Summary
Dendritic cells are highly adapted to their role of presenting antigen and directing immune responses. Developmental studies indicate that DCs originate independently from monocytes and tissue macrophages. Emerging evidence also suggests that distinct subsets of DCs have intrinsic differences that lead to functional specialisation in the generation of immunity. Comparative studies are now allowing many of these properties to be more fully understood in the context of human immunology.
Keywords: dendritic cells, haematology, therapy/immunotherapy
Introduction
The orchestration of effective immunity in vertebrates depends upon dendritic cells (DCs), a class of bone-marrow-derived cells found in the blood, epithelia and lymphoid tissues. DCs are equipped with molecular sensors and antigen-processing machinery to recognize pathogens, integrate chemical information and guide the specificity, magnitude and polarity of immune responses. Recent advances have helped to define DCs as a distinct haematopoietic lineage and to establish functional specialization between different DC subsets. The aim of this review is to present a coherent framework for understanding human DC subsets and their functional roles in vivo.
How are DCs distinct from monocytes and macrophages?
Dendritic cells, monocytes and macrophages traditionally comprise the mononuclear phagocyte system. An emerging theme is that components of this system are not as related as was presumed a decade ago. The phenotypic differences between human DCs and macrophages are discernible by immunohistochemistry1–2 and a clear functional distinction is the ability of DCs to leave tissues while macrophages remain fixed. This property may be tested directly by explanting tissue in vitro, and is mirrored by significant differences in turnover between DCs and macrophages after stem cell transplantation in vivo.3
More recently, the taxonomy of DC populations and their homology to mouse DC subsets have been scrutinized by transcriptional profiling.4–11 This has been assisted by a large and systematic effort to profile human and mouse leucocytes (Biogps; Immgen, www.biogps.org, www.immgen.org).12–13 Principal component analysis conveniently displays multidimensional data in a format familiar to flow cytometrists and reveals that quiescent primary DCs form discrete populations separate from monocytes (Fig. 1). This accords with ontogenetic studies in mice showing that most steady-state DCs are derived from committed DC-restricted precursors independently of monocytes.14
Figure 1.
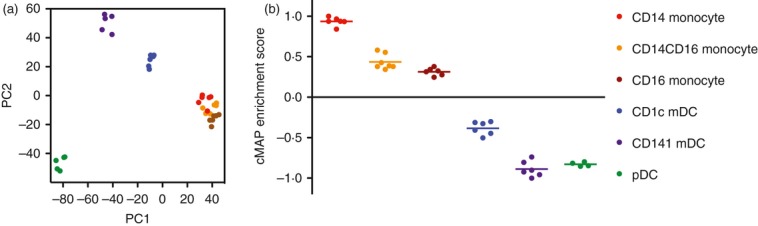
Transcriptional profiling of blood monocytes and dendritic cells (DCs). (a) Principal component analysis; (b) Connectivity mapping (cMAP) of gene set enrichment. The prinicipal components (PCs) are vectors that account for most of the variability in the populations and illustrate the clustering of all monocyte populations separately from DCs. Plasmacytoid DCs have the most distinct DC signature and cluster away from the two myeloid DCs and the monocyte cluster. The percentage variation accounted for by PC1 and PC2 in the example shown is 32·6% and 13·7%, respectively. Connectivity mapping defines the enrichment of different populations for a given gene signature. The cMAP enrichment score is a scalar quantity normalized to the index gene signature. The example shows the enrichment of monocyte and DC transcriptional profiles for the CD14 monocyte signature, illustrating the relatedness of all monocyte populations, intermediate status of CD1c+ DCs and distant relationship of CD141+ DCs and pDCs. The gene signature of each population is defined by those genes whose expression exceeds an average obtained from all the other populations. Each point represents a biological replicate (n = 6). Data replotted from ref. 8 courtesy of Pavandip Wasan, Michael Poidinger and Florent Ginhoux (Singapore Immunology Network).
The power of this approach is that it offers an unbiased assessment of relationships between cells rather than reliance upon a small selection of markers. The pitfalls in relation to DC lineage studies are that hierarchical clustering and principal component analysis are exquisitely sensitive to tissue-specific effects arising during preparation; this can easily obscure the relationships between similar DCs isolated from different sources. These effects can be minimized by bioinformatic strategies based on gene set enrichment analysis in which subset-specific signatures are identified or tissue-specific gene sets are removed before analysis. Most tissue-specific effects are the result of different states of maturation. It has recently been shown that strong activating stimuli induce convergence of DC transcriptional profiles through nuclear factor-κB and interferon-inducible pathways and naturally tend to obscure ontogenetic differences in transcriptional profiles.15
Overview of human dendritic cell lineages
Recent comparative phenotypic and functional studies have delineated a small number of distinct DC subsets that are widely distributed in all mammals (Table 1). In humans, all DCs express high levels of MHC class II (HLA-DR) and lack typical lineage markers CD3 (T cell), CD19/20 (B cell) and CD56 (natural killer cell). The classical descriptions of DCs as HLA-DR+ lineage− cells have been refined to include a number of positive DC lineage markers that identify DCs as either ‘myeloid’ or ‘plasmacytoid’ according to recent convention.16
Human mouse homology
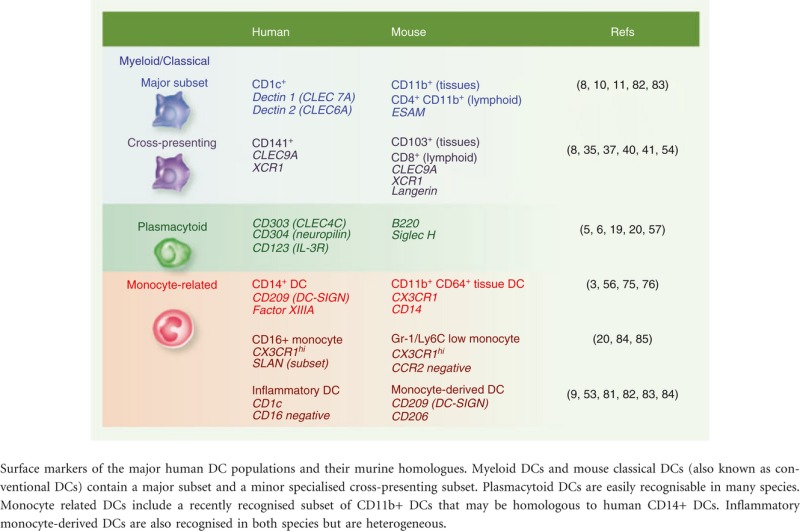 |
Myeloid DCs (mDCs)
Myeloid DCs (mDCs) express typical myeloid antigens CD11c, CD13, CD33 and CD11b, corresponding to mouse CD11c+ ‘classical’ or ‘conventional’ DCs. In humans both monocytes and mDCs express CD11c, but DCs lack CD14 or CD16 and may be split into CD1c+ and CD141+ fractions. These two fractions share homology with mouse classical DCs expressing either CD11b (CD1c+ DCs) or CD8/CD103 (CD141+ DCs).
