results for the secondary endpoint of reduced pruritus were also statistically significant, with the change in pruritus averaging 1.4 for the treatment group versus 0.7 in the placebo group on a 4-point scale (P<0.001). No topical steroid-related side effects were observed in either group.91
 |
There is a wide selection of vehicle options for TCS application. Vehicle choice is relevant to potency, bioavailability and efficacy and is an important factor in patient adherence. Ointments traditionally have been considered more potent than other formulations, though this may not necessarily be true today with modernized vehicle technology. Modern compounding of vehicles with advanced excipients has altered that convention. In an in vitro study, five clobetasol propionate formulations (foam, solution, emollient cream, cream, and lotion) were tested for skin permeation over a 24-hour period. The foam vehicle showed superior penetration without regard to body location. Patient surveys in this study and others have indicated a preference for foams and lotions over other vehicles due to ease of application when a larger body surface area requires treatment, as well as reduced irritation and cosmetic appeal.82,92 The inference from this study may be that choosing the best vehicle for the AD patient is just as important as choosing the optimal TCS.
According to the guidelines of the American Academy of Dermatology, despite extensive data on the use of TCSs, there is a dearth of data on optimal concentration, duration, frequency, and quantity of application.88 Duration of therapy for TCSs depends on the severity of the disease, the response to therapy, and the potency of the TCS. In general, ultra high-potency TCSs should be used for a shorter length of time (i.e., ≤2 weeks). Patient “fear of steroids” may also play a role in the use and duration of TCS therapy, as this may reduce adherence to therapy. Combination and intermittent therapies are strategies that have been employed successfully to increase adherence and reduce adverse effects. The idea of combination therapy is to use a TCS with another therapy with a different mechanism of action, such as a TCI or a BRMD, enabling the use of agents with a reduced potency or for a shorter duration. Intermittent therapy refers to applying a TCS (and/or TCI) to active eczematous lesions, usually with associated pruritus, to control a flare—then, after the patient is much improved (near clear or clear), transitioning to proactive or preemptive therapy using a TCS (usually a mid-potency agent) or TCI applied twice weekly to the sites at which eczematous flares commonly occur in that patient to prevent another flare.93 This treatment reduces the overall exposure to TCS (or TCI) therapy, thus averting potential adverse effects while at the same time preventing relapse.94
Various other strategies involve sequential use of different products on an intermittent basis. One such strategy is to use a TCS for a few days or weeks followed by another product for a few days or weeks.95 Another is to use the TCS only in the event of a flare. Data have shown that a short burst of a high-potency TCS for three days is as effective as a lower-potency TCS for seven days.96 This approach should be weighed against other factors, such as cost and symptom severity, as patients may get longer-term use (e.g., intermittent therapy) from a mid-potent TCS.
If all treatment strategies have been exhausted and the AD appears to be worsening, there is a small chance that the patient has an allergy to the corticosteroid molecule itself or to the formulation. A common method of testing for this allergy is the patch test using either tixocortol-21-pivalate, budesonide, or hydrocortisone-17-butyrate. The North American Contact Dermatitis Group (NACDG) reports that the incidence of contact allergies is 3.0 percent for tixocortol-21-pivalate, 1.1 percent for budesonide, and 0.5 percent for hydrocortisone-17-butyrate.97 While this is definitely something to consider in the treatment of nonresponsive individuals, the incidence remains relatively low. In the latest NACDG patch test allergen list of 65 positive tests, specific TCSs were ranked at the bottom of the list; each of the TCSs had a ≤0.6 percent positive reaction and all sterochemical (allergen) classes were represented.98 Ingredients in the vehicle may matter in contact allergy as well. A study on the reproducibility of patch tests and correlation with intradermal testing also had the objective of identifying the percentage of positive reactions to preservatives and vehicles used in commercially available TCSs. Results showed a high sensitivity to formaldehyde-based products (65%), while 10 percent had a sensitivity to propylene glycol.99
Topical Calcineurin Inhibitors. TCIs are nonsteroidal immunomodulators, and multiple studies have shown their efficacy in the treatment of AD.100 Two products are available in the US. Tacrolimus ointment is approved for moderate-to-severe AD; the 0.1% ointment is approved for patients ≥16 years of age and the 0.03% formulation is approved for patients ≥2 years of age. Pimecrolimus cream is approved for mild-to-moderate AD patients and is available as a 1% cream for patients ≥2 years of age. Both products are approved as second-line agents for the short-term treatment of active AD lesions and noncontinuous chronic treatment. Tacrolimus and pimecrolimus have the same mechanism of action, which, while not completely elucidated in AD, involves the inhibition of the phosphorylase activity of the calcium-dependent serine/threonine phosphatase calcineurin and the dephosphorylation of the nuclear factor of activated T-cell protein necessary for the expression of IL-2, IL-4, IL-5, granulocyte macrophage colony-stimulating factor (GM-CSF), and TNF-α.101,102
The key benefit of TCIs is that they offer a viable alternative to TCSs without the associated adverse events, such as atrophy, telangiectasias, purpura, and possible hypothalamic-pituitary axis suppression, especially with prolonged use and widespread application. Patients who are not candidates for TCSs due to overuse, side effects, or exaggerated patient fear are candidates for TCIs. Nevertheless, there are some considerations for their use, such as transient burning or stinging that is sometimes bothersome, particularly with initial application of tacrolimus. Also, TCIs may be less effective in moderate-to-severe disease, particularly when secondary infection is involved. Furthermore, TCIs are discouraged in some patients with defective epidermal barrier disorders, such as Netherton syndrome, in which increases in serum TCI levels can occur after cutaneous application.103 In 2005, the FDA implemented a black box warning on TCI labeling due to safety concerns related to immunosuppression. This warning was issued based on animal studies using exposures to very high doses, a limited number of sporadic case reports, knowledge of the mechanism of action of TCIs, and their potential toxicities.104,105
At the behest of the FDA, Novartis and Astellas initiated the patient registries PEER (Pediatric Eczema Elective Registry) and APPLES (Atopic Prospective Pediatric Longitudinal Evaluation Study), respectively, as part of their Phase 4 commitments to acquiring long-term safety data. PEER is a prospective, observational, parent-reported epidemiological registry for AD patients aged 2 to 17 years using topical pimecrolimus; APPLES is a prospective, observational study in patients with AD using topical tacrolimus. Endpoints are systemic malignancies and skin cancers.106 In May 2011, the FDA Pediatric Advisory Committee reviewed five observational studies published between 2005 and 2011, assessing outcomes of lymphoma, melanoma, nonmelanoma skin cancer, and other cancers in patients treated topically with tacrolimus or pimecrolimus. In general, the studies showed no increased risk, although one study showed a possible increased risk of T-cell lymphoma with tacrolimus (not specific to children).107 Study biases were acknowledged, as were other potential confounders, including the protopathic bias, referring to misdiagnosis of the cutaneous eruption as AD when in fact it had been cutaneous lymphoma all along.103 The FDA concluded that no new signals for pediatric malignancies following TCI use were identified in the postmarketing safety review and that the current TCI labeling and medication guide reflected the current understanding of potential safety risks.
Therapy regimens with TCIs are similar to those with TCSs. Intermittent application of a TCI applied to active eczematous lesions for control of AD flares has demonstrated favorable efficacy and safety primarily over durations of up to 12 weeks in pivotal trials, with some studies of prolonged duration also completed. Tacrolimus ointment has demonstrated some superiority in efficacy over pimecrolimus.100,108 During therapy, TCIs are to be applied on a twice-daily basis every day to sites of active eczematous dermatitis and not liberally to nonaffected skin, which is where a BRMD or moisturizer is to be used.
