Abstract
The neural systems underlying reward-related behaviors across development have recently generated a great amount of interest. Yet, the neurodevelopmental literature on reward processing is marked by inconsistencies due to the heterogeneity of the reward paradigms used, the complexity of the behaviors being studied, and the developing brain itself as a moving target. The present review will examine task design as one source of variability across findings by compiling this literature along three dimensions: (1) task structures, (2) cognitive processes, and (3) neural systems. We start with the presentation of a heuristic neural systems model, the Triadic Model, as a way to provide a theoretical framework for the neuroscience research on motivated behaviors. We then discuss the principles guiding reward task development. Finally, we review the extant developmental neuroimaging literature on reward-related processing, organized by reward task type. We hope that this approach will help to clarify the literature on the functional neurodevelopment of reward-related neural systems, and to identify the role of the experimental parameters that significantly influence these findings.
Keywords: Reward, Triadic Model, Adolescence, Development, Neuroimaging, Task Design, Approach, Avoidance, Motivation, Emotion, Social, Pediatric, Incentive
1. Background
The neuroimaging literature of reward-related processes across development, particularly through the adolescent period, is marked by many inconsistencies. Sources of such inconsistencies are numerous, including the complexity of reward processes and the subsequent challenge of reliably and validly modeling these processes, the diversity of strategies used to study reward-related behaviors, and the developing brain as a moving target. It is important to resolve these inconsistencies to gain a better understanding of the neural correlates of risky behaviors and risk factors for psychopathology that emerge during adolescence (Galvan, 2010; Paus et al., 2008). The goal of this review is to gather all the reward-related fMRI studies that have examined differences in reward function between adolescents and adults and examine reward task design as one source of discrepancies across studies. Many reviews on adolescent behavior address discrepancies in the literature, but given the limitations posed by issues of space and parsimony, none provide comprehensive side by side comparisons of the various paradigms, various samples (size, age and gender), and results. It is time to organize and contrast extant reward-related research findings along a heuristic that provides a model for interpretation. This review focuses solely on reward-related tasks, and does not include works specifically targeting avoidance behavior.
To this goal, the present work systematically examines this literature along three dimensions: (1) task structures, (2) cognitive processes, and (3) neural systems, and uses the triadic model (Ernst and Fudge, 2009) as the organizational framework. The triadic model considers behavior along three interacting dimensions: approach, avoidance and control. In the present review, findings from reward-related studies will be addressed along these three dimensions. The model posits that all three dimensions are engaged in all motivated behaviors but in different ways as a function of multiple factors including context (e.g., approach vs. threat), cognitive demands, and individual characteristics (e.g., age, gender, personality, psychiatric status).
The triadic model offers a dynamic rendition of the neural systems involved in goal- directed behaviors and provides a framework for organizing findings along a systematic grid, partitioning the systems involved predominantly (but not selectively) in coding response to appetitive stimuli, response to aversive stimuli, and executive function. Although this model is not optimal as these systems are not independent from one another and show large functional overlaps, it offers a relatively simple way to organize findings along a clear motivational framework, and provides more flexibility than other more restricted dual-systems models (for a comparison of neural models of motivation, see Section 2 below). We hope that this approach will help to clarify the functional neurodevelopment of reward-related neural systems, and identify the role of the experimental parameters that significantly influence the findings. This work is critical to inform the design of future studies.
Excellent reviews have recently been published on adolescent neurobehavioral changes (e.g., Casey et al., 2008; Ernst et al., 2011; Luciana, 2010; Luna et al., 2010), many of which include the description of various neural systems models explaining these changes (e.g., Casey and Jones, 2010; Galvan, 2010; Geier and Luna, 2009; Somerville and Casey, 2010; Steinberg, 2007; Wahlstrom et al., 2010), and we only provide a brief overview of these topics. However, to ground our work, we selected the triadic neural systems model (Ernst and Fudge, 2009) over a dual neural systems model (e.g., Casey et al., 2008), because it may offer greater latitude in interpreting inconsistent findings, as it allows the modulation of three rather than two systems to accommodate various and complex situations. Of note, neural systems models have been challenged recently as being too simplistic and not able to account for findings running against predictions based on these models (Pfeifer and Allen, 2012). Specifically, ventral striatal responses to reward are particularly discrepant across studies, with reports of no differences across development, in contrast to findings of hypoactivation or hyperactivation in adolescents relative to adults. The present work is a direct response to this criticism. This review seeks to provide a high level of detail about the various studies and identify parameters possibly responsible for the discrepant findings. Ultimately, we hope to facilitate the formulation of testable hypotheses about how specific task elements, such as the nature and degree of cognitive demands or rewards, can contribute to such inconsistent results.