Plasmacytoid DCs (pDCs)
Plasmacytoid DCs (pDCs) typically lack myeloid antigens and are distinguished by expression of CD123, CD303 and CD304. Although not related directly to plasma cells they retain subtle lymphoid features and unique secretory properties. Homologues are recognized in many species.
CD14+ DCs
CD14+ DCs found in tissues and lymph nodes are a third subset of CD11c+ myeloid cells originally described as ‘interstitial DCs’. They are more monocyte-like or macrophage-like than CD1c+ and CD141+ mDCs and may arise from classical monocytes. Equivalent cells have recently been found in mice as a new monocyte-derived subset of CD11b classical DCs that expresses or ESAM.
Langerhans cells (LCs) and microglia
Langerhans cells (LCs) and microglia are two specialized self-renewing DC populations found in stratified squamous epithelium and parenchyma of the brain, respectively. The LCs are capable of differentiating into migratory DCs whereas microglia are considered as a type of macrophage by many authors. Recent reviews provide excellent summaries of microglia and they will not be discussed further.17
Functional–anatomical classification of dendritic cells
A functional–anatomical classification derived from murine studies recognizes that DC function is intimately linked to location.18 Primarily this separates ‘migratory’ DCs that have trafficked through the tissues, from ‘resident’ DCs that arise in lymph nodes directly from the blood. Two further compartments also merit consideration: blood DCs and inflammatory DCs. The distribution of human DC subsets is summarized in Fig. 2.
Figure 2.
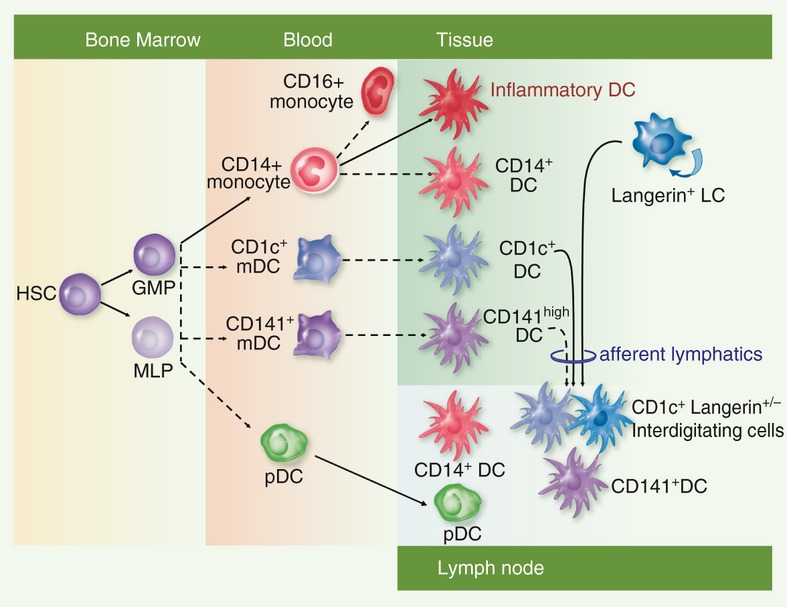
The distribution of major human dendritic cell (DC) subsets in blood, epithelial tissues and lymph nodes. Broken arrows indicate relationships that require further confirmation in humans. Human DCs can be generated either from granulocyte–macrophage progenitors (GMP) or multi-lymphoid progenitors (MLP) both of which ultimately arise from haematopoietic stem cells (HSC). Classical monocytes, blood myeloid DC (mDC) and plasmacytoid DC (pDC) are putative precursors of tissue and lymphoid DCs. Non-classical monocytes are reported to arise by conversion of classical monocytes in the mouse. Inflammatory DCs and CD14+ DCs have transcriptional profiles suggesting that they arise from monocytes; likewise tissue CD1c+ DCs and CD141+ DCs are related to their blood counterparts. Myeloid DCs and Langerhans cells (LCs) both form interdigitating cells in skin-draining lymph nodes. CD14+ DCs and pDCS are also found in nodes but may arise directly from the blood rather than by migration from tissues.