As discussed previously in the section on TCSs, proactive therapy with a TCI has been evaluated specifically with topical tacrolimus. Separate studies in adults and children have investigated whether proactive application of tacrolimus (twice to three times weekly) following clearance of active eczematous dermatitis can increase remission time between flares of active disease. These studies demonstrated more flare-free days in the tacrolimus group (compared with a vehicle group), reduction in the occurrence of AD exacerbations, and a longer time to first relapse requiring intervention.109-112 Early intervention with a TCI at the first sign of a flare may also diminish the need for TCS therapy and should be coupled with diffuse application of a BRMD or moisturizer. In a study of 543 patients with mild-to-moderate AD, twice-daily application of pimecrolimus at the onset of the first signs or symptoms of AD relapse increased the mean number of TCS-free days (compared with vehicle) and reduced the mean number of flares requiring TCS rescue therapy, with fewer unscheduled office visits.113 A similar study was conducted using pimecrolimus versus vehicle, with TCSs used for flares, in 713 pediatric patients with at least mild AD. At the endpoint of the study, patients treated with pimecrolimus experienced fewer major flares and a longer time to the first major flare.114
Phototherapy. Phototherapy and systemic therapy are primarily reserved for patients with severe or refractory AD. Phototherapy has been used in the treatment of AD for years. Broadband ultraviolet B (UVB), narrowband UVB, UVA1, UVA/B, and psoralen UVA (PUVA) are the various phototherapy options available. Jekler et al115 published the results of a small study in 1992 showing evidence of UVB radiation as an antimicrobial against S. aureus in AD patients. This study essentially solidified the benefit of UVB over sunlight for AD.115 Combination therapy of UVA/UVB was shown to be superior to broadband UVB, with the disadvantage of not being able to dose the two spectra separately. UVA1 light has some utility in AD but pales in comparison to PUVA and narrowband UVB. PUVA, also termed photochemotherapy, combines psoralen, which is taken orally, with UVA. While used for years, it has lost favor due to the discovery that melanoma risk increases in patients treated with PUVA many years earlier. Narrowband UVB is now the modality of choice. Fewer treatments are needed to achieve remission in AD, which subsequently exposes the patient to less radiation. Most recently, patients have been treated with air-conditioned narrowband UVB treatment, which assumes that patients experience worsening of itching during phototherapy due to sweating; in addition, salt from the sweat would irritate lesions.116 A small pilot study using narrowband UVB was conducted to address the hypothesis that AD is an immunologically driven disease. Not only did the patients exhibit a reduction in SCORAD by more than 50 percent, but there was also a greater than 40-percent reduction in epidermal thickness and a reversal of abnormal keratinocyte proliferation. Narrowband UVB can have direct effects on keratinocytes, but a histological reduction was also observed. Data showed a reduction in CD3 cells and dendritic cells. The cytokines and chemokines from the Th2, Th22, and Th1 pathways were also suppressed. This pilot study intimated that the AD phenotype comprises reversible objective cellular and molecular biomarkers that can be pursued in future studies.117
Systemic Therapy. Systemic therapy is generally reserved for the most severe and refractory patients. Cyclosporin is a potent immunosuppressive agent that blocks calcineurin. Multiple studies have demonstrated the benefit of cyclosporin in severe refractory AD. Success has been observed in both short-term and long-term (1 year) studies, and patients sometimes enjoy months of remission.118,119 The main disadvantages of cyclosporin are multiple drug interactions; side effects such as nausea, headache, and paresthesias; and more severe side effects of renal impairment, hypertension, and possible sequelae of chronic immunosuppression.120,121
Mycophenolate mofetil (MMF) has shown benefit in severe AD. MMF blocks T and B lymphocytes and thereby inhibits inflammatory cells involved in AD. In a small pilot study (N= 10) using MMF, SCORAD scores improved by 68 percent.122
Methotrexate is a folic acid antagonist with anti-inflammatory effects. Although its use is off label for the treatment of AD, case reports and small trials have shown efficacy in refractory patients. A prospective trial of 12 patients with moderate-to-severe AD treated with methotrexate demonstrated an average improvement of 52 percent from baseline in disease activity plus significant improvement in quality of life, affected body surface area, and loss-of-sleep and itch scores.123 Adverse effects that could limit treatment include nausea, elevated liver enzymes, and more rarely pancytopenia and hepatic and pulmonary toxicity.124
Azathioprine, initially developed for prevention of organ transplant rejection, has immunosuppressive properties shown to be useful in the treatment of severe, refractory AD. Randomized, controlled trials are limited, but a review of eight open, noncontrolled studies totaling 128 adult and pediatric patients showed overall improvement in AD.125 Results from a small (N=35, intent-to-treat population), double-blind, randomized, placebo-controlled crossover study showed a 26-percent reduction in AD severity scores with azathioprine treatment compared with three percent for the placebo group. Azathioprine was not well tolerated, with patients reporting gastrointestinal side effects (n=14). Also, abnormal liver enzymes in eight patients and leukopenia in two patients were recorded.126 Serious side effects of hepatotoxicity, myelotoxicity, and increased risk of malignancy can occur with long-term use, which may limit the value of this medication in the treatment of AD.127
A randomized, single-blind, parallel group study compared methotrexate (n=20) with azathioprine (n=22). The primary outcome was the mean change in SCORAD score at 12 weeks. At the conclusion, the methotrexate-treated patients had a relative reduction in SCORAD of 42 percent compared with 39 percent in the azathioprine-treated patients. Both groups achieved clinically relevant improvement. There were no statistically significant differences in adverse events between the two treatment groups and no serious adverse events in either group.128
Research has been conducted on other systemic treatment modalities as well, including high-dose intravenous immunoglobulin, recombinant interferon gamma, and omalizumab. Omalizumab in particular has effectively improved some, but not all, patients with AD.129-131 While promising, these agents have limited peer-reviewed evidence, and their adverse event profiles and cost may outweigh their therapeutic benefit unless there is good evidence that the treatment will likely be effective or is worthy of a trial in a given case.121
Bleach Baths and Hypochlorous Acid. Sodium hypochlorite (NaOCl), commonly known as bleach, has been used for years as a disinfectant and antibacterial agent. When diluted with water to produce a bleach bath, sodium hypochlorite chemically converts to hypochlorous acid (HOCl). A randomized, placebo-controlled, investigator-blinded, three-month study (N=31) evaluated bleach baths twice weekly as compared with plain water baths in patients (age range: 6 months to 17 years) with moderate-to-severe AD and clinical signs of bacterial skin infection. All enrolled subjects maintained their stable regimen of topical anti-inflammatory medication and emollient use, and all received a 14-day course of oral cephalexin. The study group using bleach baths also applied intranasal mupirocin ointment twice daily for five days, while the group taking plain-water baths applied intranasal petrolatum ointment (placebo control). The objective was to determine the prevalence of community-acquired methicillin-resistant S. aureus (MRSA) in AD patients and to determine whether suppression of S. aureus with bleach baths and intranasal mupirocin correlated with decreased eczema severity. Results showed greater mean reductions from baseline in EASI scores in the bleach-bath group relative to the control group. EASI scores for