Abstract
In this study, we investigated the effects of GH treatment in children with Down syndrome who had been diagnosed with GH deficiency (GHD). A total of 20 subjects were investigated in this study. Fourteen Down syndrome children (5 boys and 9 girls) with short stature due to GHD were treated with GH at Okayama Red Cross General Hospital, and 6 Down syndrome children (4 boys and 2 girls) with short stature due to GHD were registered in the Pfizer International Growth Database (KIGS). Height SD score (SDS) increased throughout the three-year GH treatment period. The overall mean height SDS increased from –3.5 at baseline to –2.5 after 3 yr of treatment. The mean change in height SDS during these 3 yr was 1.1. In addition, height assessment of SD score based on Down syndrome-specific growth data in the Japanese population revealed that the height SDS (Down syndrome) also increased across the 3-yr GH treatment period. The mean change in height SDS (Down syndrome) during these three years was 1.3. GH therapy was effective for Down syndrome short stature accompanied by GHD, and no new safety concerns were found in this study.
Keywords: Down syndrome, GH deficiency (GHD), GH treatment, height SDS
Introduction
Down syndrome is the most common chromosomal disorder, occurring in every 700 to 1000 births. The average life expectancy of Down syndrome patients has been prolonged, up to 50 yr according to recent reports (1). With this longer life expectancy, it is getting more and more important for Down syndrome patients to have a good quality of life.
Common comorbidities of Down syndrome include congenital heart disease, short stature, obesity, hypotonia, cervical instability, eye disorders (congenital cataract, nystagmus, strabismus, ametropia) and deafness. Improving care and outcomes of these comorbidities results in improved quality of life for Down syndrome patients and patient’s guardians. Relieving the burden in caring for Down syndrome patients would encourage patients to be independent.
The short stature improving effects of GH have been found not only in GHD short stature but also in Turner syndrome, Prader-Willi syndrome and small-for-gestational age (SGA) short stature, and GH has been approved for these indications. There have also been reports of GH being effective in Down syndrome short stature (2). However, GH treatment is currently approved for Down syndrome patients in Japan only when GHD is also present.
GH acts to increase the lean body mass of muscles and decrease total body fat and may have beneficial effects on the obesity and hypotonia of Down syndrome patients with GHD short stature.
We combined the treatment data on Down syndrome short stature children with GHD from the Pfizer International Growth Database (KIGS) and Okayama Red Cross General Hospital and analyzed the growth effects and safety of GH treatment over three years. We also analyzed the long-term growth-promoting effects on available data.
Subjects and Methods
We investigated data on GH therapy in Down syndrome short stature children with GHD from Okayama Red Cross General Hospital and the KIGS database.
All 20 subjects were prepubertal Down syndrome short stature children who had been diagnosed with GHD. At Okayama Red Cross General Hospital, there were 14 Down syndrome children with GHD who started receiving GH replacement therapy between June 2001 and November 2009. In the KIGS database, there were 6 Down syndrome children with GHD who started receiving GH replacement therapy between May 2000 and July 2002. All data included at least one year of GH treatment. All patients met the criteria for GHD based on a peak GH concentration level of not more than 10 ng/mL on two or three GH stimulation tests (6 subjects at Okayama Red Cross General Hospital met the criteria of a peak serum GH concentration of not more than 6 ng/mL using a GH method with recombinant GH as the reference standard).
The average GH therapy dosage at treatment initiation was 0.21 ± 0.03 mg/kg/wk among the 14 children from Okayama Red Cross General Hospital (“the Okayama group” hereafter), and the value was 0.23 ± 0.08 mg/kg/wk among the children from the KIGS database (“the KIGS group” hereafter). The dosage per unit body weight (mg/kg/wk) was adjusted as the children gained weight, and remained nearly constant throughout the treatment period.
Treatment was discontinued before reaching adult height or before epiphyseal closure in 6 children in the Okayama group and 5 children in the KIGS group. The reasons for treatment discontinuation were moving (1 patient), did not come to clinic for scheduled visit (3 patients) and refused injection (2 patients) in the Okayama group; the reasons were unknown for all 5 of the patients in the KIGS group. Five patients in the Okayama group and one patient in the KIGS group are still continuing their treatments as of today (November 2012). The mean GH treatment period was 5.2 yr (range: 1.0 to 10.4 yr).
