Summary
Secondary lymphoid organs function to increase the efficiency of interactions between rare, antigen-specific lymphocytes and antigen presenting cells, concentrating antigen and lymphocytes in a supportive environment that facilitates the initiation of an adaptive immune response. Homeostatic lymphoid tissue organogenesis proceeds via exquisitely controlled spatiotemporal interactions between haematopoietic lymphoid tissue inducer populations and multiple subsets of non-haematopoietic stromal cells. However, it is becoming clear that in a range of inflammatory contexts, ectopic or tertiary lymphoid tissues can develop inappropriately under pathological stress. Here we summarize the role of stromal cells in the development of homeostatic lymphoid tissue, and assess emerging evidence that suggests a critical role for stromal involvement in the tertiary lymphoid tissue development associated with chronic infections and inflammation.
Keywords: cytokines, inflammation, mucosal associated lymphoid tissue, spleen/lymph nodes
Introduction
Secondary lymphoid organs (SLOs) function to increase the efficiency of interactions between rare, antigen-specific lymphocytes and antigen-presenting cells, concentrating antigen and lymphocytes in a supportive environment that facilitates the initiation of an adaptive immune response. Homeostatic lymphoid tissue organogenesis proceeds via exquisitely controlled spatiotemporal interactions between haematopoietic lymphoid tissue inducer populations and multiple subsets of non-haematopoietic stromal cells. However, it is becoming clear that in a range of inflammatory contexts, ectopic or tertiary lymphoid organs (TLOs) can develop inappropriately under pathological stress. Here we summarize the role of stromal cells in the development of homeostatic lymphoid tissue, and assess emerging evidence that suggests a critical role for stromal involvement in the TLO development associated with chronic infections and inflammation.
Stromal cell–haematopoietic cell interactions govern homeostatic lymphoid tissue development
Peripheral lymphoid tissue generation occurs sequentially in the developing mouse embryo from embryonic days E11 to E16.1–2 Lymph node (LN) development is thought to be initiated by the production of retinoic acid, which acts on mesenchymal stromal cells at predetermined anatomical sites to induce expression of the chemokine CXCL133 (Fig. 1). It has been proposed that outgrowing nerves are responsible for the production of retinoic acid in development, as they express RALDH2, an enzyme required for the conversion of retinal to retinoic acid.3 A CXCL13 gradient attracts CXCR5+ haematopoietic cells to the LN anlagen; the first cells to arrive are lymphoid tissue-inducer cells (LTis),4 derived from fetal liver progenitor cells that can also give rise to B cells, T cells, natural killer cells and dendritic cells.5 The LTis express lymphotoxin (LT) α1β2 (LTα1β2), a cytokine that is the major determinant of SLO development.6,7 LTα1β2 is a heterotrimeric complex, comprising membrane-bound LTβ and soluble LTα. Together these bind to the lymphotoxin-β receptor (LTβR) that is predominantly expressed by mesenchymal stromal cells.
Figure 1.
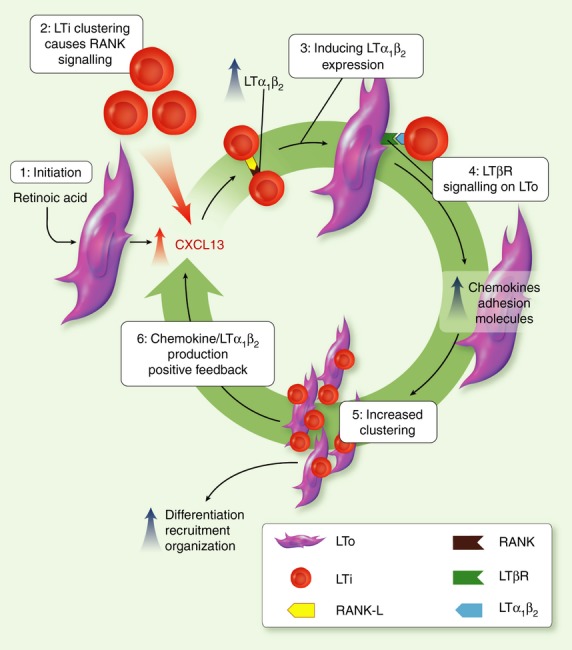
Sequential stromal cell–haematopoietic cell interactions govern secondary lymphoid organ (SLO) development (adapted from ref. 2). Lymph node (LN) development is initiated by retinoic acid acting on a population of stromal cells(s) that induces CXCL13 expression at predetermined anatomical sites (1). CXCR5+ lymphoid tissue-inducer (LTi) cells migrate on this CXCL13 gradient and cluster, with subsequent receptor activator of nuclear factor-κB (RANK)/ RANK ligand (RANKL) signalling inducing expression of lymphotoxin (LT) by LTIs (2 + 3). The LT signals through the LTβ receptor on stromal cells causing them to differentiate into lymphoid tissue organizers (LTos) (4). This activation drives the expression of chemokines and adhesion molecules that lead to the further attraction, clustering and local retention of LTis (and other lymphocytes: 5), production of more LT and the generation of a positive feedback loop that increases clustering and facilitates LN expansion (6).
