Summary
Swine leucocyte antigen (SLA) class II molecules on porcine (p) cells play a crucial role in xenotransplantation as activators of recipient human CD4+ T cells. A human dominant-negative mutant class II transactivator (CIITA-DN) transgene under a CAG promoter with an endothelium-specific Tie2 enhancer was constructed. CIITA-DN transgenic pigs were produced by nuclear transfer/embryo transfer. CIITA-DN pig cells were evaluated for expression of SLA class II with/without activation, and the human CD4+ T-cell response to cells from CIITA-DN and wild-type (WT) pigs was compared. Lymphocyte subset numbers and T-cell function in CIITA-DN pigs were compared with those in WT pigs. The expression of SLA class II on antigen-presenting cells from CIITA-DN pigs was significantly reduced (40–50% reduction compared with WT; P < 0·01), and was completely suppressed on aortic endothelial cells (AECs) even after activation (100% suppression; P < 0·01). The human CD4+ T-cell response to CIITA-DN pAECs was significantly weaker than to WT pAECs (60–80% suppression; P < 0·01). Although there was a significantly lower frequency of CD4+ cells in the PBMCs from CIITA-DN (20%) than from WT (30%) pigs (P < 0·01), T-cell proliferation was similar, suggesting no significant immunological compromise. Organs and cells from CIITA-DN pigs should be partially protected from the human cellular immune response.
Keywords: genetic engineering, immune response, MHC class II transactivator, pig, swine leucocyte antigens, xenotransplantation
Introduction
The primate T-cell response to pig antigens involves both direct and indirect pathways.1–4 Porcine (p) stimulator cells are able to directly activate human (h) T cells, reflecting productive interaction between human T-cell receptors and swine leucocyte antigen (SLA) class I and class II peptide complexes across the species barrier.5
In xenotransplantation (xenoTx), two donor cell types are likely to be the major stimulators of direct T-cell recognition: (i) the migratory passenger leucocytes known as antigen-presenting cells (APCs), including dendritic cells, and (ii) SLA class II-positive vascular endothelial cells (ECs). Although pAPCs are transient components in the graft, pig vascular ECs are usually permanently present in the xenograft. Unlike human aortic ECs (AECs), pAECs constitutively express co-stimulatory molecules (e.g. CD80/86).6–7 This is probably an important factor contributing to rejection of pig xenografts and so relatively intense immunosuppressive therapy is required in pig-to-primate xenoTx. However, intensive immunosuppression, especially long-term, results in impaired immunity, which is associated with susceptibility to infection.8 In xenoTx, there is the possibility that specific anti-graft immune responses can be reduced by genetic modification of the pig.9
MHC class II molecules on donor cells play a crucial role in allo/xenoTx as activators of recipient CD4+ T cells. The class II transactivator (CIITA) has been termed a master regulator for MHC class II.10 Some mutations of CIITA have been found to account for deficiency of MHC class II expression in patients with severe immunodeficiency, i.e. bare lymphocyte syndrome.11 Variants of the CIITA gene, especially its dominant-negative mutant (DN), have been designed and constructed to interfere with expression of the class II gene. High sequence homology (87·4%) between human and pig CIITA12 allowed us to use an hCIITA mutant to reduce MHC class II (SLA class II) expression in pigs. Human CIITA-DN transgenic pigs on a wild-type (WT) background were produced as potential sources of organs/tissues/cells for xenoTx.
The aims of this study were to investigate in vitro (i) the expression of SLA class II on pAECs (with/without activation) and on peripheral blood mononuclear cells (PBMCs) (as APCs) from CIITA-DN pigs, (ii) the hCD4+ T-cell response to cells from CIITA-DN pigs, and (iii) the immune status of CIITA-DN pigs. Our results suggest that the Tx of organs from CIITA-DN pigs into primates will be associated with reduced hCD4+ T-cell-mediated rejection.
Materials and methods
Animals
The transfection of CIITA-DN vector to porcine fetal fibroblasts and the production of the CIITA-DN pigs by nuclear transfer were carried out by Revivicor Inc. (Blacksburg, VA), as previously described.13 They were Large White/Landrace/Duroc cross-bred pigs, and were all of WT background. All CIITA-DN pigs were as healthy and free of infections as WT pigs housed under the same conditions at Revivicor. Body weight increased appropriately and by 18 months of age had reached > 250 kg, breeding capacity was normal, and there were no adverse observations at necropsy (not shown). Two CIITA-DN pigs (12 and 18 months old) and three WT siblings (not CIITA-DN transgenic) were killed to collect tissues and to culture pAECs. CIITA-DN pigs were confirmed by detecting the hCIITA-DN gene using PCR (see Supplementary material, Data S1).
All animal care procedures were in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86–23, revised 1985). All procedures had been approved by the University of Pittsburgh IACUC.
Cell culture
Porcine AECs were cultured as previously described.14 Activation of sub-confluent pAECs was carried out by co-culture with recombinant porcine interferon-γ (pIFN-γ; 40 ng/ml; Serotec, Raleigh, NC) with/without porcine tumour necrosis factor-α (TNF-α; 50 ng/ml; Serotec) for adequate periods.