the head and neck did not improve in the bleach-bath group, as these areas were not submerged in the bleach bath. Interestingly, throughout the study, skin cultures from patients in both groups continued to yield S. aureus, although it is likely that the bacterial load (quantity of organism) was reduced, which may possibly relate to improvement in AD. This study also suggests the possibility of other mechanisms of action of bleach baths in improving AD and reducing associated pruritus.132 It has been suggested that HOCl may have the ability to modulate some of the mediators of pruritus in AD discussed earlier. A known endogenous product in humans, HOCl is the end product of the polymorphonuclear leukocyte respiratory burst. HOCl may decrease proinflammatory protease activity and interleukin expression, thus contributing to interruption of the pruritus cycle.133 An open-label pilot study (N=20) evaluated the use of the combination of HOCl and NaOCl in a hydrogel in patients with mild-to-moderate AD over a seven-day period. HOCl/NaOCl hydrogel was applied twice daily as monotherapy over the first three study days and continued twice daily in combination with a designated hyaluronic acid-ceramide-based barrier repair therapy through Day 7. Investigator and subject assessments were performed on Days 1, 3, and 7. The primary endpoint of a decrease in pruritus was achieved, with a 23-percent reduction on Day 1, a 44-percent reduction on Day 3, and a 71-percent reduction on Day 7.134 Recently, the second-generation product, Aurstat® Anti-Itch Hydrogel (Onset Dermatologics, Cumberland, Rhode Island), received FDA approval as a 510(k) medical device. This product is unique in that it contains only HOCl, as compared with other products in this category, which also contain NaOCl. This patented difference allows for a reduced pH level that is compatible with the acid mantle of the skin.135
Antibiotics. AD patients are highly susceptible to skin infections due to fissured xerotic skin and an abnormal antimicrobial barrier and exhibit a higher rate of colonization by S. aureus compared with normal skin (nearly 100% vs. ≤30%, respectively).136 This higher-than-normal colonization is due, in part, to decreased levels of AMPs in skin, as previously explained. In turn, some strains of S. aureus produce superantigens that interact with the cutaneous immune system, leading to precipitation and/or prolongation of a flare. The most commonly used oral antibiotic for treatment of non-MRSA infections in AD is oral cephalexin.137
Given the extensive colonization of S. aureus on the skin of AD patients, there has been concern that these individuals may have higher rates of MRSA colonization and infection. However, this is not the case. While most hospitalized or otherwise immunosuppressed patients colonize S. aureus (quite often MRSA) in their nares, data have shown that, in actuality, resistant strains of S. aureus are present in only a very small proportion of AD patients. The prevalence of MRSA in a study of AD patients (7.4% skin, 4% nares) was negligible compared with the general population (75-85%).132
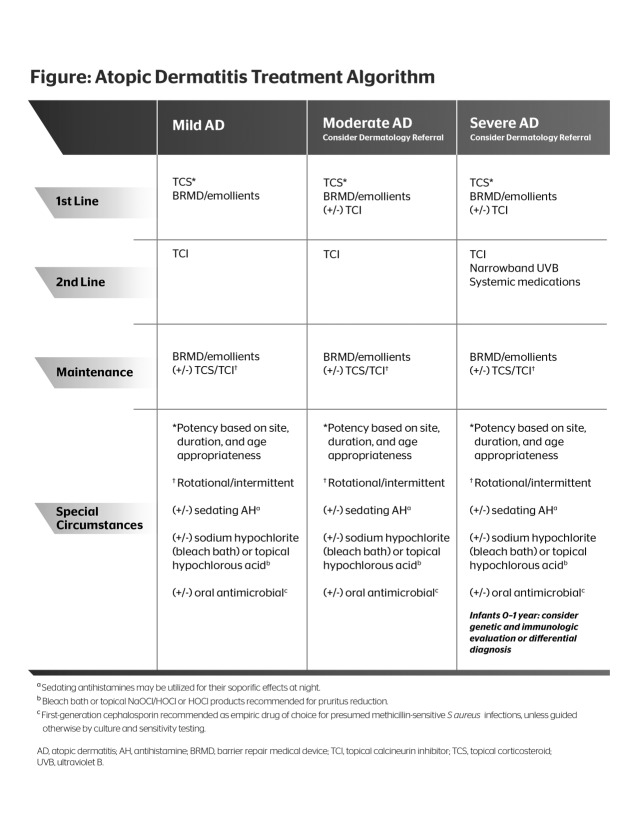
Treatment Algorithm (Figure 1)
Figure 1.
Atopic dermatitis treatment algorithm
The consensus for the treatment algorithm presented here is founded on both evidence-based medicine and clinical experience. The objective is to provide a succinct, easy-to-use model with the goal of increasing patient adherence and favorable outcomes.
All patients should be educated on skin care. Patients should be instructed to take short baths (5 to 10 minutes in duration) with a gentle soap-free cleanser, pat skin gently to dry, and follow immediately with a moisturizer or BRMD applied to the entire body. If the patient has a history of S. aureus infection, consider recommending the application of an antibacterial cleanser to the skin from the neck down for three minutes before the bath.138 Alternatively, the patient may bathe in a bleach bath (one-quarter to one-half cup of bleach in a one-quarter-filled tub of water).139
Since bleach baths could be disagreeable to some patients, and the mechanism by which bleach baths improve AD remains unknown, the packaged 510(k) products containing hypochlorous acid (leave-on anti-itch hydrogel), sodium hypochlorite (body wash), or sodium hypochlorite/hypochlorous acid (leave-on hydrogel) may be helpful alternatives. The hypochlorous acid product is available in a kit packaged with a BRMD to address the need for barrier repair along with the treatment of itch. It is important to note that while these agents contain the ingredients found in bleach or bleach baths, they have not been reviewed or approved by the FDA for use as antimicrobial agents.
Environmental triggers that may aggravate the patient’s AD should be avoided. The patient should avoid irritating clothing (e.g., wool), aeroallergens (e.g., dust, outside allergens), food to which he or she has a known allergy, and extreme temperatures (particularly indoor heat and air conditioning), and any factors that increase patient stress should be minimized.121
Before determining a treatment plan, it is essential to assess the patient’s condition and the severity of his or her disease. The SCORAD and EASI instruments are tools that may be employed for this assessment, but which are most often utilized in clinical studies.1 The clinician is encouraged to incorporate some method of consistent grading of major clinical features, symptoms, and psychosocial effects of AD in order to monitor progress. Patients who present with severe AD, and those who have moderate-to-severe AD and are under the age of one year, should be referred to a dermatologist.
The stepwise algorithm is as follows:
First Line. All patients—mild, moderate, or severe on initial presentation—should begin therapy with a TCS. The choice of TCS depends on several factors: potency, vehicle, site, and duration of therapy. It is good practice to start with the lowest potency that will achieve results, although some suggest more rapid control with an agent of greater potency used over a short duration (≤7-10 days), after which the potency of the TCS is tapered once reasonable control of the flare is achieved.90
As mentioned previously, vehicle is important and can make a difference in patient adherence as well as clinical outcomes. Patient satisfaction with the product’s vehicle leads to better adherence. For areas of the body that are particularly dry or are not as easily penetrable (e.g., the soles of the feet), an ointment or lipid-rich lotion or cream may work best. In hair-bearing areas and those involving larger body surface area, mid-potency TCS lotions with high lipid content may be preferred due to greater spreadability and cosmetic elegance as compared with thick creams or ointments. Areas of the face and intertriginous areas should use a low-potency TCS or a TCI may be considered.
Duration of treatment depends on many factors, and it is difficult to provide guidance for each circumstance. It is recognized that many clinicians prefer to customize their patients’ treatment regimens and may prescribe off label with regard to duration of TCS use. Data have shown that TCSs should be applied no more than once or twice a day. High-potency TCSs should not be used for longer than a few weeks, and should be used intermittently for control of flares, then discontinued or tapered down in frequency of use (e.g., to twice weekly).94
The TCSs are applied only to the active eczematous lesions of AD to minimize potential adverse reaction exposure area. Concurrent, diffuse application of a BRMD or an effective moisturizer to affected and nonaffected skin is recommended on a daily basis both during and between AD flares to address SC barrier integrity and function.
Second Line. AD that is not responsive to a TCS should be treated with the addition of a TCI. Data have demonstrated the effectiveness of TCIs in AD, and the steroid-sparing feature offers a valuable alternative. Patients with severe AD not responsive to either TCSs or TCIs should be considered for phototherapy or systemic therapy.
As with TCS, TCIs are applied to the active eczematous lesions of AD. In addition, concurrent diffuse application to affected and nonaffected skin of a BRMD or quality moisturizer is recommended on a daily basis both during and between AD flares.
