Abstract
Objectives
The objective of this study is to determine an optimal antibiotic-loaded bone cement (ALBC) for infection prophylaxis in total joint arthroplasty (TJA).
Methods
We evaluated the antibacterial effects of polymethylmethacrylate (PMMA) bone cements loaded with vancomycin, teicoplanin, ceftazidime, imipenem, piperacillin, gentamicin, and tobramycin against methicillin-sensitive Staphylococcus aureus (MSSA), methicillin-resistant Staph. aureus (MRSA), coagulase-negative staphylococci (CoNS), Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae. Standardised cement specimens made from 40 g PMMA loaded with 1 g antibiotics were tested for elution characteristics, antibacterial activities, and compressive strength in vitro.
Results
The ALBC containing gentamicin provided a much longer duration of antibiotic release than those containing other antibiotic. Imipenem-loading on the cement had a significant adverse effect on the compressive strength of the ALBC, which made it insufficient for use in prosthesis fixation. All of the tested antibiotics maintained their antibacterial properties after being mixed with PMMA. The gentamicin-loaded ALBC provided a broad antibacterial spectrum against all the test organisms and had the greatest duration of antibacterial activity against MSSA, CoNS, P. aeruginosa and E. coli.
Conclusion
When considering the use of ALBC as infection prophylaxis in TJA, gentamicin-loaded ALBC may be a very effective choice.
Cite this article: Bone Joint Res 2013;2:220–6.
Keywords: Bone cement, Arthroplasty, Peri-prosthetic joint infection, Gentamicin, Infection
Article focus
This study is to determine an optimal antibiotic-loaded bone cement (ALBC) for infection prophylaxis in total joint arthroplasty
Key messages
The gentamicin-loaded ALBC provided a broad antibacterial spectrum against all the test organisms and had the greatest duration of antibacterial activity against methicillin-sensitive Staphylococcus aureus, coagulase-negative staphylococci, CoNS, Pseudomonas aeruginosa and Escherichia coli as compared with the ALBC loaded with vancomycin, teicoplanin, ceftazidime, imipenem, piperacillin, or tobramycin
When considering the use of ALBC as infection prophylaxis in total joint arthroplasty, gentamicin-loaded ALBC can be a very effective choice
Strengths and limitations
This is the first study to compare the bacteria-eradication abilities of low-dose ALBCs loaded with different antibiotics
This study can provide as a reference for orthopaedic surgeons in the selection of effective ALBCs for infection prophylaxis in total joint arthroplasty
This is an in vitro study
Introduction
Deep infection is one of the most devastating complications of total joint arthroplasty (TJA). Systemic prophylactic antibiotics have been widely accepted as effective agents for reducing the rate of deep infection.1,2 However, a prevalence of deep infection between 1% and 2% is still noted in most large case series reporting on TJAs.1,2 The use of antibiotic-loaded bone cement (ALBC) has been shown to be effective in reducing the rate of early-to-intermediate deep infection after TJA.3-5
Gram-positive pathogens (including Staphylococcus aureus and coagulase-negative staphylococci (CoNS)) are the most common organisms implicated in peri-prosthetic joint infection (PJI).6-9 For gram-positive pathogen infections, vancomycin-loaded bone cement has become one of the most commonly used ALBCs in both prophylactic and therapeutic treatment of PJIs.10,11 However, gram-positive bacteria are not the only bacteria found in PJI. Although less commonly associated with PJI, gram-negative bacteria comprise between 6% and 23% of all episodes of PJI.6,7,12 When using ALBC as infection prophylaxis against both gram-positive and -negative pathogens in TJA, an antibiotic with a broad spectrum of antibacterial activity would be a more appropriate choice for PMMA bone-cement loading. To our knowledge, no study has evaluated the spectrum of bacteria-eradication abilities of bone cement loaded with different antibiotics, especially in the lower-dose ALBC used for prophylaxis in TJA.
The objective of our study was to measure the elution characteristics of vancomycin, teicoplanin, ceftazidime, imipenem, piperacillin, gentamicin, and tobramycin from low-dose ALBC specimens and compare their antibacterial activities against the most common organisms found in PJIs: methicillin-sensitive Staph. aureus (MSSA), methicillin-resistant Staph. aureus (MRSA), CoNS, Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae. We used an in vitro model to measure the antibiotic release characteristics, bacteria eradication abilities, and mechanical properties of ALBCs loaded with different antibiotics.
Materials and Methods
Antibiotic-loaded cement specimen
We used Surgical Simplex bone cement (Stryker Orthopaedics, Limerick, Ireland), to which was added vancomycin (Gentle Pharmaceutical Co., Yulin, Taiwan), teicoplanin (Sanofi-Aventis, Paris, France), ceftazidime (China Chemical & Pharmaceutical Co. Ltd, Taichung, Taiwan), imipenem (Merck Sharp & Dohme Corp., Elkton, Virginia), or piperacillin (China Chemical & Pharmaceutical Co. Ltd) was tested. The antibiotic–cement mixture comprised 1 g of antibiotic mixed with 40 g of bone cement polymer prior to the addition of the liquid component. Two commercial, premixed, low-dose ALBCs containing either 1 g of gentamicin (PALACOS R+G; Zimmer, Weheim, Germany) or 1 g of tobramycin (Simplex P; Stryker Orthopaedics) were also tested.
