Abstract
Some bacterial species can colonize humans and plants. It is almost impossible to prevent the contact of clinically pathogenic bacteria with food crops, and if they can persist there, they can reenter the human food chain and cause disease. On the leaf surface, microorganisms are exposed to a number of stress factors. It is unclear how they survive in such different environments. By increasing adhesion to diverse substrates, minimizing environmental differences, and providing protection against defence mechanisms, biofilms could provide part of the answer. Klebsiella pneumoniae subsp. pneumoniae is clinically important and also associated with fruit diseases, such as “pineapple fruit collapse.” We aimed to characterize biofilm formation and adhesion mechanisms of this species isolated from pineapple in comparison with a clinical isolate. No differences were found between the two isolates quantitatively or qualitatively. Both tested positive for capsule formation and were hydrophobic, but neither produced adherence fibres, which might account for their relatively weak adhesion compared to the positive control Staphylococcus epidermidis ATCC 35984. Both produced biofilms on glass and polystyrene, more consistently at 40°C than 35°C, confirmed by atomic force and high-vacuum scanning electron microscopy. Biofilm formation was maintained in an acidic environment, which may be relevant phytopathologically.
1. Introduction
Bacteria are unicellular microorganisms that live in many different environments but rarely as individual cells. Some species form an organized exopolysaccharide (EPS) structure around the cell wall, known as a capsule [1]. On a larger scale, clusters of bacteria sometimes form organized communities known as biofilms. Biofilm may be defined as an assemblage of microorganisms adherent to each other and/or to a surface and embedded in a matrix of exopolysaccharides (EPS) [2, 3].
Bacterial adhesion and biofilm formation are very important concepts in the area of bacterial disease and control. Not only do they aid colonisation but also often provide a degree of protection against outside stresses [4]. However, the formation and structure of biofilm communities depend on a wide variety of parameters, including species, temperature, pH, and the presence of salts [2].
Various bacterial species have been found to grow on both plant and human tissues. Klebsiella pneumoniae subsp. pneumoniae has received much attention due to an association with human and animal diseases [5, 6]. Additionally, it not only has been isolated from plants including spinach [7] and rice [8] but also has recently been implicated in a disease of pineapple (pineapple fruit collapse) [9]. It is unclear how one species could be so successful on such different substrates as fruit and human tissue that it could cause disease symptoms in both. However, biofilm formation is known to be one mechanism of clinical pathogenicity of this species [10].
We proposed to investigate the adhesion and biofilm capacity of two different isolates of the important bacterial species K. pneumoniae subsp. pneumoniae, isolated from humans and pineapples.
2. Materials and Methods
2.1. Microorganisms
The bacterial isolate, Klebsiella pneumoniae subsp. pneumoniae, was obtained from pineapple fruit diagnosed with pineapple fruit collapse and identified by biochemical and physiological tests, optimal growth temperatures, partial genetic sequencing of 16S rDNA [11, 12], and comparison with the type strain. A multidrug susceptible, clinical isolate of K. pneumoniae subsp. pneumoniae, known to form slime in plate culture, was obtained by courtesy of the Marcos Daniel Clinical Laboratory (Vitória, Brazil). Staphylococcus epidermidis ATCC 35984 and S. epidermidis ATCC 12228, positive and negative for biofilm formation, respectively, were used as controls. Bacterial isolates were maintained in slant tubes of nutrient agar at 4°C.
2.2. Biofilm Formation in Different Surfaces
Tests for biofilm formation were performed on three different materials: glass, polyester strip, and polystyrene. Glass tubes were filled with 5 mL of tryptone-soy broth (TSB) (1.7% peptone casein; 0.3% soy peptone; 0.25% glucose; 0.5% NaCl; and 0.25% K2HPO4) at two pH values (4.5 and 7.0). Broth was inoculated with 100 μL of a suspension of 107 CFU·mL−1 of each bacterial isolate. Tubes were incubated at 35°C or 40°C for 24 h. The culture was discarded and the tube washed twice with sterile distilled water. Tubes were incubated with 0.1% safranin for 1 min. Safranin was then discarded, tubes were air-dried upside down overnight, and adherence of safranin to the inner surface of the tube was assessed visually and classified as absent (0); weak (+); moderate (++); or strong (+++) [13]. Experiments were repeated three times in triplicate.