Interestingly, the first CXCR5+ LTis recruited to the site of LN formation express receptor activator of nuclear factor-κB ligand (RANKL), rather than LTα1β2.9–10 Indeed the initial clustering of LTis can occur without LTα1β2 expression by LTis9 or LTβR expression on mesenchymal stromal cells.11 Therefore, initiation of LT expression by LTis is probably through clustering and subsequent RANK/RANKL signalling. The LTα1β2 then signals via LTβR to drive mesenchymal stromal cells to differentiate into lymphoid tissue organizer cells (LTos),9 accompanied by the up-regulation of chemokine (e.g. CXCL13, CCL19 and CCL21) and adhesion molecule (e.g. vascular cell adhesion molecule-1, intercellular adhesion molecule-1, mucosal addressin cell adhesion molecule-1)12 expression in the LN anlagen. Chemokines, as well as the up-regulated expression of RANKL and interleukin-7 (IL-7) by LTos,9–10 induce the recruitment and survival of further cells to the expanding LN anlagen.13 The arrival of more LTα1β2-expressing cells, which includes few LTis14 but after birth is dominated by lymphocytes (both T and B cells),15–16 creates a positive feedback loop (Fig. 1), further increasing signalling through the LTβR and the subsequent expression of LTo-derived factors. Using conditional ablation of the Ltbr gene exclusively in VE-Cadherin+ endothelial stromal cells, Onder et al.17 recently revealed that the development of multiple peripheral LNs required LT signalling specifically into this LTβR+ stromal compartment. Interestingly, not all LNs required endothelial sensitivity to LTα1β2, as the mesenteric LNs of the intestine were fully intact in these mice, hinting at a requirement for distinct LTβR+ stromal cell populations in the development of anatomically disparate peripheral LNs in vivo. Other homeostatic SLOs develop in a fundamentally similar way to the LN with only minor differences between tissues. For instance in the Peyer's patches of the small intestine, although ligands of the receptor tyrosine kinase RET acting on a distinct population of CD45+ IL-7Rα− CD11c+ cells contributes to stromal activation in the developing anlagen,18 LTis and LTα1β2 are still important in this developmental process,4 although it is not clear if LTα1β2 expression is induced by RANK as in early LN development. However, the earliest steps in homeostatic intestinal SLO development are still under intense investigation.19
Lymphoid tissue organizers differentiate into the various non-haematopoietic stromal subtypes present in the adult SLO via LTβR signalling,20 although the ontogeny and lineage relationships of the various stromal cell subsets within the LN is still under investigation.21–22 Mesenchyme-derived stromal cells can be divided into several subsets including follicular dendritic cells (FDCs), marginal reticular cells and populations of fibroblastic reticular cells (FRCs). Lymph node stromal endothelial cells can be divided into blood endothelial cells and lymphatic endothelial cells,23 and all SLOs contain high endothelial venules composed of endothelial cells with distinct morphology and phenotype. Four CD45− stromal subsets can therefore be identified by a dual CD31 (PECAM-1) and Podoplanin (gp38) stain.23 Identification of further subsets can be achieved using a range of different surface markers (Table 1). Interestingly, the CD31− Podoplanin− double-negative population (previously of unknown function) has recently been shown to contain a novel subset of fibroblastic contractile pericytes, related to both smooth muscle cells and FRCs.24 However, this population does not account for all the stromal cells lying in the double-negative gate, and suggests further stromal subset heterogeneity within lymphoid tissue.
Secondary lymphoid organ (SLO) stromal cell subsets
| Stromal subset | Location in SLO | Selected surface markers | Function |
|---|---|---|---|
| Podoplanin (gp38) and/or CD31 positive | |||
| Fibroblastic reticular cells (gp38+ CD31−) | T-cell zone | CCL19, CCL21, IL-7, MHC Class I, laminin, desmin, fibrillin, fibronectin, collagen I, II and IV, ICAM-1, VCAM-1 | Production of reticular network and conduit system to increase antigen-presenting cell–T-cell interactions and T-cell homeostasis |
| Lymphatic endothelial cells (gp38+ CD31+) | Afferent/efferent lymphatics | ER-TR7 antigen, PROX1, LYVE1, CCL21, S1P, VE-cadherin, ICAM-1, VCAM-1 | Transport of lymph and antigen/cells it contains |
| Blood endothelial cells (gp38− CD31+) | High endothelial venules | Peripheral lymph node adressin (peripheral LN), MAdCAM-1 (mucosal LN) CCL21, CD34, JAM, ESAM1, VE-cadherin | Entry of blood cells to SLO |
| Follicular dendritic cells (gp38+ CD31−) | B-cell follicles | MAdCAM-1, CXCL12, CXCL13, BAFF, Complement receptors, including CD35–CR1) laminin, desmin, αSMA | Capture of antigen, B-cell presentation and chemokine production |
| Marginal reticular cells (gp38+ CD31−) | Subcapsular sinus | ER-TR7 antigen, ICAM1, VCAM1, MAdCAM1, RANKL, CXCL13 laminin, desmin | Conduits, chemokines and structure |
| Double negative (gp38− CD31−) | |||
| Extrathymic AIRE-expressing cells | T-cell zone | EpCAM, MHC Class II, MHC Class I | Peripheral tolerance |
| Fibroblastic contractile pericytes | Medulla and cortex | ITGA7, Unknown | Unknown |
| Bulk population | Unknown | Unknown | 5–10% of stromal population, unknown function |
Structural and functional features of SLO stromal cells
Once SLOs are formed, a major functional role of stromal cells is undoubtedly the maintenance of SLO structural integrity, and many subsets secrete large amounts of extracellular matrix (Table 1). The FRCs form collagen-rich reticular fibres, which they then surround to form conduits for afferent lymph.25 These function by allowing for the transport of low-molecular-weight antigen and so facilitate antigen presentation by antigen-presenting cells in the T-cell zone.26 Similar conduits have been found in the subcapsular sinus of the lymph node that are specialized for transport of antigen to the B-cell zone27 and may be formed by marginal reticular cells that are present at this distinct location.28
Stromal cells also play a vital role in lymphocyte trafficking by maintaining a functional separation of B-cell and T-cell zones via specific chemokine expression. The FRCs in the T-cell zone express CCL19 and CCL21, which act to recruit CCR7+ naive T cells.29 The importance of the stromal chemokine gradient induced is shown by aberrant SLO structure and T-cell distribution in the plt/plt mutant mouse,30 which lacks CCL19 and CCL21 expression. In contrast, FDCs and marginal reticular cells express CXCL13,31–32 which acts on CXCR5 to attract B cells to the B-cell zone of SLOs. As naive T cells and B cells do not express CXCR5 and CCR7, respectively (except for T-follicular helper cells, which express enough CXCR5 to enter the B-cell zone33), the stromal chemokine gradients restrict lymphocytes to their respective zones during steady-state conditions. Moreover, stromal chemokine production can even play a role in the further differentiation of lymphocytes. Recently, a key role for stromal cells in the functional activation of T helper cells in the LN has been revealed, whereby stromal cell production of CXCL9 optimizes the polarization of CXCR3+ T cells toward an interferon-γ+ T helper type 1 phenotype in vivo.34 Multiple stromal subsets also provide vital survival signals to peripheral lymphocytes, e.g. FRC and lymphatic endothelial cell-derived IL-7 for T cells23–35 and FDC-derived BAFF for B cells.36
Stromal cells control the influx and retention of naive lymphocytes to SLOs via chemokines, yet they may also control the egress of lymphocytes via sphingosine-1-phosphate (S1P) signalling.37 Levels of S1P are much lower in SLOs than in the circulation because of increased SLO expression of S1P-lyase.38 Cyclic expression of the S1P receptor on lymphocytes competes with CCR7 or CXCR5 signalling to determine lymphocyte retention versus egress.39 It is highly plausible that SLO stromal cells constitutively express S1P-lyase to maintain this S1P gradient. Furthermore, differential stromal subset expression of oxysterol determines B-cell positioning within lymphoid tissue,40 adding a further level of complexity to the regulation of lymphocyte localization by stromal cells within SLOs.