Antibiotic broth elution assay
Each cement cylinder was immersed in a polypropylene tube with 5 ml phosphate buffered saline (PBS; pH 7.3) and shaken in a rotator at 37°C. The study lasted for 14 days, with the daily transfer of the cement cylinder into a test tube with PBS after saline washings. Elution samples of 2 ml of PBS were collected at ten time points over a period of 14 days (days zero, one, two, three, four, five, six, seven, ten, and 14).
The concentrations of antibiotics were determined using high-performance liquid chromatography (HPLC). The method of HPLC analyses was modified from previously published methods.13,14 The details of the HPLC analyses are given in Table I.
Table I.
Summary of chromatography assay. All of the chromatography assay was performed using a model ALC 717 chromatograph (Waters Associates, Milford, Massachusetts). The concentrations of antibiotics in the cement cylinders were obtained from the high-performance liquid chromatography (HPLC) analysis through comparisons made with the prepared daily standard curves where related the peak areas with antibiotic concentrations
| Antibiotic | Column (dimensions) | Particle size | Mobile phase | Detection sensitivity (µg/ml) | |||
|---|---|---|---|---|---|---|---|
| Vancomycin | RP18 (100 mm × 4.6 mm) | 5 μm | Water/Acetonitrile/100 mM ammonium formate (78/12/10) | 0.5 | |||
| Teicoplanin | C18 (250 mm × 4.6 mm) | 5 μm | Sodium dihydrogen phosphate/Acetonitrile (90/10) | 0.25 | |||
| Imipenem | Atlantis T3 (150 mm × 4.6 mm) | 5 μm | Water/Methanol (93/7) | 0.5 | |||
| Ceftazidime | Atlantis T3 (150 mm × 4.6 mm) | 5 μm | Acetonitrile/25 mM KH2PO4-Na2HPO4 (10/90) | 0.25 | |||
| Piperacillin | Atlantis T3 (150 mm × 4.6 mm) | 5 μm | Acetonitrile/0.05 M phosphate buffer (50/50) | 0.5 | |||
| Gentamicin | C18 (300 mm × 3.9 mm) | 5 μm | Acetonitrile/0.05% aqueous trifluoroacetic acid (70/30) | 0.2 | |||
| Tobramycin | C18 (300 mm × 3.9 mm) | 5 μm | Water/Acetonitrile (6/94) | 0.2 |
Bioassay of antibiotic activity
A modified microtube dilution bioassay was used to measure the biological activity of the released antibiotics in the sample aliquots. The following strains were selected as test organisms: MSSA strain (ATCC 25923), MRSA strain (ATCC43300), CoNS (ATCC14990), P. aeruginosa (ATCC27853), E. coli (ATCC 25922), and K. pneumoniae (ATCC 700603).
The samples were inoculated with tested bacteria of 105 colony forming units (CFUs)/ml in 96-well cultured dishes and incubated at 37°C for 24 hours. The growth was visually compared by an author (YC) with the positive control (without the antibiotic) and standard samples with different concentrations of antibiotics.
Ultimate compressive test
The cement specimens (both before and after the antibiotic broth elution) in each group were tested for failure in axial compression by a material testing system machine (Bionix 858; MTS Corp., Eden Prairie, Minnesota). A specially designed pushrod with a self-alignment function was used as a plunger to ensure full surface contact between the specimen and the pushrod. Each specimen was compressed at a displacement rate of 0.1 mm/s. Force, displacement, and time were recorded simultaneously for each specimen in increments of 0.05 mm by the MTS Test star software (MTS Corp.). Ultimate compressive force was compared between groups. A cement specimen without antibiotics was used as a control.
Statistical analysis
The antimicrobial concentration of elution samples and the ultimate compressive force of cements with different preparations were tested in triplicate. The results are reported as mean with range. We used analysis of variance (ANOVA) to determine the statistical difference in the ultimate compression force and antibiotic release efficacy between cements with different preparation. A p-value of < 0.01 was considered significant.
Results
On the basis of the HPLC results, all tested samples showed a burst release on the first day. The release rates rapidly decreased during the following few days. During the 14-day study period, imipenem-loaded ALBCs showed a significantly worse cumulative antibiotic release efficacy compared with the other tested antibiotics (all p < 0.001) (Table II). The ALBCs containing different antibiotics exhibited different release durations and different additional daily releases (Table II). The ALBCs containing gentamicin had much longer release durations (ten days), than those containing ceftazidime (six days), tobramycin (five days), and vancomycin, teicoplanin, imipenem and piperacillin (two days each) (Table II).
Table II.
