Summary
Indomethacin is a cyclo-oxygenase inhibitor, and shows therapeutic potential for various eosinophilic skin diseases, particularly eosinophilic pustular folliculitis. One of the unique characteristics of indomethacin is that, unlike other non-steroidal anti-inflammatory drugs, it is a potent agonist of chemoattractant receptor-homologous molecule expressed on T helper type 2 cells (CRTH2), a receptor for prostaglandin D2 (PGD2). This study investigated the pharmacological actions of indomethacin on eosinophil migration to clarify the actual mechanisms underlying the therapeutic effects of indomethacin on eosinophilic pustular folliculitis. Eosinophils exhibited chemokinetic and chemotactic responses to both PGD2 and indomethacin through CRTH2 receptors. Pre-treatment of eosinophils with indomethacin greatly inhibited eosinophil migration to PGD2 and, to a much lesser extent, to eotaxin (CCL11); these effects could be mediated by homologous and heterologous desensitization of eosinophil CRTH2 and CCR3, respectively, by agonistic effects of indomethacin on CRTH2. Indomethacin also cancelled a priming effect of Δ12-PGJ2, a plasma metabolite of PGD2, on eosinophil chemotaxis to eotaxin. Indomethacin down-modulated cell surface expression of both CRTH2 and CCR3. Hair follicle epithelium and epidermal keratinocytes around eosinophilic pustules together with the eccrine apparatus of palmoplantar lesions of eosinophilic pustular folliculitis were immunohistochemically positive for lipocalin-type PGD synthase. Indomethacin may exert therapeutic effects against eosinophilic skin diseases in which PGD2-CRTH2 signals play major roles by reducing eosinophil responses to PGD2.
Keywords: CCR3, chemoattractant receptor-homologous molecule expressed on T helper type 2 cells, desensitization, eotaxin, Lipocalin-type prostaglandin D synthase
Introduction
Eosinophilic pustular folliculitis (EPF) is an eosinophilic inflammatory skin disease characterized by pruritic follicular papulopustules that tend to form in an annular configuration on the face, and occasionally on the trunk and extremities. Histopathologically, a number of eosinophils infiltrate around and into hair follicles. Uncommonly, EPF also affects the palms and soles, which lack the hair follicle apparatus.1 Our recent study demonstrated that, in palmoplantar lesions of EPF, intra-epidermal eccrine ducts may be the sites that are predominantly affected.2 Occasionally EPF is resistant to topical or systemic corticosteroids, but, importantly, shows a consistently good response to systemic indomethacin.3–4 Indomethacin can even be used as a diagnostic tool for EPF,5 although the underlying therapeutic mechanisms have not been fully elucidated.
Prostaglandin D2 (PGD2) is one of the cyclo-oxygenase metabolites of arachidonic acid. It is synthesized by the isomerization of PGH2 through the enzymatic activity of PGD synthase. Two types of PGD synthase have been identified: haematopoietic-type PGD synthase (H-PGDS); and lipocalin-type PGD (L-PGDS).6–7 H-PGDS is expressed by haematopoietic cells, such as mast cells,8–9 T helper type 2 (Th2) cells,10 dendritic cells,11 eosinophils12 and basophils.13 These cells produce PGD2 in response to a variety of stimuli. L-PGDS is principally present in meningeal cells, epithelial cells of the choroid plexus, and oligodendrocytes in the brain.14 Prostaglandin D2 shows a wide range of biological activities, including vasodilatation, bronchoconstriction, inhibition of platelet aggregation and regulation of the sleep–wake cycle.14–18 A number of recent lines of evidence have indicated that PGD2 is also involved in allergic inflammation. Mice that over-produce PGD2 exhibit enhanced allergic lung inflammation with eosinophilia and Th2-type cytokine production.19 Prostaglandin D2 promotes skin inflammation of IgE-mediated chronic skin responses and the elicitation phase of contact hypersensitivity.20–21
Biological activities of PGD2 are mediated by the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) and the D prostanoid receptor. These receptors are members of the G protein-coupled, seven transmembrane receptor family. In eosinophils, CRTH2 signals induce calcium mobilization and cell migration.22 Eosinophil degranulation is also promoted by CRTH2 stimulation.23 Expression of CRTH2 in eosinophils is increased in patients with atopic dermatitis, chronic urticaria or prurigo.24 Antagonists of CRTH2 ameliorate skin inflammation in IgE-mediated chronic skin responses, contact hypersensitivity and cedar pollen dermatitis in accordance with reduced numbers of dermal eosinophils.20,21
Recent studies have identified indomethacin as a potent agonist of CRTH2,26 causing PGD2-like and eotaxin (CCL11) -like responses in eosinophils.27 These findings are somewhat inconsistent, offering clinical evidence that indomethacin is a useful therapeutic tool for EPF3–4 and other eosinophilic skin diseases, such as angiolymphoid hyperplasia with eosinophilia28 and recurrent cutaneous eosinophilic vasculitis.29 In this respect, our previous evidence might provide one explanation for this discrepancy.30 Treatment of eosinophils and lymphocytes with indomethacin resulted in reduced cell surface expression of CRTH2 on these cells. However, functional modulations in eosinophils by indomethacin have yet to be fully determined. The present study sought to elucidate the pharmacological effects of indomethacin on eosinophil migration in response to PGD2 and eotaxin (CCL11), to obtain insights into mechanisms for the amelioration of eosinophilic skin inflammation by indomethacin.