Another significant criticism is that these models do not explicitly incorporate critical changes in social and affective processing that take place during adolescence (Crone and Dahl, 2012a). Only one currently available study directly manipulates social context during an incentive-based task as a means of addressing developmental differences in the effects of peer influence on the neural substrates of reward (Chein et al., 2011). Certainly, more studies of this kind are needed to refine existing neural systems models of adolescent reward processing, taking into account broader social and affective changes as factors influencing reward processing and motivation. In addition, models that incorporate intrinsic connectivity across networks may soon complement the relatively more static neural systems models used here.
This review is organized into three sections. First, we present the triadic neural systems framework of reward-related processes along which the review of extant findings will be organized; second, we lay out the principles underlying the development of reward processing paradigms; and third, as the main goal of this work, we review the developmental functional neuroimaging studies of reward-related behavior and point to variability in reward task parameters as potential sources of discrepant results. This review focuses selectively on studies that directly examine either developmental differences or maturational changes in reward-related neural systems, spanning childhood and/or adolescence through adulthood. We define a study as “developmental” if it makes direct comparisons among different age groups, including at least one or more pediatric samples.
2. Neural systems framework of reward-related behavior
Before examining the neurodevelopmental mechanisms governing changes in reward processing, it is first important to clearly define what is meant by “reward” and to review what is known about its neural basis based on extant animal and human neuroimaging research on healthy adults. Rewards can be defined by their function. A stimulus is rewarding if it activates an integrated set of neural responses that are translated into positive emotions (liking) and is associated with increased motivation to approach/consume (wanting) the stimulus (Berridge and Kringelbach, 2008). The neural correlates of reward processing and reward-related behavior in humans have been extensively studied (see reviews, Bechara, 2005; Ernst and Paulus, 2005; McClure et al., 2004). Briefly, the reward circuitry is a distributed network, classically described as part of the cortico-basal ganglia system (Alexander and Crutcher, 1990; Haber, 2003; Parent and Hazrati, 1995). Its central core includes the ventral striatum and midbrain area, particularly the ventral tegmental area. Reward-related information is processed across a circuitry involving large glutamatergic projections from the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC), as well as massive dopaminergic projections from the midbrain onto the ventral striatum. The ventral striatum integrates this information and sends it back to the prefrontal cortex indirectly through the ventral pallidum and midbrain area, and, in turn, medial dorsal (MD) nucleus of the thalamus. The MD nucleus of the thalamus filters this information and directs it back to the prefrontal cortex, where it is translated into action. Other important structures, such as the amygdala, hippocampus, lateral habenular nucleus and regions of the raphe, modulate this circuitry.
A number of theoretical models have been used to study the neural correlates of motivated behavior, particularly from a developmental perspective (e.g., Casey et al., 2008; Luciana, 2010; Luna et al., 2010). The dual neural systems models have been advocated by a number of investigators (e.g., Casey and Jones, 2010; Galvan, 2010; Geier and Luna, 2009; Somerville and Casey, 2010; Steinberg, 2007; Wahlstrom et al., 2010) and are widely used to model the neural underpinnings of adolescent behavior. In essence, these models describe a cognitive regulatory system that modulates the activity of an emotional/motivational system. The cognitive regulatory system is supported by the prefrontal cortex which exerts top-down influences on subcortical regions, and the emotional/motivational system is driven by subcortical structures, mainly including the striatum and less centrally the amygdala. The developmental dynamics of this dual-systems model focus on a less efficient control system that does not optimally regulate an overreactive emotional/motivational system in adolescence relative to adults. This dual-system framework is very similar to the triadic framework, as described below. The main difference is that the triadic system separates the emotional/motivational system into a positive (approach) and a negative (avoidance) system, and posits different qualitative and quantitative patterns of functioning within these two emotional/motivational systems during adolescence compared to adulthood. This triangulation permits a more sensitive account for the different neural mechanisms driving specific aspects of motivated behavior, including responses to either appetitive or aversive stimuli, as they have been shown to differ across age groups (Spear, 2011). Finally, an alternative theoretical viewpoint of the neurobiology of motivated behaviors includes neurochemical modulators of neural activity. This perspective can be quite informative given the known changes in neurotransmitter function with age, particularly within the dopamine system (Ernst et al., 2009; Ernst and Spear, 2009; Spear, 2000; Wahlstrom et al., 2010). However, this latter perspective is outside the scope of this review.
2.1. The Triadic Model
According to the Triadic Model, three neural systems collaborate to generate and modulate motivated behaviors. Their respective functions consist of a reward/approach module, an aversion/avoidance module, and a regulatory/control module. These functional modules are supported by distributed neural systems, which are distinct but largely overlapping, and linked by reciprocal interactions (Ernst and Fudge, 2009). The neural substrates of the triadic modules are summarized in Table 1 and Figure 1.