The polystyrene adhesion test was performed using a microplate of 96 wells [14]. Aliquots of 180 μL of TSB broth and 20 μL of bacterial suspension (107 CFU·mL−1) of bacterial isolate were added to each well, and TSB broth alone was used as negative control. All sets were incubated at 35 or 40°C for 24 h in one of two pH values (4.5 and 7.0). Media were removed from the microplate by inversion, wells were gently washed with sterile distilled water, and cells adhered to the microplate were stained with 200 μL of violet crystal solution (0.1%) for 30 min. The dye was discarded, and microplates dried at 40°C for 15 min. Biofilm was quantified by adding 200 μL of 95% of ethanol to each well and the OD was measured at 595 nm using Elisa reader (Thermo Plate, model TP NM, Brazil) after the adjustment to zero of the negative control. Strains were considered as efficient in biofilm formation when absorbance at 595 nm was equal or the greater than 0.15 [15].
Biofilm formation was also investigated by the colony diameter test [16]. Bacterial isolate was inoculated in the center of a Petri dish containing semisolid TSB media (0.5% agar). Plates were incubated at 35 and 40°C and the diameter of the colony was measured after 5 days. The colony diameters of bacterial isolate and the negative control, S. epidermidis ATCC 12228, were compared, and if greater by 30% or more, the isolate was considered positive.
2.3. Capsule Presence by Light Microscopy
Capsule formation was assessed by the Congo red method [17]. The isolates were incubated in TSB broth at 35°C for 24 h. After this period, 2 drops of the cell suspension were mixed with 2 drops of 0.5% Congo red solution on a glass slide, and the mixture was smeared and air-dried. The material was stained with Maneval solution (1 min.), washed with distilled water, and air dried. Slides were observed under light microscope (Leica, model DMLS, Leica Microsystems, Germany) using oil immersion lens. A nonstained region around central red bacterial cells on a blue background indicated the presence of a capsule.
2.4. Adhesion Fiber Formation
Ability to produce adherence fibers (curli) was evaluated using a previously published protocol [18]. Plates of diluted nutrient broth (1 : 10) with 1.5% agar, Congo red (40 mg·L−1) and Coomassie blue (20 mg·L−1), were inoculated by streaking the colonies and incubated at 25°C for 48 h. Dark red or black colonies were indicative of adhesion fibers while white or pink colonies were indicative that fibers were not produced.
2.5. Bacterial Hydrophobicity
Bacterial hydrophobicity was assayed by the ammonium sulfate method. Bacterial suspension (15 μL) was combined with different concentrations of ammonium sulfate (0.5 M; 1 M; 1.5 M; 2 M; 2.5 M; and 3 M) (15 μL) on a glass slide. The suspension was gently mixed and observed for aggregate formation for 2 min. Agglutination in salt concentrations of less than 1.0 M indicated surface hydrophobicity. Conversely, surface hydrophilicity was indicated by the agglutination of bacteria in high salt concentrations, from 2.0 to 4.0 M [19].
2.6. Sample Preparation for High-Vacuum Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM)
Biofilm formation on glass and polyester strips was also monitored visually by high-vacuum scanning electron microscopy (SEM) and atomic force microscopy (AFM). Bacterial isolate was inoculated in glass tubes with TSB as described above, and a 1 cm length dentistry polyester strip or glass coverslip was added to be settled on by the cells for microscopy experiments. Microorganisms were incubated for 20 h at 35°C. After this time, glass and polyester strips were removed from each tube, washed three times with sterile saline (0.9% NaCl), placed in sterile Petri dishes with cheesecloth, and air dried at room temperature [20].
For SEM, each sample was mounted on aluminum stubs and sputter-coated with 20 nm gold (Bal-Tec Sputter Coater SCD-050, Capovani Brothers Inc., Scotia, USA). Samples were observed using a Shimadzu SSX 550 high-vacuum scanning electron microscope (Shimadzu, Kyoto, Japan) operated at 20.0 kV.