Materials and methods
Isolation of eosinophils
Peripheral blood anti-coagulated with EDTA was obtained from healthy volunteers with informed consent. After sedimentation of red blood cells using 6% Dextran-T500 (Sigma-Aldrich, St Louis, MO) in physiological saline, eosinophils (density > 1·085) were semi-purified by Percoll (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) gradient centrifugation.31 Eosinophils were further purified by negative selection with CD16 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of eosinophils was > 99%. This study was approved by the Ethics Committee of Tokyo Medical and Dental University (No. 887).
Chemotaxis assay
Twenty-five-microlitre aliquots of eosinophils at 4 × 107 to 6 × 107/ml (in PBS containing 10 mm HEPES, 0·1% BSA, 1 mm Ca2+, 1 mm Mg2+ and 10 mm glucose) were placed on the top filter membrane of a 96-well micro-chemotaxis chamber with 5-μm pores (ChemoTx® Disposable Chemotaxis System; NeuroProbe, Gaithersburg, MD).27–32 These plates were incubated at 37° in a humidified CO2 incubator for 60 min. Cells in the upper and lower chambers were collected and stored at − 80° until use.
Migration of eosinophils was assessed by photometric assay using eosinophil peroxidase activity, as described previously with modifications.33–36 In brief, eosinophil samples were thawed and mixed with an equal volume of 1% hexadecyltrimethylammonium bromide (Wako Pure Chemical Industries, Osaka, Japan) in 50-mm potassium phosphate buffer (pH 6·4) to entirely lyse eosinophils. Samples were then reacted with twice the volume of 4·5-mmo-phenylenediammonium dichloride in 50 mm HEPES containing 4·5 mm KBr and 3·3 mm H2O2 for 15 min. The reaction was stopped with 1M H2SO4. Optical density was read at 490 nm with a MicroReader (Model 680; Bio-Rad Laboratories, Hercules, CA). A standard curve was drawn by plotting the eosinophil peroxidase activities of serially diluted eosinophil samples for assessment of the region of linear response. Results were expressed as percentages of eosinophil migration [(cells in lower chambers)/(cells in lower chambers + cells in upper chambers) × 100].
Flow cytometric analyses
Single-cell suspensions in PBS/5% fetal calf serum were stained with FITC-conjugated CCR3 (R&D Systems, Minneapolis, MN) and/or phycoerythrin-conjugated anti-CD294 (CRTH2; Miltenyi Biotec) antibodies. FITC-conjugated or phycoerythrin-conjugated mouse IgG1 antibodies (Dako, Glostrup, Denmark) were used as negative controls. Cells were analysed using a FACSCalibur flow cytometer (Becton Dickinson and Co., Franklin Lakes, NJ).
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections were incubated with mouse anti-human L-PGDS monoclonal antibody (1B7) or rabbit anti-human L-PGDS polyclonal antibody (kindly provided by Urade and Aritake; Osaka Bioscience Institute, Osaka, Japan) overnight following heat treatment with Dako Real Target Retrieval Solution (Dako) and inactivation of internal peroxidase activity with H2O2. These sections were then reacted with horseradish peroxidase-labelled EnVision polymer (Dako). Reactive products were visualized by diaminobenzidine.
Statistical analyses
An analysis of variance test followed by Scheffe's F-test was performed to assess the statistical significance of differences between means.
Results
Indomethacin and PGD2 induce both chemokinetic and chemotactic responses in eosinophils via CRTH2 receptors
To begin to understand the direct effects of indomethacin on eosinophils, migration of eosinophils to indomethacin was assessed. Indomethacin (Wako Pure Chemical Industries) in the upper chambers induced weak eosinophil migration to medium (lower chambers), which became more marked when indomethacin was added to both upper and lower chambers (Fig. 1a). This indicated that indomethacin induced chemokinetic responses in eosinophils. In addition, restriction of indomethacin to only the lower chambers resulted in more significant migration of eosinophils, in a dose-dependent manner. Indomethacin therefore has both chemokinetic and chemotactic effects on eosinophils.
Figure 1.
Indomethacin and prostaglandin D2 (PGD2) induced chemotaxis in eosinophils through chemoattractant receptor-homologous molecule expressed on T helper type 2 cells (CRTH2) receptors. Cells were mixed with either indomethacin, PGD2, or medium, and then applied to the upper chamber. Eosinophil migration to the lower chamber containing either indomethacin, PGD2, or medium was assessed by photometric assay using eosinophil peroxidase activity (a, b). Eosinophil migration in response to indomethacin and PGD2 (lower chambers) was assessed in the presence of CAY10471 (3 nm), a CRTH2 antagonist, in the upper chamber (c). Representative results of two independent experiments are shown.