The height SD score (SDS) and weight SDS were calculated based on the sex and age standard heights and weights for Japanese children in 2000 (3). The height SDS (Down syndrome) was calculated from the standard values for Japanese Down syndrome using the Japanese Down syndrome growth curve reported by Kimura et al. (4).
Results
The patients clinical characteristics at baseline are described below. The mean age for the entire cohort was 4.6 yr (range: 2 to 11 yr). The mean age in the Okayama group was 5.1 yr (range: 4 to 11 yr), and the mean age in the KIGS group was 3.5 yr (range: 2 to 5 yr). The children in the KIGS group were therefore younger on average than the children in the Okayama group. The height SDS (mean ± SD) at treatment initiation was –3.5 ± 1.3 and was below –3 SD in both groups (–3.3 ± 0.5 in the Okayama group; –4.1 ± 2.2 in the KIGS group). The height SDS (Down syndrome), based on the Japanese Down syndrome disease-specific standard values, was –1.9 ± 1.2 (–1.7 ± 0.7 in the Okayama group and –2.3 ± 1.8 in the KIGS group). The mean height velocity (cm/yr) before treatment initiation was 5.7 ± 2.0 cm/yr. The weight SDS (mean ± SD) at treatment initiation was –1.8 ± 1.2 (–1.5 ± 0.5 in the Okayama group and –2.4 ± 2.2 in the KIGS group).