Maintenance Therapy. Because AD is a chronic condition and there are inherent SC barrier deficiencies even in the presence of normal-appearing skin, maintenance therapy is vital to the long-term control of AD. Consistency with gentle cleansing and constant skin hydration with a moisturizer or BRMD each day is imperative, with overwashing avoided.67 In addition, early incorporation of a TCS and/or TCI for control of an active flare is very important, with the options of a proactive or rotational schedule for prevention of recurrences of AD. Use of hypochlorous acid, either in the form of bleach baths or in a topically applied vehicle, is safe for long-term use, and both bleach baths and topical hypochlorous acid in a hygrogel have been shown to reduce itch in AD in controlled trials.132,134
Special Circumstances. AD cases that are complicated by infections require an antibiotic. Most cases of S. aureus infection are responsive to 10 to 14 days of oral cephalexin. Localized S. aureus infections may be treated with a seven-day course of topical mupirocin or a five-day course of topical retapamulin.
Oral antihistamines have not been conclusively shown to be effective in relieving pruritus, but sedating antihistamines may be helpful in promoting sleep. A sedating antihistamine, such as diphenhydramine, doxepin, or hydroxyzine, is recommended. In children, diphenhydramine may result in a paradoxical hyperactive state; hence, hydroxyzine is often the first choice of antihistamine in children. Infants under the age of one year who present with severe AD and do not respond to first- or second-line therapy should be considered for genetic or immunological evaluation or differential diagnosis evaluation.
Future Directions in the Treatment of Atopic Dermatitis. Treatment for moderate-to-severe AD that is both effective and safe is lacking. Systemic therapies currently in use are riddled with side effects and drug interactions. Research is beginning to focus on molecular medicine and disease pathogenesis to develop new treatments. Clinical trials are emerging with specific immune antagonists. Studies are targeting the Th2 axis, IgE, Th22/IL-22, and anti-p40 antibodies that block IL-12 and IL-23, in addition to phosphodiesterase 4 (PDE4) inhibitors that block inflammation. There are several targets within the Th2 pathways. Dupilumab, a fully human monoclonal antibody, targets IL-4Rα, allowing for dual IL-4/IL-13 antagonism, and potently inhibits the Th2 pathway. IL-4/IL-13 is responsible for inhibiting keratinocyte differentiation, driving Th2 differentiation, activating B cells/IgE class switching, and recruiting eosinophils. Analysis of pooled phase lb studies has shown significant improvement in AD severity and pruritus with dupilumab and improvement in Th2 inflammatory markers in the skin.17,18 Finally, there is interest in the thymic stromal lymphopoietin (TSLP) receptor, a mediator of the Th2 cell. Blockade of this receptor is under investigation.140
The mechanism of action of recently described topical therapies has also been studied. Specifically, hypochlorous acid has been shown to neutralize IL-6 and LTB4 while it increases inhibitory α2-macroglobulin binding to IL-2, TNF-α, and IL-6 trials.133 It also chlorammates histamine, effectively impeding its biological reactivity. Each of these pathways may underlie the pruritic pathways involved in AD.133 Additionally, hypochlorous acid may decrease protease binding and modulate interleukins involved in the inflammatory cascade in a dose-dependent manner.133
Biographies








Footnotes
During the 2013 South Beach Symposium in Miami Beach, Florida, eight dermatology thought leaders convened for a roundtable meeting to discuss atopic dermatitis. The primary objective of this meeting was to bring together a panel of experts in atopic dermatitis to discuss, develop, and publish atopic dermatitis treatment guidelines. The consensus recommendations describe evidence-based treatment approaches for atopic dermatitis in an algorithm that is both therapeutically comprehensive and practical for utility in everyday practice.
The roundtable discussion and this supplement were supported by Onset Dermatologics. See author disclosures on page S18.
Author disclosures:Dr. Lebwohl has been a consultant and investigator for Celgene, Janssen Biotech, Novartis, and Leo Pharma, and a consultant for Galderma, Onset, Pfizer, and Taro. Dr. Del Rosso has served as a consultant, advisory board participant, clinical investigator, and speaker for Allergan, Bayer, Dermira, Eisai, Galderma, Leo Pharma, Medicis (Valeant), Onset Dermatologics, PharmaDerm, Quinnova, Primus, Promius, Ranbaxy, Taro, TriaBeauty, Unilever, Valeant, and Warner-Chilcott. Dr. Abramovits has been an advisor for Abbott, Anacor Pharm, Galderma, Janssen Biotech, Leo Pharma, PharmaDerm, Quinnova, Stiefel (GSK), Taro, and Valeant; is a consultant for Galderma, Kikaku America International, Quinnova, and Stiefel (GSK); an investigator for Abbott, Amgen, Astellas, Celgene, Conversant Labs, Galderma, Janssen Biotech, Leo Pharma, Novartis, Pfizer, Quinnova, Regeneron, Tolmar, Novum, Eli Lilly, and Otsuka; and on the speakers bureau for Abbott, Galderma, Janssen Biotech, Leo Pharma, PharmaDerm, Quinnova, and Stiefel (GSK). Dr. Berman is a consultant for Onset. Dr. Cohen has served as a consultant and advisory board participant for Onset, Ferndale, and Galderma, and is on the board of directors or has stock ownership in Brickell Biotech, Dermira, Topica, and Dr. Tattoff. Dr. Guttman-Yassky receives research support/consulting/lecture fees from Regeneron, Merck, Stiefel, GSK, Pfizer, Bristol-Myers Squibb, Celgene, Leo Pharma, Janssen, Medimmune, and Genentech, and has no patents, ownership, or financial gain from any atopic dermatitis drug. Dr. Mancini is a consultant for Galderma, Onset, Promius, Top MD, and Anacor, and is on the speakers bureau for Galderma. Dr. Schachner is an investigator for Astellas, Ferndale Labs, Novartis, Organogenesis, Inc., and Stiefel Laboratories, and is a consultant for Beiersdorf and Lexington.
Contributor Information
Mark G. Lebwohl, Professor & Chairman, Department of Dermatology The Icahn School of Medicine at Mount Sinai, New York, NY.
James Q. Del Rosso, Clinical Professor (Dermatology) Adjunct Faculty Section of Medicine, Touro University College of Osteopathic Medicine Henderson, NV.
William Abramovits, Assistant Professor Dermatology, Baylor University Medical Center, Dallas TX.
Brian Berman, Voluntary Prof. of Derm. and Cutaneous Surgery, Univ. of Miami Miller School of Medicine; Co-Director, Center for Clin. and Cosm. Research, Aventura, FL.
David E. Cohen, Charles C. and Dorothea E. Harris Professor of Dermatology, NYU Langon Medical Center, New York, NY.
Emma Guttman-Yassky, Associate Professor of Dermatology & Immunology, Mount Sinai Hospital, New York, NY.
Anthony J. Mancini, Head, Div. of Dermatology, Lurie Children's Hospital of Chicago; Prof. of Pediatrics and Derm., Northwestern University Feinberg School of Medicine.
Lawrence A. Schachner, Chairman and Harvey Blank Professor of Dermatology, The University of Miami Miller School of Medicine, Miami, FL.