Prostaglandin D2 is known as a chemotactic factor for eosinophils.22 However, another study denied PGD2-induced chemotactic responses, but not chemokinetic responses.23 This study therefore attempted to verify actual effects of PGD2 on eosinophil migration. Eosinophils exhibited marked migration to PGD2 (Cayman Chemical, Ann Arbor, MI) in the lower chambers, whereas PGD2 in the upper chambers and in upper/lower chambers also induced weak and moderate cell migration, respectively (Fig. 1b). Checkerboard analysis clearly revealed the chemotactic action of PGD2 on eosinophils (Table 1). These effects of indomethacin and PGD2 were dependent on CRTH2, as cell migration was almost entirely abolished by pre-treatment with CAY10471 (Cayman Chemical), a specific CRTH2 antagonist (Fig. 1c).
Checkerboard analysis for migration to prostaglandin D2 (PGD2)
| PGD2 in upper chamber (nm) | |||||
|---|---|---|---|---|---|
| 0 | 1 | 5 | 10 | ||
| PGD2 in lower chamber (nm) | |||||
| 0 | 0·4 ± 0·7 | 0·2 ± 0·3 | 2·5 ± 2·0 | 3·9 ± 1·4 | |
| 1 | 0·5 ± 0·9 | 0·6 ± 0·8 | 3·3 ± 2·0 | 2·3 ± 0·6 | |
| 5 | 22·1 ± 3·8 | 8·7 ± 2·2 | 7·4 ± 1·3 | 6·5 ± 1·0 | |
| 10 | 32·0 ± 5·5 | 19·2 ± 3·1 | 19·9 ± 5·2 | 15·7 ± 2·6 | |
Percentage of migrated eosinophils.
Indomethacin pre-treatment inhibits eosinophil migration to PGD2 and eotaxin (CCL11)
We next sought to determine the effects of indomethacin on eosinophil migration to PGD2 and eotaxin (CCL11). Pre-treatment of eosinophils with indomethacin (37°, 90 min) inhibited PGD2-induced chemotaxis in a dose-dependent manner, whereas low-dose indomethacin (10−10 m) appeared to exert weak stimulatory effects on eosinophil migration (Fig. 2a). Chemotactic response to eotaxin (CCL11) was more mildly inhibited by indomethacin than the response to PGD2 (Fig. 2b). Again, a low dose of indomethacin weakly stimulated cell migration to eotaxin. On the other hand, the chemotactic response of eosinophils was not suppressed by pre-treatment with diclofenac (Wako Pure Chemical Industries), a cyclo-oxygenases inhibitor (Fig. 2c,d).
Figure 2.

Eosinophil chemotaxis is inhibited by pre-treatment with indomethacin, but not diclofenac. Eosinophils were pre-treated with indomethacin or diclofenac for 90 min at 37°, followed by centrifugation and application to the upper chamber. Eosinophil migration to prostaglandin D2 (PGD2) (a, c) and eotaxin (CCL11) (b, d) in the lower chambers was assessed by eosinophil peroxidase photometric assay. *P < 0·05. Representative results of at least three independent experiments are shown.
Previous studies have suggested that eotaxin and/or eotaxin-3 (CCL26), both of which are ligands for CCR3 on eosinophils, may contribute to eosinophil accumulation in EPF lesions.37–38 Eotaxin synthesis, in principle, is stimulated by interleukin-4 and interleukin-13, which are Th2-type cytokines.39–40 Th2-type immune responses are promoted by PGD219–41 and a number of H-PGDS+ cells have been demonstrated in EPF.30–38 Prostaglandin D2 and eotaxin may therefore co-exist in the lesional skin. We next assessed the effects of indomethacin on chemotaxis to PGD2 in combination with eotaxin. Addition of eotaxin to PGD2 in lower chambers showed enhancing effects on eosinophil migration (Fig. 3). Pre-treatment of eosinophils with indomethacin suppressed eosinophil chemotactic responses to PGD2/eotaxin in a dose-dependent manner.
Figure 3.

Effects of indomethacin on chemotaxis to prostaglandin D2 (PGD2) in combination with eotaxin. Eosinophils were pre-treated with indomethacin for 90 min at 37°, followed by centrifugation and application to the upper chamber. In lower chambers, both PGD2 and eotaxin (CCL11) were added, and eosinophil migration was assessed. *P < 0·05. Representative results of at least three independent experiments are shown.
Indomethacin cancels priming effects of Δ12-PGJ2
As a plasma metabolite of PGD2, Δ12-PGJ2 has been shown to exert priming effects on eotaxin-induced chemotaxis.32 We confirmed this, as evidenced by increased eosinophil migration to eotaxin when Δ12-PGJ2 (5 × 10−8 m) was added to the upper chambers (Fig. 4a). This priming effect was almost completely inhibited by pre-treatment with a CRTH2 antagonist (CAY10471) (Fig. 4a), indicating that Δ12-PGJ2 exerted its actions via the CRTH2 receptor. Similarly, indomethacin, a CRTH2 agonist, completely cancelled the priming effects of Δ12-PGJ2 on eosinophil chemotaxis to eotaxin in a dose-dependent manner (Fig. 4b).