Human CD4+ T-cell proliferation assays
The PBMCs and hCD4+ T cells were isolated as previously described.15 Mixed lymphocyte reactions were carried out as previously described.4–15 Briefly, isolated hCD4+ T cells (as responders) (2 × 105 cells/well) were co-cultured with irradiated (2500 cGy) pAECs (2 × 104 cells/well) with/without activation by pIFN-γ, or isolated hCD4+ T cells (as responders) (1 × 105 cells/well) were co-cultured with irradiated (2500 cGy) pPBMCs (4 × 105 cells/well). The cells were cultured at 37° in 5% CO2 for 7 days (pAEC stimulators) or 5 days (pPBMC stimulators).
Statistical methods
All results are expressed as mean ± SEM. The statistical significance of differences was determined by Student's t-test or non-parametric tests, as appropriate, using graphpad prism version 4 (Graphpad Software, San Diego, CA). A P-value < 0·05 was considered to be statistically significant.
Results
Expression of hCIITA-DN mRNA in CIITA-DN pig organs
An hCIITA-DN transgene under a CAG promoter with an endothelium-specific enhancer (Tie 2) was constructed (Fig. 1) and CIITA-DN pigs were generated by nuclear transfer of the hCIITA-DN transfected porcine fetal fibroblasts. Significant levels of hCIITA-DN mRNA were confirmed in tissues obtained from heart, lung, liver, kidney and aorta in CIITA-DN pigs (see Supplementary material, Fig. S1).
Figure 1.
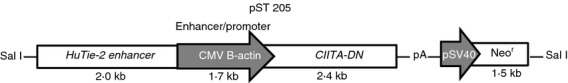
Human dominant-negative mutant class II transactivator (CIITA-DN) construct. A 9·1 kb SalI fragment containing the human Tie-2 enhancer a dominant negative CIITA mutant cDNA driven by the cytomegalovirus enhancer/B-actin promoter (CAG), and an antibiotic resistance cassette, was used to transfect porcine fetal fibroblasts for subsequent nuclear transfer.
Human CIITA-DN suppresses the expression of pCIITA mRNA and SLA class II on pig tissues (e.g. aorta) and cultured pAECs
The expression of SLA class II on the vascular endothelium (aorta, spleen, pancreas, lymph nodes) and leucocytes (in the spleen and lymph nodes) from CIITA-DN pigs was reduced compared with that from WT pigs (Fig. 2).
Figure 2.
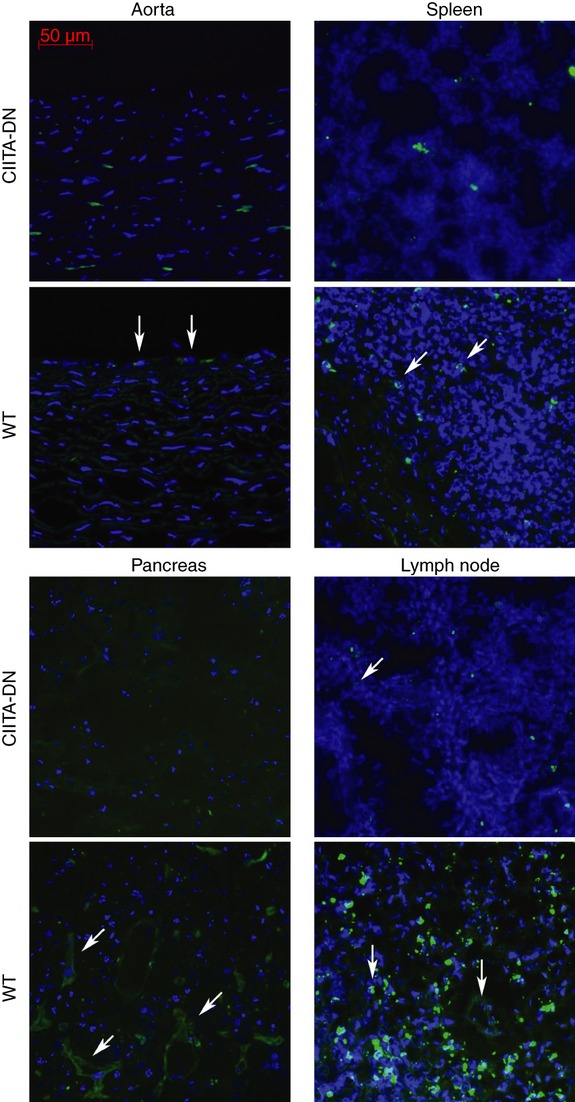
Expression of swine leucocyte antigen (SLA) class II in dominant-negative mutant class II transactivator (CIITA-DN) pig organs. Staining for SLA class II DR (FITC: green) and nuclei (DAPI: blue) in tissues from CIITA-DN and wild-type (WT) pigs was determined by fluorescence microscopy. Expression of SLA class II DR in CIITA-DN pigs was considerably weaker than in WT pigs in all tissues. Results are representative of at least two independent experiments.
Cultured CIITA-DN pAECs were activated with pIFN-γ for 72 hr. Expression of pCIITA mRNA was investigated by reverse transcription PCR and quantitative real-time PCR, and the results were compared with WT pAECs. Reverse transcription PCR demonstrated that pCIITA mRNA was significantly suppressed on CIITA-DN pAECs even after activation with pIFN-γ (Fig. 3a). Minimal increases in pCIITA mRNA were detected during activation, but the levels were still lower than in WT pAECs (Fig. 3b).
Figure 3.