Daily and cumulative release of antibiotics from the cement specimens in the broth elution assay over a period of 14 days (-, below detection limit). The imipenem values showed a significantly worse cumulative antibiotic release efficacy compared with all other tested antibiotics (all p < 0.001)
| Antibiotic | ||||||||
|---|---|---|---|---|---|---|---|---|
| Vancomycin | Teicoplanin | Ceftazidime | Imipenem | Piperacillin | Gentamicin | Tobramycin | ||
| Mean (sem) daily release (µg/ml) | ||||||||
| Day 1 | 38.5 (5.9) | 59.5 (8.4) | 66.0 (6.0) | 1.7 (0.1) | 68.4 (4.0) | 56.4 (10.7) | 53.8 (7.4) | |
| Day 2 | 5.2 (1.0) | 6.5 (1.7) | 7.0 (1.0) | 0.4 (0.1) | 1.4 (0.8) | 3.6 (1.1) | 10.0 (1.3) | |
| Day 3 | - | - | 1.7 (0.1) | - | - | 3.3 (1.0) | 3.3 (1.0) | |
| Day 4 | - | - | 1.4 (0.1) | - | - | 1.9 (0.8) | 2.1 (0.8) | |
| Day 5 | - | - | 1.2 (0.1) | - | - | 1.3 (0.1) | 1.3 (0.1) | |
| Day 6 | - | - | 1.3 (0.1) | - | - | 1.1 (0.1) | - | |
| Day 7 | - | - | - | - | - | 0.6 (0.1) | - | |
| Day 10 | - | - | - | - | - | 0.7 (0.1) | - | |
| Day 14 | - | - | - | - | - | - | - | |
| Mean (sem) cumulative total release (µg/ml) | 218.6 (34.8) | 330.1 (51.0) | 393.3 (26.1) | 10.5 (0.8) | 349.5 (24.5) | 343.6 (54.2) | 352.6 (25.6) | |
The organisms in this study were found to be susceptible to different concentrations of the test antibiotics. Vancomycin and teicoplanin did not exhibit anti-bacterial effects against the gram-negative organisms (including P. aeruginosa, E. coli, and K. pneumoniae) at a concentration up to 256 µg/ml (see Supplementary Material).
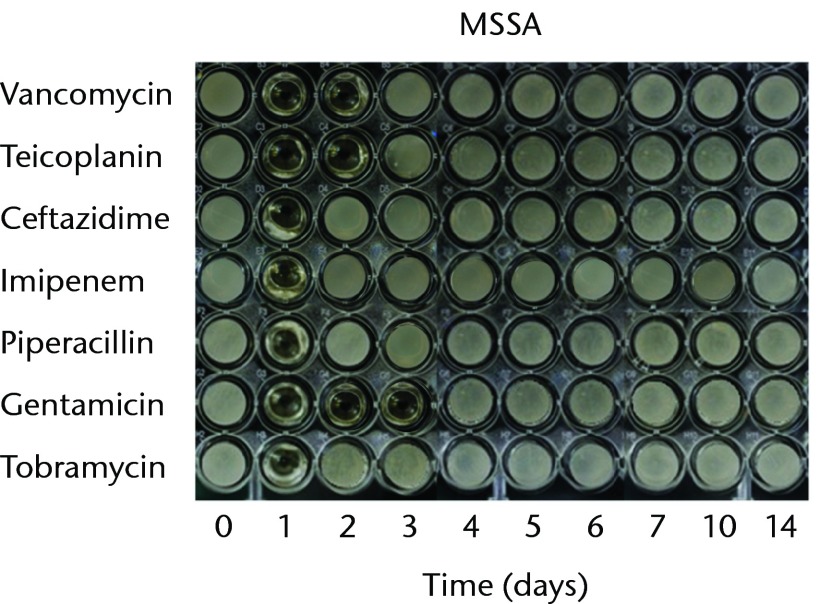
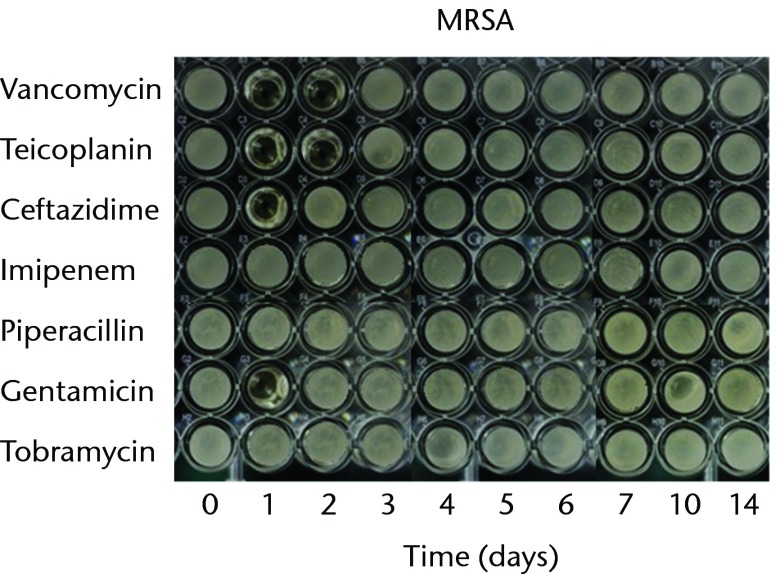
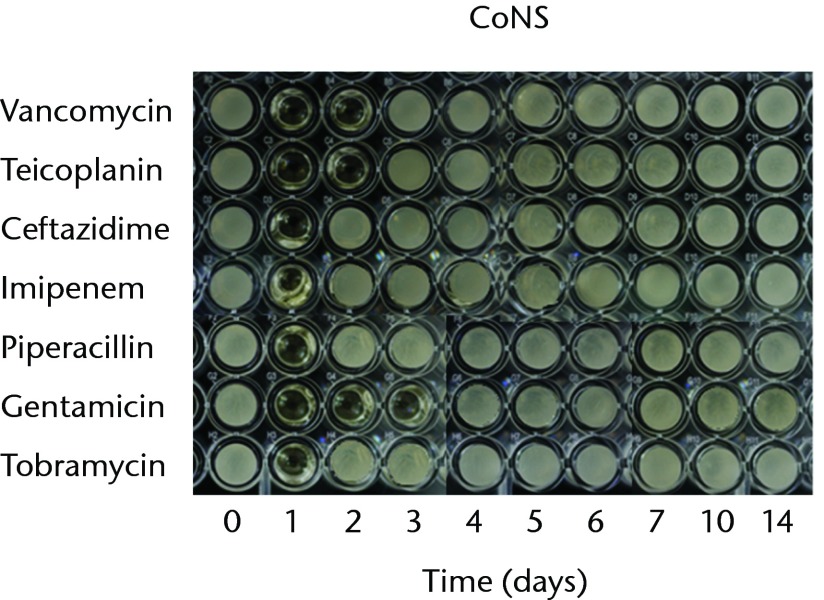