Figure 4.

Chemoattractant receptor-homologous molecule expressed on T helper type 2 cells (CRTH2) -mediated eosinophil priming by Δ12-prostaglandin J2 (PGJ2) was cancelled by indomethacin. (a) Eosinophils were pre-treated with CAY10471 for 10 min at 37°. After centrifugation, they were mixed with Δ12-PGJ2, then applied to the upper chamber. Chemotaxis to eotaxin (lower chamber) was assessed in the presence of Δ12-PGJ2. Pre-treatment with CAY10471 cancelled the priming effects of Δ12-PGJ2 on eosinophil migration to eotaxin. (b) Eosinophils were pre-treated with indomethacin for 90 min at 37°. After centrifugation, they were mixed with Δ12-PGJ2, then applied to the upper chamber, and subjected to chemotactic assay for eotaxin. *P < 0·05. Representative results of at least three independent experiments are shown.
Down-modulation of CRTH2 and CCR3 by indomethacin
Treatment of eosinophils with indomethacin clearly down-modulated cell surface expression of CRTH2 (Fig. 5), consistent with our previous report.30 Similarly, indomethacin suppressed CCR3 expression on eosinophils. However, these modulatory effects on CRTH2 and CCR3 were barely detectable when eosinophils were treated with low doses of indomethacin, i.e. at 10−8 m for CRTH2 (data not shown) and 10−7 m for CCR3 (Fig. 5, left lower panel). Suppressive effects of indomethacin on chemotaxis to PGD2 and eotaxin therefore did not seem to be simply mediated by down-modulation of cell surface expressions of these receptors, but could instead be a result of altered functions.
Figure 5.
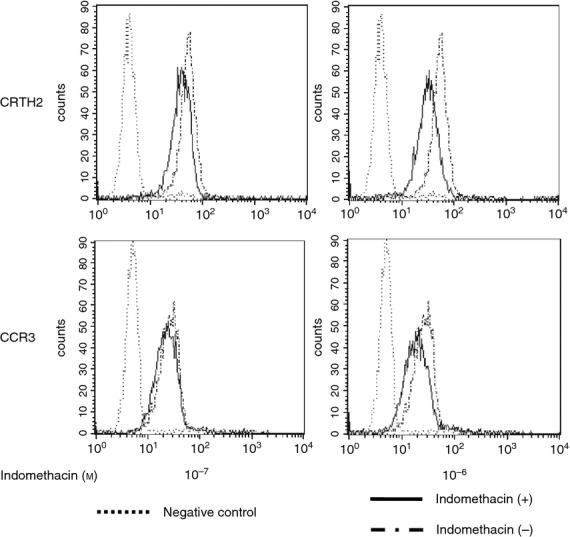
Down-modulation of chemoattractant receptor-homologous molecule expressed on T helper type 2 cells (CRTH2) and CCR3 by indomethacin. Eosinophils were incubated with indomethacin (10−7 or 10−6 m) for 90 min. Cell surface expression of CRTH2 and CCR3 was assessed by flow cytometry. Representative results of three independent experiments are shown.
Continuous exposure of eosinophils to indomethacin inhibits eosinophil migration in response to PGD2, but enhances eotaxin-induced migration
Next, we were interested in the migration of eosinophils under continuous exposure to indomethacin. Indomethacin was added to both upper and lower chambers. As expected, spontaneous migration to the lower chambers (chemokinesis) increased in parallel with doses of indomethacin (Fig. 6a), whereas migration in response to PGD2 was markedly suppressed by continuous exposure of eosinophils to indomethacin. These findings were in striking contrast to the results of eotaxin-induced cell migration. Migration in response to eotaxin did not decrease, but rather increased at higher doses of indomethacin (Fig. 6b). Eosinophil migration to PGD2/eotaxin decreased in the presence of indomethacin in a dose-dependent manner (Fig. 6c).
Figure 6.

Effects of continuous exposure to indomethacin on eosinophil migration in response to prostaglandin D2 (PGD2) and eotaxin. Indomethacin was added to both upper and lower chambers. Migration to PGD2 (a), eotaxin (b) and PGD2/eotaxin (c) was assessed. Chemokinesis (eosinophil migration to medium, open column) markedly increased under exposure to indomethacin. On the other hand, eosinophil migration to PGD2 significantly decreased, and eotaxin-induced migration was enhanced at high doses of indomethacin along with increased chemokinesis. (c) Eosinophil migration to PGD2/eotaxin was dose-dependently suppressed under continuous exposure to indomethacin. *P < 0·05. Representative results of at least three independent experiments are shown.