Stromal cells and SLO plasticity
During inflammation or infection, SLO stromal networks have a degree of plasticity. For example T-cell and B-cell networks grow and remodel41–42 accompanied by changes to homeostatic chemokine expression43 and lymphatics,44,45 enabling lymphocyte motility. Data have revealed a key role for IL-7-expressing stromal cells in the infection-induced remodelling of murine LN, with lymphatic endothelial cells found to be the major producers of IL-7 using an in vivo IL-7 fate-mapping system and the staining of human LN sections.35 Importantly, the in vivo ablation of IL-7-expressing stromal cells abolished infection-driven changes in LN architecture, highlighting the crucial role that these cells play in both the development and subsequent remodelling of the LN. Interestingly FRCs are capable of directly modifying LN endothelial cell growth and expansion,45 suggesting that both stromal–stromal and stromal–leucocyte interactions regulate the processes underlying the formation and remodelling of lymphoid tissues.
In addition to the developmentally imprinted homeostatic tissues discussed above, ‘intermediate’ lymphoid tissues exist that can be considered as somewhere between predetermined and inflammatory lymphoid tissues. Isolated lymphoid follicles (ILFs) are primarily B-cell follicle-containing lymphoid structures that form at predetermined sites along the length of the mesenteric wall of the small intestine.47 The ILFs develop from cryptopatches, clusters of LTi cells seen in both mouse48 and human49 intestine. As with the LN, LTi–stromal interactions are vital in ILF formation50 mediated via LTβR signalling,47–51 which is aided by the recruitment of naive LTα1β2-expressing B cells.52 Recent work has also revealed that the cytokine IL-22 may also be involved in the maintenance of ILFs during bacterial-induced inflammation.53 Mice kept in a specific-pathogen-free environment develop few and small ILFs,51 whereas infection with Salmonella enterica greatly enlarges individual ILFs, but importantly does not increase their overall number.54 The ILFs therefore represent a partially programmed lymphoid tissue lying between ectopic and predetermined. Their anatomical location is predetermined and their developmental processes show many similarities to LN expansion, yet their formation is dependent upon environmental signals, namely microbial stimulation.54–55
Stromal cells: a crucial role in ectopic TLO development?
Truly distinct from developmentally encoded lymphoid tissue are ectopic or TLOs, also known as tertiary lymphoid tissue. The TLOs spontaneously develop as a result of chronic inflammation,56 normally due to chronic infection, autoimmunity or tumours. Such tissues can rapidly form stable structures during inflammation, and yet equally as easily regress, as seen in the dynamic development of TLOs during chronic Helicobacter pylori infection.57 The fundamentals underpinning SLO development also lie at the heart of TLO development: inflammatory cytokine expression (LT/tumour necrosis factor-α); stromal activation and chemokine production; and high endothelial venule development.58–59 As seen in transplantation studies,60–61 activated stromal cells alone are capable of initiating TLO formation in some instances, indicating their overriding capacity to contribute to TLO development. Nevertheless, the precise signals leading to stromal activation during TLO development in vivo are still unclear. The majority of mechanistic data on the development of TLOs are derived from transgenic mice expressing molecules in ectopic sites. Although these are narrow models that lack the complexity that undoubtedly underpins in vivo TLO generation, they do offer a glimpse into TLO development that would otherwise be hard to observe. Table 2 highlights animal models of TLO development that use either LTβR signalling, homeostatic chemokine or non-homeostatic cytokine transgenic expression.
Transgenic ectopic expression models used to study tertiary lymphoid organ (TLO) development
| Model | TLO characteristics | Reference |
|---|---|---|
| LTα | Lymphotoxin α (LTα) expressed under control of the rat insulin promoter (RIP-LTα) developed functional secondary lymphoid organ (SLO)-like tissue organized by stromal cells via CXCL13 and CCL21, in both kidney and pancreas | 120 |
| LTα/LTβ | Double LTα and LTβ transgenic under the control of the rat insulin promoter mouse saw more developed TLO in the kidney and pancreas than a single RIP-LTα transgenic model | 6 |
| LIGHT | RIP-LIGHT transgenic mice on a NOD (diabetic) background rapidly develop ectopic pancreatic TLOs via LTβR and HVEM signalling | 121 |
| CXCL13 | RIP-BLC transgenic mice developed very well defined TLO in the pancreas with clear T-cell and B-cell zones, stromal cells expressing CCL21 and high endothelial venules | 122 |
| CCL21 | RIP-SLC and TG-SLC (CCL21 expression under the thyroglobulin promoter) transgenic mice did produce TLO in the pancreas and thyroid, respectively, but to a less developed degree, with mainly haematopoietic infiltrates. CCL21 less important in stromal network development? But CCL21 is still vital in T-cell recruitment and extravasation, showing that at least some of the molecular interactions in TLO and SLOs are preserved | 123,124 |
| IL-5 | Constitutive interleukin-5 expression under the promoter of a lung-derived protein, CC10, causes the formation of inducible bronchial-associated lymphoid tissue (iBALT). However, interleukin-5 is an aggressive effector cytokine, therefore TLO formation is probably secondary to the observed lung pathology | 126 |
If TLO and SLO development is conceptually similar, what is the source of LTα1β2 in TLO development? One possibility is that TLOs are formed by LTis in much the same way as in SLOs, but there is conflicting evidence to support this hypothesis. Interleukin-7 (a key survival factor for LTis in developing SLOs) transgenic mice develop a large number of LNs and Peyer's patches, as well as the formation of organized TLOs after immunization with antigen, in a process that is dependent upon LTα1β2 and the LTi-associated transcription factor retinoic acid-related orphan receptor γt (RORγt).62 However, a CCL21 transgenic model of TLO development lacking LTis still develops TLOs,63 with CD3+ CD4+ T cells the first to arrive at the site of TLO development, indicating an LTi-independent mechanism that may be unique to TLOs. Formation of TLOs during inflammation of the intestine is able to occur in the absence of RORγt (and hence LTis),64–65 although with the recent identification of multiple innate lymphoid cell (ILC) populations, which express similar levels of LTα1β2 to their LTi cousins,66–67 the extent to which RORγt-independent ILCs can contribute to intestinal TLO generation requires further investigation.68 As B and T cells both express LTα1β2,69 are relatively much more abundant in chronically inflamed tissues than LTis (or other ILCs), and activated conventional lymphocytes are known to play a role in TLO generation in the skin,60 it is likely that B and T cells contribute significantly to TLO development during inflammation. In addition, there is emerging evidence for a potential role of Th17 cells in the development of TLOs during experimental autoimmune encephalomyelitis, a murine model of central nervous system inflammation.70–71 Nevertheless a definitive comparison between the TLO-inducing capacities of ILCs versus T and/or B cells in vivo has not yet been attempted.