Table 1.
Neural substrates of the triadic model: The anatomy, function, and role of the striatum, amygdala, and prefrontal cortex
| Modules of the Triadic Model | ||
|---|---|---|
| Approach | Avoidance | Regulation |
| Main Structures | ||
| Striatum | Amygdala | Dorsolateral PFC |
| Orbitofrontal Cortex | Hippocampus | Ventral PFC |
| Insula | Anterior Cingulate Cortex | |
| Function | ||
| Appetitive Stimuli | Aversive Stimuli | Salience Detection |
| Valence/Salience value | Valence/Salience value | Executive Attention |
| Motivation | Fear Responses | Motor Control |
| Motor Response | Threat Avoidance | Conflict Detection |
| Positive Affect | Negative Affect | Conflict Monitoring |
| Conflict Resolution Inhibition | ||
Figure 1.
The Triadic Model. The prefrontal cortex (PFC) has a reciprocal relationship with the striatum and amygdala, and the amygdala projects directly to the striatum. Within the triadic model the striatum represents the reward system, and is associated with approach; the amygdala represents the emotion system, particularly responses to aversive (e.g., fearful) stimuli, and plays a significant role in avoidance; and the prefrontal cortex is the regulatory center, which serves to control approach and avoidance behaviors. Of the four behaviors typically observed in adolescence, the striatum is chiefly responsible for risk seeking and cognitive impulsivity; the amygdala for emotional intensity and lability. Social reorientation involves interactions among all three systems.
The approach module refers to the reward-related neural system. This neural system comprises subcortical and cortical structures that are major sites of dopamine action, and include primarily the striatum (caudate nucleus, putamen and nucleus accumbens) (e.g., Di Chiara, 2002; Di Chiara and Bassareo, 2007; McClure et al., 2004; Schultz et al., 1998; Wise, 2004) and the medial and orbital prefrontal cortices (Jensen et al., 2003; Kringelbach, 2005). Behaviorally, appetitive motivational processes seem to follow a curvilinear developmental trajectory, whereby reward sensitivity peaks in adolescence (Ernst and Spear, 2009).
The avoidance module refers to the aversion-related neural system. This module is comprised of the amygdala, hippocampus, and insula, which consistently respond to aversive stimuli (Hardin et al., 2009b; Rauch et al., 2003). Although distinctly implicated in threat-related processes (e.g., LeDoux, 2000), this system is involved in both positive and negative emotions. Behaviorally, emotion-related processes also seem to follow a curvilinear trajectory, by which emotional responses peak in intensity and lability in adolescence (Arnett, 1999; Larson et al., 2002; Silk et al., 2003; Weinstein et al., 2007). There is a relative dearth of research examining avoidance behavior in the context of incentives; however, the available evidence seems to map onto a quadratic function indicating a dip in avoidance response during adolescence when there is some probability of reward (Ernst et al., 2011).
The control module refers to regulatory processes that modulate subcortical function, (i.e., the approach and the avoidance systems), through “top-down” cognitive control. This module relies on prefrontal cortical structures that carry specialized functions, such as inhibition (right inferior prefrontal cortex) (Aron et al., 2004; Chikazoe et al., 2007; Liddle et al., 2001), working memory and cognitive salience detection (dorsolateral prefrontal cortex) (Rubia et al., 2010), and conflict detection, monitoring, and resolution (anterior cingulate cortex) (Amodio and Frith, 2006; Bush et al., 2000; Carter and van Veen, 2007). It is not clear whether these specialized cognitive processes directly modulate subcortical function (through direct corticostriatal projections (Haber and Knutson, 2010)), or whether they use an indirect path to exert top-down regulatory function (such as the modulation of ventromedial prefrontal cortical regions, coding for valuation, by dorsolateral prefrontal regions, coding for self-control (Hare et al., 2009)). Behaviorally, in contrast to the curvilinear and quadratic trajectories of reward-related and aversion-related processes (respectively), control processes (e.g., sustained attention, behavioral inhibition) seem to mature linearly with age (Marsh et al., 2006; Rubia et al., 2007b; Rubia et al., 2006).
Another dimension to be considered in the future is connectivity. This dimension is embedded in the terms “networks” or “systems” associated with each module. However, in addition to connectivity within a given system, we also need to understand connectivity across networks. Very little has been done developmentally to examine within and, particularly, between networks connectivity, although this research is rapidly expanding. Most recent advances have been made using task-independent intrinsic connectivity using resting state methodology (Dosenbach et al., 2010; Fair et al., 2008; Fair et al., 2009; Kelly et al., 2009; Supekar et al., 2009; Uddin et al., 2010; Carlisi et al., in press). The most consistent findings have been the increase with development of long-range connections at the expense of short-term connections, and the enhanced selectivity and specialization of connections.