References
- 1.Garnacho-Saucedo G, Salido-Vallejo R, Moreno-Giménez JC. Atopic dermatitis: update and proposed management algorithm. Actas Dermosifiliogr. 2013;104(1):4–16. doi: 10.1016/j.ad.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Eichenfield LF, Ellis CN, Mancini AJ, Paller AS, Simpson EL. Atopic dermatitis: epidemiology and pathogenesis update. Semin Cutan Med Surg. 2012;31(3) suppl:S3–S5. doi: 10.1016/j.sder.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Watson W, Kapur S. Atopic dermatitis. Allergy Asthma Clin Immunol. 2011;7(suppl 1):S4. doi: 10.1186/1710-1492-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams HC. Clinical practice: atopic dermatitis. N Engl J Med. 2005;352(22):2314–2324. doi: 10.1056/NEJMcp042803. [DOI] [PubMed] [Google Scholar]
- 5.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(6):S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Ozkaya E. Adult-onset atopic dermatitis. J Am Acad Dermatol. 2005;52(4):579–582. doi: 10.1016/j.jaad.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 7.European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Dermatology. 1993;186(1):23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 8.Rullo VE, Segato A, Kirsh A, Sole D. Severity scoring of atopic dermatitis: a comparison of two scoring systems. Allergol Immunopathol (Madr). 2008;36(4):205–211. [PubMed] [Google Scholar]
- 9.Emerson RM, Williams HC, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. Br J Dermatol. 1998;139(1):73–76. doi: 10.1046/j.1365-2133.1998.02316.x. [DOI] [PubMed] [Google Scholar]
- 10.Novak N, Bieber T, Leung DYM. Immune mechanisms leading to atopic dermatitis. J Allergy Clin Immunol. 2003;112(6) suppl:S128–S139. doi: 10.1016/j.jaci.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-mside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121(6):1337–1343. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugarman JL. The epidermal barrier in atopic dermatitis. Semin Cutan Med Surg. 2008;27(2):108–114. doi: 10.1016/j.sder.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Heimall J, Spergel JM. Filaggrin mutations and atopy: consequences for future therapeutics. Expert Rev Clin Immunol. 2012;8(2):189–197. doi: 10.1586/eci.11.100. [DOI] [PubMed] [Google Scholar]
- 14.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis—part I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127(5):1110–1118. doi: 10.1016/j.jaci.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 15.Kim BE, Leung DYM, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by the Th2 cytokines through STAT-6. Clin Immunol. 2008;126(3):332–337. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis—part II: immune cell subsets and therapeutic concepts. J Allergy Clin Immunol. 2011;127(6):1420–1432. doi: 10.1016/j.jaci.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 17.Beck LA, Thaci D, Hamilton JD, et al. Systemic treatment of patients with severe atopic dermatitis (AD) with an anti IL-4Rα mAb (REGN668/SAR231893) results in rapid and sustained improvements in disease signs and symptoms. Abstract presented at: International Investigative Dermatology Meeting; May 8-11, 2013; Edinburgh, Scotland.
- 18.Hamilton JD, Suárez-Fariñas M, Kostic A, et al. Blocking IL-4Rα signaling with REGN668/SAR231893 rapidly suppresses major pathogenic pathways in severe atopic dermatitis. Abstract presented at: International Investigative Dermatology Meeting; May 8-11, 2013; Edinburgh, Scotland.
- 19.Suárez-Fariñas M, Dhingra N, Gittler J, et al. Intrinsic atopic dermatitis (AD) shows similar Th2 and higher Th17 immune activation compared to extrinsic AD. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2013.04.046. [manuscript accepted]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347(15):1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 21.Leung DYM, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113(5):651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nograles KE, Zaba LC, Shemer A, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123(6):1244–1252. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howell MD, Fairchild HR, Kim BE, et al. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol. 2008;128(9):2248–2258. doi: 10.1038/jid.2008.74. [DOI] [PubMed] [Google Scholar]
- 24.Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of TH2/TH22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130(6):1344–1354. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011;2(3):110. doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornelissen C, Marquardt Y, Czaja K, et al. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol. 2012;129(2):426–433. doi: 10.1016/j.jaci.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 27.Cardona ID, Cho SH, Leung DYM. Role of bacterial superantigens in atopic dermatitis: implications for future therapeutic strategies. Am J Clin Dermatol. 2006;7(5):273–279. doi: 10.2165/00128071-200607050-00001. [DOI] [PubMed] [Google Scholar]
- 28.Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128(11):2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 29.Toda M, Leung DYM, Molet S, et al. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J Allergy Clin Immunol. 2003;111(4):875–881. doi: 10.1067/mai.2003.1414. [DOI] [PubMed] [Google Scholar]
- 30.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, et al. Low expression of the IL-23/Thl7 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181(10):7420–7427. doi: 10.4049/jimmunol.181.10.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langan SM, Silcocks P, Williams HC. What causes flares of eczema in children? Br J Dermatol. 2009;161(3):640–646. doi: 10.1111/j.1365-2133.2009.09320.x. [DOI] [PubMed] [Google Scholar]
- 32.Teplitsky V, Mumcuoglu KY, Babai I, Cohen R, Tanay A. House dust mites on skin, clothes, and bedding of atopic dermatitis patients. Int J Dermatol. 2008;47(8):790–795. doi: 10.1111/j.1365-4632.2008.03657.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee SI, Kim J, Han Y, Ahn K. A proposal: Atopic Dermatitis Organizer (ADO) guideline for children. Asia Pac Allergy. 2011;1(2):53–63. [Google Scholar]
- 34.Morgenstern V, Zutavern A, Cyrys J, et al. LISA Study Group. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Grit Care Med. 2008;177(12):1331–1337. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- 35.Riedler J, Braun-Fahrländer C, Eder W, et al. ALEX Study Team. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358(9288):1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 36.Mailhol C, Lauwers-Cances V, Rancé F, et al. Prevalence and risk factors for allergic contact dermatitis to topical treatment in atopic dermatitis: a study in 641 children. Allergy. 2009;64(5):801–806. doi: 10.1111/j.1398-9995.2008.01890.x. [DOI] [PubMed] [Google Scholar]
- 37.De Waard-van der Spek FB, Oranje AP. Patch tests in children with suspected allergic contact dermatitis: a prospective study and review of the literature. Dermatology. 2009;218(2):119–125. doi: 10.1159/000165629. [DOI] [PubMed] [Google Scholar]
