Abstract
Several studies have described brain white matter abnormalities on magnetic resonance imaging (MRI) in children and adults with congenital adrenal hyperplasia (CAH), while the brain MRI findings of newborn infants with CAH have not been clarified. We report a newborn boy with CAH who presented brain white matter abnormality on MRI. He was diagnosed as having salt-wasting CAH with a high 17-OHP level at neonatal screening and was initially treated with hydrocortisone at 8 days of age. On day 11 after birth, he had a generalized tonic seizure. No evidence of serum electrolyte abnormalities was observed. Brain MRI revealed white matter abnormalities that consisted of bilateral small diffuse hyperintensities on T1-weighted images with slightly low intensity on T2-weighted images in the watershed area. Several factors associated with brain white matter abnormalities in adults with CAH, such as increasing age, hypertension, diabetes and corticosteroid replacement, were not applicable. Although the cause of the phenomenon in this case is unclear, brain white matter abnormality could be observed in newborn infants with CAH as well as in adult patients.
Keywords: aldosterone, brain white matter, congenital adrenal hyperplasia, magnetic resonance imaging, newborn infant
Introduction
The most common cause of congenital adrenal hyperplasia (CAH) is 21-hydroxylase deficiency, which is an autosomal recessive disease. This deficiency leads to a considerable cascade of hormonal modifications including underproduction of cortisol and aldosterone and overproduction of adrenocorticotropic hormone and, as a consequence, adrenal androgen. Replacement of the deficient hormones, glucocorticoid and mineralocorticoid, is necessary for patients with CAH (1).
Several studies have described brain white matter abnormalities on magnetic resonance imaging (MRI) in children and adults with CAH (2,3,4,5,6,7). However, the brain MRI findings in newborn infants with CAH have not been clarified except in one case report (8).
Case Report
The patient was the first child of healthy, non-consanguineous Japanese parents and was born at 41 wk of gestation with a birth weight of 3278 g. He had no significant prenatal history and was born after an uncomplicated delivery with Apgar scores of 9 at 1 min and 9 at 5 min. The patient was admitted to Tohoku University Hospital at 8 d of age because of an elevated 17-hydroxyprogesterone level in neonatal screening (>100 ng/mL). On admission, he was hypoactive and had pigmentation. Laboratory investigations showed a low serum Na+ (132 mEq/L), a high serum K+ (6.1 mEq/L) and a normal serum blood glucose level. The level of plasma cortisol (4.0 μg/dL; reference range [N]: #7.1–17.7 μg/dL) was decreased, whereas the levels of plasma adrenocorticotropic hormone (559 pg/mL; N: #7.2–63.3 pg/mL), dehydroepiandrosterone sulfate (5530 ng/mL; N: #59-87 ng/mL), renin activity (507.4 ng/mL/hr; N: #3.66–11.1 ng/mL/h) and testosterone (973 ng/dL; N: #59.0-408 ng/dL) were markedly increased. The patient was diagnosed as having salt-wasting CAH and was initially treated with hydrocortisone (200 mg/m2/day), and fludrocortisone and NaCl were added 10 days and 15 days after admission, respectively. He recovered gradually, and his serum Na+ and K+ levels were normalized by the second day of treatment.
On day 11 after birth (fourth day in the hospital), the patient had a generalized tonic seizure with conjugate deviation of the eyes to the right. No evidence of inflammatory reaction, serum electrolyte abnormalities including calcium or coagulation disorders were observed. Head ultrasonography and computed tomography showed no intracranial hemorrhage. Brain MRI on the day after the seizure revealed white matter abnormalities that consisted of bilateral small diffuse hyperintensities on T1-weighted images (WI) with a slightly low intensity on T2WI in the watershed area (Fig. 1). Electroencephalography showed no epileptiform discharges. The patient had no further seizures on anticonvulsant medication with phenobarbital.
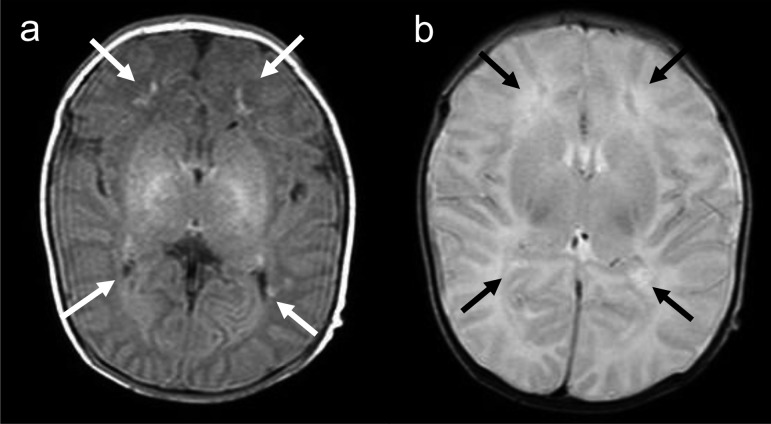
Fig. 1.