With regard to the bioactivities of ALBCs loaded with different antibiotics against test organisms, all of the antibiotics retained their antibacterial effects after incorporation into PMMA. In order to test the bioactivity against gram-positive bacteria, three staphylococci strains (MSSA, MRSA, and CoNS) were used. The antibacterial effects against MSSA lasted for one day in the cements loaded with ceftazidime, imipenem, piperacillin, or tobramycin; two days in the cements loaded with vancomycin, or teicoplanin; three days in the cements loaded with gentamicin (Fig. 1a). The antibacterial effects against MRSA lasted for one day in the cements loaded with ceftazidime or gentamicin and two days in the cements loaded with vancomycin and teicoplanin; neither imipenem-, tobramycin-, nor piperacillin-loaded cement provided any bioactivity against MRSA (Fig. 1b). The antibacterial effects against CoNS lasted for one day in the cements loaded with ceftazidime, imipenem, piperacillin, or tobramycin; two days in the cements loaded with vancomycin or teicoplanin; and three days in the cement loaded with gentamicin (Fig. 1c).
Figs. 1a - 1c.
Micro-tube dilution bioassays showing the antibacterial activities against gram-positive bacteria in broth elution samples from the low-dose antibiotic loaded cements (1 g antibiotic in 40 g polymethylmethacrylate) over a 14-day elution period for a) methicillin-sensitive Staphylococcus aureus (MSSA), b) methicillin-resistant Staph. aureus (MRSA) and c) coagulase-negative staphylococci (CoNS).
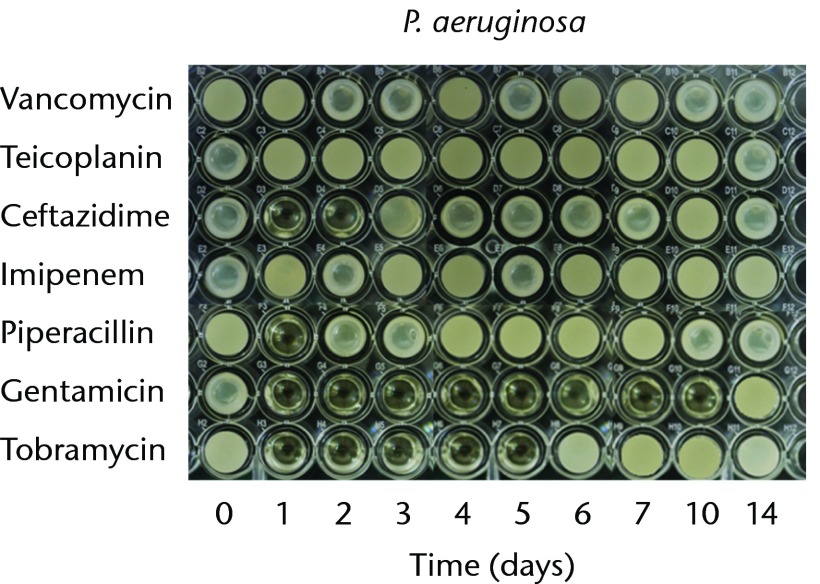
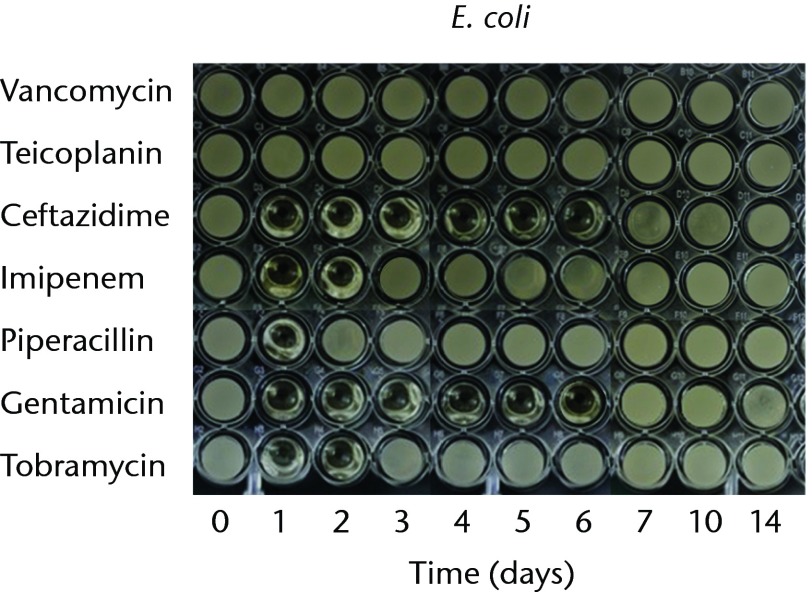
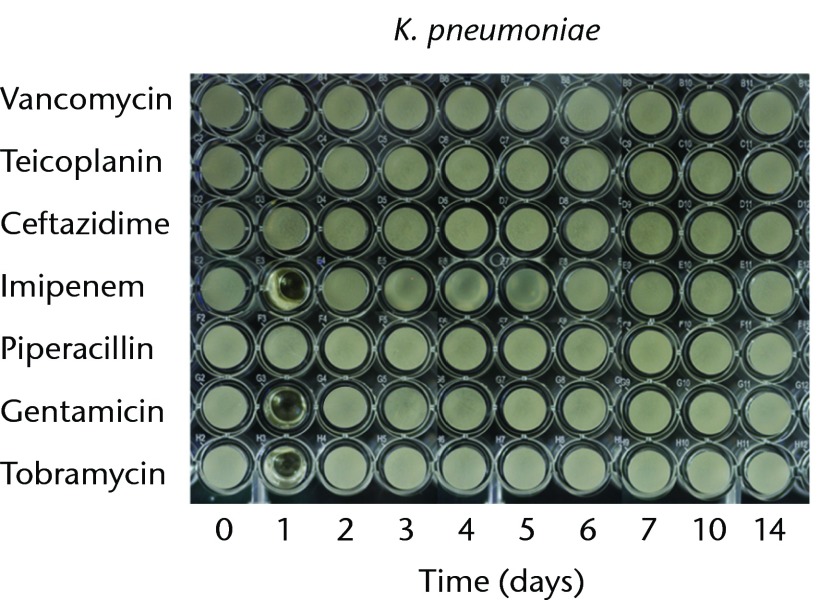