To our knowledge, only one activation study has been devoted to examining connectivity of a core reward network in adolescents and adults during a reward task (Cho et al., 2012). This study used a causal modeling approach (dynamic causal modeling) and reported distinct adolescent and adult patterns of connectivity strengths in a discrete network including ventral striatum, thalamus and insula. We expect that the increased knowledge in changes of functional connectivity with age will lead to new conceptualizations of how the brain develops and underlies changes in behavior, as well as optimized neural systems models.
Based on a given level of regional function and connectivity, the integrated activity of the triad reaches an equilibrium that determines behavioral output. This equilibrium is specific to a given situation (e.g., deciding on a course of action), but is modulated by both transient and sustained factors. Transient factors may include individual mental state (e.g., depressed, stressed), physical state (e.g., drug action, sleep-deprived), or context (e.g., school vs. home, social vs. non-social). Sustained factors may include individual mental traits (e.g., inhibited temperament), maturation level (age, puberty), genetic make-up, past experiences, or gender. These factors are critical as they contribute to the large inter-individual variability of behavioral responses. However, with the exception of age, transient and sustained factors will not be addressed specifically in this review, although they certainly need to be considered when interpreting the neuroimaging studies presented below. Along these lines, the effects of pubertal changes also need to be considered, particularly when studying changes in neural functioning and behavior across the adolescent developmental period. Paradoxically, this area of research has been relatively ignored (see Blakemore et al., 2010; Crone and Dahl, 2012b; Ernst et al., 2009), and while interest is growing, only one study so far has examined pubertal effects on neurodevelopmental changes in the reward system (Forbes and Dahl, 2010). Future work will hopefully provide data to parse the effects of age versus pubertal status on reward-related processes. Similarly, sex differences in functional changes in reward-related neural systems during adolescence have not been studied systematically, and sample sizes are usually too small to examine sex effects. This is an obvious gap that will need to be addressed. Finally, the role of social context on motivated behavior in adolescence is paramount (Blakemore, 2008; Crone and Dahl, 2012b; Nelson et al., 2005; Steinberg, 2008). Here again, so far, only one study addresses social context on reward-related neural systems across development (Chein et al., 2011).
Central to the Triadic Model is the notion of a shift in the triadic balance during adolescence that varies across contexts. This shift putatively facilitates preferential recruitment of the reward module at the expense of the control module in the context of incentives (Ernst et al., 2006), and underlies the peak in reward-related traits and behaviors (e.g., reward sensitivity, risk-taking, novelty seeking) that typically emerges during adolescence. Indeed, a number of studies have reported enhanced reward-related activation within the striatum among adolescents relative to adults or pre-pubertal children (e.g., Ernst et al., 2005; Eshel et al., 2007; Galvan et al., 2006). However, some studies have provided conflicting reports of reduced recruitment of reward structures in response to rewarding stimuli among adolescents compared to adults or children (Bjork et al., 2004; Bjork et al., 2010). Possible reasons for these inconsistencies may be the variability in the type of rewards (e.g., points, candies, money; abstract vs. concrete) and the experimental paradigms that have been used in functional neuroimaging studies to probe the neural basis of reward functioning, in addition to sample characteristics.
Of note, this shift in the triadic balance may also underlie a unique adolescent-related sensitivity to uncertainty and ambiguity, as suggested by Tymula et al. (2012). In this recent behavioral study, adolescents appeared ambiguity-tolerant and, paradoxically, uncertainty-averse compared to adults. These two dimensions, which characterize most daily life events, have been shown to involve the modulation of amygdala, prefrontal cortex and striatum (e.g., Hsu et al., 2005), the key nodes of the triadic model. The paradoxical uncertainty-averse finding in adolescents is provocative, given the substantial evidence of adolescence as a period of peak in risk taking. It will be important to examine further this question using the same task in different contexts (e.g., social, nonsocial), and perhaps also variants of the task to understand which parameters (e.g., magnitude of reward; primary vs. secondary rewards) could modulate such developmental behavioral differences. Subsequent neuroimaging studies may elucidate the role of developmental changes in the triadic balance in determining adolescents’ unique aversion to uncertainty.
This review focuses selectively on the issue of task designs, in contrast to other reviews that provide more comprehensive coverage of the potential reasons underlying discrepant results (e.g., Galvan, 2010). The following section presents an overview of basic principles underlying the design of reward-related paradigms for fMRI, and describes three key categories of reward tasks that frequently have been used in the literature.