L-PGDS expression in lesional skin of EPF
Previous reports have demonstrated a number of inflammatory cells expressing H-PGDS in lesional skin of EPF.30–38 However, the involvement of L-PGDS in EPF lesions has not been clarified. An immunohistochemical study using mouse monoclonal antibody (1B7) detected L-PGDS in hair follicle epithelium with eosinophilic pustules (Fig. 7a). Rarely, EPF affects palms and soles that lack the hair follicle apparatus. In such situations, the acrosyringium may be the site predominantly affected in these lesions.2 Interestingly, epidermal keratinocytes surrounding eosinophilic pustules were positive for L-PGDS, together with positive staining in acrosyringeal ducts in the stratum corneum (Fig. 7c). More importantly, eccrine sweat glands exhibited a strongly positive reaction for L-PGDS (Fig. 7d). Similar data were obtained when rabbit polyclonal antibody to L-PGDS was used (data not shown).
Figure 7.

Immunohistochemical localization of lipocalin-type prostaglandin D synthase (L-PGDS) in skin lesions of eosinophilic pustular folliculitis (EPF). (a) Hair follicle epithelium with eosinophil infiltration was positive for L-PGDS. (b) Isotype control of (a). (c) Palmar lesions of EPF. Epidermal keratinocytes around pustules and eccrine ostia in the stratum corneum showed positive results for L-PGDS. (d) L-PGDS in eccrine ducts and glands. Basal (clear) cells in secretory portion and ductal cells were positive for L-PGDS.
Discussion
Indomethacin is an inhibitor of cyclo-oxygenases,42 exerting therapeutic effects on certain eosinophilic diseases, particularly EPF.3,4 The present study revealed inhibitory effects of indomethacin on eosinophil migratory functions in vitro, whereas diclofenac, another cyclo-oxygenase inhibitor, was unable to suppress eosinophil migration.
Prostaglandin D2 induced both chemokinesis and chemotaxis in eosinophils via CRTH2 stimulation, and this was also the case for indomethacin. Nevertheless, indomethacin clearly inhibited eosinophil chemotaxis to PGD2 within a therapeutic range (10−7–10−6 m). The priming effects of Δ12-PGJ2, a plasma metabolite of PGD2, on eotaxin-induced migration were also cancelled by indomethacin. These actions are probably attributable to homologous and functional desensitization of the CRTH2 receptor by the agonistic actions of indomethacin. Down-modulation of cell surface expression of CRTH2 on eosinophils by indomethacin could also contribute to low responsiveness to PGD2. These findings could explain the apparently inconsistent evidence that indomethacin has an anti-inflammatory effect on eosinophilic diseases such as EPF despite being a CRTH2 agonist, rather than an antagonist. Th2 cells and basophils are known to express CRTH2.22 Hence, not only eosinophils, but also Th2 cells and basophils could be targets of indomethacin. Our previous report found that a significant number of basophils infiltrate the skin lesions of EPF to a similar degree to eosinophils.43
Indomethacin also weakly suppressed eotaxin-induced eosinophil migration. This is possibly a result of cross-desensitization between CRTH2 and a receptor for eotaxin, CCR3.44 Mutual and functional modulation between seven-transmembrane G protein-coupled chemoattractant receptors has also been demonstrated in C5a, N-formyl-methionyl-leucyl-phenylalanine and interleukin-8 receptors on neutrophils.45
Interestingly, low-dose indomethacin (10−10 m) had a weak priming effect on PGD2-induced and eotaxin-induced eosinophil migration. Although the data shown here were not statistically significant (Fig. 2a,b), we observed a reproducible trend toward promotion of chemotactic responses by low-dose indomethacin in repeated experiments, some of which produced statistically significant results. Such findings suggest that CRTH2 stimulation by agonists results in a bell-shaped priming response for chemotaxis, and administration of doses of indomethacin below the therapeutic range may carry a potential risk of eosinophil activation that may lead to exacerbation of PGD2-mediated or eotaxin-mediated inflammation.
To further elucidate the pharmacological actions of indomethacin, this study assessed eosinophil migration in response to PGD2 and eotaxin in the presence of indomethacin in both upper and lower chambers. Continuous exposure of eosinophils to indomethacin exerted marked inhibitory effects on PGD2-induced chemotaxis, but caused more apparent chemokinesis than that seen with indomethacin pre-treatment. Conversely, and intriguingly, eotaxin-induced cell migration was dose-dependently enhanced along with promotion of chemokinesis. These findings imply that indomethacin may not necessarily inhibit eotaxin-induced eosinophilic inflammation, due to the promotion of chemokinesis when eosinophils are persistently exposed to indomethacin. Whether this recapitulates the circumstances at local inflammatory sites in vivo of individuals administered with indomethacin is uncertain, but cross-desensitization of CCR3 receptor may not be the sole explanation for the therapeutic mechanisms of indomethacin. In general, indomethacin is not necessarily effective for Th2-predominant inflammation, including atopic dermatitis and bronchial asthma, where eotaxin is probably produced. Indomethacin can therefore be assumed to exert therapeutic effects in diseases where PGD2–CRTH2 interactions offer greater contributions to the pathological mechanisms than Th2-type cytokines and chemokines such as eotaxin.