Blood/precursor DCs
Blood DCs are well defined in humans, and are likely to be precursors of tissue and lymphoid organ DCs. In support of this, blood contains pDCs, CD1c+ and CD141+ mDCs in immature forms of those found in tissues and lymph nodes.19–20 Mice also have blood pDCs and circulating precursors of classical DCs known as pre-cDCs. Pre-cDCs are blood mDCs in all but name and comprise multiple subsets that may correspond to the two human myeloid blood DCs.21
Non-lymphoid/tissue/migratory DCs
Most epithelial tissues contain ‘non-lymphoid’ or ‘migratory’ DCs whose function is to acquire antigen and migrate via the afferent lymphatics to lymph nodes. Quiescent interstitial tissues contain CD1c+ mDCs, CD141+ mDCs and CD14+ DCs but few pDCs.8–22 Epidermal LCs also migrate to form a component of afferent lymphatic DCs23 but it remains uncertain whether CD14+ DCs are migratory.24
Lymphoid/resident DCs
Lymphoid tissue also contains a large cohort of blood-derived non-migratory ‘lymphoid’ or ‘resident’ DCs. In the steady state, these may be difficult to separate from migratory DCs derived from the tissues. Human lymphoid tissue is less well described than mouse but contains CD1c+ mDCs, CD141+ mDCs and pDC in the steady state, in addition to a number of CD14+ populations.8–22 The contingent of resident lymphoid and migratory DCs in lymph nodes increases markedly during inflammation.
Inflammatory DCs
The content of tissues and lymphoid organs is dramatically altered during inflammation principally by the recruitment of granulocytes, classical monocytes and pDCs. Steady-state DC populations become more difficult to detect either because they migrate or are diluted by recruited cells. CD14+ classical monocytes are the putative precursors of inflammatory DCs. It is not known whether blood DCs are also recruited during inflammation but expression of CD62L and CXCR3, (receptor for interferon-γ-inducible chemokines CXCL9,10,11) suggests that they are competent to extravasate. Recent work confirms that inflammatory exudates contain two populations with polarized DC and macrophage properties.9 The relative contributions of migrating tissue DCs and newly recruited inflammatory DCs to the initiation of immunity, is a critical unresolved problem in humans.
CD1c+ myeloid DCs
CD1c+ mDCs are the major population of human mDCs in blood, tissues and lymphoid organs. They were originally recognized in the blood as a fraction of HLA-DR+ lineage− cells expressing myeloid antigens CD11b, CD11c, CD13, CD33, CD172 (SIRPa) and CD45RO25 and comprise approximately 1% of mononuclear cells, usually slightly lower than pDCs. CD1c was identified as a useful marker by the commercial antibody BDCA-1.19
In tissues, dermal CD1c+ DCs were originally described as the HLA-DR+ ‘indeterminate’ cell by electron microscopy (EM)26 and later characterized as CD1a+ DCs migrating from in vitro explants.27–28 Although dermal CD1c+ DCs and LCs both express CD1a, LCs may be identified by Langerin, epithelial cell adhesion molecule (EpCAM) and higher CD1a expression.1,3
Human tissue CD1c+ DCs appear more activated than their blood counterparts in terms of expression of CD80, CD83, CD86 and CD40. They have lost expression of homing receptors CLA and CD62L but up-regulated CCR7.1,29 A potential precursor–progeny relationship between blood and tissue CD1c+ DCs is supported by recent in vitro differentiation and gene expression analysis.8 This model is not universally accepted on the grounds that simple hierarchical clustering fails to discern any lineage relationship between blood and skin.11
In lymph nodes, CD1c+ DCs are found as ‘interdigitating cells’ of T-cell areas. Variable expression of CD1a and Langerin in skin-draining nodes suggests that both CD1c+ DCs and LCs contribute to this population.31,32 Tonsil and spleen also contain CD1c+ DCs.34,35 As these tissues do not receive afferent lymph, it is concluded that some CD1c+ DCs are ‘resident DCs’ originating directly from the blood.8–37
CD1c+ DCs are equipped with a wide range of lectins, toll-like receptors (TLRs) and other pattern recognition receptors for general purpose antigen uptake, transport and presentation. Through TLRs 1–8 they respond well to lipopolysaccharide, flagellin, poly(IC) and R848.38 The potential of CD1c and CD1a to present the glycolipid antigens of mycobacteria and other pathogens is often overlooked.39 Dectin-1 (CLEC7A) and Dectin-2 (CLEC6A) are highly expressed, suggesting a role in fungal recognition.10–11 DEC205 (CD205; CLEC13B) and macrophage mannose receptor (CD206; CLEC13D) are variable.22 CD1c+ DCs are good stimulators of naive CD4 T cells but have inferior capacity to cross-present antigen to CD8 T cells compared with CD141+ DCs.8–41 They secrete tumour necrosis factor-α (TNF-α), interleukin-8 (IL-8) and IL-10 when stimulated and produce IL-12 in response to TLR7/8 ligation by R848.36 A small amount of IL-23 can be detected after a range of stimuli.42 This implies a dual role in T helper type 1 (Th1) and Th17 sensitization and highlights the plasticity of DCs in different contexts. Deletion of the equivalent CD11b DC subset of mice leads to a range of functional deficits in Th1, Th2 and Th17 immunity (reviewed in ref. 43).102
CD141high myeloid dendritic cells
The expression of CD141 or thrombomodulin on approximately 10% of human blood mDCs (0·1% of mononuclear cells) was noted over a decade ago with the development of the blood DC antibody BDCA-3.19–20 CD141+ DCs have also been found among resident DCs of lymph node, tonsil, spleen and bone marrow22–37 and non-lymphoid tissues, skin, lung and liver.8
CD141+ DCs are difficult to identify in situ owing to their small numbers and the wide expression of CD141 on other cells.37 In particular, CD141 is found on migratory CD14+ DCs and on CD1c+ DCs and monocytes cultured with vitamin D.44 Differentiation from the major CD1c+ DC subset by flow cytometry is aided by the observation that CD141+ DCs express less CD11b and CD11c.8–45
The realisation that human CD141+ DCs are homologous to mouse CD8+ lymph node DCs35–41 and CD103+ tissue DCs8 is something of a Rosetta stone in DC biology, enabling the systematic alignment of human and mouse DC subsets across lymphoid and non-lymphoid tissues. Both CD141+ and CD8+ DCs have acquired the status of ‘cross-presenting’ DCs in comparison with other myeloid or classical DCs.46 Comparative biology approaches have also identified CLEC9A,47,48 XCR1,50 Necl251 and TRL3 expression52 as a concise functional profile that species barriers. DEC205, a marker of CD8+ mouse DCs, is not restricted to human CD141+ DCs but XCR1 and CLEC9A have emerged as universal markers of cross-presenting DCs in multiple species.45–50
CD141+ mDCs have an enhanced ability to take up dead or necrotic cells via CLEC9A,40–49 sense viral nucleic acids with TLR3 and TLR8 and to cross-present antigen to CD8+ T-cell clones in vitro.8–40 This function is consistent with their homology to mouse CD8+/CD103+ DCs, although CD141+ DCs show less enrichment for gene transcripts controlling class I presentation than the homologous mouse DCs.6 CD141+ DCs readily secrete TNF-α, CXCL10 and interferon-λ but surprisingly little IL-12 p70, in contrast to mDCs and CD1c+ DCs.8–54 Notably, other populations of human DCs derived in vitro are also capable of cross-presentation, especially LC-like cells differentiated in granulocyte–macrophage colony-stimulating factor (GM-CSF) and TNF-α.55–56 Overall, the division of labour between CD141+ DCs and other myeloid DCs appears less sharply demarcated than in the mouse, a reminder that functional cross-species comparison is an imprecise science.