Magnetic resonance imaging of the brain, performed on day 12 after birth, reveals white matter abnormalities that consisted of (a) bilateral small diffuse hyperintensities (white arrow) on T1-weighted images and (b) hypointensities (black arrow) on T2-weighted images in the watershed area.
At 1 yr of age, the patient exhibited favorable growth and no neurological sequelae. On a follow-up brain MRI, the small hyperintensities on T1WI had decreased in intensity, whereas these areas presented with a high intensity on T2WI (Fig. 2).
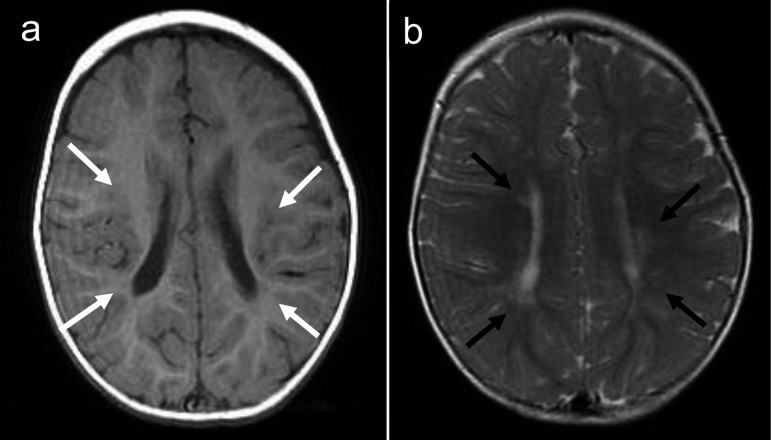
Fig. 2.
Magnetic resonance imaging of the brain, performed at 1 yr of age, reveals (a) bilateral small diffuse hyperintensities (white arrow) on T1-weighted images and (b) hypointensities (black arrow) on T2-weighted images in the watershed area.
Discussion
The brain white matter abnormalities in children and adults with CAH are considered to be associated with increasing age, hypertension, diabetes and corticosteroid replacement (2,3,4,5,6,7). Samia et al. reported brain MRI abnormalities in a 15-yr-old CAH patient who presented with tremors and hypertension (7). Recently, a newborn infant with CAH was reported as showing brain white matter abnormalities on MRI, the mother of which received dexamethasone therapy at between 8 and 12 wk of gestation (8). The patient we reported was young, did not have hypertension or diabetes and did not receive glucocorticoid in utero.
The patient that Winfeld et al. reported showed multiple foci with reduced intensity in the periventricular white matter and corpus callosum on diffusion-weighted imaging, in addition to T1 hyperintensity and T2 hyperintensity in the corresponding area on day 11 of life (8). The patient had seizures with hypocalcemia, and his mother was treated with prenatal dexamethasone during 8 and 12 wk of gestation. Our case had no evidence of serum electrolyte abnormalities including calcium, and he did not receive a massive dose of glucocorticoid prior to the MRI examination. Thus we speculate that the abnormal white matter findings on MRI may be associated with CAH itself.
Experimental studies have revealed that aldosterone is a major stimulator of vascular collagen synthesis and fibroblast proliferation via the activation of local mineralocorticoid receptors (3). Because of the presence of such receptors in large arteries and of the endogenous vascular synthesis of aldosterone, this hormone appears to play a significant role in the regulation of arterial structure independently of its action on blood pressure. Aldosterone is also synthesized de novo in the brain and could play a direct local regulatory role with regard to the cerebral arterial structure (3). Therefore, aldosterone deficiency could lead to subclinical cerebral ischemia (3, 5). Bhangoo et al. reported three children with lipoid CAH on brain MRI findings; one case had evidence of frontal and temporal atrophy, another had white matter lesions consistent with demyelination, and the third case had Chiari-I malformation (9). They speculated that StAR might serve an important function in brain development or function. But it could also be possible that aldosterone deficiency might have some influence on brain development in lipoid CAH.
Another possible cause of white matter abnormality is periventricular leukomalacia (PVL). The pathogenesis of PVL is related to a remarkable confluence of maturation-dependent pathogenetic factors that conspire to render the cerebral white matter of the human premature infant vulnerable to injury (10). The result is a propensity to injury initiated by two major upstream mechanisms, ischemia and infection/inflammation (10). The current case had no remarkable prenatal history and was born at term after an uncomplicated delivery. No evidence of an inflammatory reaction or hemorrhage was noted in a macroscopic examination of the placenta and umbilical blood gas analysis. Hypoxia was not observed at any time after admission. We considered that PVL was unlikely in the patient because he did not experience ischemia or infection/inflammation during the pre- and perinatal periods.