In one patient with EPF, we measured serum levels of PGD2. During disease onset, the patient showed 4·9 × 10−10 m of PGD2 in the blood, considerably higher than the PGD2 levels of three healthy volunteers (1·8 ± 0·84 × 10−10 m). Elevated PGD2 levels normalized (1·1 × 10−10 m) after successful treatment with indomethacin. Although a further study with larger sample size is required, these data suggest that PGD2 is actually produced in the EPF. Indeed, in EPF lesions, our group and others detected H-PGDS-expressing cells in inflammatory infiltrates as cellular sources of PGD2.30–38 The present study also found that hair follicle epithelium stained positive by immunohistochemistry for L-PGDS, another enzyme synthesizing PGD2, which may account for hair follicle accumulation of eosinophils. Interestingly, dermal eccrine glands/ducts, eccrine ostia in stratum corneum and epidermal keratinocytes around eosinophilic pustules were also positive for L-PGDS. Lipocalin-type PGDS is a multifunctional protein showing a lipocalin-type structure.46 This protein functions as a PGD2-producing enzyme, but also binds various lipophilic substances and can be secreted into various body fluids, such as cerebrospinal fluid and urine. In this respect, we lack direct evidence for local synthesis of L-PGDS by keratinocytes and eccrine apparatuses. However, the presence of L-PGDS may contribute to local production of PGD2 and accumulation of CRTH2-expressing cells, such as eosinophils, Th2 cells and basophils in hair follicles and acrosyringium. These findings may be consistent with our recent finding that acrosyringium appears to be the principal site affected in EPF with palmoplantar lesions, where hair follicles are lacking.2
A recent study revealed that PGD2 stimulates sebocytes to produce eotaxin-3 (CCL26) via peroxisome proliferator-activated receptor γ, but not CRTH2, leading to the accumulation of eosinophils in sebaceous hair follicles.38 That study illustrated upstream PGD2 signals and downstream CCR3 signals in the cascade of mechanisms underlying eosinophil accumulation. Hence, several inflammatory pathways (i.e. CRTH2-dependent and independent pathways) appear to be involved in the pathological mechanisms underlying EPF. Therefore, it is assumed that indomethacin exerts its effects through desensitization of CRTH2 signals in eosinophils as well as through inhibition of PGD2 synthesis in local tissue. The latter case may be limited to some types of eosinophilic inflammation where PGD2 production is a major contributor in the pathogenesis of the disease.
Acknowledgments
We wish to thank C. Miyagishi for providing technical assistance. This work was partly supported by the Japan Society for the Promotion of Science (22591238) and by the grant of the Ministry of Health, Labour and Welfare (H-21-114 and H-22-179), Japan.
Glossary
- CRTH2
chemoattractant receptor-homologous molecule expressed on T helper type 2 cells
- EPF
eosinophilic pustular folliculitis
- H-PGDS
haematopoietic prostaglandin D synthase
- L-PGDS
lipocalin-type prostaglandin D synthase
- PGD2
prostaglandin D2
- Th2
T helper type 2
Disclosures
The authors declare that they have no conflicts of interest.
References
- Aoyama H, Tagami H. Eosinophilic pustular folliculitis starting initially only with palmoplantar pustular lesions. Report of a case and review of the literature. Dermatology. 1992;185:276–80. doi: 10.1159/000247468. [DOI] [PubMed] [Google Scholar]
- Satoh T, Ikeda H, Yokozeki H. Acrosyringeal involvement of palmoplantar lesions of eosinophilic pustular folliculitis. Acta Derm Venereol. 2013;93:99. doi: 10.2340/00015555-1372. [DOI] [PubMed] [Google Scholar]
- Ishiguro N, Shishido E, Okamoto R, et al. Ofuji's disease: a report on 20 patients with clinical and histopathologic analysis. J Am Acad Dermatol. 2002;46:827–33. doi: 10.1067/mjd.2002.120533. [DOI] [PubMed] [Google Scholar]
- Lee ML, Tham SN, Ng SK. Eosinophilic pustular folliculitis (Ofuji's disease) with response to indomethacin. Dermatology. 1993;186:210–2. doi: 10.1159/000247348. [DOI] [PubMed] [Google Scholar]
- Ota T, Hata Y, Tanikawa A, et al. Eosinophilic pustular folliculitis (Ofuji's disease): indomethacin as a first choice of treatment. Clin Exp Dermatol. 2001;26:179–81. doi: 10.1046/j.1365-2230.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- Urade Y, Eguchi N. Lipocalin-type and hematopoietic prostaglandin D synthases as a novel example of functional convergence. Prostaglandins Other Lipid Mediat. 2002;68–69:375–82. doi: 10.1016/s0090-6980(02)00042-4. [DOI] [PubMed] [Google Scholar]