The precise mechanisms leading to stromal activation and TLO generation in multiple tissue sites are not yet fully defined. This includes doubt as to whether tissue stromal cells simply convert to a ‘lymphoid-like’ phenotype during inflammation,72 or whether LTos in TLOs arise from distinct progenitors. The tools to begin assessing this second hypothesis have only recently been developed, with sophisticated genetic lineage tracing and ablation systems leading to the identification of a pro-fibrotic stromal cell population in murine skin that arises during inflammation from a fetal progenitor developmentally distinct from muscle and skin tissue cells.73 In addition, recent work has revealed that FDCs arise from perivascular platelet-derived growth factor receptor β+ stromal progenitors in lymphoid and non-lymphoid tissues, with this process occurring during chronic inflammation.74 Interestingly, the development of LN stromal cell subsets from adipocyte precursors has been recently reported.75 As chronic inflammation of the intestine is associated both with TLOs76 and substantial mesenteric fat deposits around the inflamed organ77 it is possible that inflamed adipose tissue may provide precursors that subsequently develop into TLO-associated stromal networks in the gut. The specific precursor(s) responsible for differentiating into the various stromal subsets remain elusive, but may well be tissue-specific and disease-specific. Fibroblast-like cells are a potential candidate; fibrocytes are capable of differentiating into FDCs and have been implicated in human inflammatory disease;78–81 fibroblasts themselves are capable of expressing adhesion molecules and producing homeostatic chemokines (so mimicking SLO stroma);82 and large numbers of intestinal fibroblast-like cells up-regulate Podoplanin expression during intestinal inflammation.72 Nevertheless, there is still much to be revealed about the specific stromal subsets and/or stromal alterations that underlie TLO generation during inflammation, including in the gut.83
As Table 3 shows, the structural make up of TLOs varies. Most TLOs will develop supportive and effective B-cell zones, sometimes capable of antigen-driven B-cell maturation, somatic hypermutation and class-switching.84 This can occur via FDC expression of activation-induced cytidine deaminase,85 with these processes accompanied by significant lymphangiogenesis86,87 and vascular remodelling.56 The level of T-cell zone development varies greatly; although the CCL21 expression often observed in TLOs would suggest that T-cell-zone-associated LTos may be present. Indeed the TLOs associated with rheumatoid arthritis appear to have distinct spatial segregation of T-cell and B-cell zones akin to that observed in SLOs, with this micro-anatomical localization governed by CXCL13 and CCL21 expression.85 Whether the corresponding LTo stromal subsets are present in these TLOs is not entirely clear.
Examples of ectopic tertiary lymphoid organ (TLO) formation in human autoimmune/chronic inflammatory diseases
| Disease | Prevalence (%) | Characteristics |
|---|---|---|
| Rheumatoid arthritis | 10–35 | Well-developed TLO appears in synovial membrane, separated functional areas, high endothelial venules (HEVs), stromal chemokines (CXCL13, CCL21) |
| Hashimoto's thyroiditis | 100 | Well developed TLO in thyroid, functional germinal centres with stromal support [CCL21, CXCL12, CXCL13, peripheral lymph node addressin (PNAd)] |
| Sjögren's syndrome | 17 | TLO appears in salivary glands, well-developed germinal centres with follicular dendritic cell support and chemokines (CXCL13, CCL21, CXCL12, PNAd) |
| Multiple sclerosis | 30–40 | Seen in brains of patients with secondary progressive multiple sclerosis. Mainly less developed, with germinal centres, CCL19 and CCL21 as well as lymphangiogenesis |
| Crohn's disease | – | Development of both T-cell and B-cell compartments with HEVs in inflamed bowel. Could potentially be hyperplasia of gut mucosa-associated lymphoid tissue, undetermined |
| Atherosclerosis | 32 | Develops in arteries. Highly developed, conduits, separated into functional T-cell and B-cell areas. Evidence to show that smooth muscle cells differentiate into various stromal-like subsets and produce stromal chemokines (CXCL13, CCL19, CXCL16). |
| Helicobacter pylori infection | 27–100 | Gastric mucosa-associated lymphoid tissue forms in stomach, CXCL13 expression as well as the formation of HEVs expressing PNAd and mucosal addressin cell adhesion molecule 1 |
| Graft rejection | – | TLO seen in human kidney and heart transplants, with the development of germinal centres and lymphangiogenesis |
Beneficial and detrimental properties of TLOs to the host
The importance of SLO stromal cells in microbial defence is well documented. During inflammation, FRCs up-regulate anti-microbial genes24 and the disruption of stromal networks (via viral infection) leaves the host susceptible to secondary infection,43 an immunodeficiency that is reversed by the restoration of stromal architecture via LT expression by LTis.89 Whether specific stromal populations in TLOs versus SLOs have a differential capacity to induce an antimicrobial state is not known. However, viral infection models hint at a major role for TLOs in the defence against pathogens. Well-developed inducible bronchial-associated lymphoid tissue (iBALT) is a form of TLO formed during acute influenza infection,90 via stromal chemokine expression91 in a process that is stabilized by myeloid cells.92 Other processes, including the expression of IL-17 by T cells, appear to contribute to iBALT generation in some experimental contexts,93 however, the absolute requirement for this cytokine in iBALT generation is unclear.94–95 Interestingly mice that lack SLOs, but retain iBALT, can withstand higher inoculations of virus90 and have a fully intact memory CD8+ T-cell compartment in the context of influenza infection.96 Hence TLOs can assume a host-protective role in some infectious contexts by providing a microenvironment that supports the local generation of a protective immune response. Further support for a role of TLOs in a protective response to infectious insult, comes from evidence that antigen persistence in itself is important for the maintenance of TLO structure during chronic infection. So the eradication of Helicobacter pylori antigen via antibiotics leads to drastic mucosa-associated lymphoid tissue regression,57 presumably because the TLO has performed its function.