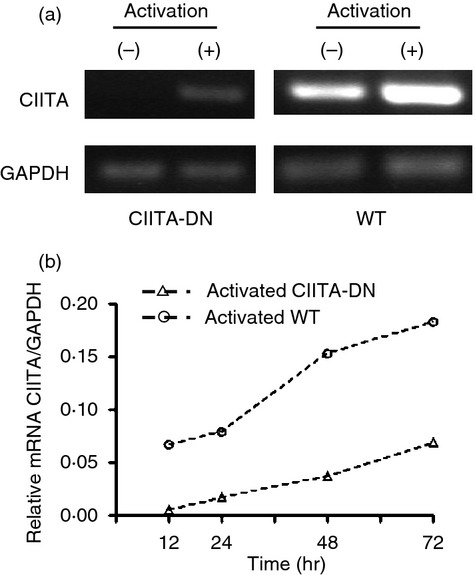
Class II transactivator (CIITA) expression on dominant-negative CIITA (CIITA-DN) and wild-type (WT) porcine aortic endothelial cells (pAECs). Detection of mRNA levels for CIITA in quiescent and activated pAECs. CIITA-DN and WT pAECs were activated with porcine interferon-γ (pIFN-γ; 50 ng/ml) for 48 hr. (a) The expression of CIITA in pAECs was investigated by reverse transcription-PCR using specific primers for pCIITA. Significant levels of mRNA for CIITA were constitutively detected in quiescent WT pAECs, and were markedly increased in activated WT pAECs. In contrast, CIITA-DN mRNA was undetectable in pAECs when cells were quiescent, and minimally increased when cells were activated. (b) Quantitative real-time PCR showed a minimal increase of CIITA mRNA in CIITA-DN pAECs after activation with pIFN-γ (50 ng/ml) for 72 hr.
Although the levels of pCIITA mRNA were increased in CIITA-DN pAECs after activation (Fig. 3), the expression of SLA class II (DR and DQ) on pAECs was strongly suppressed (Fig. 4a,b) even after activation with pIFN-γ ± pTNF-α, indicating that hCIITA-DN strongly inhibits the function of pCIITA. In contrast to SLA class II, the expression of SLA class I on pAECs was up-regulated similarly on WT and CIITA-DN pAECs after activation with pIFN-γ (data not shown).
Figure 4.
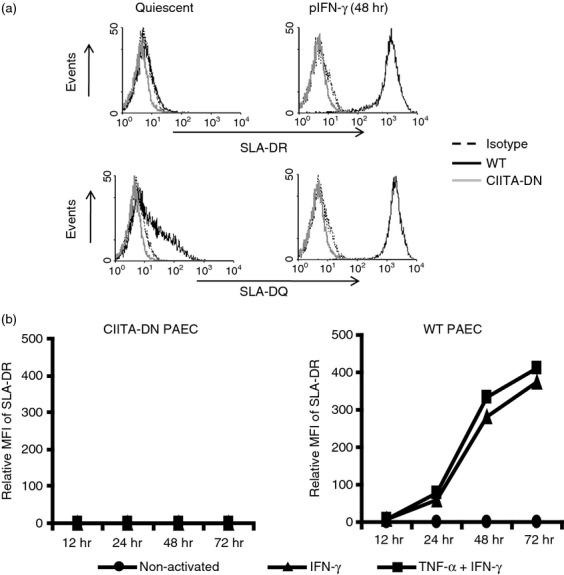
Significant down-regulation of swine leucocyte antigen (SLA) class II expression on cells from dominant-negative mutant class II transactivator (CIITA-DN) pigs. (a) The expression of SLA-class II DR and DQ on CIITA-DN porcine aortic endothelial cells (pAECs) was compared with that on wild-type (WT) pAECs. The pAECs were activated with porcine interferon- γ (pIFN-γ; 50 ng/ml) for 48 hr. The pAECs were stained with specific anti-SLA-DR or DQ monoclonal antibodies. Results are representative of at least three independent experiments. There was no expression of either SLA-DR or DQ on CIITA-DN pAECs, even after activation. Isotype control (dotted line), CIITA-DN (grey line) and WT (solid line). (b) The expression of SLA class II (DR) on quiescent WT pAECs was absent or minimal. However, expression was up-regulated when pAECs were activated with pIFN-γ (50 ng/ml) ± porcine tumour necrosis factor-α (pTNF-α; 50 ng/ml). In contrast to WT pAECs, CIITA-DN pAECs showed complete absence of expression of SLA-DR and DQ (not shown) even when the cells were activated with both pIFN-γ and pTNF-α for 72 hr.
Reduction of anti-pig hCD4+ T-cell responses by constitutive suppression of SLA class II expression
The hCD4+ T-cell response to CIITA-DN pAECs was significantly lower than to WT (sibling) pAECs in both non-activated and activated states (Fig. 5a) (P < 0·01). The reductions in the response of hCD4+ T cells to non-activated and activated CIITA-DN pAECs compared with those of WT pAECs were 60% and 70%, respectively. It is important to note that the suppressive effect of CIITA-DN was consistently found throughout the 9-day period of mixed lymphocyte culture (Fig. 5b).
Figure 5.