Seven of the fourteen children in the Okayama group had a medical history of congenital heart disease that could affect growth. The disposition of these diseases was atrial septal defect (3 patients), Fallot’s tetralogy (2 patients) and ventricular septal defect (2 patients). Information was not available on medical history of congenital heart disease for the children in the KIGS group. One of the children in the KIGS group had congenital hypothyroidism. Two of the children in the Okayama group had hematologic diseases (transient abnormal myelopoiesis and acute myeloid leukemia) in their past treatment histories. These diseases had been cured before GH treatment initiation, and no exacerbations occurred during the treatment period.
Table 1 shows the growth parameter profile for the 3 yr of GH treatment. The mean height velocity increased appreciably, from 5.7 cm/yr at baseline to 8.1 cm/yr after 1 yr of treatment. Although the height velocity decreased in the second and third years of treatment compared with the 1 yr of treatment, it remained the same as or greater than the baseline height velocity.
Table 1. Change in growth parameters during GH treatment.
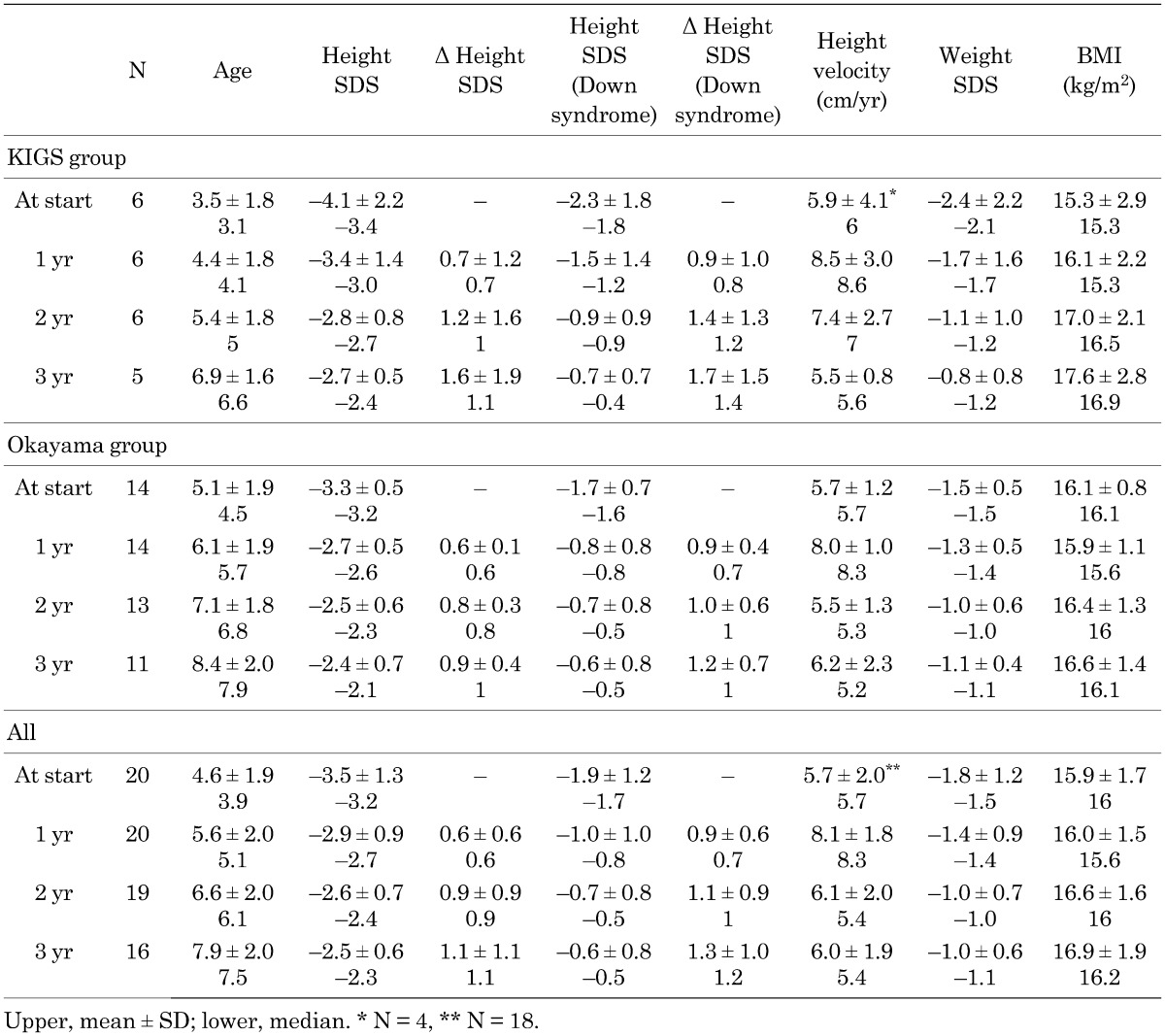
Height SDS increased across the 3-yr GH treatment period. The mean height SDS increased from –3.5 at treatment initiation to –2.5 after 3 yr of treatment. The mean change in height SDS for the 3 yr was 1.1 (0.9 in the Okayama group, but 1.6 in the KIGS group).