In order to test the bioactivities against gram-negative bacteria, we used P. aeruginosa, E. coli, and K. pneumoniae. The antibacterial effects against P. aeruginosa lasted for one, two, five and ten days in the cements loaded with piperacillin, ceftazidime, tobramycin, and gentamicin, respectively. The imipenem-loaded cement did not exhibit antibacterial activity against P. aeruginosa (Fig. 2a). The antibacterial effects against E. coli lasted for one day in the cements loaded with piperacillin, and two days in cement loaded with imipenem or tobramycin. The gentamicin- and ceftazidime-loaded cements provided similar antibacterial durations (six days) against E. Coli (Fig. 2b). The anti-bacterial activity against K. pneumoniae lasted for one day for imipenem-, gentamicin and tobramycin-loaded cements. Ceftazidime- or piperacillin-loaded cements did not exhibit antibacterial activities against K. pneumoniae (Fig. 2c). In addition, vancomycin- or teicoplanin-loaded cement did not provide bioactivity against any of the tested gram-negative bacteria (Fig. 2).
Figs. 2a - 2c.
Micro-tube dilution bioassays showing the antibacterial activities against gram-negative bacteria in broth elution samples from the low-dose antibiotic loaded cements (1 g antibiotic in 40 g polymethylmethacrylate) over a 14-day elution period for a) Pseudomonas aeruginosa, b) Escherichia coli and c) Klebsiella pneumoniae.
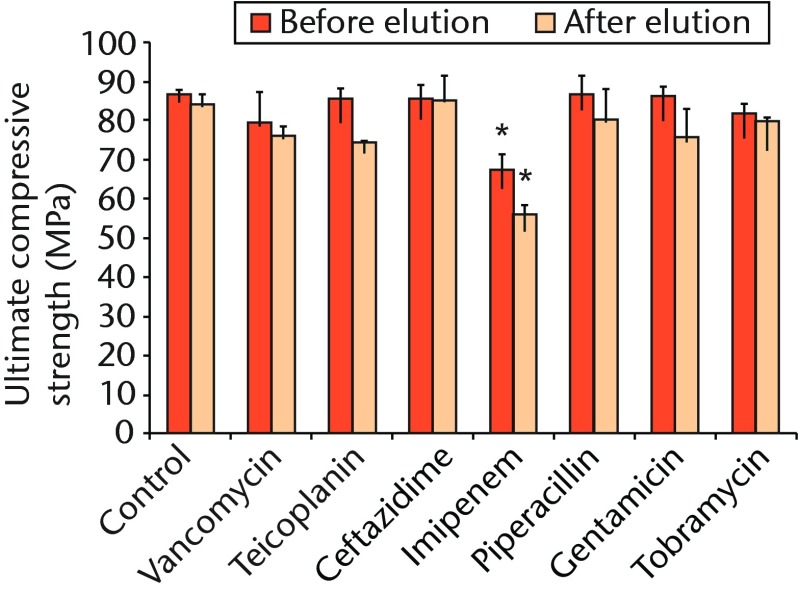
Regarding the mechanical strength of cement samples, with the exception of the imipenem-loaded ALBC, there were no significant differences in ultimate compressive strength between the cement samples loaded without or with different antibiotics, and all pre- and post-elution compression testing showed a mean cement strength > 70 MPa (Table III, Fig. 3). In contrast, the mean compression modulus was significantly lower for the specimens containing imipenem than it was for the control specimens and those containing other preparations either before or after broth elution (p = 0.004 and p < 0.001, respectively) (Table III, Fig. 3).