3. Basic principles underlying task design of reward paradigms for fMRI
3.1. Processes involved in reward-related behavior
Reward stimuli can be defined operationally as stimuli that are positively reinforcing. Accordingly, these stimuli increase the probability that individuals will engage in a behavior to approach or obtain the reward. Rewards are desirable stimuli that are either innate (e.g., food, water, sex) or learned (e.g., money), and can be characterized in multiple ways (e.g., complexity, magnitude, timing, ambiguity or probability, to cite only a few). Thus, research questions probing the neural basis of different aspects of reward processing necessarily require tailored reward paradigms that can (1) manipulate the factors under scrutiny and (2) control for factors of no interest.
The cognitive neuroscience approach to the study of complex behaviors parses out these behaviors into more elementary functional units of behavior/cognition. These elemental units are more easily manipulated and measurable, and more amenable to scientific scrutiny. In the context of reward, one strategy is to decompose a simple “reward-related behavior” into its sequential parts (including pre- and post-reward receipt), which are often referred to as different stages of reward processing, (i.e., anticipation stage or feedback stage). These stages, as well as additional stages in more complex reward-related paradigms, are detailed below, drawing from tasks already published in the literature.
3.2. Basic principles underlying reward-related paradigms
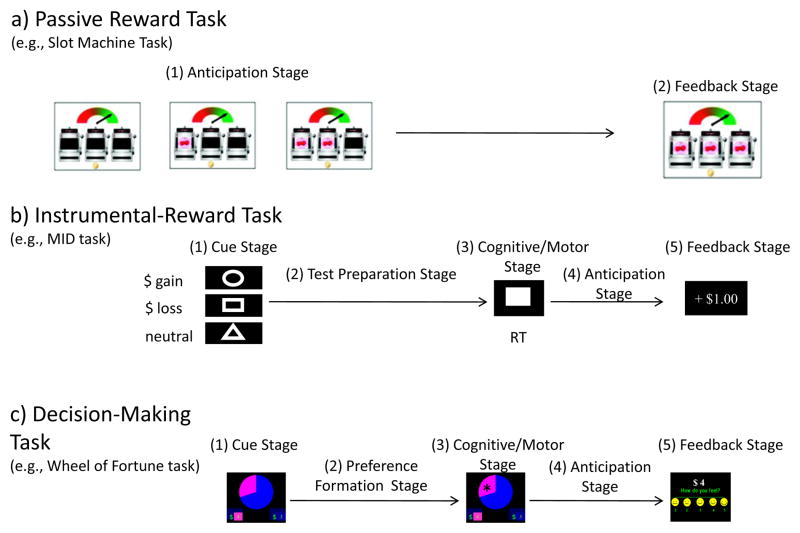
Figure 2 summarizes the general approach used to study the neural correlates of reward-related processes in the fMRI environment. This approach is based on the decomposition of complex motivated behaviors into smaller parts. Three basic task structures, outlined below, have been used to contrast reward-related processes in youths relative to adults: (1) passive reward tasks, (2) instrumental-reward tasks and (3) reward decision-making tasks.
Figure 2.
Reward Paradigm Stages: An appraisal cue stage (provides trial information or signals participants to prepare to respond), a preference/preparation stage (formation of preference for decision-making paradigms and performance preparation for the instrumental-reward-tasks), a cognitive/motor stage (execute the selection or the test); an anticipation stage; and a reward feedback stage.
3.2.1. Passive reward tasks
The first and simplest task consists of the passive presentation of rewards without any active action on the part of the individual to obtain the rewards (see Figure 2a). One example of a passive reward presentation paradigm is the slot-machine task (Van Leijenhorst et al., 2009). This task has been used to contrast adult and adolescent neural functioning during the passive receipt of probabilistic gains. For this task, participants view three slot machines which display pictures of fruit one at a time. Participants are rewarded at the end of the trial if the three pieces of fruit are all the same. The neuroimaging analysis focuses on the anticipation phase of this task.
We also include an emotion task in this category, which presents three types of emotionally salient stimuli (positive, negative, and neutral) in the form of facial expressions (Guyer et al., 2008). Although used in the context of emotion processing rather than reward/motivation processing, this task is a typical example of a passive viewing paradigm of appetitive (happy expression), aversive (angry or fearful expressions), and neutral (neutral expression) stimuli.
Of note, a major limitation of passive viewing tasks is the absence of behavioral or subjective measures that could provide additional information to interpret individual differences in neural activation. As such, more complex, but more nuanced tasks allow for more refined interpretations of the neural basis of reward processing and reward-related behavior.