When we use the Japanese Down syndrome growth curve as the standard, the height SDS (Down syndrome) increased across the 3-yr GH treatment period. The mean change in height SDS (Down syndrome) for the 3 yr was 1.3 (1.2 in the Okayama group and 1.7 in the KIGS group), which was slightly higher than the mean change in height SDS (1.1).
The mean BMI remained unchanged from baseline (15.9 kg/m2) to 3 yr after initiation of GH treatment (16.9 kg/m2).
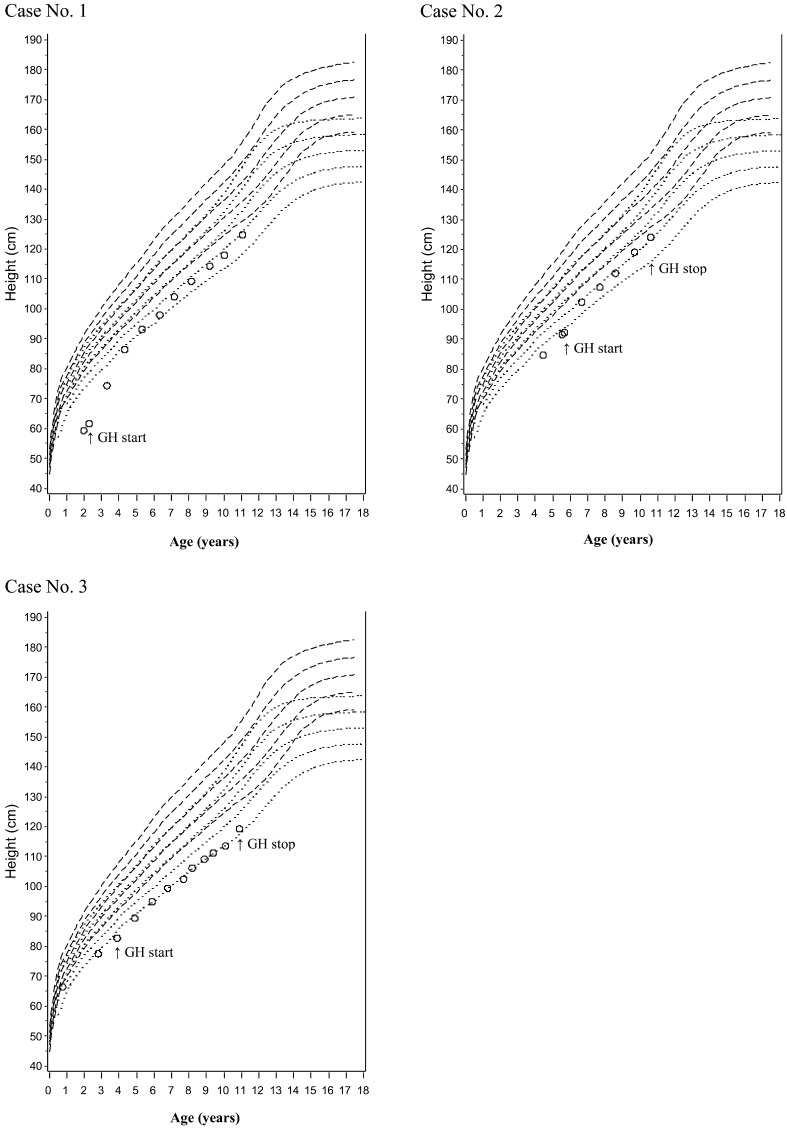
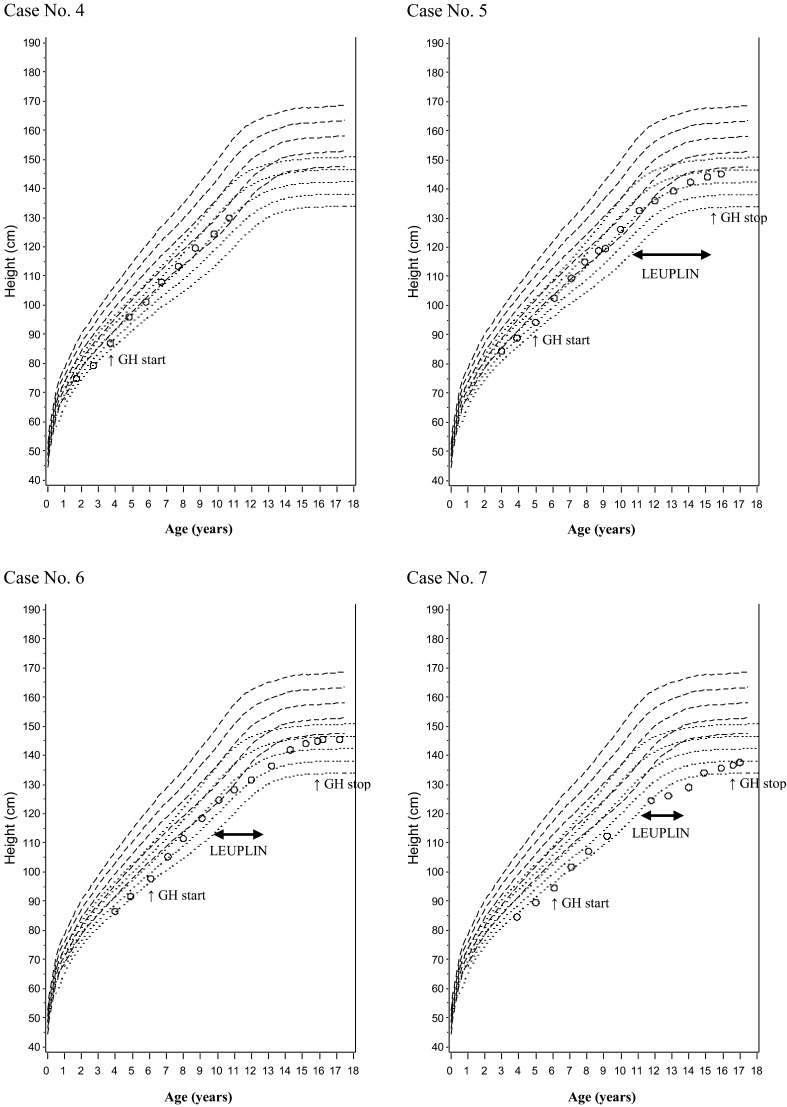
In Figs. 1 and 2, we plotted growth curves for patients receiving long-term GH therapy over the standard growth curves of Japanese healthy children and that of Japanese Down syndrome children (for 7 patients in all, 3 boys and 4 girls). The growth curves during GH therapy paralleled the standard curves overall, and growth persisted even in late puberty, while the standard curve flattens at that time. Three patients (cases 5, 6 and 7 [all girls]) also received an LH-RH analogue (Leuplin®) temporarily. The rate of height increase flattened in a manner similar to that seen in healthy children receiving LH-RH analogue therapy. After LH-RH therapy was discontinued, the height increase returned to the pace seen prior to LH-RH analogue therapy or an even greater pace.