- Kanaoka Y, Urade Y. Hematopoietic prostaglandin D synthase. Prostaglandins Leukot Essent Fatty Acids. 2003;69:163–7. doi: 10.1016/s0952-3278(03)00077-2. [DOI] [PubMed] [Google Scholar]
- Christ-Hazelhof E, Nugteren DH. Purification and characterisation of prostaglandin endoperoxide D-isomerase, a cytoplasmic, glutathione-requiring enzyme. Biochim Biophys Acta. 1979;572:43–51. doi: 10.1016/0005-2760(79)90198-x. [DOI] [PubMed] [Google Scholar]
- Urade Y, Fujimoto N, Ujihara M, et al. Biochemical and immunological characterization of rat spleen prostaglandin D synthetase. J Biol Chem. 1987;262:3820–5. [PubMed] [Google Scholar]
- Tanaka K, Ogawa K, Sugamura K, et al. Cutting edge: differential production of prostaglandin D2 by human helper T cell subsets. J Immunol. 2000;164:2277–80. doi: 10.4049/jimmunol.164.5.2277. [DOI] [PubMed] [Google Scholar]
- Shimura C, Satoh T, Igawa K, et al. Dendritic cells express hematopoietic prostaglandin D synthase and function as a source of prostaglandin D2 in the skin. Am J Pathol. 2010;176:227–37. doi: 10.2353/ajpath.2010.090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Gomes T, Magalhaes KG, Mesquita-Santos FP, et al. Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation. J Immunol. 2011;187:6518–26. doi: 10.4049/jimmunol.1101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugajin T, Satoh T, Kanamori T, et al. FcεRI, but not FcγR, signals induce prostaglandin D2 and E2 production from basophils. Am J Pathol. 2011;179:775–82. doi: 10.1016/j.ajpath.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urade Y, Hayaishi O. Prostaglandin D2 and sleep regulation. Biochim Biophys Acta. 1999;1436:606–15. doi: 10.1016/s0005-2760(98)00163-5. [DOI] [PubMed] [Google Scholar]
- Beasley CR, Robinson C, Featherstone RL, et al. 9 α,11 β-prostaglandin F2, a novel metabolite of prostaglandin D2 is a potent contractile agonist of human and guinea pig airways. J Clin Invest. 1987;79:978–83. doi: 10.1172/JCI112909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi H, Uehara Y, Kanai F, et al. Prostaglandin D2 inhibits inducible nitric oxide synthase expression in rat vascular smooth muscle cells. Circ Res. 1998;82:204–9. doi: 10.1161/01.res.82.2.204. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Toda N. Different responsiveness of prostaglandin D2-sensitive systems to prostaglandin D2 and its analogues. Br J Pharmacol. 1985;85:367–75. doi: 10.1111/j.1476-5381.1985.tb08870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle BJ, Moncada S, Vane JR. Comparison of the effects of prostacyclin (PGI2), prostaglandin E1 and D2 on platelet aggregation in different species. Prostaglandins. 1978;16:373–88. doi: 10.1016/0090-6980(78)90216-2. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Kanaoka Y, Aritake K, et al. Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. J Immunol. 2002;168:443–9. doi: 10.4049/jimmunol.168.1.443. [DOI] [PubMed] [Google Scholar]
- Satoh T, Moroi R, Aritake K, et al. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol. 2006;177:2621–9. doi: 10.4049/jimmunol.177.4.2621. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Otani S, Hirai H, et al. Dual functions of prostaglandin D2 in murine contact hypersensitivity via DP and CRTH2. Am J Pathol. 2011;179:302–14. doi: 10.1016/j.ajpath.2011.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Tanaka K, Yoshie O, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–61. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais FG, Cruz RP, Chateauneuf A, et al. Selective modulation of chemokinesis, degranulation, and apoptosis in eosinophils through the PGD2 receptors CRTH2 and DP. J Allergy Clin Immunol. 2001;108:982–8. doi: 10.1067/mai.2001.119919. [DOI] [PubMed] [Google Scholar]
- Yahara H, Satoh T, Miyagishi C, et al. Increased expression of CRTH2 on eosinophils in allergic skin diseases. J Eur Acad Dermatol Venereol. 2010;24:75–6. doi: 10.1111/j.1468-3083.2009.03267.x. [DOI] [PubMed] [Google Scholar]
- Oiwa M, Satoh T, Watanabe M, et al. CRTH2-dependent, STAT6-independent induction of cedar pollen dermatitis. Clin Exp Allergy. 2008;38:1357–66. doi: 10.1111/j.1365-2222.2008.03007.x. [DOI] [PubMed] [Google Scholar]
- Hirai H, Tanaka K, Takano S, et al. Cutting edge: agonistic effect of indomethacin on a prostaglandin D2 receptor, CRTH2. J Immunol. 2002;168:981–5. doi: 10.4049/jimmunol.168.3.981. [DOI] [PubMed] [Google Scholar]
- Stubbs VE, Schratl P, Hartnell A, et al. Indomethacin causes prostaglandin D2-like and eotaxin-like selective responses in eosinophils and basophils. J Biol Chem. 2002;277:26012–20. doi: 10.1074/jbc.M201803200. [DOI] [PubMed] [Google Scholar]