Although it is clear that TLO formation can help to increase the efficiency of antigen presentation to lymphocytes for a protective immune response, TLOs can also initiate immune responses that may be responsible for inducing or exacerbating an autoimmune response. Although there is no definitive causal link between TLO presence and disease, in certain autoimmune diseases such as multiple sclerosis (or the murine model experimental autoimmune encephalomyelitis), TLO presence correlates with increased disease severity.97–98 TLOs in the pancreas skew B cells toward an autoreactive phenotype during diabetes99 and a recently described model of murine salivary gland pathology is characterized by TLO formation, ectopic stromal chemokine expression and GL7+ germinal centre development that initiates autoimmunity by breaking self-tolerance to antigen.59 Similarly, the TLOs in salivary glands of patients suffering from Sjögren syndrome sustain high levels of the B-cell survival factor BAFF,100 potentially leading to the expansion of self-reactive B-cells escaping peripheral negative selection, that can then promote pathology.
Tertiary lymphoid organs also form in diseases that may be inflammatory but are (at least partially) antigen independent. For example; TLO formation and aberrant stromal chemokine expression in the terminal ileum of colitic TNFΔRE mice, which lack a negative regulator of tumour necrosis factor-α signalling and are therefore predisposed to joint and gut inflammation, drives the accumulation of effector T-cell populations and exacerbates disease;101 and multiple stromal-derived factors contribute to TLO generation and the perpetuation of inflammation during rheumatoid arthritis.82 The TLOs can also develop during atherosclerosis, and intriguingly the development of these structures coincides with the attraction/retention of both effector and regulatory T-cell populations in the artery, highlighting the potential for TLOs to simultaneously localize potentially damaging and protective immune cell types to the same tissue site.102
TLO stromal cells as therapeutic targets in inflammatory disease
The stromal cell networks of TLOs could be a future therapeutic target for (auto)immune disease. First, blocking the stromal-led development or maintenance of TLOs is a possibility; this has been shown in pre-clinical models by inhibiting LTβR signalling via administration of a LTβR-immunoglobulin fusion protein.103 This strategy has reduced clinical symptoms in experimental autoimmune encephalomyelitis,104–105 decreased insulitis in NOD mice,106 reduced corneal pathology in a model of Sjögren syndrome,107 inhibited the development of intestinal pathology in models of inflammatory bowel disease108 and ameliorated pathology in collagen-induced arthritis.109 However, efficacy data for this approach in humans are currently lacking. Beyond the targeting of lymphotoxin, recent pre-clinical data have revealed that biological CXCL13 blockade can disrupt splenic germinal centre structures after immunization, and ameliorate pathology during collagen-induced arthritis.110 However, administration of a therapeutic anti-CXCL13 monoclonal antibody in a distinct model of inflammation had no impact on the structure of established ectopic follicles (e.g. in salivary glands), presumably because of functional redundancy in pathways downstream of this stromal chemokine. In some inflammatory contexts adjunctive blockade of multiple stromal pathways may therefore be required to modulate TLO formation.
Stromal cells also appear to be naturally immunosuppressive. As well as maintaining peripheral tolerance via tissue-specific antigen expression,111 in SLOs they have been shown to directly suppress T-cell proliferation via nitric oxide production112 and regulate CD8+ T-cell function via PD-L1 expression during viral infection.113 In addition it appears that stromal cells of multiple organs are naturally predisposed to the generation of immunoregulatory myeloid cell populations.114 Therefore techniques could be developed to selectively activate or target stromal cells for the initiation of tolerogenic or regulatory responses, although much needs to be revealed regarding the differential mechanisms underlying inflammatory and tolerogenic stromal cell activation before this becomes a realistic option. Nevertheless, a similar conceptual approach is under intense investigation in the field of tumour therapeutics, where antibody–drug conjugates targeting tumour stroma for therapeutic manipulation have been developed and show promise in pre-clinical models.115
Conclusion and future directions
A critical outstanding question is to define the relative contribution of inflammatory lymphoid tissue (i.e. TLOs) versus homeostatic lymphoid tissue (i.e. SLOs) to inflammatory pathology. As is clear from this review, many of the developmental pathways between TLOs and SLOs are shared, particularly at the stromal cell and chemokine level, and so differentiating between them functionally will prove challenging. Interestingly it would appear that many features of immune responses generated from SLOs versus TLOs differ significantly, at least in the context of chronic allograft rejection,116 but the specific contributions of stromal cells to these differences are not known. Unravelling the ontogeny of stromal cell subsets in homeostatic and inflammatory lymphoid tissues is another important area for future research. Newly developed tools73–117 offer the promise of developmentally tracking and functionally manipulating the stromal cell networks that underlie lymphoid organogenesis, yet multiple outstanding questions remain as to the precise functions of these critical cell populations during homeostasis and inflammatory disease. Extending our knowledge of stromal cell biology will enable the development of novel therapeutic strategies for severely debilitating inflammatory conditions, treatments for which are currently lacking or sub-optimal.
Acknowledgments
We thank Dr Claire Pearson for critical review of this manuscript. No specific funds were received for the support of this work.
Disclosure
BMJO is in receipt of an Oxford - UCB Pharma Fellowship.