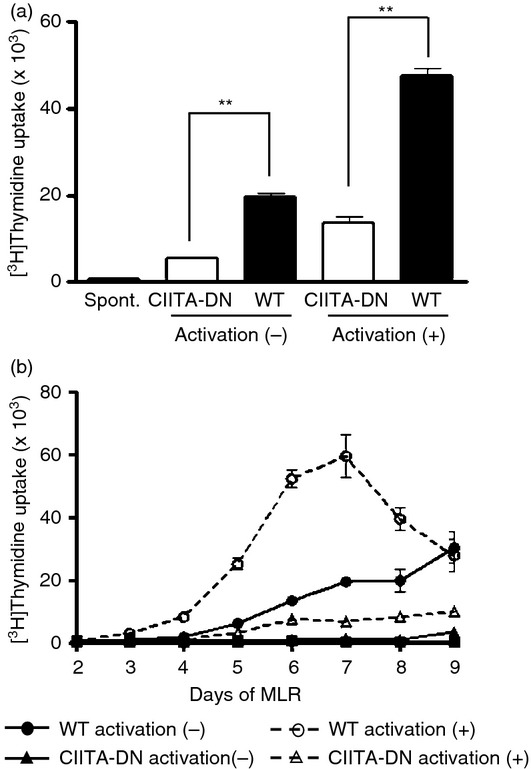
Reduction of the hCD4+ T-cell response to dominant-negative mutant class II transactivator (CIITA-DN) porcine aortic endothelial cells (pAECs). Aortic endothelial cells from CIITA-DN and wild-type (WT; sibling of CIITA-DN) pigs were either non-activated (quiescent) or activated with porcine interferon-γ (IFN-γ; 50 ng/ml) for 48 hr. (a) Human (h) CD4+ T cells were co-cultured with pAECs for 7 days (n = 6). The response of hCD4+ T cells was evaluated by [3H]thymidine incorporation. As a negative control, hCD4+ T cells were cultured with culture medium (spont). There was a significantly lower hCD4+ T-cell response to CIITA-DN pAECs compared with WT pAECs (**P < 0·01). (b) The hCD4+ T-cell responses to both CIITA-DN and WT pAECs were evaluated over a period of 9 days in mixed lymphocyte reaction (2–9 days co-culture). Results are representative of three independent experiments. The hCD4+ T-cell response to CIITA-DN pAECs remained minimal throughout the 9 days of culture.
SLA class II expression on PBMCs from CIITA-DN pigs was down-regulated and resulted in suppression of the hCD4+ T-cell response
In the generation of CIITA-DN pigs, an endothelial-specific enhancer was used to enhance the tissue-specific expression hCIITA-DN in pAECs. As well as suppression of SLA class II in pAECs, CIITA-DN pigs also showed significantly down-regulated SLA class II expression on APCs (B cells) (Fig. 6a,b). A moderate reduction (40–50%) in expression of SLA class II on B cells from CIITA-DN pigs was observed compared with expression on WT B cells (100% expression) (Fig. 6a). However, the percentage reduction of relative mean fluorescence intensity of SLA class II expression on B cells from CIITA-DN pigs was 90% compared with WT pigs (Fig. 6b). There was a significantly lower response of hCD4+ T cells to CIITA-DN pPBMCs compared with WT pig and human PBMCs (P < 0·01) (Fig. 6c). The percentage reductions of the hCD4+ T-cell responses to CIITA-DN stimulators were 50% and 30%, respectively, compared with WT pig and human PBMC stimulators.
Figure 6.
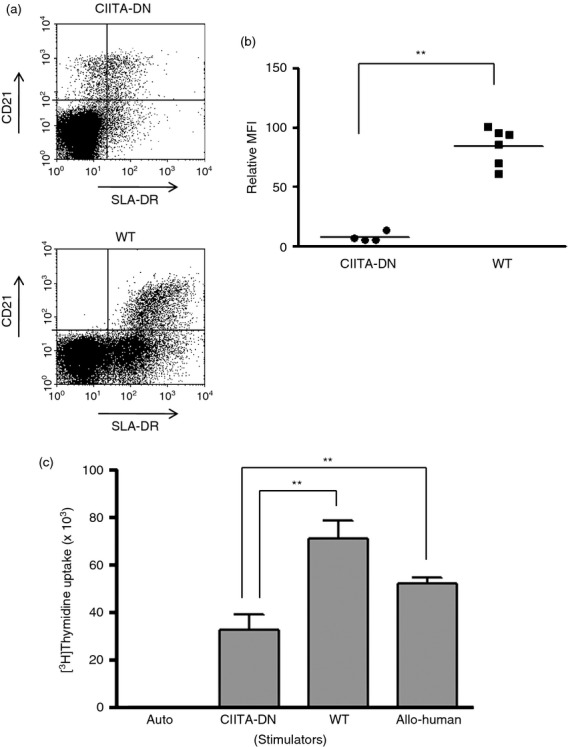
Significant reduction of the human (h) CD4+ T-cell response to dominant-negative mutant class II transactivator (CIITA-DN) antigen-presenting cells. Expression of swine leucocyte antigen (SLA) class II (DR) on CD21+ B cells in peripheral blood mononuclear cells (PBMCs) from CIITA-DN (n = 4) and wild-type (WT) pigs (n = 6) was compared. (a) The expression of SLA class II (DR) on CD21+ B cells in CIITA-DN porcine (p) PBMCs was down-regulated compared with that on WT pPBMCs. Results are representative of four independent experiments. (b) There was significantly lower expression of SLA class II (DR) on CD21+ B cells from CIITA-DN pPBMCs than WT pPBMCs (**P < 0·01). (c) Human CD4+ T cells were co-cultured with CIITA-DN and WT (sibling with CIITA-DN pigs) pPBMCs (n = 6), and allogeneic hPBMCs (n = 6) for 6 days. The responses of hCD4+ T cells were measured by [3H]thymidine incorporation. As a negative control, hCD4+ T cells were cultured with autologus PBMCs (auto). There was a significantly lower hCD4+ T-cell response to CIITA-DN pPBMCs than to WT and allogeneic hPBMCs (**P < 0·01).