- 38.Boussault P, Léauté-Labrèze C, Saubusse E, et al. Oat sensitization in children with atopic dermatitis: prevalence, risks and associated factors. Allergy. 2007;62(11):1251–1256. doi: 10.1111/j.1398-9995.2007.01527.x. [DOI] [PubMed] [Google Scholar]
- 39.Cramer C, Link E, Koletzko S, et al. The hygiene hypothesis does not apply to atopic eczema in childhood. Chem Immunol Allergy. 2012;96:15–23. doi: 10.1159/000331805. [DOI] [PubMed] [Google Scholar]
- 40.Chaumont A, Voisin C, Sardella A, Bernard A. Interactions between domestic water hardness, infant swimming and atopy in the development of childhood eczema. Environ Res. 2012;116:52–57. doi: 10.1016/j.envres.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Schoefer Y, Zutavern A, Brockow I, et al. LISA Study Group. Health risks of early swimming pool attendance. Int J Hyg Environ Health. 2008;211(3-4):367–373. doi: 10.1016/j.ijheh.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131(10):1974–1980. doi: 10.1038/jid.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gern JE, Reardon CL, Hoffjan S, et al. Effects of dog ownership and genotype on immune development and atopy in infancy. J Allergy Clin Immunol. 2004;113(2):307–314. doi: 10.1016/j.jaci.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 44.Kurosaka F, Nakatani Y, Terada T, et al. Current cat ownership may be associated with the lower prevalence of atopic dermatitis, allergic rhinitis, and Japanese cedar pollinosis in schoolchildren in Himeji, Japan. Ped Allergy Immunol, 2006;17(1):22–28. doi: 10.1111/j.1399-3038.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 45.Noh J, Noh G, Kim HS, Kim A-R, Choi WS. Allergen-specific responses of CD19(+)CD5(+)Foxp3(+) regulatory B cells (Bregs) and CD4(+)Foxp3(+) regulatory T cell (Tregs) in immune tolerance of cow milk allergy of late eczematous reactions. Cell Immunol. 2012;274(1-2):109–114. doi: 10.1016/j.cellimm.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Hill DJ, Hosking CS, de Benedictis FM, Oranje AP, Diepgen TL, Bauchau V. EPAAC Study Group. Confirmation of the association between high levels of immunoglobulin E food sensitization and eczema in infancy: an international study. Clin Exp Allergy. 2008;38(1):161–168. doi: 10.1111/j.1365-2222.2007.02861.x. [DOI] [PubMed] [Google Scholar]
- 47.Sinagra JL, Bordignon V, Ferraro C, et al. Unnecessary milk elimination diets in children with atopic dermatitis. Pediatr Dermatol. 2007;24(1):1–6. doi: 10.1111/j.1525-1470.2007.00323.x. [DOI] [PubMed] [Google Scholar]
- 48.Snijders BEP, Thijs C, van Ree R, van den Brandt PA. Age at first introduction of cow milk products and other food products in relation to infant atopic manifestations in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics. 2008;122(l):ell5–el22. doi: 10.1542/peds.2007-1651. [DOI] [PubMed] [Google Scholar]
- 49.Greer FR, Sicherer SH, Burks AW. American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Allergy and Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121(1):183–191. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 50.Duncan JM, Sears MR. Breastfeeding and allergies: time for a change in paradigm? Curr Opin Allergy Clin Immunol. 2008;8(5):398–405. doi: 10.1097/ACI.0b013e32830d82ed. [DOI] [PubMed] [Google Scholar]
- 51.Karino S, Okuda T, Uehara Y, Toyo-oka T. Breastfeeding and prevalence of allergic diseases in Japanese university students. Ann Allergy Asthma Immunol. 2008;101(2):153–159. doi: 10.1016/S1081-1206(10)60203-7. [DOI] [PubMed] [Google Scholar]
- 52.Wickens K, Black P, Stanley TV, et al. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin Exp Allergy. 2012;42(7):1071–1079. doi: 10.1111/j.1365-2222.2012.03975.x. [DOI] [PubMed] [Google Scholar]
- 53.Kalliomäki M, Salminen, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119(4):1019–1021. doi: 10.1016/j.jaci.2006.12.608. [DOI] [PubMed] [Google Scholar]
- 54.Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics. 2008;121(4):e850–e856. doi: 10.1542/peds.2007-1492. [DOI] [PubMed] [Google Scholar]
- 55.Osborn DA, Sinn JKH. Prebiotics in infants for prevention of allergic disease and food allergy. Cochrane Summaries. March 2013. [May 29, 2013]. http://summaries.cochrane.org/CD006474/prebiotics-in-infants-for-prevention-of-allergic-disease-and-food-allergy
- 56.Lowe AJ, Abramson MJ, Hosking CS, et al. The temporal sequence of allergic sensitization and onset of infantile eczema. Clin Exp Allergy. 2007;37(4):536–542. doi: 10.1111/j.1365-2222.2007.02691.x. [DOI] [PubMed] [Google Scholar]
- 57.Boyce JA, Assa’ad A, Burks AW, et al. NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-Sponsored Expert Panel report. J Allergy Clin Immunol. 2010;126(6):1105–1118. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keck LE, Simpson EL, Berry TM, Hanifin JM. Is food allergy testing reliable in pediatric atopic dermatitis? A population-based study. Chem Immunol Allergy. 2012;96:108–112. doi: 10.1159/000331906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bockelbrink A, Heinrich J, Schäfer I, et al. LISA Study Group. Atopic eczema in children: another harmful sequel of divorce. Allergy. 2006;61(12):1397–1402. doi: 10.1111/j.1398-9995.2006.01186.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Lam S-P, Li SX, Li AM, Wing Y-K. The longitudinal course and impact of non-restorative sleep: a five-year community-based follow-up study. Sleep Med. 2012;13(6):570–576. doi: 10.1016/j.sleep.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Yosipovitch G, Papoiu DP. What causes itch in atopic dermatitis? Curr Allergy Asthma Rep. 2008;8(4):306–311. doi: 10.1007/s11882-008-0049-z. [DOI] [PubMed] [Google Scholar]
- 62.Steinhoff M, Neisius U, Ikoma A, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23(15):6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han L, Ma C, Liu Q, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16(2):174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chamlin SL, Kao J, Frieden IJ, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002;47(2):198–208. doi: 10.1067/mjd.2002.124617. [DOI] [PubMed] [Google Scholar]
- 65.Lucky AW, Leach AD, Laskarzewski P, Wenck H. Use of an emollient as a steroid-sparing agent in the treatment of mild to moderate atopic dermatitis in children. Pediatr Dermatol. 1997;14(4):321–324. doi: 10.1111/j.1525-1470.1997.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 66.Msika P, De Belilovsky C, Piccardi N, et al. New emollient with topical corticosteroid-sparing effect in treatment of childhood atopic dermatitis: SCORAD and quality of life improvement. Pediatr Dermatol. 2008;25(6):606–612. doi: 10.1111/j.1525-1470.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 67.Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aestfuet Dermatol, 2011;4(9):22–42. [PMC free article] [PubMed] [Google Scholar]
- 68.Draelos ZD. Therapeutic moisturizers. Dermatol Clin. 2000;18(4):597–607. doi: 10.1016/s0733-8635(05)70210-2. [DOI] [PubMed] [Google Scholar]
- 69.Elias PM, Ansel JC, Woods LD, Feingold KR. Signaling networks in barrier homeostasis: the mystery widens. Arch Dermatol. 1996;132(12):1505–1506. [PubMed] [Google Scholar]