Plasmacytoid dendritic cells
Plasmacytoid DCs have unique specialized functions that have been extensively reviewed.57 The most numerous blood DCs, they lack myeloid antigens CD11b, CD11c, CD13 and CD33 but express CD45RA, variable CD2 and CD7 and may harbour T-cell receptor and immunoglobulin rearrangements. The morphological epithet ‘plasmacytoid’ reflects abundant secretory capacity and avoids the anatomical connotations of ‘lymphoid’. Plasmacytoid DCs are now separable from mDCs by positive markers CD123 (IL-3R), CD303 (CLEC4C; BDCA-2) and CD304 (neuropilin; BDCA-4).19 They are not abundant in quiescent tissues but are present in LN at about 20% of MHC class II positive cells and are rapidly recruited to both sites in inflammation.58
The propensity of pDCs to release type 1 interferon in response to viruses was one of the first specialized DC functions to be described.59,60 They express very high levels of TLR7 and TLR9, which transduce signals from viral and self nucleic acids.62–63 Freshly isolated blood pDCs do not prime naive T cells efficiently and appear less ‘mature’ than mDCs until activated.60,64 Their ability to polarize CD4 responses towards Th1 or Th2 is variable and may be context-dependent.66 In other systems pDC have been reported to induce regulatory T cells or tolerance, possibly related to their ability to sense DNA released from apopotic cells and notable expression of ILT7.67–68 Failure of tolerance is linked to the autoimmune diseases systemic lupus erythematosus and psoriasis.63
CD14+ dendritic cells
Many non-lymphoid tissues contain a modest population of CD14+ HLA-DR+ cells that express CD11c in common with monocytes but lack typical mDC markers such as CD1c or CD141, co-stimulatory molecules and CCR7.3,29 These cells were originally identified as migrant CD14+ populations from human skin.27–28 They were previously referred to as ‘interstitial-type’ or ‘dermal-type’ DCs (to contrast with epidermal LCs) but this term is misleading because it ignores the major population of CD1c+ interstitial mDCs.
CD14+ DCs express DC-SIGN (CD209) and the macrophage markers FXIIIA and CD163, which may also be found on monocytes or monocyte-derived DCs and macrophages, especially those generated under tolerogenic conditions such as IL-10 or vitamin D.3,69 In situ, it can be difficult to separate CD14+ DCs from macrophages by antigen expression and recourse to the physical properties of side scatter, autofluorescence and ability to migrate from explanted skin, is required.3 Somewhat confusingly, CD14+ DCs acquire CD141 in culture but otherwise they do not resemble cross-presenting CD141+ DCs, which are typically CD14 negative and cannot be made from monocytes.
CD14+ DCs migrating from explanted human skin are less mature or more macrophage-like than CD1c+ DCs and do not stimulate naive T cells efficiently.8–72 CD14+ DCs retain the ability to differentiate into LC-like cells or more mature DC-like cells in vitro.42,71 Unstimulated they have proven tolerogenic functions that can be recapitulated by treating monocytes with vitamin D.44 Together these properties suggest that CD14+ DCs may be extravasated, quiescent ‘tissue monocytes’. Their turnover in bone marrow transplantation is rapid, and parallels blood myeloid engraftment, in contrast to long-lived macrophages.3
As CD14+ DCs express little CCR7, their migration to lymph nodes is questionable3–29 although discrete, possibly blood-derived CD14+ CD209+ cells are found in the paracortex.22–32 Important functions have been ascribed to CD14+ DCs in the formation of follicular helper T cells56 or in providing direct B-cell help.74
The mouse equivalent of CD14+ DCs is currently under scrutiny. As suspected from human biology, non-lymphoid tissue CD11b+ DCs are heterogeneous and contain monocyte-derived cells with properties similar to CD14+ DCs. New markers including CD24, CD26, CD64 and CX3CR1 are allowing a monocyte-derived component of the CD11b DC population to be identified.75–76 Further confirmation of this would suggest by correlation, that CD14+ human DCs are indeed monocyte-derived and shed new light on their in vivo function.