For AFM, samples were observed using a Shimadzu SPM-9600 series Microscope (Shimatzu, Kyoto, Japan). Si3N4 cantilever tips (model OMCL-TR, Olympus, Tokyo, Japan) with a nominal constant of 32 N·m−1 and resonance frequency of ≈300 kHz were used with scan rates of 0.3–1.0 Hz and scan size of 2,000–10,000 nm and imaging analysis were performed with the accompanying software.
2.7. Statistical Analysis
All experiments were conducted in triplicate, and repeated three times. Statistical analysis was performed by ANOVA using Multistat software by comparing the mean values by Tukey test with 5% probability [21].
3. Results
3.1. Biofilm Formation Assessment by Routine Laboratory Tests
Colony diameter tests showed that both Klebsiella isolates were positive for biofilm formation. Colonies of K. pneumoniae subsp. pneumoniae isolated from pineapple fruit and the clinical isolate were 152.9% and 135.3% larger, respectively, compared to negative control S. epidermidis ATCC 12228. The Klebsiella isolates were both significantly different to the control (P < 0.001), but not to each other (P > 0.05).
K. pneumoniae subsp. pneumoniae isolated from pineapple fruit and the clinical isolate were positive for adherence to the inner surface of glass tubes and on polystyrene microtiter plates as was the positive control S. epidermidis ATCC 35984. Microtiter plate tests performed with both Klebsiella isolates demonstrated low OD values (0.130–0.212) compared to the positive control S. epidermidis ATCC 35984 (1.525), indicating the presence of a biofilm but at a relatively low level.
Klebsiella isolates formed a more consistent biofilm at 40°C than 35°C as measured by both glass adhesion and polystyrene microtiter plate assays (Table 1). There was no difference between the two Klebsiella isolates. Biofilm formation by both was greater than S. epidermidis ATCC 35984 at pH 4.5 but considerably less at pH 7.0.
Table 1.
Biofilm formation by microorganisms on glass in different pH and temperature.
| Microorganisms | pH 4.5 | pH 7.0 | ||
|---|---|---|---|---|
| 35°C | 40°C | 35°C | 40°C | |
| K. pneumoniae subsp. pneumoniae (pineapple isolate) | +* | ++ | + | ++ |
| K. pneumoniae subsp. pneumoniae (clinical isolate) | + | ++ | + | ++ |
| S. epidermidis ATCC 35984 | 0 | 0 | ++ | +++ |
*Absent (0); weak (+); moderate (++); or strong (+++), Stepanović et al., 2000 [13].
3.2. Capsule and Adhesion Fimbriae Formation
K. pneumoniae subsp. pneumoniae isolated from pineapple fruit and the clinical isolate were both able to form capsules as shown by the Congo red test as was the positive control S. epidermidis ATCC 35984. However, neither of the K. pneumoniae subsp. pneumoniae isolates was able to produce adhesion fibers (curli), in contrast to S. epidermidis ATCC 35984.
3.3. Hydrophobicity
Both K. pneumoniae subsp. pneumoniae isolates demonstrated surface hydrophobicity in aggregation tests in different concentrations of ammonium sulfate, aggregating in concentrations >3.0 M. This was higher than the positive control, S. epidermidis ATCC 35984, which aggregated in concentrations >2.0 M.
3.4. Atomic Force Microscopy (AFM) and High-Vacuum Scanning Electron Microscopy (SEM)
In situ biofilm monitoring by high-vacuum scanning electron (Figures 1(a) and 1(b)) and atomic force microscopy (Figures 1(c) and 1(d)) showed that K. pneumoniae subsp. pneumoniae isolated from pineapple was able to form a biofilm on the surface of glass (Figures 1(a) and 1(c)) and polyester (Figures 1(b) and 1(d)).
Figure 1.