Immune status of CIITA-DN pigs is similar to that of WT pigs
Lymphocyte subset numbers and T-cell function were compared between CIITA-DN and WT pigs. Although there was no significant difference in the frequencies of CD3+ T cells, CD21+ B cells, CD8+ T cells and CD4+ CD25high regulatory T cells in PBMCs between CIITA-DN pigs and non-CIITA (WT) sibling pigs, there was a significantly lower frequency of CD4+ T cells in PBMCs from CIITA-DN pigs (20%) compared with non-CIITA (WT) sibling pigs (30%) (P < 0·01) (see Supplementary material, Fig. S2A). However, T-cell responses to hPBMCs were equivalent between CIITA-DN and sibling WT pigs (see Supplementary material, Fig. S2B). (CIITA-DN pigs are as healthy as WT pigs housed at Revivicor.)
Discussion
As both pigs and humans normally express MHC class II on their ECs upon activation,16–17 donor MHC class II molecules might play a crucial role in graft rejection by activation of the recipient's direct T-cell response. The accessory interactions for direct T-cell recognition of pAPCs by human T cells are largely intact.18 It has been shown that direct xenorecognition of SLA class II by human T cells is greater than direct allorecognition of HLA class II by human T cells.4 AECs are permanent components of grafts. However, unlike hAECs, pAECs constitutively express co-stimulatory molecules.6,7 This species difference would be likely to result in greater immunogenicity of pig xenografts compared with human allografts. In addition, donor-derived passenger leucocytes, including APCs, migrate into host lymphoid tissues after xenograft placement and may be responsible for initiating graft rejection through the direct pathway (as in allograft rejection).20,21 Therefore, the direct route of xenoantigen presentation (activation of human T cells by both pAPCs and ECs) will be functional after pig-to-primate xenoTx.
There are several potential strategies for reducing the human T-cell immune response to pig xenografts.23–26 Suppression of these interactions by administering co-stimulatory blockade agents has been widely used in experimental models.23–29 In contrast to non-specific immune suppression, it would be ideal if only the donor-specific responses could be suppressed, possibly, for example, by the xenoTx of organs from pCTLA4-Ig transgenic pigs.13–19
One potential strategy would be to suppress MHC class II expression on ECs in transplanted organs,12 with a view to diminishing their long-term immunogenicity. We constructed a mutated hCIITA gene lacking the coding regions for the N-terminal domain, with the objective of creating DN mutants. The aim was that the mutated hCIITA proteins, lacking transcription activation activity, would bind to the promoter regions of SLA class II genes and competitively inhibit the binding of endogenous pCIITA. However, high levels of constitutive expression of hCIITA-DN, particularly in the thymus and APCs (e.g. by using a CIITA-DN construct under the control of a widely expressed constitutive promoter or by gene knock-out), is associated with a decreased number of pCD4+ T cells in the blood, with concomitant risks of infection.30 Such pigs would be immunodeficient, as are patients with the bare lymphocyte syndrome.11 Therefore, an hCIITA-DN transgene under a CAG promoter with an endothelium-specific enhancer was constructed to ensure high-level expression of hCIITA-DN on endothelium but modest expression on lymphocytes. Indeed, the expression of SLA class II on pACEs was significantly suppressed even after activation by cytokines.
Because of the limited number of pigs available to us, the number of CIITA-DN pigs used for the investigation of SLA class II expression on pAECs was small (n = 2). It will be important to confirm the effect of CIITA-DN on the down-regulation of SLA class II on pAECs when this genetic modification is combined with others, e.g. in α1,3-galactosyltransferase gene-knockout (GTKO) pigs transgenic for one or more human complement-regulatory proteins.
In contrast to surface expression of SLA class II, there was still a significant level of pCIITA mRNA in CIITA-DN pAECs although the level was less than that in WT pAECs. The reduced mRNA expression of pCIITA in both non-activated and activated CIITA-DN pAECs is explained by the effect of hCIITA-DN. However, the mechanism of remaining expression and up-regulated expression of pCIITA after activation is unclear.
Interferon-γ inhibits the functional activity of CMV promoter/enhancers,31 which were used for hCIITA-DN, and therefore it is possible that IFN-γ might interfere in the functional activity of hCIITA-DN. The extent of suppression of SLA class II expression would be associated with the level of competitive inhibitors of hCIITA-DN. Whether other mechanisms of down-regulation of SLA class II in CIITA-DN pigs, e.g. auto-regulation, or competitive inhibition by hCIITA-DN binding to transactivator receptors, result in down-regulation of transactivator expression is unknown.