Fig. 3.
Bar chart showing the mean ultimate compressive strength of the cement samples before and after the 14-day broth elution assay compared with cement without antibiotics (control). The bars denote the mean of three tests for each antibiotic with the standard error of the mean. Asterisks denote a significant difference (p < 0.01) when compared with the control.
Table III.
Results of ultimate compressive strength testing
| Mean ultimate compressive strength (MPa) (range) | |||||
|---|---|---|---|---|---|
| Pre-elution | p-value vs control | Post-elution | p-value vs control | ||
| Control | 86 (85 to 87) | - | 84 (81 to 86) | - | |
| Vancomycin | 79 (73 to 88) | 0.692 | 75 (72 to 77) | 0.707 | |
| Teicoplanin | 85 (82 to 87) | 1.000 | 74 (73 to 74) | 0.530 | |
| Ceftazidime | 85 (87 to 81) | 1.000 | 85 (77 to 88) | 1.000 | |
| Imipenem | 67 (65 to 68) | 0.004 | 55 (53 to 58) | < 0.001 | |
| Piperacillin | 87 (82 to 91) | 1.000 | 80 (71 to 85) | 0.994 | |
| Gentamicin | 86 (84 to 88) | 1.000 | 75 (70 to 82) | 0.723 | |
| Tobramycin | 82 (79 to 83) | 0.958 | 79 (79 to 80) | 0.989 | |
Discussion
The ALBCs have been widely used in the prophylaxis and treatment of skeletal infection.15,16 In the treatment of skeletal infections, a high-dose ALBC (≥ 3.6 g of antibiotic per 40 g of PMMA powder) is desirable for effective elution kinetics and sustained therapeutic levels of the antibiotic.17 In contrast to therapeutic measures, prophylaxis requires low-dose antibiotics in the bone cement to avoid adverse mechanical effects on cement that is intended for fixation of the prosthesis. In general, low-dose ALBC is defined as ≤ 1 g of powdered antibiotic per 40 g of PMMA powder. International industrial standards state that bone cement used for definitive fixation must have an ultimate compressive strength of ≥ 70 MPa.18,19 This standard was established to reduce the incidence of premature cement breakdown.18,19 In this study, we found that the ultimate compressive strengths of cement specimens are not significantly compromised after being loaded with low-dose antibiotics, with the exception of imipenem-loaded-cement. The ultimate compressive strength of imipenem-loaded ALBC was < 70 MPa both before and after broth elution. This finding indicated that imipenem-loaded low-dose ABLC does not provide sufficient mechanical strength for use in prosthesis fixation in TJA. High doses of antibiotics in bone cement have been shown to significantly reduce the mechanical strength of the cement and cannot be used in prosthesis fixation.20 However, the result of this study implied that not only the loading dose but also the type of antibiotic may cause significant adverse effects on mechanical strength of bone cement. Therefore, not all low-dose ALBCs can provide sufficient mechanical strength for prosthesis fixation. However, with the exception of imipenem, the mechanical strengths of all cement-sample preparations in this study were acceptable according to industrial standards both before and after the antibiotic elution. These findings partially support the use of the low-dose ALBCs as the definitive choice for joint prosthesis fixation.
In our previous study, we showed that the use of ALBC loaded with vancomycin or oxacillin in total knee arthroplasty (TKA) was able to prolong the antibacterial activity in the joint fluid after the discontinuation of intravenous prophylactic antibiotics.15 This longer duration of antibacterial activity in joint fluid may contribute to a lower infection rate in TKA performed using ALBC. However, only resistant or non-resistant strains of Staph. aureus were selected as test organisms in the experiment.15 The bioactivity of joint fluid against other organisms, especially against gram-negative organisms, is still unclear. Gram-negative bacteria accounted for 53 (15%) of 346 first-time episodes of culture-positive PJI encountered in our institute during the period from 2000 through 2006.12 Of these, P. aeruginosa (39.6%) was the most commonly isolated pathogen in patients with gram-negative PJI in 21 (40%), followed by E. coli in ten (19%) and K. pneumoniae in eight (15%).12 Similar results were reported by Zmistowski et al,21 who found that E. coli was the most common gram-negative pathogen (30.2%), followed by P. aeruginosa (25.5%) in a total of 43 patients with hip or knee PJI. Therefore, when considering the use of ALBC as a prophylactic measure for infection in TJA, the antibiotics incorporated into bone cements should have a broad antibacterial spectrum that include gram-positive and gram-negative pathogens.