Langerhans cells
Langerhans cells reside in the supra-basal epidermis and other stratified squamous epithelia (bronchus, oral and genital mucous membrane) where they form a network. Human LCs express high levels of the C-type lectin Langerin and CD1a, a non-polymorphic class I MHC molecule. Both antigen capture and presentation molecules are found together in a specialized endosomal compartment, visible by EM as the Birbeck granule. Other markers include CD36, ATPase and FcεR1.23–77
Langerhans cells epitomize migratory tissue DC and have facilitated many studies of functional DC specialization across several species.23 Their derivatives can easily be detected in skin-draining lymph nodes, especially in inflammatory skin disorders. They occupy the lymph node paracortex as langerin+ CD1ahigh interdigitating cells.78
The function of LCs in immunity has been surprisingly difficult to pin down. They can be matured into potent cross-presenting DCs39 but also lack critical TLRs and can induce regulatory T cells and IL-22 production through CD1a-restricted antigen to autologous T cells.79 Overall, LCs appear to maintain epidermal health and tolerance to commensals, while retaining the ability to respond to selected intracellular pathogens and viruses under inflammatory conditions.
Inflammatory dendritic cells
It has been known for many years that highly functional DCs can be derived from classical CD14+ blood monocytes, using GM-CSF and IL-480 or more subtle in vitro tissue models.24 Monocyte-derived DCs have a wide range of properties including potent stimulation of naive CD4+ T cells, cross-presentation to CD8+ T cells and production of key cytokines IL-1, IL-6, TNF-α, IL-12 and IL-23.53
With correlative evidence that most primary DCs are probably not derived from monocytes, the challenge is to understand the role of inflammatory monocyte recruitment to human DC biogenesis in vivo. Monocytes are highly plastic and their differentiation into DCs or different forms of macrophages (M1/M2) in vitro provides a conceptual framework for inflammation. Human inflammatory exudates contain distinct inflammatory DC-like and macrophage-like cells and transcriptional profiling suggests a common monocyte origin.9 Key features of these cells are the expression of CD1c, CD1a, CD206, FcεR1, SIRPα but lack of CD16 and CD209. In vitro they synthesize IL-1β, TNF-α, IL-6 and IL-23 and stimulate Th17 responses. Monocyte-derived DCs and inflammatory DCs in this study both express transcription factor zbtb46, in common with CD1c+ DCs.81–82 Previous descriptions of inflammatory DCs include inflammatory dendritic epidermal cells (IDECs), found in Th2-mediated atopic dermatitis, and TNF and inducible nitric oxide synthase-producing (TiP) DCs, found in psoriasis.83–84 Together these data suggest that different inflammatory environments will generate monocyte-derived DC subsets with distinct functions. Many questions remain, including whether blood DCs also form inflammatory cells directly and whether inflammatory DCs migrate to lymph nodes or differentiate into steady-state resident cells after resolution of inflammation.
Non-classical monocytes and SLAN DCs
Human monocyte populations are heterogeneous16 and CD16+ monocytes possess distinct characteristics including higher MHC class II and co-stimulatory antigen expression that have led some authors to classify them as a type of blood DC.20 In agreement with this view, a subset of CD16+ monocytes characterized by expression of the antigen 6-Sulpho LacNAc (SLAN) is reported to secrete large amounts of TNF-α, IL-1β and IL-12 and to respond rapidly to inflammatory stimuli.84 There is a lack of consensus, however. Gene expression analysis and functional studies suggest that CD16+ CD14dim non-classical monocytes, including SLAN+ cells, have low inflammatory activity and are homologous to murine Gr-1/Ly6C low ‘patrolling’ monocytes.85 In this report, cytokine secretion and pro-inflammatory activity were attributed to intermediate CD14+ CD16+ monocytes. Anatomically, non-classical CD16+ monocytes are well positioned to infiltrate tissues but it remains uncertain that they should be classified as DCs. Unsupervised hierarchical clustering of gene expression data is unequivocal that all monocytes cluster independently of DCs.5–11
Origin of human dendritic cells
Human DCs arise from the bone marrow through a series of currently undefined precursors that may have both myeloid and lymphoid ancestry.86 Bone marrow transplantation and human DC deficiency states indicate that continual replenishment from blood-borne precursors is required for tissue populations of CD1c+, CD141+ and CD14+ DCs.3,87 The potential role of blood DCs as precursors to tissue DCs has been outlined but there is a gap in knowledge between the bone marrow and peripheral blood compartments. Although Flt-3 and MHC class II expression has been useful for tracking restricted DC precursors in the mouse, they are less informative markers in humans because the entire CD34+ progenitor compartment is positive.
Studies in humans with DC deficiency have begun to shed some light on the cellular pathways and genetic regulation of DC haematopoiesis.88 By analogy with mice, it is likely that the early transcription factors Ikaros, PU.1, Gfi1 and Id2 are required for DC development in humans.89–90 Ikaros and GFi1 defects have been characterized in humans but have much broader haematopoietic defects than suggested by murine models. Heterozygous GATA-2 mutation induces a specific defect in mononuclear cell development, known as DC, monocyte, B and NK lymphoid (DCML) deficiency.91
Dendritic cell production from human progenitors is promoted by Flt-3 ligand, GM-CSF and IL-4 in vitro35 and Flt-3, macrophage colony-stimulating factor (M-CSF) and GM-CSF signalling via signal transducers and activators of transcription 3 and 5 is likely to play an important role in vivo. Flt-3 is more highly expressed on DCs than monocytes or macrophages, in inverse relationship with M-CSF receptor.8 Functional GM-CSF deficiency due to an autoimmune neutralizing antibody exists and causes defective function of alveolar macrophages but DC profiling has not been performed in detail.92
More specific defects in classical DCs have been reported in mice lacking interferon regulatory factor 4 (IRF4), IRF8 and Batf3 and in pDCs due to loss of E2-2.89–90 In particular the balance between Id2 and E2-2 is critical in specifying myeloid or plasmacytoid DC development and E2-2-deficient humans have impaired pDC function.57–93 Bi-allelic IRF8 deficiency in humans causes loss of all DCs and monocytes, in keeping with a broader pattern of human IRF8 expression than in mouse.94 Batf3 knock-down by lentiviral short hairpin RNA inhibits the formation of CD141+ DCs, a result that is more closely aligned to the selective loss of CD8+ DCs in knockout mice.45 The recently described transcription factor zbtb46 is specifically expressed in mDCs but is not required for the development of murine classical DCs.81–82
Human LCs have some self-renewal capacity independently of the bone marrow. Several reports have shown that they are proliferating cells95–96 maintained in a hair follicle niche.97 Human limb transplants show that they remain of donor origin, independent of the bone marrow,98 although after haematopoietic stem cell transplantation, they are gradually replaced by bone-marrow-derived cells.87 Humans lacking monocytes and all identifiable DC populations also maintain reasonable LC populations, ruling against monocytes or CD14+ dermal DCs as steady-state precursors.91 The ready derivation of LC-like cells from CD34+ bone marrow progenitors or monocytes in vitro99–100 is in keeping with observations in mice that blood-borne progenitors may be recruited to renew LCs after severe inflammation has destroyed their epidermal niches.101 The development of LCs reflects a more macrophage-like ancestry than other DC populations. Treansforming growth factor-β is required for the acquisition of langerin in humans and M-CSF receptor rather than Flt-3 is critical for their development in mice.