3.2.2. Instrumental-reward tasks
The second type of task, the instrumental-reward task, requires participants to complete an action or test (instrumental) correctly in order to obtain the reward (see Figure 2b). These instrumental actions/tests are linked to a single expected value, in contrast to the decision-making tasks (detailed below) that require participants to take action in response to the presentation of more than one expected value on any given trial. Instrumental-reward tasks can involve perceptual, cognitive and/or motor processes. For example, the test could consist of a memory challenge (e.g., the Pirate’s Paradigm; Galvan et al., 2006), a perceptual judgment (e.g., the Cake Gambling Task; Van Leijenhorst et al., 2006), and/or a timed button press response (e.g., the Monetary Incentive Delay task (MID; Knutson et al., 2001) illustrated in Figure 2b). Performance on these instrumental tests is determined by criteria that are independent of the incentives, although incentives modulate performance.
For many instrumental-reward tasks, each trial includes five stages. First, a cue (i.e., the cue stage) is presented and indicates the nature of the trial to come (e.g., reward vs. loss) or the amount of reward associated with the trial. Second, a delay period is provided for the participant to prepare for the upcoming test (i.e., the preparation stage). Third, the test is presented and the participant must execute the correct action (i.e., cognitive/motor stage). Fourth, another delay period allows participants to anticipate the outcome of their action (i.e., the anticipation stage). Fifth, the outcome is finally presented (i.e., the feedback stage). Of note, the “preparation” stage is often labeled as anticipation. Indeed, this stage clearly includes both anticipation and preparation-for-action, both processes being often impossible to differentiate within existing instrumental-reward task designs. However, one may reason that the more difficult the test performance will be, the more preparation may be necessary; therefore, orthogonal manipulations of the cognitive demands (effort level) and the reward level may be a way to dissociate the contribution of both types of processes (preparation-for-action vs. anticipation) during the “preparation” stage. A few studies have started to examine these two types of processes separately by separating them temporally, but none have compared adolescents to adults (Bjork et al., 2012; Bjork et al., 2008).
These instrumental-reward paradigms offer flexibility in allowing for the manipulation of many parameters of great interest, such as difficulty level of the test, which can inform motivation, or the nature of the actions involved in the test (e.g., cognitive, motor, perceptual). Instrumental-reward tasks lend themselves to the examination of interactions between reward processes and cognitive/motor processes, probing both top-down and bottom-up processes (e.g., Geier et al., 2009b; Hardin et al., 2009a; Jazbec et al., 2005). However, these instrumental-reward tasks do not inform decision-making per se, a fundamental aspect of motivated behavior.
3.2.3. Reward decision-making task
The third type of task is the reward decision-making task (see Figure 2c). In this type of task, participants are required to select one of several options, each associated with a distinct likelihood of reward. This type of task is probably the most complex task as it involves a number of different behavioral reward-related processes, each providing opportunities for experimental manipulations.
Structurally, reward decision-making tasks are quite similar to instrumental-reward tasks, with the exception that the test-preparation stage (see section 3.2.2. “Instrumental-reward tasks”) is replaced by a preference-formation stage. The formation of a preference is the process that is uniquely associated with decision-making. This process implies weighting competing options of different expected values, benefits and costs. It involves the ability to integrate the various characteristics of these values into a common currency and to take action according to a plan that optimizes outcome. A key neural substrate underlying value integration and planning is the lateral prefrontal cortex (Kable and Glimcher, 2009), and activation of this region would be expected to play a different role in tasks of decision-making vs. instrumental-reward tasks. However, the boundary between an instrumental-reward task and a decision-making task can be subtle and subject to controversy. For example, the first version of the Cake Gambling task (Van Leijenhorst et al., 2006) has been framed as making a decision for the reward underlying the largest piece of cake, leading this task to be referred to as a “reward decision-making task”; however, we place this task in the category of “instrumental-reward tasks”, because there is an uncontroversial correct and incorrect response, the correct response being the largest slice of the cake. This selection, therefore, does not depend on subjects’ preference, but on a perceptual discrimination of sizes. This distinction is important for predicting the neural correlates engaged in this task. For example, this task is not expected to modulate substantially the dorsolateral prefrontal cortex, which is involved in planning and self-control (Bunge et al., 2002; Hare et al., 2010; Kable and Glimcher, 2009). As noted below, age did not modulate activation of DLPFC in this task, suggesting that the DLPFC, notable for its prolonged maturation (Gogtay et al., 2004; Paus, 2005), was not critical to the test. Overall, decision-making reward tasks, as defined here, permit researchers to test the neural basis of varying preferences among a range of risky (i.e., probabilistic), novel, or delayed options, rather than execution of an objectively correct or incorrect response.