Vancomycin and teicoplanin, two glycopeptide antibiotics, remain the standard therapeutic agents used in most gram-positive pathogen infections, including resistant and non-resistant strains.22 Vancomycin and teicoplanin have both been shown to be stable in PMMA, and both are released in a microbiologically active form.22 Vancomycin-loaded bone cement is one of the most commonly used ALBCs in both prophylactic and therapeutic measures for PJI.10,11 However, based on the bioassay results of this study, we found that neither vancomycin nor teicoplanin exhibited anti-bacterial effects against gram-negative pathogens (including P. aeruginosa, E. coli, and K. pneumoniae). Imipenem (subgroup: carbapenems),23 ceftazidime (third-generation cephalosporin),24 and piperacillin (extended spectrum β-lactam)25 all have a broad spectrum of activity against both gram-positive and -negative bacteria. Theoretically, bone cement loaded with a broad-spectrum antibiotic should provide similar spectrum of antibacterial activity. However, in this study, bone cement loaded with broad-spectrum antibiotics (ceftazidime, imipenem or piperacillin) did not show antibacterial activity against all the tested organisms. Piperacillin is active against gram-positive and gram-negative aerobic and anaerobic bacteria. However, piperacillin-loaded bone cement was unable to provide antibacterial duration > 24 hours against the tested micro-organisms. This may be due to the piperacillin with relative higher minimal inhibitory concentrations against the tested organisms. In contrast, the imipenem exhibits relative lower minimal inhibitory concentrations against the tested organisms. However, a short anti-bacterial activity is also observed in the cement loaded with imipenem. This may be due to the poor elusion efficacy of imipenem from PMMA. Imipenem-loaded ALBCs showed a significantly worse antibiotic release efficacy as compared with ALBCs with other preparations. Therefore, in addition to the spectrum of antibiotic loaded in the ALBC, the release efficacy of antibiotics from the bone cement and the minimal inhibitory concentrations of antibiotic are all the critical factors which determine antibacterial activities of ALBCs.
Several different commercial premixed ALBC products have been approved for use by the United States Food and Drug Administration.20 All of these commercial premixed ALBCs contain aminoglycoside combined with either tobramycin or gentamicin. Today, surgeons continue to mix ALBC by hand. The primary drawback of using commercially premixed ALBC is that the antibiotic used is dictated by the chosen bone cement, and consequently surgeons are unable to tailor the antibiotic to a specific organism. To date, there are no comparative studies determining the superiority of anti-bacterial activities of commercially premixed or hand mixed ALBCs. Two commercial premixed ALBCs were tested in this study (PALACOS R+G, containing 1 g gentamicin in 40 g PMMA; and Simplex P, containing 1 g tobramycin in 40 g PMMA). The premixed gentamicin-loaded ABLC showed a broad antibacterial spectrum against all of the tested bacteria, including gram-positive and gram-negative strains. It also provides the longest antibacterial duration against MSSA, CoNS, P. aeruginosa and E. coli. The premixed tobramycin ABLC also provided a broad antibacterial spectrum against all of the test bacteria, with the exception of MRSA. As compared with hand-mixed ALBCs, the commercial premixed gentamicin-loaded ALBC provided an equal or longer antibacterial duration against different bacteria in this study. However, this superiority in antibacterial activity was not observed in the premixed tobramycin ALBC as compared with hand-mixed ALBCs. The results indicated that commercial premixed ABLCs did not always exhibit superior antibacterial activity over hand-mixed ALBCs.
Our study has certain limitations. First, this is an in vitro study of specimens prepared in a laboratory environment, which does not necessarily reflect actual clinical circumstances. The quantity and flow of body fluid, limb mobility, host response, and antibiotic stability in vivo were not taken into consideration. Secondly, the bone cement used for hand-mixed ALBCs was different than the commercial premixed ALBCs. Third, ATCC bacteria were selected as test organisms in the experiment. We do not know the bioactivities of the cement specimens against other clinical isolates, especially biofilm-forming organisms.
In conclusion, vancomycin, teicoplanin, ceftazidime, imipenem, and piperacillin all retained antibacterial activities after loading into PMMA. Further, compared with the hand mixed low-dose ALBCs, the commercial premixed low-dose ALBCs did not definitively provide significantly better mechanical strength or superior antibacterial activities. The imipenem loaded low-dose ABLC was unable to provide sufficient mechanical strength to be used for prosthesis fixation in TJA. Vancomycin- or teicoplanin-loaded ABLCs should not be used as a prophylactic measure against gram-negative bacteria. In this experiment, the premixed gentamicin-loaded ABLC provided an equal or longer antibacterial activity against MSSA, MRSA, CoNS, P. aeruginosa, E. coli, and K. pneumoniae than ALBCs loaded with other antibiotics. When considering the use of ALBC as a prophylactic measure against infection in TJA, the premixed gentamicin-loaded ALBC may be an effective choice.
Supplementary material
Micro-tube dilution bioassays showing the different concentrations of the antibiotics exhibiting inhibitory effects against each bacteria are available as supplementary material on our website www.bjr.boneandjoint.org.uk.
Funding Statement
This work was supported by internal funding (Grant no. CMRPG3C0851).