It was important to investigate the immunological profile of CIITA-DN pigs because SLA class II down-regulation on the CIITA-DN pig cells, especially APCs, may affect the frequencies of the lymphocyte populations, particularly by reducing the number of CD4+ T cells, as occurs in MHC class II-knockout mice.32–33 In such cases, they would develop impaired immunity, associated with a susceptibility to infection, resulting in it not being possible to maintain them in good health. Although CIITA-DN pigs showed a lower frequency of CD4+ T cells in the blood compared with sibling WT pigs, the difference was only 10%, and there was no difference in the frequency of CD3+ T cells. Furthermore, CIITA-DN pigs had a normal T-cell proliferative response to human stimulator cells, suggesting that CIITA-DN pigs are not immunologically compromised. In support of this conclusion, GTKO pigs transgenic for CD46 and CIITA-DN have been developed by breeding by Revivicor; these pigs are not at increased risk of infection, and breed and grow normally compared with WT pigs housed under the same conditions.
In the present study, we demonstrated that a mutated form of the hCIITA gene, coding for a protein lacking the N-terminal 335 amino acid, acts as a potent and constitutive DN suppressor of SLA class II expression in pAECs. It is important to note that total suppression of SLA class II expression on APCs (e.g. dendritic cells) is not required for substantial reduction in their antigen-presenting capacity, as discussed above. The expression of SLA class II on CD21+ B cells was evaluated with regard to the suppressive effect of CIITA-DN because (i) there is a high level of SLA class II expression on B cells compared with other cells (e.g. CD14+ monocyte/macrophage), (ii) there is a large population of B cells compared with dendritic cells in PBMCs,34–35 and (iii) B cells can also stimulate the T cells as APCs. (In contrast, for in vitro study, dendritic cells have to be generated from monocytes, necessitating the application of several conditioning media.)
The results demonstrated that, in contrast to CIITA-DN pAECs, moderate SLA class II expression remained on PBMCs, especially on B cells. However, the residual low level of SLA class II expression (< 30–50% compared with WT) significantly reduced the stimulation associated with direct xenorecognition by hCD4+ T cells, even when compared with the alloresponse, suggesting that sensitization by passenger leucocytes might be reduced. CIITA-DN pigs may also prevent SLA class II-specific sensitization through the indirect pathway because of down-regulation of SLA class II expression. Therefore, CIITA-DN pigs may reduce SLA class II-mediated rejection through both direct and indirect pathways. However, primate T cells may proliferate against various peptides (not only SLA class I and II) derived from pig cells through the indirect pathway.1,2 Therefore, additional strategies (e.g. co-stimulatory blockade) to suppress sensitization to pig antigens expressed on host APCs through the indirect pathway will be required even when organs from CIITA-DN pigs have been transplanted.
Although the hCD4+ T-cell response to CIITA-DN pAECs was significantly reduced, characterization of hCD4+ T cells after co-culture with CIITA-DN pAECs would provide valuable information with regard to the underlying mechanism by which this effect is obtained (e.g. anergy, regulatory phenotype, or apoptosis). However, it would be preferable to carry out this investigation in an in vivo model (CIITA-DN pig to non-human primate organ Tx) rather than in vitro.
The induction of donor-specific tolerance is a recognized ultimate goal in xenoTx.37 Down-regulation of SLA class II might affect the development of SLA class II-related tolerance. In the present study, the expression of SLA class II on APCs (B cells) in contrast to pAECs was preserved, although the level of SLA class II expression was lower than on APCs of WT pigs. In addition, sufficient numbers of CD4+ T cells were detected in the blood of CIITA-DN pigs to suggest that SLA class II expression on haematopoietic cells and in the thymus had been preserved. In addition, donor-specific tolerance would be associated with the expression not only of SLA class II, but also with other pig antigens. Therefore, it would be necessary to carry out further experiments to specifically investigate whether the Tx of organs from CIITA-DN pigs might interfere with the development of donor-specific tolerance in xenoTx.
CIITA has also been shown to play a role in up-regulating the expression of MHC class I.38–39 However, in our studies, suppression of CIITA did not have a consistent effect on SLA class I expression. Furthermore, although CIITA-independent expression of HLA-DQ has been demonstrated in Epstein–Barr virus-transformed B cells,40 we did not see a differential effect of CIITA-DN on SLA-DR and SLA-DQ expression.
Taken together, our data suggest that (i) organs/tissue/cells from CIITA-DN pigs (under the control of a constitutive promoter and an EC-specific enhancer) will be associated with a reduced hCD4+ T-cell response, and (ii) CIITA-DN pigs are not immunocompromised. Although CIITA-DN pigs on a GTKO/CD46 background have been produced, these pigs remain suboptimal as sources of organs because thrombotic microangiopathy still occurs. XenoTx of vascularized organs from CIITA-DN pigs on a background of GTKO transgenic for a human coagulation-regulatory protein (e.g. GTKO/thrombomodulin) will be necessary to confirm the beneficial effect of the CIITA-DN transgene. We suggest that (iii) organs from GTKO pigs transgenic for both human complement-regulatory and coagulation-regulatory proteins and with the CIITA-DN mutation should be significantly protected from complement-mediated lysis, the development of thrombotic microangiopathy, and the CD4+ T-cell response, and (iv) long-term pig xenograft survival with clinically acceptable levels of immunosuppression might be facilitated.