Concluding remarks and future directions
Many years after the mononuclear phagocyte system was conceived and monocyte-derived DCs were discovered in humans, we perceive monocytes, macrophages and DCs more clearly but acknowledge ever more complexity in their origins and complementary functions. The specialized functions of human DC subsets and their derivation from DC-restricted precursors is beginning to be established and should lead to new therapeutic opportunities. However, much remains to be learned about DC haematopoiesis in humans in the steady state, and the pathological consequences of inflammatory generation of DCs from monocytes.
Acknowledgments
The authors thank Pavandip Wasan, Michael Poidinger and Florent Ginhoux (Singapore Immunology Network) and Venetia Bigley (Newcastle University) for providing figures and comment. The authors are supported by Leukaemia and Lymphoma Research, The Wellcome Trust and Bright Red.
Disclosures
The authors declare that they have no conflicts of interests.
References
- Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117:2517–25. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel CE, George E, Ostrovsky LL, Dunbar PR. Comprehensive analysis of MHC-II expression in healthy human skin. Immunol Cell Biol. 2007;85:363–9. doi: 10.1038/sj.icb.7100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniffa M, Ginhoux F, Wang XN, et al. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J Exp Med. 2009;206:371–85. doi: 10.1084/jem.20081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt M, Lundberg K, Borrebaeck CA. Gene family clustering identifies functionally associated subsets of human in vivo blood and tonsillar dendritic cells. J Immunol. 2005;175:4839–46. doi: 10.4049/jimmunol.175.8.4839. [DOI] [PubMed] [Google Scholar]
- Robbins SH, Walzer T, Dembele D, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozat K, Guiton R, Guilliams M, Henri S, Baranek T, Schwartz-Cornil I, Malissen B, Dalod M. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunol Rev. 2010;234:177–98. doi: 10.1111/j.0105-2896.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- Guilliams M, Henri S, Tamoutounour S, Ardouin L, Schwartz-Cornil I, Dalod M, Malissen B. From skin dendritic cells to a simplified classification of human and mouse dendritic cell subsets. Eur J Immunol. 2010;40:2089–94. doi: 10.1002/eji.201040498. [DOI] [PubMed] [Google Scholar]
- Haniffa M, Shin A, Bigley V, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E, Touzot M, Bohineust A, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 2013;38:336–48. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Lundberg K, Albrekt AS, Nelissen I, Santegoets S, de Gruijl TD, Gibbs S, Lindstedt M. Transcriptional profiling of human dendritic cell populations and models – unique profiles of in vitro dendritic cells and implications on functionality and applicability. PLoS ONE. 2013;8:e52875. doi: 10.1371/journal.pone.0052875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman AN, Bye CR, Nasr N, et al. Identification of lineage relationships and novel markers of blood and skin human dendritic cells. J Immunol. 2013;190:66–79. doi: 10.4049/jimmunol.1200779. [DOI] [PubMed] [Google Scholar]
- Miller JC, Brown BD, Shay T, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–99. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–28. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- Manh TP, Alexandre Y, Baranek T, Crozat K, Dalod M. Plasmacytoid, conventional and monocyte-derived dendritic cells undergo a profound and convergent genetic reprogramming during their maturation. Eur J Immunol. 2013 doi: 10.1002/eji.201243106. ; doi: 10.1002/eji.201243106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–61. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–20. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–71. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- Segura E, Valladeau-Guilemond J, Donnadieu MH, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med. 2012;209:653–60. doi: 10.1084/jem.20111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N, Lenz A, Glassel H, Stossel H, Stanzl U, Majdic O, Fritsch P, Schuler G. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989;93:600–9. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–3. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Czernielewski JM, Schmitt D, Faure MR, Thivolet J. Functional and phenotypic analysis of isolated human Langerhans cells and indeterminate cells. Br J Dermatol. 1983;108:129–38. doi: 10.1111/j.1365-2133.1983.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Lenz A, Heine M, Schuler G, Romani N. Human and murine dermis contain dendritic cells. Isolation by means of a novel method and phenotypical and functional characterization. J Clin Invest. 1993;92:2587–96. doi: 10.1172/JCI116873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993;151:6535–45. [PubMed] [Google Scholar]
- Angel CE, George E, Brooks AE, Ostrovsky LL, Brown TL, Dunbar PR. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J Immunol. 2006;176:5730–4. doi: 10.4049/jimmunol.176.10.5730. [DOI] [PubMed] [Google Scholar]
- McLellan AD, Heiser A, Sorg RV, Fearnley DB, Hart DN. Dermal dendritic cells associated with T lymphocytes in normal human skin display an activated phenotype. J Invest Dermatol. 1998;111:841–9. doi: 10.1046/j.1523-1747.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Asagoe K, Zaishun J, Yanai H, Yoshino T, Hayashi K, Akagi T. Heterogeneity of dendritic cells in human superficial lymph node: in vitro maturation of immature dendritic cells into mature or activated interdigitating reticulum cells. Am J Pathol. 1998;153:745–55. doi: 10.1016/S0002-9440(10)65618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel CE, Chen CJ, Horlacher OC, et al. Distinctive localization of antigen presenting cells in human lymph nodes. Blood. 2009;113:1257–67. doi: 10.1182/blood-2008-06-165266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven R, van den Hout MF, Lindenberg JJ, et al. Characterization of four conventional dendritic cell subsets in human skin-draining lymph nodes in relation to T-cell activation. Blood. 2011;118:2502–10. doi: 10.1182/blood-2011-03-344838. [DOI] [PubMed] [Google Scholar]