References
- Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin α/β complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal R, Mebius RE. Stromal cell–immune cell interactions. Annu Rev Immunol. 2011;29:23–43. doi: 10.1146/annurev-immunol-031210-101357. [DOI] [PubMed] [Google Scholar]
- van de Pavert SA, Olivier BJ, Goverse G, et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol. 2009;10:1193–9. doi: 10.1038/ni.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORγ(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Miyamoto T, Christensen J, Domen J, Cupedo T, Weissman IL, Akashi K. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+ CD4+ CD3− cells, as well as macrophages. J Immunol. 2001;166:6593–601. doi: 10.4049/jimmunol.166.11.6593. [DOI] [PubMed] [Google Scholar]
- Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT αβ directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197:1153–63. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks TA, Rouse BT, Kerley MK, et al. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–93. [PubMed] [Google Scholar]
- Boulianne B, Porfilio EA, Pikor N, Gommerman JL. Lymphotoxin-sensitive microenvironments in homeostasis and inflammation. Front Immunol. 2012;3:243. doi: 10.3389/fimmu.2012.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vondenhoff MF, Greuter M, Goverse G, et al. LTβR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J Immunol. 2009;182:5439–45. doi: 10.4049/jimmunol.0801165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Mebius RE, MacMicking JD, et al. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. 2000;192:1467–78. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénézech C, White A, Mader E, et al. Ontogeny of stromal organizer cells during lymph node development. J Immunol. 2010;184:4521–30. doi: 10.4049/jimmunol.0903113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–664. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- Schmutz S, Bosco N, Chappaz S, Boyman O, Acha-Orbea H, Ceredig R, Rolink AG, Finke D. Cutting edge: IL-7 regulates the peripheral pool of adult rorγ+ lymphoid tissue inducer cells. J Immunol. 2009;183:2217–21. doi: 10.4049/jimmunol.0802911. [DOI] [PubMed] [Google Scholar]
- Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptorα, and CCR7 ligands in lymph node development. J Exp Med. 2003;197:1191–8. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel KM, Ngo VN, Hyman PL, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–14. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Lund FE, Ngo VN, et al. Initiation of cellular organization in lymph nodes is regulated by non-B cell-derived signals and is not dependent on CXC chemokine ligand 13. J Immunol. 2004;173:4889–96. doi: 10.4049/jimmunol.173.8.4889. [DOI] [PubMed] [Google Scholar]
- Onder L, Danuser R, Scandella E, Firner S, Chai Q, Hehlgans T, Stein JV, Ludewig B. Endothelial cell-specific lymphotoxin-β receptor signaling is critical for lymph node high endothelial venule formation. J Exp Med. 2013;210:465–73. doi: 10.1084/jem.20121462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Coles MC, Foster KE, et al. Tyrosine kinase receptor RET is a key regulator of Peyer's patch organogenesis. Nature. 2007;446:547–51. doi: 10.1038/nature05597. [DOI] [PubMed] [Google Scholar]
- Ferreira M, Domingues RG, Veiga-Fernandes H. Stroma cell priming in enteric lymphoid organ morphogenesis. Front Immunol. 2012;3:219. doi: 10.3389/fimmu.2012.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin Immunol. 2008;20:26–42. doi: 10.1016/j.smim.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendolan A, Caamano JH. Mesenchymal cell differentiation during lymph node organogenesis. Front Immunol. 2012;3:381. doi: 10.3389/fimmu.2012.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning JJ, Mebius RE. Interdependence of stromal and immune cells for lymph node function. Trends Immunol. 2012;33:264–70. doi: 10.1016/j.it.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–65. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Fletcher AL, Astarita J, et al. Transcriptional profiling of stroma from inflamed resting lymph nodes defines immunological hallmarks. Nat Immunol. 2012;13:499–510. doi: 10.1038/ni.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajenoff M. Stromal cells control soluble material and cellular transport in lymph nodes. Front Immunol. 2012;3:304. doi: 10.3389/fimmu.2012.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–29. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, Mebius RE, von AU, Carroll MC. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–76. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakai T. Marginal reticular cells: a stromal subset directly descended from the lymphoid tissue organizer. Front Immunol. 2012;3:200. doi: 10.3389/fimmu.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajenoff M, Glaichenhaus N, Germain RN. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J Immunol. 2008;181:3947–54. doi: 10.4049/jimmunol.181.6.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Mori S, Yonekawa H, Nariuchi H, Matsuzawa A, Kakiuchi T. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood. 1998;91:2886–95. [PubMed] [Google Scholar]
- Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- Katakai T, Suto H, Sugai M, et al. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181:6189–200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–65. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- Groom JR, Richmond J, Murooka TT, et al. CXCR3 chemokine receptor–ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity. 2012;37:1091–103. doi: 10.1016/j.immuni.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder L, Narang P, Scandella E, et al. IL-7-producing stromal cells are critical for lymph node remodeling. Blood. 2012;120:4675–83. doi: 10.1182/blood-2012-03-416859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland RT, Schmidt MR, Thompson CB. BLyS and B cell homeostasis. Semin Immunol. 2006;18:318–26. doi: 10.1016/j.smim.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–9. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- Yi T, Wang X, Kelly LM, et al. Oxysterol gradient generation by lymphoid stromal cells guides activated B cell movement during humoral responses. Immunity. 2012;37:535–48. doi: 10.1016/j.immuni.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med. 2004;200:783–95. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Scandella E, Danuser R, et al. Global lymphoid tissue remodeling during a viral infection is orchestrated by a B cell-lymphotoxin-dependent pathway. Blood. 2010;115:4725–33. doi: 10.1182/blood-2009-10-250118. [DOI] [PubMed] [Google Scholar]
- Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, Matloubian M. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317:670–4. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- Soderberg KA, Payne GW, Sato A, Medzhitov R, Segal SS, Iwasaki A. Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc Natl Acad Sci U S A. 2005;102:16315–20. doi: 10.1073/pnas.0506190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyou S, Ekland EH, Carpenter AC, Tzeng TC, Tian S, Michaud M, Madri JA, Lu TT. Fibroblast-type reticular stromal cells regulate the lymph node vasculature. J Immunol. 2008;181:3887–96. doi: 10.4049/jimmunol.181.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton JE, Maroof A, Owens BM, et al. Inhibition of receptor tyrosine kinases restores immunocompetence and improves immune-dependent chemotherapy against experimental leishmaniasis in mice. J Clin Invest. 2010;120:1204–16. doi: 10.1172/JCI41281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Hiroi T, Nishiyama Y, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–59. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lügering A, Ross M, Sieker M, Heidemann J, Williams IR, Domschke W, Kucharzik T. CCR6 identifies lymphoid tissue inducer cells within cryptopatches. Clin Exp Immunol. 2010;160:440–9. doi: 10.1111/j.1365-2249.2010.04103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M, Suzuki K, Kitamura H, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–71. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin β receptor, and TNF receptor I function. J Immunol. 2003;170:5475–82. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- McDonald KG, McDonough JS, Newberry RD. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J Immunol. 2005;174:5720–8. doi: 10.4049/jimmunol.174.9.5720. [DOI] [PubMed] [Google Scholar]
- Ota N, Wong K, Valdez PA, Zheng Y, Crellin NK, Diehl L, Ouyang W. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat Immunol. 2011;12:941–8. doi: 10.1038/ni.2089. [DOI] [PubMed] [Google Scholar]
- Halle S, Bumann D, Herbrand H, Willer Y, Dähne S, Förster R, Pabst O. Solitary intestinal lymphoid tissue provides a productive port of entry for Salmonella enterica serovar Typhimurium. Infect Immun. 2007;75:1577–85. doi: 10.1128/IAI.01392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–10. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33:297–305. doi: 10.1016/j.it.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genta RM, Hamner HW, Graham DY. Gastric lymphoid follicles in Helicobacter pylori infection: frequency, distribution, and response to triple therapy. Hum Pathol. 1993;24:577–83. doi: 10.1016/0046-8177(93)90235-9. [DOI] [PubMed] [Google Scholar]
- Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–53. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- Stranford S, Ruddle NH. Follicular dendritic cells, conduits, lymphatic vessels, and high endothelial venules in tertiary lymphoid organs: parallels with lymph node stroma. Front Immunol. 2012;3:350. doi: 10.3389/fimmu.2012.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupedo T, Jansen W, Kraal G, Mebius RE. Induction of secondary and tertiary lymphoid structures in the skin. Immunity. 2004;21:655–67. doi: 10.1016/j.immuni.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Suematsu S, Watanabe T. Generation of a synthetic lymphoid tissue-like organoid in mice. Nat Biotechnol. 2004;22:1539–45. doi: 10.1038/nbt1039. [DOI] [PubMed] [Google Scholar]
- Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R, Acha-Orbea H, Finke D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26:643–54. doi: 10.1016/j.immuni.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Marinkovic T, Garin A, Yokota Y, Fu YX, Ruddle NH, Furtado GC, Lira SA. Interaction of mature CD3+ CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest. 2006;116:2622–32. doi: 10.1172/JCI28993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner M. Tertiary lymphoid tissues in the colon: friend and foe. Gut microbes. 2011;2:193–7. doi: 10.4161/gmic.2.3.16732. [DOI] [PubMed] [Google Scholar]
- Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, Eberl G. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORγt and LTi cells. J Exp Med. 2011;208:125–34. doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Artis D, Colonna M, et al. Innate lymphoid cells – a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- Bernink JH, Peters CP, Munneke M, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–9. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- Pearson C, Uhlig HH, Powrie F. Lymphoid microenvironments and innate lymphoid cells in the gut. Trends Immunol. 2012;33:289–96. doi: 10.1016/j.it.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- Peters A, Pitcher LA, Sullivan JM, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35:986–96. doi: 10.1016/j.immuni.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan JL, Ouyang W. A role for Th17 cells in the regulation of tertiary lymphoid follicles. Eur J Immunol. 2012;42:2255–62. doi: 10.1002/eji.201242656. [DOI] [PubMed] [Google Scholar]
- Peduto L, Dulauroy S, Lochner M, Späth GF, Morales MA, Cumano A, Eberl G. Inflammation recapitulates the ontogeny of lymphoid stromal cells. J Immunol. 2009;182:5789–99. doi: 10.4049/jimmunol.0803974. [DOI] [PubMed] [Google Scholar]
- Dulauroy S, Di CS, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262–70. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- Krautler NJ, Kana V, Kranich J, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150:194–206. doi: 10.1016/j.cell.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezech C, Mader E, Desanti G, et al. Lymphotoxin-β receptor signaling through NF-κB2-RelB pathway reprograms adipocyte precursors as lymph node stromal cells. Immunity. 2012;37:721–34. doi: 10.1016/j.immuni.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sura R, Colombel JF, van Kruiningen HJ. Lymphatics, tertiary lymphoid organs and the granulomas of Crohn's disease: an immunohistochemical study. Aliment Pharmacol Ther. 2011;33:930–9. doi: 10.1111/j.1365-2036.2011.04605.x. [DOI] [PubMed] [Google Scholar]
- Desreumaux P, Ernst O, Geboes K, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology. 1999;117:73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- Lindhout E, van EM, van PM, Lindeman J, Dinant HJ, de Groot C. Fibroblast-like synoviocytes from rheumatoid arthritis patients have intrinsic properties of follicular dendritic cells. J Immunol. 1999;162:5949–56. [PubMed] [Google Scholar]
- Braun A, Takemura S, Vallejo AN, Goronzy JJ, Weyand CM. Lymphotoxin β-mediated stimulation of synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2004;50:2140–50. doi: 10.1002/art.20356. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427–35. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone F, Nayar S, Buckley CD. The role of non-hematopoietic stromal cells in the persistence of inflammation. Front Immunol. 2012;3:416. doi: 10.3389/fimmu.2012.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens BM, Simmons A. Intestinal stromal cells in mucosal immunity and homeostasis. Mucosal Immunol. 2013;6:224–34. doi: 10.1038/mi.2012.125. [DOI] [PubMed] [Google Scholar]
- Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjögren's syndrome. J Clin Invest. 1998;102:938–46. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardieri M, Barone F, Humby F, et al. Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjogren's syndrome. J Immunol. 2007;179:4929–38. doi: 10.4049/jimmunol.179.7.4929. [DOI] [PubMed] [Google Scholar]
- Kaiserling E. Newly-formed lymph nodes in the submucosa in chronic inflammatory bowel disease. Lymphology. 2001;34:22–9. [PubMed] [Google Scholar]
- Paavonen K, Mandelin J, Partanen T, Jussila L, Li TF, Ristimaki A, Alitalo K, Konttinen YT. Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium. J Rheumatol. 2002;29:39–45. [PubMed] [Google Scholar]
- Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116:3183–94. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandella E, Bolinger B, Lattmann E, et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–75. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–34. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Kusser K, Randall TD. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc Natl Acad Sci USA. 2007;104:10577–82. doi: 10.1073/pnas.0700591104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeurtsvanKessel CH, Willart MA, Bergen IM, et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206:2339–49. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Moreno J, Carragher DM, De la Luz Garcia-Hernandez M, et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12:639–46. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupedo T. An unexpected role for IL-17 in lymphoid organogenesis. Nat Immunol. 2011;12:590–2. doi: 10.1038/ni.2058. [DOI] [PubMed] [Google Scholar]
- Fleige H, Haas JD, Stahl FR, Willenzon S, Prinz I, Förster R. Induction of BALT in the absence of IL-17. Nat Immunol. 2012;13:1. doi: 10.1038/ni.2167. author reply 2. [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–54. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- Kuerten S, Schickel A, Kerkloh C, Recks MS, Addicks K, Ruddle NH, Lehmann PV. Tertiary lymphoid organ development coincides with determinant spreading of the myelin-specific T cell response. Acta Neuropathol. 2012;124:861–73. doi: 10.1007/s00401-012-1023-3. [DOI] [PubMed] [Google Scholar]
- Kendall PL, Yu G, Woodward EJ, Thomas JW. Tertiary lymphoid structures in the pancreas promote selection of B lymphocytes in autoimmune diabetes. J Immunol. 2007;178:5643–51. doi: 10.4049/jimmunol.178.9.5643. [DOI] [PubMed] [Google Scholar]
- Szodoray P, Jellestad S, Teague MO, Jonsson R. Attenuated apoptosis of B cell activating factor-expressing cells in primary Sjogren's syndrome. Lab Invest. 2003;83:357–65. [PubMed] [Google Scholar]
- McNamee EN, Masterson JC, Jedlicka P, Collins CB, Williams IR, Rivera-Nieves J. Ectopic lymphoid tissue alters the chemokine gradient, increases lymphocyte retention and exacerbates murine ileitis. Gut. 2013;62:53–62. doi: 10.1136/gutjnl-2011-301272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weih F, ner R, Hu D, Beer M, Habenicht AJ. Control of dichotomic innate and adaptive immune responses by artery tertiary lymphoid organs in atherosclerosis. Front Physiol. 2012;3:226. doi: 10.3389/fphys.2012.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JL. Inhibition of the lymphotoxin pathway as a therapy for autoimmune disease. Immunol Rev. 2008;223:202–20. doi: 10.1111/j.1600-065X.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- Columba-Cabezas S, Griguoli M, Rosicarelli B, Magliozzi R, Ria F, Serafini B, Aloisi F. Suppression of established experimental autoimmune encephalomyelitis and formation of meningeal lymphoid follicles by lymphotoxin β receptor-Ig fusion protein. J Neuroimmunol. 2006;179:76–86. doi: 10.1016/j.jneuroim.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Gommerman JL, Browning JL. Lymphotoxin/light, lymphoid microenvironments and autoimmune disease. Nat Rev Immunol. 2003;3:642–55. doi: 10.1038/nri1151. [DOI] [PubMed] [Google Scholar]
- Wu Q, Salomon B, Chen M, Wang Y, Hoffman LM, Bluestone JA, Fu YX. Reversal of spontaneous autoimmune insulitis in nonobese diabetic mice by soluble lymphotoxin receptor. J Exp Med. 2001;193:1327–32. doi: 10.1084/jem.193.11.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava RA, Kennedy SM, Wood SG, et al. Lymphotoxin-β receptor blockade reduces CXCL13 in lacrimal glands and improves corneal integrity in the NOD model of Sjogren's syndrome. Arthritis Res Ther. 2011;13:R182. doi: 10.1186/ar3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F, Browning JL, Lawton P, et al. Both the lymphotoxin and tumor necrosis factor pathways are involved in experimental murine models of colitis. Gastroenterology. 1998;115:1464–75. doi: 10.1016/s0016-5085(98)70025-3. [DOI] [PubMed] [Google Scholar]
- Fava RA, Notidis E, Hunt J, et al. A role for the lymphotoxin/LIGHT axis in the pathogenesis of murine collagen-induced arthritis. J Immunol. 2003;171:115–26. doi: 10.4049/jimmunol.171.1.115. [DOI] [PubMed] [Google Scholar]
- Finch DK, Ettinger R, Karnell JL, Herbst R, Sleeman MA. Effects of CXCL13 inhibition on lymphoid follicles in models of autoimmune disease. Eur J Clin Invest. 2013;43:501–9. doi: 10.1111/eci.12063. [DOI] [PubMed] [Google Scholar]
- Fletcher AL, Lukacs-Kornek V, Reynoso ED, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207:689–97. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, Collier AR, Turley SJ. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol. 2011;12:1096–104. doi: 10.1038/ni.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Matloubian M, Clemens DM, Sharpe AH, Freeman GJ, Gangappa S, Larsen CP, Ahmed R. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci USA. 2007;104:15430–5. doi: 10.1073/pnas.0702579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens BM, Kaye PM. Stromal cell induction of regulatory dendritic cells. Front Immunol. 2012;3:262. doi: 10.3389/fimmu.2012.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y. Cancer stromal targeting (CAST) therapy. Adv Drug Deliv Rev. 2012;64:710–9. doi: 10.1016/j.addr.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Thaunat O, Graff-Dubois S, Brouard S, et al. Immune responses elicited in tertiary lymphoid tissues display distinctive features. PLoS ONE. 2010;5:e11398. doi: 10.1371/journal.pone.0011398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder L, Scandella E, Chai Q, et al. A novel bacterial artificial chromosome-transgenic podoplanin-cre mouse targets lymphoid organ stromal cells in vivo. Front Immunol. 2011;2:50. doi: 10.3389/fimmu.2011.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley SJ, Fletcher AL, Elpek KG. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat Rev Immunol. 2010;10:813–25. doi: 10.1038/nri2886. [DOI] [PubMed] [Google Scholar]
- Mabbott NA, Kenneth BJ, Kobayashi A, et al. Expression of mesenchyme-specific gene signatures by follicular dendritic cells: insights from the meta-analysis of microarray data from multiple mouse cell populations. Immunology. 2011;133:482–98. doi: 10.1111/j.1365-2567.2011.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183:1461–72. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Chin RK, Christiansen P, Sun Y, Tumanov AV, Wang J, Chervonsky AV, Fu YX. Recruitment and activation of naive T cells in the islets by lymphotoxin β receptor-dependent tertiary lymphoid structure. Immunity. 2006;25:499–509. doi: 10.1016/j.immuni.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–81. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–33. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- Furtado GC, Marinkovic T, Martin AP, et al. Lymphotoxin β receptor signaling is required for inflammatory lymphangiogenesis in the thyroid. Proc Natl Acad Sci USA. 2007;104:5026–31. doi: 10.1073/pnas.0606697104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170:4638–48. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- Lee JJ, McGarry MP, Farmer SC, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–56. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–17. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- Lötzer K, Döpping S, Connert S, et al. Mouse aorta smooth muscle cells differentiate into lymphoid tissue organizer-like cells on combined tumor necrosis factor receptor-1/lymphotoxin β-receptor NF-κB signaling. Arterioscler Thromb Vasc Biol. 2010;30:395–402. doi: 10.1161/ATVBAHA.109.191395. [DOI] [PMC free article] [PubMed] [Google Scholar]