Fig. 1.
Growth curves of GHD short stature Down syndrome children (3 boys) receiving long-term GH therapy. Case No. 1 was still continuing GH treatment as of November 2012.
Fig. 2.
Growth curves of GHD short stature Down syndrome children (4 girls) receiving long-term GH therapy. Case No. 4 was still continuing GH treatment as of November 2012.
Precocious puberty was observed in one boy. This patient was diagnosed with precocious puberty 3.4 yr after GH treatment initiation (when he was 9.1 yr old) based on increased testicular volume (7 mL). It is not considered an adverse event related to GH treatment. In this cohort, the age of onset of puberty during GH therapy ranged from 11 to 13 yr in boys and 9 to 14 yr in girls, and we did not observe early onset of puberty. Otitis media was reported as an adverse event in four children, and growing pain was reported in one patient, but neither of these adverse events were related to GH treatment. TSH was elevated in one boy. This patient received oral levothyroxine sodium hydrate (THYRADIN-S), and his thyroid function was monitored.
Bone age was also measured periodically during GH therapy, but no excessive progression in bone age was noted when the values were compared with chronological age.
Most of the patients had comorbid congenital heart diseases, and GH therapy did not result in any clinically significant changes in these diseases.
Discussion
This report showed that at the baseline, the mean height SDS in the Down syndrome short stature children with GHD was –3.5, which was even lower than that seen with untreated Turner syndrome or GHD short stature, for which GH therapy is approved in Japan. Also noted in this report was that height velocity 1 yr prior to treatment initiation of this cohort was slow as well. We presume that if these children were left untreated, the height velocity would have deviated even further from the standard height. The height SDS (Down syndrome) of this cohort was –1.9 relative to the standard value for the Japanese Down syndrome population, and it is therefore believed that, in addition to Down syndrome, GHD also contributed to their short statures.
As seen in growth disorders such as GHD short stature and Turner syndrome, for which GH therapy is approved in Japan, height velocity was highest in the first yr of therapy and decreased from the second yr and later. However, it remained at a level equal to or greater than that seen before treatment initiation. Although the mean height velocity (8.1 cm/yr) in the first yr of treatment in this study was lower than the results obtained for GH in GHD short stature (11.0 cm/yr), it was greater than the height velocity of 6.4 cm/yr that was obtained for the first year of treatment in Turner syndrome (Genotropin® package insert).
The mean change in height SDS for the three yr was 0.9 for the Okayama group and 1.6 in the KIGS group. This difference may be attributed to 1) the lower average age at treatment initiation in the KIGS group (age of 3.5), 2) the higher incidence of comorbidities such as congenital heart disease and 3) the higher incidence of poor GH treatment compliance in the Okayama group. With the sample size being so small, it is difficult to conclude if these three factors contributed to the height SDS change or not.
Despite the improvement in height SDS, the mean height SDS at the third yr was –2.4 in the Okayama group and –2.7 in the KIGS group. Neither of these values exceeded –2 SD, the definition of short stature. Further study is needed regarding whether or not initiating treatment earlier would increase treatment efficacy.