3.2.4. Other Reward-Related tasks
Combining aspects of these three types of reward tasks can enable researchers to address additional questions, such as the influence of self-agency on reward processes (Bar-Haim et al., 2009b; Bar-Haim et al., 2009a). That is, by including both a passive condition (e.g., passive reward task), which is devoid of agency, and an active condition (e.g., decision-making), which includes agency, the two conditions may be contrasted with one another to isolate the role of agency in reward processing. This approach was taken by Jarcho and colleagues (2012). This study found that neural activity differed by age when the reward-receipt was prescribed by the task (no-choice condition), but was not influenced by age when decision-making was required (choice condition). Given that the no-choice and choice trials were randomly distributed across runs, behavioral and neural responses in one condition were relative to the responses in the other condition. Therefore, the results cannot be easily addressed in one or the other category used below, and for the sake of parsimony, will not be discussed further.
Other tasks have been used to examine aspects of contingency, including direct manipulation of the action-reward contingency (Liljeholm et al., 2011), or social exchange paradigms, in which participants make a choice that leads to an outcome either for self or a confederate. A step further could be to parameterize the level of contingency, by manipulating the number of the recipients of the reward (e.g., self, self and various numbers of confederates, and confederates only). Finally, for completeness, we note that a number of decision-making reward tasks, which are becoming quite influential, have not yet been used in developmental neuroimaging studies. Among them is a modified version of the Balloon Analogue Risk Task (BART) (Lejuez et al., 2003). The BART simulates risk-taking behavior indexed by pumping a balloon (each pump represents a discrete monetary gain), but stopping before the balloon “explodes”, in which case the accrued gain would be lost.
We now proceed with a review of the neurodevelopmental literature on reward processing, organized by the distinct types of task paradigms that have been described above. This review includes only reward-related studies of healthy individuals that include both pediatric and adult samples.
4. Findings of reward-related fMRI studies
This section reviews the developmental neuroimaging literature, organized by type of reward task, and addresses the studies summarized in Table 2a and Table 2b. Table 2a lists the studies by type of reward task and includes sample characteristics, paradigm names, and the reward-stages analyzed. Table 2b presents the major developmental findings, classified by type of reward task, stage, and neuroactivation areas. In addition, we provide the peak activation coordinates, when available, using either the Talairach (Tal) or standard Montreal Neurological Institute (MNI) coordinates. We organize this fMRI review of reward-related studies by type of paradigms (as described above) and stage of the reward process (see Figure 2). Rarely are the trials analyzed as whole trials, without separating the different stages (events) within trials (e.g., anticipation vs. feedback).
Table 2a.
Tasks used to examine developmental neural correlates of reward. The studies are arranged according to type of reward task and include authors, population, task used, and stage(s) analyzed
| Authors | Participants | Task | Stages analyzed |
|---|---|---|---|
| TYPE-1 PASSIVE REWARD TASKS | |||
| Guyer et al., 2008 | 31 adolescents (9–17) 30 adults (21–40) | passive emotion task | feedback |
| Van-Leijenhorst, 2009 | 18 adolescents (14–15) 15 adults (18–23) 17 children (10–12) | Slot Machine task | cue/anticipation |
| TYPE-2 INSTRUMENTAL-REWARD TASKS | |||
| Van Leijenhorst, 2006 | 14 adults (18–26) 12 children (9–12) | Cake Gambling task | selection and feedback |
| Galvan et al. 2006 | 13 adolescents (13–17) 12 adults (23–29) 16 children (7–11) | Pirate task | whole trial |
| Bjork et al., 2004 | 12 adolescents (12–17) 12 adults (22–28) | Monetary Incentive Delay task | anticipation and feedback |