Footnotes
Author contributions:Y. Chang: Study design, Conducting of the experiment, Data collection, Writing the paper
C-L. Tai: Biomechanical testing
P-H. Hsieh: Data analysis
S. W. N. Ueng: Study concept
ICMJE Conflict of Interest:None declared
References
- 1.Norden CW. Prevention of bone and joint infections. Am J Med 1985;78:229–232 [DOI] [PubMed] [Google Scholar]
- 2.Hill C, Flamant R, Mazas F, Evrard J. Prophylactic cefazolin versus placebo in total hip replacement: report of a multicentre double-blind randomised trial. Lancet 1981;1:795–796 [DOI] [PubMed] [Google Scholar]
- 3.Josefsson G, Gudmundsson G, Kolmert L, Wijkström S. Prophylaxis with systemic antibiotics versus gentamicin bone cement in total hip arthroplasty: a five-year survey of 1688 hips. Clin Orthop Relat Res 1990;253:173–178 [PubMed] [Google Scholar]
- 4.Chiu FY, Chen CM, Lin CF, Lo WH. Cefuroxime-impregnated cement in primary total knee arthroplasty: a prospective, randomized study of three hundred and forty knees. J Bone Joint Surg [Am] 2002;84-A:759–762 [PubMed] [Google Scholar]
- 5.Chiu FY, Lin CF, Chen CM, Lo WH, Chaung TY. Cefuroxime-impregnated cement at primary total knee arthroplasty in diabetes mellitus: a prospective, randomised study. J Bone Joint Surg [Br] 2001;83-A:691–695 [DOI] [PubMed] [Google Scholar]
- 6.Zimmerli W, Ochsner PE. Management of infection associated with prosthetic joints. Infection 2003;31:99–108 [DOI] [PubMed] [Google Scholar]
- 7.Tattevin P, Crémieux AC, Pottier P, Huten D, Carbon C. Prosthetic joint infection: when can prosthesis salvage be considered? Clin Infect Dis 1999;29:292–295 [DOI] [PubMed] [Google Scholar]
- 8.Davis JS. Management of bone and joint infections due to Staphylococcus aureus. Intern Med J 2005;35(Suppl 2):S79–S96 [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg DL. Septic arthritis. Lancet 1998;351:197–202 [DOI] [PubMed] [Google Scholar]
- 10.Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J Trauma 2009;66:804–808 [DOI] [PubMed] [Google Scholar]
- 11.Nowinski RJ, Gillespie RJ, Shishani Y, et al. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg 2012;21:324–328 [DOI] [PubMed] [Google Scholar]
- 12.Hsieh PH, Lee MS, Hsu KY, et al. Gram-negative prosthetic joint infections: risk factors and outcome of treatment. Clin Infect Dis 2009;49:1036–1043 [DOI] [PubMed] [Google Scholar]
- 13.MacLeod CM, Bartley EA, Galante JO, Friedhoff LT, Dhruv R. Aztreonam penetration into synovial fluid and bone. Antimicrob Agents Chemother 1986;29:710–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verdier MC, Tribut O, Tattevin P, et al. Simultaneous determination of 12 beta-lactam antibiotics in human plasma by high-performance liquid chromatography with UV detection: application to therapeutic drug monitoring. Antimicrob Agents Chemother 2011;55:4873–4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueng SW, Hsieh PH, Shih HN, et al. Antibacterial activity of joint fluid in cemented total-knee arthroplasty: an in vivo comparative study of polymethylmethacrylate with and without antibiotic loading. Antimicrob Agents Chemother 2012;56:5541–5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanssen AD, Rand JA, Osmon DR. Treatment of the infected total knee arthroplasty with insertion of another prosthesis: the effect of antibiotic-impregnated bone cement. Clin Orthop Relat Res 1994;309:44–55 [PubMed] [Google Scholar]
- 17.Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty 1996;11:939–944 [DOI] [PubMed] [Google Scholar]
- 18.Bridgens J, Davies S, Tilley L, Norman P, Stockley I. Orthopaedic bone cement: do we know what we are using? J Bone Joint Surg [Br] 2008;90-B:643–647 [DOI] [PubMed] [Google Scholar]
- 19.Pelletier MH, Malisano L, Smitham PJ, Okamoto K, Walsh WR. The compressive properties of bone cements containing large doses of antibiotics. J Arthroplasty 2009;24:454–460 [DOI] [PubMed] [Google Scholar]
- 20.Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg [Am] 2006;88-A:2487–2500 [DOI] [PubMed] [Google Scholar]
- 21.Zmistowski B, Fedorka CJ, Sheehan E, et al. Prosthetic joint infection caused by gram-negative organisms. J Arthroplasty 2011;26:104–108 [DOI] [PubMed] [Google Scholar]
- 22.Chang Y, Chen WC, Hsieh PH, et al. In vitro activities of daptomycin-, vancomycin, and teicoplanin-loaded polymethylmethacrylate against methicillin-susceptible, methicillin-resistant, and vancomycin-intermediate strains of Staphylococcus aureus. Antimicrob Agents Chemother 2011;55:5480–5484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhanel GG, Wiebe R, Dilay L, et al. Comparative review of the carbapenems. Drugs 2007;67:1027–1052 [DOI] [PubMed] [Google Scholar]
- 24.Nordmann P, Mammeri H. Extended-spectrum cephalosporinases: structure, detection and epidemiology. Future Microbiol 2007;2:297–307 [DOI] [PubMed] [Google Scholar]
- 25.Lau WK, Mercer D, Itani KM, et al. Randomized, open-label, comparative study of piperacillin-tazobactam administered by continuous infusion versus intermittent infusion for treatment of hospitalized patients with complicated intra-abdominal infection. Antimicrob Agents Chemother 2006;50:3556–3561 [DOI] [PMC free article] [PubMed] [Google Scholar]