The delta height SDS results obtained in our study were nearly identical to the delta height SDS values reported by Fujieda et al. in the first and third yr after the start of treatment in GHD short stature children with peak GH levels of not more than 10 ng/mL in GH stimulation tests (5). This suggests that similar efficacy can be obtained from GH treatment in patients with GHD short stature associated with Down syndrome.
The BMI of the subjects in this cohort remained virtually unchanged across three yr. It is known that obesity in Down syndrome patients is not pronounced until the age of six, but the incidence of obesity increases after the age of six (6). The mean obesity (%), calculated from the standard weights by height for children reported by Ito et al. (7), was around 5% in both groups. Both groups were therefore found to be slightly overweight. The effects of GH on body composition are hard to distinguish from natural processes of growth, and clear conclusions cannot be drawn. More study will be required with untreated control groups.
The delta height SDS (Down syndrome) for three yr using the Down syndrome standard value was greater than the delta height SDS relative to the standard value for healthy children. This shows that the growth curve for Down syndrome patients gets ever farther away from the standard growth curve for healthy children as age increases, suggesting the need for early treatment initiation.
In patients treated for a longer period, the height SDS decreased when compared with the standard height in healthy children. However, compared with the standard value for Down syndrome, height SDS was improved. The growth-promoting effects of GH were confirmed in patients with Down syndrome over a longer term.
No specific events were observed in association with GH treatment in Down syndrome in this study. However, many of the subjects of this study had comorbid diseases, such as congenital heart disease and thyroid disease. Down syndrome is also known to be accompanied by hematological malignancy comorbidities. Careful monitoring is encouraged in order to detect progression of these life-threatening comorbidities in the early phase.
Conclusions
Twenty cases of Down syndrome short stature children with GHD were treated with GH for three yr, and the growth-promoting effects and safety were investigated. The height SDS increased across the three yr of GH treatment, and the mean height SDS increased from –3.5 at treatment initiation to –2.5 after three yr of treatment. The growth-promoting effects of GH across a long period were confirmed as well. GH treatment for Down syndrome short stature with GHD was as effective as GH treatment for GHD short stature, and no new safety concerns were found.
Acknowledgments
We thank the physicians who participated in KIGS Japan and the KIGS Japan national board members.
References
- 1.Masaki M, Higurashi M, Iijima K, Ishikawa N, Tanaka F, Fujii T, et al. Mortality and survival for Down syndrome in Japan. Am J Hum Genet 1981;33: 629–39 [PMC free article] [PubMed] [Google Scholar]
- 2.Torrado C, Bastian W, Wisniewski KE, Castells S. Treatment of children with Down syndrome and growth retardation with recombinant human growth hormone. J Pediatr 1991;119: 478–83 [DOI] [PubMed] [Google Scholar]
- 3.Ito Y, Kato N, Tachibana K, Fujieda K. “Table of standard height” and “standard growth curve” in conformity with the height standard adopted in Research into Treatment for Specific Child Chronic Diseases, 2000 edition. The Journal of Pediatric Practice 2005;68: 1343–51(Shounika Sinryo) [Google Scholar]
- 4.Kimura J, Tachibana K, Imaizumi K, Kurosawa K, Kuroki Y. Longitudinal growth and height velocity of Japanese children with Down’s syndrome. Acta Paediatr 2003;92: 1039–42 [DOI] [PubMed] [Google Scholar]
- 5.Fujieda K, Hanew K, Hirano T, Igarashi Y, Nishi Y, Tachibana K, et al. Three-year growth response to growth hormone therapy in patients with different degrees of growth hormone deficiency: Analysis of the Japanese International Cooperative Growth Study. Clin Pediatr Endocrinol 1999;8(Suppl 13): 23–8 [Google Scholar]
- 6.Takano T. Down syndrome –Recent topics– 2. Clinical features. Japanese Journal of Pediatrics 2011;64: 2103–15(Shounika Rinsyo). [Google Scholar]
- 7.Ito Y. 1. Commentary: Explanation of standard growth curve and disease-specific growth curve, explanation of obesity assessment curve. In: Fujieda K, editor. Revelations of the growth curve: Pediatric diseases causing growth disturbance –cases and explanations–. 1st ed. Tokyo: Shindan-to-chiryo-sha Co., ltd; 2005. p. 39–43. [Google Scholar]