From a clinical perspective, it is uncertain if the seizure at 11 days after birth was associated with the white matter abnormalities because the MRI findings did not correspond to the symptoms. A couple of studies described brain atrophy on MRI in some patients with CAH and lipoid CAH, although these patients had no neurological sequelae, developmental delay or reduced intelligence quotient (2, 9). At 1 year of age, the psychomotor development of the patient was normal without any neurological signs. Since the brain MRI findings were similar to that of PVL, careful neurodevelopmental follow-up is necessary.
A few studies investigated children with CAH and acute infection-related encephalopathy (11, 12). Brain MRI disclosed various patterns of white matter lesions, suggesting different types of acute encephalopathy, such as clinically mild encephalitis/encephalopathy with a reversible splenial lesion or hemiconvulsion-hemiplegia syndrome (11). Although no MRI data were available before the onset of encephalopathy, there might have been some brain abnormalities that were not necessarily involved in encephalopathy in the CAH patients.
In summary, we reported a newborn patient with CAH who presented white matter abnormality on brain MRI and speculated that it could be observed in young children with the disease. To elucidate if the finding is related to CAH itself or not, a substantial evaluation with additional cases is needed.
References
- 1.Migeon CJ, Wisniewski AB. Congenital adrenal hyperplasia owing to 21-hydoroxylase deficiency. Endocr Metabol Cl North Am 2001;30: 193–206 [DOI] [PubMed] [Google Scholar]
- 2.Nass R, Heier L, Moshang T, Oberfield S, George A, New MI, et al. Magnetic resonance imaging in the congenital adrenal hyperplasia population: increased frequency of white-matter abnormalities and temporal lobe atrophy. J Child Neurol 1997;12: 181–6 [DOI] [PubMed] [Google Scholar]
- 3.Verpillat P, Alpérovitch A, Cambien F, Besancon V, Desal H, Tzourio C. Aldosterone synthase (CYP11B2) gene polymorphism and cerebral white matter hyperintensities. Neurology 2001;56: 673–5 [DOI] [PubMed] [Google Scholar]
- 4.Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, Giedd JN. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. J Clin Endocrinol Metab 2003;88: 1760–5 [DOI] [PubMed] [Google Scholar]
- 5.Bergamaschi R, Livieri C, Uggetti C, Candeloro E, Egitto MG, Pichiecchio A, et al. Brain white matter impairment in congenital adrenal hyperplasia. Arch Neurol 2006;63: 413–6 [DOI] [PubMed] [Google Scholar]
- 6.Gaudiano C, Malandrini A, Pollazzon M, Murru S, Mari F, Renieri A, et al. Leukoencephalopathy in 21-beta hydroxylase deficiency: report of a family. Brain Dev 2010;32: 421–4 [DOI] [PubMed] [Google Scholar]
- 7.Samia YM, Mahdi K, Baha Z, Saida JO, Tahar SM, Habid SM. Congenital adrenal hyperplasia and brain magnetic resonance imaging abnormalities. Clin Pediatr Endocrinol 2010;19: 109–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winfeld M, Patel P, Shah B, Nass R, Milla S. Early occurrence of cerebral white matter abnormality detected in a neonate with salt-wasting congenital adrenal hyperplasia. J Pediatr Endocrinol Metab 2013;26:13–7 [DOI] [PubMed] [Google Scholar]
- 9.Bhangoo A, Gu WX, Pavlakis S, Anhalt H, Heier L, Ten S, et al. Phenotypic features associated with mutations in steroidogenic acute regulatory protein. J Clin Endocrinol Metab 2005;90: 6303–9 [DOI] [PubMed] [Google Scholar]
- 10.Volpe JJ. Periventricular leukomalacia. Hypoxic-ischemic encephalopathy. In: Volpe JJ, editor. Neurology of the Newborn, 5th ed. Philadelphia: WB Saunders;2008. p. 359-79. [Google Scholar]
- 11.Lee S, Sanefuji M, Watanabe K, Uematsu A, Torisu H, Baba H, et al. Clinical and MRI characteristics of acute encephalopathy in congenital adrenal hyperplasia. J Neurol Sci 2011;306: 91–3 [DOI] [PubMed] [Google Scholar]
- 12.Kawashima Y, Hanaki M, Kinoshita T, Nagaishi J, Kanzaki S. A nation wide survey for the incidence of the central nervous system complications in patients treated for congenital adrenal hyperplasia. Horumon To Rinsho 2002;50: 1165–9(in Japanese). [Google Scholar]