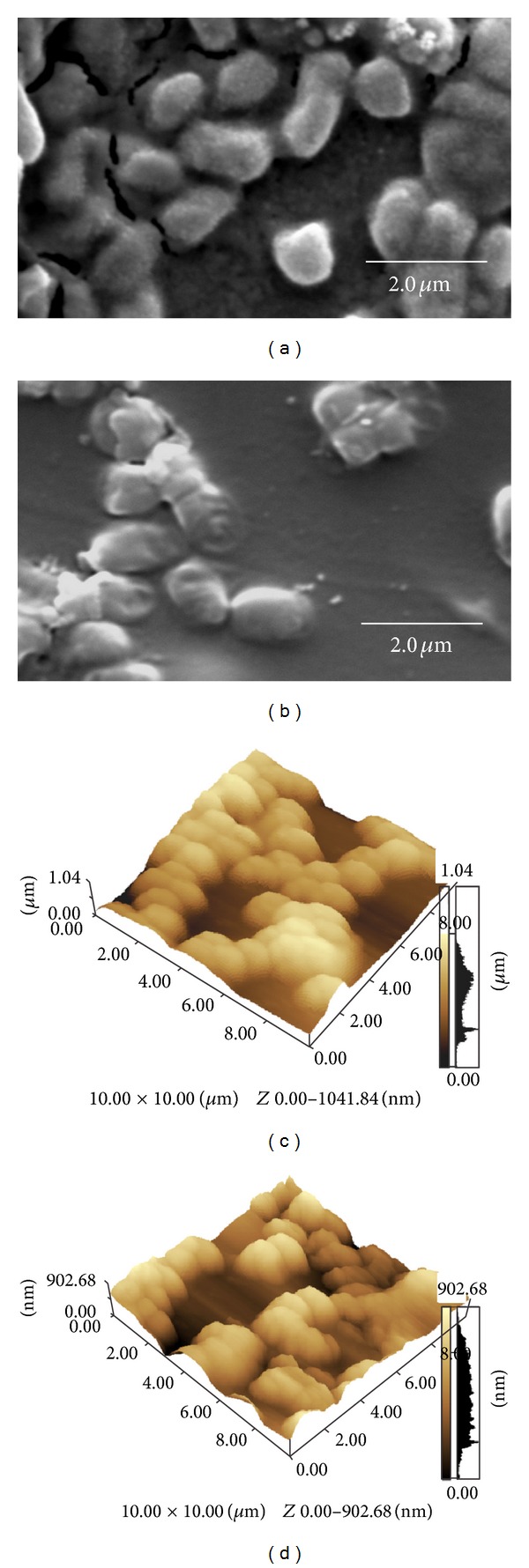
High-vacuum scanning electron micrographs and atomic force microscopy of K. pneumonia subsp. pneumoniae. SEM ((a) and (b)) and AFM in 3D ((c) and (d)) micrographs showing cell aggregates and biofilm on glass ((a) and (c)) and polyester ((b) and (d)).
In AFM plan view, EPS was clearly visible as cloudy areas around the cells (Figures 2(a) and 2(b)). Topographic profiles indicated that biofilm height on glass and polyester was similar (Figures 1(c), 1(d) and Figure 2); the average height of biofilm on polyester was 85.21% of the average obtained on glass. The profile of cells on glass (Figure 2(c)) was smoother whilst divisions between cells were more evident on polyester (Figure 2(d)).
Figure 2.