Acknowledgments
The authors thank Seung Eun and Jason Fang for technical help in performing in vitro assays. Work in our laboratory is supported in part by National Institutes of Health grants U01-AI068642 (DKCC), R21-A1074844 (DKCC), U19-AI090959 (DKCC),and RO3 (AI096296, HH) and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor Inc., Blacksburg, VA, USA.
Glossary
- AEC
aortic endothelial cell
- CIITA-DN
MHC Class II transactivator dominant-negative mutant
- EC
endothelial cell
- GTKO
α1,3-galactosyltransferase gene-knockout
- h
human
- p
porcine
- PBMC
peripheral blood mononuclear cell
- SLA
swine leucocyte antigen
- Tx
transplantation
- WT
wild-type
Authors' contribution
HH participated in research design, in the performance of the research, interpretation of the results and in writing of the manuscript. TC, WW, CL, KI and LF participated in the performance of the research and in review of the manuscript. TES and YD suggested this approach, participated in research design, in the interpretation of the results and in review of the manuscript; in addition, YD supervised the construction and testing of the hCIITA-DN vector. CJP and DA supervised the nuclear transfer/embryo transfer of the genetically engineered pigs, and participated in review of the manuscript. DKCC participated in research design, in interpretation of the results, and in writing of the manuscript.
Disclosures
CJP and DA are employees of Revivicor Inc., Blacksburg, VA, USA. YD is an employee of Lung Biotechnology Nanjing, Nanjing, China. The other authors have no conflict of interest in this study.
Supporting Information
Figure S1. Expression of human dominant-negative mutant class II transactivator (CIITA-DN) mRNA in CIITA-DN pig organs.
Figure S2. Immune status of dominant-negative mutant class II transactivator (CIITA-DN) pigs.
References
- Dorling A, Lombardi G, Binns R, Lechler RI. Detection of primary direct and indirect human anti-porcine T cell responses using a porcine dendritic cell population. Eur J Immunol. 1996;26:1378–87. doi: 10.1002/eji.1830260630. [DOI] [PubMed] [Google Scholar]
- Brouard S, Gagne K, Blancho G, Soulillou JP. T cell response in xenorecognition and xenografts: a review. Hum Immunol. 1999;60:455–68. doi: 10.1016/s0198-8859(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Davila E, Byrne GW, Labreche PT, et al. T-cell responses during pig-to-primate xenotransplantation. Xenotransplantation. 2006;13:31–40. doi: 10.1111/j.1399-3089.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Hara H, Tai HC, et al. Suppressive efficacy and proliferative capacity of human regulatory T cells in allogeneic and xenogeneic responses. Transplantation. 2008;86:1452–62. doi: 10.1097/TP.0b013e318188acb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravery CA, Batten P, Yacoub MH, Rose ML. Direct recognition of SLA- and HLA-like class II antigens on porcine endothelium by human T cells results in T cell activation and release of interleukin-2. Transplantation. 1995;60:1024–33. [PubMed] [Google Scholar]
- Murray AG, Khodadoust MM, Pober JS, Bothwell AL. Porcine aortic endothelial cells activate human T cells: direct presentation of MHC antigens and costimulation by ligands for human CD2 and CD28. Immunity. 1994;1:57–63. doi: 10.1016/1074-7613(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Rogers NJ, Jackson IM, Jordan WJ, Hawadle MA, Dorling A, Lechler RI. Cross-species costimulation: relative contributions of CD80, CD86, and CD40. Transplantation. 2003;75:2068–76. doi: 10.1097/01.TP.0000069100.67646.08. [DOI] [PubMed] [Google Scholar]
- Corcoran PC, Horvath KA, Singh AK, Hoyt RF, Jr, Thomas ML, 3rd, Eckhaus MA, Mohiuddin MM. Surgical and nonsurgical complications of a pig to baboon heterotopic heart transplantation model. Transplant Proc. 2010;42:2149–51. doi: 10.1016/j.transproceed.2010.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, Bottino R, Trucco M, Cooper DK. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–83. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- Reith W, Leibundgut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol. 2005;5:793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu Rev Immunol. 2001;19:331–73. doi: 10.1146/annurev.immunol.19.1.331. [DOI] [PubMed] [Google Scholar]
- Yun S, Gustafsson K, Fabre JW. Suppression of human anti-porcine T-cell immune responses by major histocompatibility complex class II transactivator constructs lacking the amino terminal domain. Transplantation. 1998;66:103–11. doi: 10.1097/00007890-199807150-00016. [DOI] [PubMed] [Google Scholar]
- Phelps CJ, Ball SF, Vaught TD, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16:477–85. doi: 10.1111/j.1399-3089.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- Hara H, Long C, Lin YJ, Tai HC, Ezzelarab M, Ayares D, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–74. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- Hara H, Koike N, Long C, et al. Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs. Invest Ophthalmol Vis Sci. 2011;52:5278–86. doi: 10.1167/iovs.10-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo JK, Seebach JD, Nickeleit V, Shimizu A, Lei H, Sachs DH, Madsen JC. Species differences in the expression of major histocompatibility complex class II antigens on coronary artery endothelium: implications for cell-mediated xenoreactivity. Transplantation. 1997;64:1315–22. doi: 10.1097/00007890-199711150-00014. [DOI] [PubMed] [Google Scholar]