- Summers KL, Hock BD, McKenzie JL, Hart DN. Phenotypic characterization of five dendritic cell subsets in human tonsils. Am J Pathol. 2001;159:285–95. doi: 10.1016/S0002-9440(10)61694-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin LF, Salio M, Griessinger E, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8α+ dendritic cells. J Exp Med. 2010;207:1261–71. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittag D, Proietto AI, Loudovaris T, Mannering SI, Vremec D, Shortman K, Wu L, Harrison LC. Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J Immunol. 2011;186:6207–17. doi: 10.4049/jimmunol.1002632. [DOI] [PubMed] [Google Scholar]
- Jongbloed SL, Kassianos AJ, McDonald KJ, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–60. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Aar AM, Sylva-Steenland RM, Bos JD, Kapsenberg ML, de Jong EC, Teunissen MB. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178:1986–90. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- van Rhijn I, Ly D, Moody DB. CD1a, CD1b, and CD1c in immunity against mycobacteria. Adv Exp Med Biol. 2013;783:181–97. doi: 10.1007/978-1-4614-6111-1_10. [DOI] [PubMed] [Google Scholar]
- Bachem A, Guttler S, Hartung E, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–81. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozat K, Guiton R, Contreras V, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8α+ dendritic cells. J Exp Med. 2010;207:1283–92. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli AE, Rubin JP, Erdos G, et al. CD4+ T cell responses elicited by different subsets of human skin migratory dendritic cells. J Immunol. 2005;175:7905–15. doi: 10.4049/jimmunol.175.12.7905. [DOI] [PubMed] [Google Scholar]
- Bar-On L, Jung S. Defining dendritic cells by conditional and constitutive cell ablation. Immunol Rev. 2010;234:76–89. doi: 10.1111/j.0105-2896.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- Chu CC, Ali N, Karagiannis P, et al. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J Exp Med. 2012;209:935–45. doi: 10.1084/jem.20112583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin LF, Reyal Y, Uronen-Hansson H, et al. DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues. Blood. 2012;119:6052–62. doi: 10.1182/blood-2012-01-406967. [DOI] [PubMed] [Google Scholar]
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–69. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- Caminschi I, Proietto AI, Ahmet F, et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112:3264–73. doi: 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysamen C, Willment JA, Dennehy KM, Brown GD. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem. 2008;283:16693–701. doi: 10.1074/jbc.M709923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner BG, Dorner MB, Zhou X, et al. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity. 2009;31:823–33. doi: 10.1016/j.immuni.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Galibert L, Diemer GS, Liu Z, et al. Nectin-like protein 2 defines a subset of T-cell zone dendritic cells and is a ligand for class-I-restricted T-cell-associated molecule. J Biol Chem. 2005;280:21955–64. doi: 10.1074/jbc.M502095200. [DOI] [PubMed] [Google Scholar]
- Schulz O, Diebold SS, Chen M, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–92. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- Ebner S, Ratzinger G, Krosbacher B, et al. Production of IL-12 by human monocyte-derived dendritic cells is optimal when the stimulus is given at the onset of maturation, and is further enhanced by IL-4. J Immunol. 2001;166:633–41. doi: 10.4049/jimmunol.166.1.633. [DOI] [PubMed] [Google Scholar]
- Lauterbach H, Bathke B, Gilles S, et al. Mouse CD8α+ DCs and human BDCA3+ DCs are major producers of IFN-λ in response to poly IC. J Exp Med. 2010;207:2703–17. doi: 10.1084/jem.20092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzinger G, Baggers J, de Cos MA, et al. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173:2780–91. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- Klechevsky E, Morita R, Liu M, et al. Functional specializations of human epidermal langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol. 2011;29:163–83. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K, North M, Burke M, Singhal H, Renton S, Aqel N, Islam S, Knight SC. Plasmacytoid dendritic cells (PDC) are the major DC subset innately producing cytokines in human lymph nodes. J Leukoc Biol. 2005;78:1142–52. doi: 10.1189/jlb.1103532. [DOI] [PubMed] [Google Scholar]
- Perussia B, Fanning V, Trinchieri G. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro α interferon production in response to viruses. Nat Immun Cell Growth Regul. 1985;4:120–37. [PubMed] [Google Scholar]
- Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–11. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- Doherty U, Peng M, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, Steinman RM. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–93. [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Barragan L, Lewis KL, Firner S, Thiel V, Hugues S, Reith W, Ludewig B, Reizis B. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci U S A. 2012;109:3012–17. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–42. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- Ito T, Yang M, Wang YH, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–15. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AE, Sayers BL, Haniffa MA, Swan DJ, Diboll J, Wang XN, Isaacs JD, Hilkens CM. Differential regulation of naive and memory CD4+ T cells by alternatively activated dendritic cells. J Leukoc Biol. 2008;84:124–33. doi: 10.1189/jlb.1107744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa MT, Loncaric A, Krutzik SR, Becker TC, Modlin RL. “Dermal Dendritic Cells” comprise two distinct populations: CD1+ dendritic cells and CD209+ macrophages. J Invest Dermatol. 2008;128:2225–31. doi: 10.1038/jid.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gruijl TD, Sombroek CC, Lougheed SM, Oosterhoff D, Buter J, van den Eertwegh AJ, Scheper RJ, Pinedo HM. A postmigrational switch among skin-derived dendritic cells to a macrophage-like phenotype is predetermined by the intracutaneous cytokine balance. J Immunol. 2006;176:7232–42. doi: 10.4049/jimmunol.176.12.7232. [DOI] [PubMed] [Google Scholar]