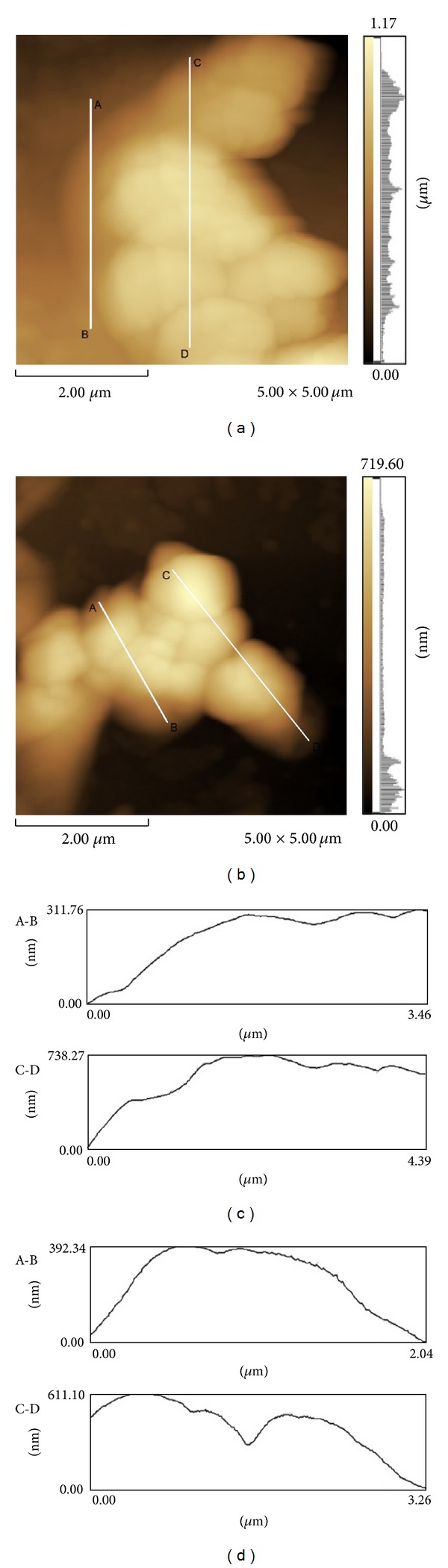
Atomic force microscopy of K. pneumoniae subsp. pneumoniae. Topographic profile of cell aggregates and biofilm on glass ((a) and (c)) and polyester ((b) and (d)). Plan view ((a) and (b)). Cell aggregate topographical profile along the lines A-B and C-D ((c) and (d)).
4. Discussion
Tests performed on K. pneumoniae subsp. pneumoniae isolated from both pineapple and human tissue, for adhesion to polystyrene and glass and comparative colony diameter, showed that these isolates are able to form biofilms. However, results suggested a lower level of biofilm formation compared to the positive control S. epidermidis ATCC 35984, and Congo red tests indicated that the K. pneumoniae subsp. pneumoniae isolates did not form adhesion fibers (curli). In hydrophobicity tests, the isolates were hydrophilic compared to S. epidermidis ATCC 35984. Together these results explain the relatively low adhesion to polystyrene microtiter plates [18, 22].
We found that in every respect the biofilm and surface characteristics of the two K. pneumoniae subsp. pneumoniae isolates were identical despite their very different origins. Both isolates of Klebsiella formed a consistent biofilm in both acidic (pH 4.5) and neutral (pH 7.0) environments whilst S. epidermidis ATCC 35984 only produced biofilm at pH 7.0. In a phytopathological context, biofilms may help bacteria survive in the highly acidic pineapple environment as the nature of the gel-like structure prevents rapid diffusion of ions and allows considerable pH gradients to develop within the matrix [23]. In contrast, it has been suggested that alkaline environments are inhibitory to K. pneumoniae so that the toxicity of various bioactive glasses to this species may be partly due to the release of ions in aqueous solutions, and thus an increase in pH [24].
Acidity, temperature, and ion concentration have all been shown to influence biofilm formation by microorganisms in different conditions [25]. The isolates tested here were also affected by temperature. Both Klebsiella isolates and S. epidermidis ATCC 35984 formed a more consistent biofilm at 40 than at 35°C. In a similar context, the plant rhizobacteria Pseudomonas putida has also been reported to tolerate temperatures of 40°C by enhanced biofilm production [26].
The survival of pathogenic bacteria outside of the human host is of crucial clinical importance, and an association with plants and plant based products is becoming more apparent. For example, plant polysaccharides, increasingly used in food packaging, have been shown to be adhesion sites for the pathogens Escherichia coli and Staphylococcus aureus [27]. Additionally, it is almost impossible to prevent the contact of clinically pathogenic bacteria with food crops. They can be applied with contaminated manure or irrigation water or via wild animals [28]. If clinically pathogenic bacteria can survive on food plants they can reenter the human food chain and cause disease [29, 30]. Bacteria on the plant surface are exposed to UV light, desiccation, and, conversely, high flow from rain and irrigation and so tend not to maintain a presence for long [31, 32]. However, if they can enter plant tissues via injuries, survival is greatly improved with contamination lasting at least 3 weeks in lettuce and chives, for example [32].
Overall, our results showed a similar profile of biofilm and adhesion characteristics between the two K. pneumoniae subsp. pneumoniae isolates despite the differences in original substrates. This suggests a flexibility in choice of mechanisms within isolates as both adjusted equally to glass and plastic after their respective tissues, which may account for the success of this species in humans and plants and for the health problems that result.