- Houser SL, Benjamin LC, Wain JC, Madsen JC, Allan JS. Constitutive expression of major histocompatibility complex class II antigens in pulmonary epithelium and endothelium varies among different species. Transplantation. 2004;77:605–7. doi: 10.1097/01.tp.0000114285.63313.e7. [DOI] [PubMed] [Google Scholar]
- Yamada K, Sachs DH, Dersimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;155:5249–56. [PubMed] [Google Scholar]
- Koshika T, Phelps C, Fang J, et al. Relative efficiency of porcine and human cytotoxic T-lymphocyte antigen 4 immunoglobulin in inhibiting human CD4+ T-cell responses co-stimulated by porcine and human B7 molecules. Immunology. 2011;134:386–97. doi: 10.1111/j.1365-2567.2011.03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustman DL, Steinman RM, Gebel HM, Hauptfeld V, Davie JM, Lacy PE. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proc Natl Acad Sci USA. 1984;81:3864–8. doi: 10.1073/pnas.81.12.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–14. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenda V, Le Mauff B, Boeffard F, Cassard A, Jugeau N, Soulillou JP, Anegon I. Intact pancreatic islet function despite humoral xenorecognition in the pig-to-monkey combination. Transplantation. 1998;66:1485–95. doi: 10.1097/00007890-199812150-00012. [DOI] [PubMed] [Google Scholar]
- Buhler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69:2296–304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- Vaughan AN, Malde P, Rogers NJ, Jackson IM, Lechler RI, Dorling A. Porcine CTLA4-Ig lacks a MYPPPY motif, binds inefficiently to human B7 and specifically suppresses human CD4+ T cell responses costimulated by pig but not human B7. J Immunol. 2000;165:3175–81. doi: 10.4049/jimmunol.165.6.3175. [DOI] [PubMed] [Google Scholar]
- Plege A, Schwinzer R. Stimulatory and inhibitory receptor interactions in xenotransplantation. Curr Opin Organ Transplant. 2010;15:219–23. doi: 10.1097/MOT.0b013e328336b8e5. [DOI] [PubMed] [Google Scholar]
- Ekser B, Kumar G, Veroux M, Cooper DK. Therapeutic issues in the treatment of vascularized xenotransplants using gal-knockout donors in nonhuman primates. Curr Opin Organ Transplant. 2011;16:222–30. doi: 10.1097/MOT.0b013e3283446c3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in α1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–12. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekser B, Rigotti P, Gridelli B, Cooper DK. Xenotransplantation of solid organs in the pig-to-primate model. Transpl Immunol. 2009;21:87–92. doi: 10.1016/j.trim.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Charerntantanakul W, Roth JA. Biology of porcine T lymphocytes. Anim Health Res Rev. 2006;7:81–96. doi: 10.1017/S1466252307001235. [DOI] [PubMed] [Google Scholar]
- Harms JS, Splitter GA. Interferon-γ inhibits transgene expression driven by SV40 or CMV promoters but augments expression driven by the mammalian MHC I promoter. Hum Gene Ther. 1995;6:1291–7. doi: 10.1089/hum.1995.6.10-1291. [DOI] [PubMed] [Google Scholar]
- Williams GS, Malin M, Vremec D, Chang CH, Boyd R, Benoist C, Mathis D. Mice lacking the transcription factor CIITA – a second look. Int Immunol. 1998;10:1957–67. doi: 10.1093/intimm/10.12.1957. [DOI] [PubMed] [Google Scholar]
- Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci USA. 1999;96:10338–43. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Parkhouse RM. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunology. 1996;89:76–83. doi: 10.1046/j.1365-2567.1996.d01-705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamin A, Gorin S, Le Potier MF, Kuntz-Simon G. Characterization of conventional and plasmacytoid dendritic cells in swine secondary lymphoid organs and blood. Vet Immunol Immunopathol. 2006;114:224–37. doi: 10.1016/j.vetimm.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Dorling A, Lechler RI. T cell-mediated xenograft rejection: specific tolerance is probably required for long term xenograft survival. Xenotransplantation. 1998;5:234–45. doi: 10.1111/j.1399-3089.1998.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Sachs DH, Sykes M, Yamada K. Achieving tolerance in pig-to-primate xenotransplantation: reality or fantasy. Transpl Immunol. 2009;21:101–5. doi: 10.1016/j.trim.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BK, Chin KC, Olsen JC, et al. Induction of MHC class I expression by the MHC class II transactivator CIITA. Immunity. 1997;6:591–600. doi: 10.1016/s1074-7613(00)80347-7. [DOI] [PubMed] [Google Scholar]
- Gobin SJ, Peijnenburg A, Keijsers V, van den Elsen PJ. Site α is crucial for two routes of IFN γ-induced MHC class I transactivation: the ISRE-mediated route and a novel pathway involving CIITA. Immunity. 1997;6:601–11. doi: 10.1016/s1074-7613(00)80348-9. [DOI] [PubMed] [Google Scholar]
- Zhou H, Su HS, Zhang X, Douhan J, 3rd, Glimcher LH. CIITA-dependent and -independent class II MHC expression revealed by a dominant negative mutant. J Immunol. 1997;158:4741–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression of human dominant-negative mutant class II transactivator (CIITA-DN) mRNA in CIITA-DN pig organs.
Figure S2. Immune status of dominant-negative mutant class II transactivator (CIITA-DN) pigs.