- Penel-Sotirakis K, Simonazzi E, Peguet-Navarro J, Rozieres A. Differential capacity of human skin dendritic cells to polarize CD4+ T cells into IL-17, IL-21 and IL-22 producing cells. PLoS ONE. 2012;7:e45680. doi: 10.1371/journal.pone.0045680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larregina AT, Morelli AE, Spencer LA, Logar AJ, Watkins SC, Thomson AW, Falo LDJ. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat Immunol. 2001;2:1151–8. doi: 10.1038/ni731. [DOI] [PubMed] [Google Scholar]
- Matthews K, Chung NP, Klasse PJ, Moore JP, Sanders RW. Potent induction of antibody-secreting B cells by human dermal-derived CD14+ dendritic cells triggered by dual TLR ligation. J Immunol. 2012;189:5729–44. doi: 10.4049/jimmunol.1200601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlet C, Tamoutounour S, Henri S, Luche H, Ardouin L, Gregoire C, Malissen B, Guilliams M. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J Immunol. 2012;188:1751–60. doi: 10.4049/jimmunol.1102744. [DOI] [PubMed] [Google Scholar]
- Plantinga M, Guilliams M, Vanheerswynghels M, et al. Conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–35. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701–8. doi: 10.1172/JCI19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Dieu-Nosjean MC, Dezutter C, et al. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J Exp Med. 2002;196:417–30. doi: 10.1084/jem.20020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong A, Pena-Cruz V, Cheng TY, Clark RA, van Rhijn I, Moody DB. CD1a-autoreactive T cells are a normal component of the human αβ T cell repertoire. Nat Immunol. 2010;11:1102–9. doi: 10.1038/ni.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MM, Liu K, Darrasse-Jeze G, et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med. 2012;209:1153–65. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy AT, KC W, Albring JC, et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–52. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg A, Mommaas M, Oppel T, Schottdorf EM, Gunther S, Moderer M. Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J Invest Dermatol. 2002;118:327–34. doi: 10.1046/j.0022-202x.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- Hansel A, Gunther C, Ingwersen J, et al. Human SLAN (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong TH17/TH1 T-cell responses. J Allergy Clin Immunol. 2011;127:787–94. doi: 10.1016/j.jaci.2010.12.009. .e1–9. [DOI] [PubMed] [Google Scholar]
- Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–86. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulatov S, Notta F, Eppert K, Nguyen LT, Ohashi PS, Dick JE. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol. 2010;11:585–93. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- Collin MP, Hart DN, Jackson GH, Cook G, Cavet J, Mackinnon S, Middleton PG, Dickinson AM. The fate of human Langerhans cells in hematopoietic stem cell transplantation. J Exp Med. 2006;203:27–33. doi: 10.1084/jem.20051787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M, Bigley V, Haniffa M, Hambleton S. Human dendritic cell deficiency: the missing ID? Nat Rev Immunol. 2011;11:575–83. doi: 10.1038/nri3046. [DOI] [PubMed] [Google Scholar]
- Watowich SS, Liu YJ. Mechanisms regulating dendritic cell specification and development. Immunol Rev. 2010;238:76–92. doi: 10.1111/j.1600-065X.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–35. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigley V, Haniffa M, Doulatov S, et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J Exp Med. 2011;208:227–34. doi: 10.1084/jem.20101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell BC, Carey BC, Uchida K, Suzuki T. Pulmonary alveolar proteinosis, a primary immunodeficiency of impaired GM-CSF stimulation of macrophages. Curr Opin Immunol. 2009;21:514–21. doi: 10.1016/j.coi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse B, Caton ML, Lehner M, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton S, Salem S, Bustamante J, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365:127–38. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernielewski JM, Demarchez M. Further evidence for the self-reproducing capacity of Langerhans cells in human skin. J Invest Dermatol. 1987;88:17–20. doi: 10.1111/1523-1747.ep12464659. [DOI] [PubMed] [Google Scholar]
- Chorro L, Sarde A, Li M, et al. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med. 2009;206:3089–100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam AC, Kremer IB, Yoshida Y, Stevens SR, Tootell E, Teunissen MB, Hammerberg C, Cooper KD. The human hair follicle: a reservoir of CD40+ B7-deficient Langerhans cells that repopulate epidermis after UVB exposure. J Invest Dermatol. 1998;110:422–7. doi: 10.1046/j.1523-1747.1998.00162.x. [DOI] [PubMed] [Google Scholar]
- Kanitakis J, Morelon E, Petruzzo P, Badet L, Dubernard JM. Self-renewal capacity of human epidermal Langerhans cells: observations made on a composite tissue allograft. Exp Dermatol. 2011;20:145–6. doi: 10.1111/j.1600-0625.2010.01146.x. [DOI] [PubMed] [Google Scholar]
- Strobl H, Riedl E, Scheinecker C, Bello-Fernandez C, Pickl WF, Rappersberger K, Majdic O, Knapp W. TGF-β1 promotes in vitro development of dendritic cells from CD34+ hemopoietic progenitors. J Immunol. 1996;157:1499–507. [PubMed] [Google Scholar]
- Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor β1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med. 1998;187:961–6. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Tacke F, Angeli V, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–73. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlitzer A, McGovern N, Teo P, et al. IRF4 Transcription Factor-Dependent CD11b+ Dendritic Cells in Human and Mouse Control Mucosal IL-17 Cytokine Responses. Immunity. 2013;38:970–83. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