Acknowledgments
The authors thank Professor Ana Paula Nunes (Universidade Federal do Espírito Santo (UFES)) for providing the S. epidermidis strains and Professor Paulo Moscon (UFES) for his invaluable help with the atomic force and electron microscopy. They also acknowledge the financial support provided by the Brazilian agencies CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FINEP (Financiadora de Estudos e Projetos), and FAPES (Fundação de Amparo à Pesquisa do Espírito Santo).
References
- 1.Brock TD, Madigan MT, Martinko JM, Parker J. Biology of Microorganisms. Englewood Cliffs, NJ, USA: Prentice Hall; 1994. [Google Scholar]
- 2.Morris E, Monier J. The ecological significance of biofilm formation by plant-associated bacteria. Annual Review of Phytopathology. 2003;41:429–453. doi: 10.1146/annurev.phyto.41.022103.134521. [DOI] [PubMed] [Google Scholar]
- 3.Ramey BE, Koutsoudis M, von Bodman SB, Fuqua C. Biofilm formation in plant-microbe associations. Current Opinion in Microbiology. 2004;7(6):602–609. doi: 10.1016/j.mib.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. The lancet. 2001;358(9276):135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 5.Erasmus LD. Friedlander bacillus infection of the lung. Quarterly Journal Of Medicine. 1956;25(4):507–521. [PubMed] [Google Scholar]
- 6.Wei H, Shen J, Pang X, et al. Fatal infection in human flora-associated piglets caused by the opportunistic pathogen Klebsiella pneumoniae from an apparently healthy human donor. Journal of Veterinary Medical Science. 2008;70:715–717. doi: 10.1292/jvms.70.715. [DOI] [PubMed] [Google Scholar]
- 7.Lavizzari T, Breccia M, Bover-Cid S, Vidal-Carou MC, Veciana-Nogués MT. Histamine, cadaverine, and putrescine produced in vitro by enterobacteriaceae and pseudomonadaceae isolated from spinach. Journal of Food Protection. 2010;73:385–389. doi: 10.4315/0362-028x-73.2.385. [DOI] [PubMed] [Google Scholar]
- 8.Zhang GX, Peng GX, Wang ET, et al. Diverse endophytic nitrogen-fixing bacteria isolated from wild rice Oryza rufipogon and description of Phytobacter diazotrophicus gen. nov. sp. nov. Archive Microbiology. 2007;189(5):431–439. doi: 10.1007/s00203-007-0333-7. [DOI] [PubMed] [Google Scholar]
- 9.Korres AMN, Ventura JA, Fernandes PMB. First report of bacteria and yeasts associated with pineapple fruit collapse in espírito santo state, Brazil. Plant Disease. 2010;54(12):p. 1509. doi: 10.1094/PDIS-04-10-0276. [DOI] [PubMed] [Google Scholar]
- 10.Ohgaki N. Bacterial biofilm in chronic airway infection. Kansenshogaku Zasshi. 1994;68(1):138–151. doi: 10.11150/kansenshogakuzasshi1970.68.138. [DOI] [PubMed] [Google Scholar]
- 11.Neder RN. Microbiologia: Manual De LaboratóRio. São Paulo, Brazil: Nobel; 1992. [Google Scholar]
- 12.Nubel U, Engelen B, Felske A, et al. Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. Journal of Bacteriology. 1996;178(19):5636–5643. doi: 10.1128/jb.178.19.5636-5643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stepanović S, Vuković D, Dakić I, Savić B, Švabić-Vlahović M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. Journal of Microbiological Methods. 2000;40(2):175–179. doi: 10.1016/s0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 14.O'Toole GAO, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Molecular Microbiology. 1998;28(3):449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 15.Di Martino P, Cafferini N, Joly B, Darfeuille-Michaud A. Klebsiella pneumoniae type 3 pili facilitate adherence and biofilm formation on abiotic surfaces. Research Microbiology. 2003;154(1):9–16. doi: 10.1016/s0923-2508(02)00004-9. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds TB, Fink GR. Baker's yeast, a model for fungal biofilm formation. Science. 2001;291(5505):878–881. doi: 10.1126/science.291.5505.878. [DOI] [PubMed] [Google Scholar]