- 70.Harding CR. The stratum corneum: structure and function in health and disease. Dermatol Ther. 2004;17(suppl 1):6–15. doi: 10.1111/j.1396-0296.2004.04s1001.x. [DOI] [PubMed] [Google Scholar]
- 71.Bouwstra JA, de Graaff A, Gooris GS, et al. Water distribution and related morphology in human stratum corneum at different hydration levels. J Invest Dermatol. 2003;120(5):750–758. doi: 10.1046/j.1523-1747.2003.12128.x. [DOI] [PubMed] [Google Scholar]
- 72.Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol, 1998;78(1):27–30. doi: 10.1080/00015559850135788. [DOI] [PubMed] [Google Scholar]
- 73.Kezic S, Kemperman PMJH, Koster ES, et al. Loss-offunction mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J Invest Dermatol. 2008;128(8):2117–2119. doi: 10.1038/jid.2008.29. [DOI] [PubMed] [Google Scholar]
- 74.O’Regan GM, Irvine AD. The role of filaggrin in the atopic diathesis. Clin Exp Allergy. 2010;40(7):965–972. doi: 10.1111/j.1365-2222.2010.03522.x. [DOI] [PubMed] [Google Scholar]
- 75.Ananthapadmanabhan KP, Moore DJ, Subramanyan K, et al. Cleansing without compromise: the impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol Ther. 2004;17(suppl 1):16–25. doi: 10.1111/j.1396-0296.2004.04s1002.x. [DOI] [PubMed] [Google Scholar]
- 76.Lodén M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol, 2003;4(11):771–788. doi: 10.2165/00128071-200304110-00005. [DOI] [PubMed] [Google Scholar]
- 77.Rawlings AV, Canestrari DA, Dobkowski B. Moisturizer technology versus clinical performance. Dermatol Ther. 2004;17(suppl 1):49–56. doi: 10.1111/j.1396-0296.2004.04s1006.x. [DOI] [PubMed] [Google Scholar]
- 78.Park KY, Kim DH, Jeong MS, et al. Changes of antimicrobial peptides and transepidermal water loss after topical application of tacrolimus and ceramide-dominant emollient in patients with atopic dermatitis. J Korean Med Sci. 2010;25(5):766–771. doi: 10.3346/jkms.2010.25.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee YB, Park HJ, Kwon MJ, et al. Beneficial effects of pseudoceramide-containing physiologic lipid mixture as a vehicle for topical steroids. Eur J Dermatol, 2011;21(5):710–716. doi: 10.1684/ejd.2011.1447. [DOI] [PubMed] [Google Scholar]
- 80.Mao-Qiang M, Brown BE, Wu-Pong S, et al. Exogenous nonphysiologic vs physiologic lipids: divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol. 1995;131(7):809–816. doi: 10.1001/archderm.131.7.809. [DOI] [PubMed] [Google Scholar]
- 81.Draelos ZD. New treatments for restoring impaired epidermal barrier permeability: skin barrier repair creams. Clin Dermatol. 2012;30(3):345–348. doi: 10.1016/j.clindermatol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 82.Draelos ZD. A clinical evaluation of the comparable efficacy of hyaluronic acid-based foam and ceramide-containing emulsion cream in the treatment of mild-to-moderate atopic dermatitis. J Cosmet Dermatol. 2011;10(3):185–188. doi: 10.1111/j.1473-2165.2011.00568.x. [DOI] [PubMed] [Google Scholar]
- 83.Cash K, High W, de Sterke J. An evaluation of barrier repair foam on the molecular concentration profiles of intrinsic skin constituents utilizing confocal Raman spectroscopy. J Clin Aesthet Dermatol. 2012;5(8):14–17. [PMC free article] [PubMed] [Google Scholar]
- 84.Abramovits W, Hebert AA, Boguniewicz M, et al. Patient-reported outcomes from a multicenter, randomized, vehicle-controlled clinical study of MAS063DP (Atopiclair) in the management of mild-to-moderate atopic dermatitis in adults. J Dermatolog Treat. 2008;19(6):327–332. doi: 10.1080/09546630802232799. [DOI] [PubMed] [Google Scholar]
- 85.Simpson EL. Atopic dermatitis: a review of topical treatment options. Curr Med Res Opin. 2010;26(3):633–640. doi: 10.1185/03007990903512156. [DOI] [PubMed] [Google Scholar]
- 86.Sugarman JL, Parish LC. Efficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J Drugs Dermatol. 2009;8(12):1106–1111. [PubMed] [Google Scholar]
- 87.Frankel A, Sohn A, Patel RV, Lebwohl M. Bilateral comparison study of pimecrolimus cream 1% and a ceramide-hyaluronic acid emollient foam in the treatment of patients with atopic dermatitis. J Drugs Dermatol. 2011;10(6):666–672. [PubMed] [Google Scholar]
- 88.Hanifin JM, Cooper KD, Ho VC, et al. Guidelines of care for atopic dermatitis. J Am Acad Dermatol, 2004;50(3):391–404. doi: 10.1016/j.jaad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 89.Del Rosso J, Friedlander SF. Corticosteroids: options in the era of steroid-sparing therapy. J Am Acad Dermatol. 2005;53(l) suppl 1:S50–S58. doi: 10.1016/j.jaad.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 90.Ference JD, Last AR. Choosing topical corticosteroids. Am Family Phys. 2009;79(2):135–140. [PubMed] [Google Scholar]
- 91.Matheson R, Kempers S, Breneman D, et al. Hydrocortisone butyrate 0.1% lotion in the treatment of atopic dermatitis in pediatric subjects. J Drugs Dermatol. 2008;7(3):266–271. [PubMed] [Google Scholar]
- 92.Huang X, Tanojo H, Lenn J, et al. A novel foam vehicle for delivery of topical corticosteroids. J Am Acad Dermatol. 2005;53(1) Suppl 1:S26–S38. doi: 10.1016/j.jaad.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 93.Schmitt J, von Kobyletzki, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2011;164(2):415–428. doi: 10.1111/j.1365-2133.2010.10030.x. [DOI] [PubMed] [Google Scholar]
- 94.Hanifin J, Gupta AK, Rajagopalan R. Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients. Br J Dermatol. 2002;147(3):528–537. doi: 10.1046/j.1365-2133.2002.05006.x. [DOI] [PubMed] [Google Scholar]
- 95.Nakahara T, Koga T, Fukagawa S, et al. Intermittent topical corticosteroid/tacrolimus sequential therapy improves lichenification and chronic papules more efficiently than intermittent topical corticosteroid/emollient sequential therapy in patients with atopic dermatitis. J Dermatol. 2004;31(7):524–528. doi: 10.1111/j.1346-8138.2004.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 96.Thomas KS, Armstrong S, Avery A, et al. Randomised controlled trial of short bursts of a potent topical corticosteroid versus prolonged use of a mild preparation for children with mild or moderate atopic eczema. BMJ. 2002;324(7340):768. doi: 10.1136/bmj.324.7340.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amin N, Brancaccio R, Cohen D. Cutaneous reactions to injectable corticosteroids. Dermatitis. 2006;17(3):143–146. doi: 10.2310/6620.2006.05047. [DOI] [PubMed] [Google Scholar]
- 98.Fransway AF, Zug KA, Belsito DV, et al. North American Contact Dermatitis Group patch test results for 2007-2008. Dermatitis. 2013;24(1):10–21. doi: 10.1097/DER.0b013e318277ca50. [DOI] [PubMed] [Google Scholar]
- 99.Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17(3):137–142. doi: 10.2310/6620.2006.05048. [DOI] [PubMed] [Google Scholar]
- 100.Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. doi: 10.1007/s40272-013-0013-9. 2013 Apr 3 [Epub]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Elidel [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2011.
- 102. Protopic [package insesrt]. Northbrook, IL: Astellas Pharma US Inc.; 2012.