- Nomura K, Sasaki C, Murai T, et al. Angiolymphoid hyperplasia with eosinophilia: successful treatment with indomethacin farnesil. Br J Dermatol. 1996;134:189–90. doi: 10.1111/j.1365-2133.1996.tb07873.x. [DOI] [PubMed] [Google Scholar]
- Tanglertsampan C, Tantikun N, Noppakun N, et al. Indomethacin for recurrent cutaneous necrotizing eosinophilic vasculitis. J Med Assoc Thai. 2007;90:1180–2. [PubMed] [Google Scholar]
- Satoh T, Shimura C, Miyagishi C, et al. Indomethacin-induced reduction in CRTH2 in eosinophilic pustular folliculitis (Ofuji's disease): a proposed mechanism of action. Acta Derm Venereol. 2010;90:18–22. doi: 10.2340/00015555-0759. [DOI] [PubMed] [Google Scholar]
- Cramer R, Dri P, Zabucchi G, et al. A simple and rapid method for isolation of eosinophilic granulocytes from human blood. J Leukoc Biol. 1992;52:331–6. doi: 10.1002/jlb.52.3.331. [DOI] [PubMed] [Google Scholar]
- Heinemann A, Schuligoi R, Sabroe I, et al. Delta 12-prostaglandin J2, a plasma metabolite of prostaglandin D2, causes eosinophil mobilization from the bone marrow and primes eosinophils for chemotaxis. J Immunol. 2003;170:4752–8. doi: 10.4049/jimmunol.170.9.4752. [DOI] [PubMed] [Google Scholar]
- Moshfegh A, Hallde nG, Lundahl J. Methods for simultaneous quantitative analysis of eosinophil and neutrophil adhesion and transmigration. Scand J Immunol. 1999;50:262–9. doi: 10.1046/j.1365-3083.1999.00594.x. [DOI] [PubMed] [Google Scholar]
- Nagase H, Yamaguchi M, Jibiki S, et al. Eosinophil chemotaxis by chemokines: a study by a simple photometric assay. Allergy. 1999;54:944–50. doi: 10.1034/j.1398-9995.1999.00184.x. [DOI] [PubMed] [Google Scholar]
- Varga SM, Beckman NA, Chu M, et al. Sensitive detection and quantitation of mouse eosinophils in tissues using an enzymatic eosinophil peroxidase assay: its use to rapidly measure pulmonary eosinophilia during experimental respiratory syncytial virus infection of mice. J Immunol Methods. 2002;262:111–20. doi: 10.1016/s0022-1759(02)00014-5. [DOI] [PubMed] [Google Scholar]
- Satoh T, Yokozeki H, Nishioka K. Pathogenic roles of eosinophils in guinea-pig contact sensitivity: regulation of dermal eosinophilia with remotely administered IL-5. Clin Exp Immunol. 2000;122:300–7. doi: 10.1046/j.1365-2249.2000.01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amerio P, Verdolini R, Proietto G, et al. Role of Th2 cytokines, RANTES and eotaxin in AIDS-associated eosinophilic folliculitis. Acta Derm Venereol. 2001;81:92–5. doi: 10.1080/00015550152384191. [DOI] [PubMed] [Google Scholar]
- Nakahigashi K, Doi H, Otsuka A, et al. PGD2 induces eotaxin-3 via PPARγ from sebocytes: a possible pathogenesis of eosinophilic pustular folliculitis. J Allergy Clin Immunol. 2012;129:536–43. doi: 10.1016/j.jaci.2011.11.034. [DOI] [PubMed] [Google Scholar]
- Hoeck J, Woisetschlager M. Activation of eotaxin-3/CCLl26 gene expression in human dermal fibroblasts is mediated by STAT6. J Immunol. 2001;167:3216–22. doi: 10.4049/jimmunol.167.6.3216. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Bartels J, Mallet AI, et al. IL-4 induces eotaxin: a possible mechanism of selective eosinophil recruitment in helminth infection and atopy. J Immunol. 1998;160:60–8. [PubMed] [Google Scholar]
- Tanaka K, Hirai H, Takano S, et al. Effects of prostaglandin D2 on helper T cell functions. Biochem Biophys Res Commun. 2004;316:1009–14. doi: 10.1016/j.bbrc.2004.02.151. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Akarasereenont P, Thiemermann C, et al. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci USA. 1993;90:11693–7. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Ito Y, Miyagishi C, et al. Basophils infiltrate skin lesions of eosinophilic pustular folliculitis (Ofuji's disease) Acta Derm Venereol. 2011;91:371–2. doi: 10.2340/00015555-1052. [DOI] [PubMed] [Google Scholar]
- Ali H, Richardson RM, Haribabu B, et al. Chemoattractant receptor cross-desensitization. J Biol Chem. 1999;274:6027–30. doi: 10.1074/jbc.274.10.6027. [DOI] [PubMed] [Google Scholar]
- Sabroe I, Williams TJ, Hebert CA, et al. Chemoattractant cross-desensitization of the human neutrophil IL-8 receptor involves receptor internalization and differential receptor subtype regulation. J Immunol. 1997;158:1361–9. [PubMed] [Google Scholar]
- Nagata N, Fujimori K, Okazaki I, et al. De novo synthesis, uptake and proteolytic processing of lipocalin-type prostaglandin D synthase, β-trace, in the kidneys. FEBS J. 2009;276:7146–58. doi: 10.1111/j.1742-4658.2009.07426.x. [DOI] [PubMed] [Google Scholar]