- 17.Seeley JR, Vandemark PJ, Lee JJ. Microbes in Action: A Laboratory Manual Of Microbiology. New York, NY, USA: W. H. Freeman; 1991. [Google Scholar]
- 18.Rivas L, Dykes GA, Fegan N. A comparative study of biofilm formation by Shiga toxigenic Escherichia coli using epifluorescence microscopy on stainless steel and microtitre plate method. Journal of Microbiological Methods. 2007;69(1):44–51. doi: 10.1016/j.mimet.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Sherman PM, Houston WL, Boedeker EC. Functional heterogeneity of intestinal Escherichia coli strains expressing type 1 somatic pili (fimbriae): assessment of bacterial adherence to intestinal membranes and surface hydrophobicity. Infection Immunity. 1985;49(3):797–804. doi: 10.1128/iai.49.3.797-804.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyde FW, Alberg M, Smith K. Comparison of fluorinated polymers against stainless steel, glass and polypropylene in microbial biofilm adherence and removal. Journal of Industrial Microbiology and Biotechnology. 1997;19(2):142–149. doi: 10.1038/sj.jim.2900448. [DOI] [PubMed] [Google Scholar]
- 21.Shane WW. Multistat: a multiple range and ANOVA statistics program. ver. 3. 0. OSU, Ohio, USA, 1989.
- 22.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nature Reviews. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 23.Vroom JM, De Grauw KJ, Gerritsen HC, et al. Depth penetration and detection of pH gradients in biofilms by two-photon excitation microscopy. Applied and Environmental Microbiology. 1999;65(8):3502–3511. doi: 10.1128/aem.65.8.3502-3511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munukka E, Lepparanta O, Korkeamaki M, et al. Bactericidal effects of bioactive glasses on clinically important aerobic bacteria. Journal of Materials Science. 2008;19:27–32. doi: 10.1007/s10856-007-3143-1. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher M. Attachment of Pseudomonas fluorescens to glass and influence of electrolytes on bacterium-substratum separation distance. Journal of Bacteriol. 1988;170(5):2027–2030. doi: 10.1128/jb.170.5.2027-2030.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava S, Yadav A, Seem K, Mishra S, Chaudhary V, Nautiyal CS. Effect of high temperature on Pseudomonas putida NBRI0987 biofilm formation and expression of stress sigma factor RpoS. Current Microbiology. 2008;56(5):453–457. doi: 10.1007/s00284-008-9105-0. [DOI] [PubMed] [Google Scholar]
- 27.Prokopovich P, Perni S. An investigation of microbial adhesion to natural and synthetic polysaccharide-based films and its relationship with the surface energy components. Journal of Materials Science Materials in Medicine. 2009;20(1):195–202. doi: 10.1007/s10856-008-3555-6. [DOI] [PubMed] [Google Scholar]
- 28.Brackett RE. Incidence, contributing factors, and control of bacterial pathogens in produce. Postharvest Biology Technology. 1999;15(3):305–311. [Google Scholar]
- 29.Center for Disease Control Prevention. Outbreak of Salmonella serotype Saintpaul infections associated with multiple raw produce items. Morbidity and Mortality Weekly Report. 2008;57:929–934. [PubMed] [Google Scholar]
- 30.Grant J, Wendelboe AM, Wendel A, et al. Spinach-associated Escherichia coli O157:H7 outbreak, Utah and New Mexico, 2006. Emerging Infectious Diseases. 2008;14:1633–1636. doi: 10.3201/eid1410.071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell RG, Bole JB. Elimination of fecal coliform bacteria from reed canary grass irrigated with municipal sewage lagoon effluent. Journal of Environmental Quality. 1976;5(4):417–418. [Google Scholar]
- 32.Harapus D, Premier R, Tomkins B, Franz P, Ajlouni S. Persistence of Escherichia coli on injured vegetable plants. International Journal of Food Microbiology. 2010;138(3):232–237. doi: 10.1016/j.ijfoodmicro.2010.01.022. [DOI] [PubMed] [Google Scholar]