- 103.Fonacier L, Spergel J, Charlesworth EN, et al. American College of Allergy, Asthma and Immunology; American Academy of Allergy, Asthma and Immunology. Report of the Topical Calcineurin Inhibitor Task Force of the American College of Allergy, Asthma and Immunology and the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2005;115(6):1249–1253. doi: 10.1016/j.jaci.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 104.Food and Drug Administration. Information for healthcare professionals: pimecrolimus (marketed as Elidel) [May 29, 2013]. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm153525.htm
- 105.Food and Drug Administration. Tacrolimus (marketed as Protopic Ointment) information. [May 29, 2013]. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm107845.htm
- 106.Qureshi AA, Fisher MA. Topical calcineurin inhibitors for atopic dermatitis: balancing clinical benefit and possible risks. [May 29, 2013]. http://nationaleczema.drupalgardens.com/sites/nationaleczema.drupalgardens.com/files/topical_calcineuri n_inhibitors_for_ad_quereshi.pdf [DOI] [PubMed]
- 107.Hui RL, Lide W, Chan J, et al. Association between exposure to topical tacrolimus or pimecrolimus and cancers. Ann Pharmacother. 2009;43(12):1956–1963. doi: 10.1345/aph.1M278. [DOI] [PubMed] [Google Scholar]
- 108.Chen SL, Yan J, Wang FS. Two topical calcineurin inhibitors for the treatment of atopic dermatitis in pediatric patients: a meta-analysis of randomized clinical trials. J Dermatolog Treat. 2010;21(3):144–156. doi: 10.3109/09546630903401470. [DOI] [PubMed] [Google Scholar]
- 109.Wollenberg A, Reitamo S, Atzori F, et al. European Tacrolimus Ointment Study Group. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy. 2008;63(6):742–750. doi: 10.1111/j.1398-9995.2008.01683.x. [DOI] [PubMed] [Google Scholar]
- 110.Thaçi D, Reitamo S, Gonzalez Ensenat MA, et al. European Tacrolimus Ointment Study Group. Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: results of a randomized, multicentre, comparative study. Br J Dermatol. 2008;159(6):1348–1356. doi: 10.1111/j.1365-2133.2008.08813.x. [DOI] [PubMed] [Google Scholar]
- 111.Breneman D, Fleischer AB, Abromovits W, et al. Intermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a reandomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicle. J Am Acad Derm. 2008;58(6):990–999. doi: 10.1016/j.jaad.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 112.Paller AS, Eichenfield LF, Kirshner RS, et al. Three times weekly tacrolimus ointment reduces relapse in stabilized atopic dermatitis: a new paradigm for use. Pediatrics. 2008;122(6):1210–1218. doi: 10.1542/peds.2008-1343. [DOI] [PubMed] [Google Scholar]
- 113.Gollnick H, Kaufmann R, Stough D, et al. Pimecrolimus Cream 1% in (Adult) Eczema: Prevention of Progression Multicentre Investigator Study Group. Pimecrolimus cream 1% in the long-term management of adult atopic dermatitis: prevention of flare progression: a randomized controlled trial. Br J Dermatol. 2008;158(5):1083–1093. doi: 10.1111/j.1365-2133.2008.08484.x. [DOI] [PubMed] [Google Scholar]
- 114.Wahn U, Bos JD, Goodfield M, et al. Flare Reduction in Eczema with Elidel (Children) Multicenter Investigator Study Group. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110(1 pt 1):e2. doi: 10.1542/peds.110.1.e2. [DOI] [PubMed] [Google Scholar]
- 115.Jekler J, Bergbrant IM, Faergemann J, Larkö O. The in vivo effect of UVB radiation on skin bacteria in patients with atopic dermatitis. Acta Derm Venereol. 1992;72(1):33–36. [PubMed] [Google Scholar]
- 116.Grundmann SA, Beissert S. Modern aspects of phototherapy for atopic dermatitis. J Allergy (Cairo). 2012;2012:121797. doi: 10.1155/2012/121797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128(3):583–593. doi: 10.1016/j.jaci.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sowden JM, Berth-Jones J, Ross JS, et al. Double-blind, controlled, crossover study of cyclosporin in adults with severe refractory atopic dermatitis. Lancet. 1991;338(8760):137–140. doi: 10.1016/0140-6736(91)90134-b. [DOI] [PubMed] [Google Scholar]
- 119.Harper JI, Ahmed I, Barclay G, et al. Cyclosporin for severe childhood atopic dermatitis: short course versus continuous therapy. Br J Dermatol. 2000;142(1):52–58. doi: 10.1046/j.1365-2133.2000.03241.x. [DOI] [PubMed] [Google Scholar]
- 120.Berth-Jones J, Graham-Brown RA, Marks R, et al. Long-term efficacy and safety of cyclosporin in severe adult atopic dermatitis. Br J Dermatol. 1997;136(1):76–81. [PubMed] [Google Scholar]
- 121.Boguniewicz M, Eichenfield F, Hultsch T. Current management of atopic dermatitis and interruption of the atopic march. J Allergy Clin Immunol. 2003;112(6):S140–S150. doi: 10.1016/j.jaci.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 122.Neuber K, Schwartz I, Itschert G, Dieck AT. Treatment of atopic eczema with oral mycophenolate mofetil. Br J Dermatol. 2000;143(2):385–391. doi: 10.1046/j.1365-2133.2000.03667.x. [DOI] [PubMed] [Google Scholar]
- 123.Weatherhead SC, Wahie S, Reynolds NJ, Meggitt SJ. An open-label, dose-ranging study of methotrexate for moderate-to-severe adult atopic eczema. Br J Dermatol. 2007;156(2):346–351. doi: 10.1111/j.1365-2133.2006.07686.x. [DOI] [PubMed] [Google Scholar]
- 124.Gelbard CM, Hebert AA. New and emerging trends in the treatment of atopic dermatitis. Patient Prefer Adherence. 2008;2:387–392. [PMC free article] [PubMed] [Google Scholar]
- 125.Meggitt SJ, Reynolds NJ. Azathioprine for atopic dermatitis. Clin Exp Dermatol. 2001;26(5):369–375. doi: 10.1046/j.1365-2230.2001.00837.x. [DOI] [PubMed] [Google Scholar]
- 126.Berth-Jones J, Takwale A, Tan E, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol. 2002;147(2):324–330. doi: 10.1046/j.1365-2133.2002.04989.x. [DOI] [PubMed] [Google Scholar]
- 127.Food and Drug Administration. FDA drug safety communication: safety review update on reports of hepatosplenic T-cell lymphoma in adolescents and young adults receiving tumor necrosis factor (TNF) blockers, azathioprine and/or mercaptopurine. [May 29, 2013]. http://www.fda.gov/ Drugs/DrugSafety/ucm250913.htm
- 128.Schram ME, Roekevisch E, Leeflang MMG, et al. A randomized trial of methotrexate versus azathioprine for severe atopic eczema. J Allergy Clin Immunol. 2011;128(2):353–359. doi: 10.1016/j.jaci.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 129.Park SY, Choi MR, Na JI, et al. Recalcitrant atopic dermatitis treated with omalizumab. Ann Dermatol. 2010;22(3):349–352. doi: 10.5021/ad.2010.22.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Amrol D. Anti-immunoglobulin E in the treatment of refractory atopic dermatitis. South Med J. 2010;103(6):554–558. doi: 10.1097/SMJ.0b013e3181de0cf6. [DOI] [PubMed] [Google Scholar]
- 131.Fernández-Antón Martinez MC, Leis-Dosil V, Alfageme-Roldán, et al. Omalizumab for the treatment of atopic dermatitis. Actas Dermosifiliogr. 2012;103(7):624–628. doi: 10.1016/j.ad.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 132.Huang JT, Abrams M, Tlougan B, et al. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123(5):e808–e814. doi: 10.1542/peds.2008-2217. [DOI] [PubMed] [Google Scholar]
- 133.Friedman A, Cash K, Berman B. Hypochlorous acid is anti-inflammatory and immunomodulatory. Poster presented at: 2013 Winter Clinical Dermatology Conference; January 18-23, 2013; Kauai, HI.
- 134.Draelos Z, Cash K. Evaluation of a gel formulation of hypochlorous acid and sodium hypochlorite to reduce pruritus in mild to moderate atopic dermatitis. Poster presented at: 2013 Winter Clinical Dermatology Conference; January 18-23, 2013; Kauai, HI.
- 135. Aurstat Anti-Itch Hydrogel [package insert]. Cumberland, RI: Onset Dermatologics; 2013.
- 136.Breuer K, Haussler S, Kapp A, Werfel T. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol. 2002;147(1):55–61. doi: 10.1046/j.1365-2133.2002.04872.x. [DOI] [PubMed] [Google Scholar]
- 137.Niebuhr M, Mai U, Kapp A, Werfel T. Antibiotic treatment of cutaneous infections with Staphylococcus aureus in patients with atopic dermatitis: current antimicrobial resistances and susceptibilities. Exp Dermatol. 2008;17(11):953–957. doi: 10.1111/j.1600-0625.2008.00734.x. [DOI] [PubMed] [Google Scholar]
- 138.Schachner LA. Barrier repair devices & emollients. Presented at: Pathways to Managing AD: Consensus From the Experts Roundtable; April 12, 2013; Miami, FL.
- 139.Fisher RG, Chain RL, Hair PS, Cunnion KM. Hypochlorite killing of community-associated methicillin-resistant Staphylococcal aureus. Ped Inf Dis J. 2008;27(10):934–935. doi: 10.1097/INF.0b013e318175d871. [DOI] [PubMed] [Google Scholar]
- 140.Guttman E. Atopic dermatitis: pathogenic concepts and emerging therapeutics. Presented at: Pathways to Managing AD: Consensus From the Experts Roundtable; April 12, 2013; Miami, FL.
- 141.National Eczema Association. Living with eczema: topical corticosteroids: myths & facts. [May 29, 2013]. http://www.national eczema.org/eczema-treatments/topical-corticosteroids