| Bjork et al., 2010 | 24 adolescents (12–17) 24 adults (22–42) | Monetary Incentive Delay task | anticipation and feedback |
| Cho et al., 2012 | 24 adolescents (10–17) 30 adults (22–48) | Monetary Incentive Delay task | anticipation |
| Smith et al., 2011 | 35 adolescents (10–17) 35 adults (18–43) | continuous performance task | whole trial |
| Geier et al., 2009 | 18 adolescents (13–17) 16 adults (18–30) | Antisaccade task | cue/anticipation, and cognitive/motor |
| Padmanabhan et al., 2011 | 10 adolescents (14–17) 10 adults (18–25) 10 children (8–13) | Antisaccade task | Whole trial and 1st half of the trial |
| Somerville et al., 2011 | 19 adolescents (13–17) 25 adults (18–29) 18 children (6–12) | Go/No-go task | cue |
| Cohen et al., 2010 | 16 adolescents (14–19) 11 adults (25–30) 18 children (8–12) | Probabilistic Learning | selection and feedback |
| TYPE-3 REWARD DECISION-MAKING TASKS | |||
| Eshel et al., 2006 | 16 adolescents (9–17) 14 adults (20–40) | Wheel of Fortune task | selection |
| Ernst et al., 2005 | 18 adolescents (9–17) 16 adults (20–40) | Wheel of Fortune task | selection and feedback |
| Van Leijenhorst et al., 2010 | 15 pubertal adolescents (12–14) 15 post-pubertal adolescents (16–17) 15 adults (19–26) 13 children (8–10) | Modified Cake Gambling task | selection and feedback |
| Chein et al., 2011 | 14 adolescents (14–18) 14 young adults (19–22) 12 adults (24–29) | Stoplight task | selection stage |
| Christakou et al., 2011 | 19 male adolescents (11–17) 21 male adults (18–31) | Hypothetical Delay Discounting | selection stage |
| Forbes et al., 2010 | 26 pre/early pub (11.4) 51 mid/late pub (12.2) 19 adults (45.5) | Card Guessing Game | Anticipation and win-feedback |
| Bjork et al., 2007 | 20 adolescents (12–17) 20 adults (23–33) | Game of Chicken | Cue appraisal |
| MIXED TASK | |||
| Jarcho et al., 2012 | 26 adolescents (14.1, 2.4), 26 adults (31.3, 8.2) | Choice/No-Choice | Cue and feedback |
Table 2b.
Main results from fMRI reward studies showing between group differences, organized by type of reward task, stage analyzed, and brain areas activated. Green refers to the approach network, red to the avoidance network, and blue to the regulation network; MNI and Talaraich coordinates included when available
| Cue appraisal/Selection/Performance Preparation | Cognitive/Motor performance execution | Feedback | Whole trial | |
|---|---|---|---|---|
| PASSIVE REWARD TASKS | ||||
| Striatum | Favor: Adol. > Adult (VL-09); MNI 12, 9, −15 | |||
| OFC | Unfav: Adult > Adol. (VL-09); MNI −27, 48,−3 | |||
| Amygdala | Unfav: Adol. > Adult (G-08); MNI 16, −4, −16; −20, −8, −6 | |||
| Insula | Favor: Adol. > Adult (VL-09); MNI 42, 12, −3 | |||
| ACC | ||||
| IFG | ||||
| DLPFC | ||||
| INSTRUMENTAL-REWARD TASKS | ||||
| Striatum | Favor: Adult > Adol. (B−04;B-10; Cho−11); Tal (B−04) −9, 10, 0; 11, 12, 0; Favor: Adol. > Adult (Ge−09); Tal 11, 8, −7 Favor: Adult > Adol. (Ge-09); Tal 14, 2, −7 Favor (happy face): Adol. > Adult/Child (S-10); Tal −4, 11, −9 Favor: Adol.>Adult=Child (P-11) Tal −10,8,−4 |
Decision Value: Adol.> Adult = Child (C-10); MNI 14, 16, 4 | Favor: Adol. > Adult/Child (G-06) Favor: Adol. > Adult (Sm-11); Tal 29, −7, 4 |
|
| OFC | Unfav (neutral) Adol. > Adult (Ge-09); Tal −25, 44, −4 | Unfav (Lowest prob): Child > Adult (VL-06); MNI 40, 46, −12 | Favor: Child > Adol.=Adult (G-06) Favor: Adult>Adol.=Child (P-11) |
|
| Amygdala | ||||
| Insula | ||||
| ACC/mPFC | Decision Value: Adol. > Adult (C-10); MNI 0, 50, −8 Unfav. Child > Adult (VL-06); MNI 0, 6, 20 Favor: Child>Adol.=adult (P-11) Tal 2, −2, 52; 8, 7, 3 |
|||
| IFG | Unfav (calm vs happy) Child > Adol. > Adult (S-10); Tal 32, 23, 3 | |||
| DLPFC | Favor: Adol. > Adult (Sm-11); Tal 40, 44, 15 | |||
| REWARD DECISION-MAKING TASKS | ||||
| Striatum | Social > Nonsocial: Adol. > Adult (Che-11); MNI 9, 12, −8 Immediate > Delay: Adol. > Adult (Chr-11); Tal −7, 26, −13 |
Post-selection and Pre-feedback: MidLate=Adult=PreEarly (F-10) | Favor: Adol. > Adult (E-05); MNI −16, 20, −4 Favor: Puberty: MidLate<Adult<PreEarly (F-10)*only MidLate<PreEarly MNI 10, −5, 15 Favor: Adol. > Adult/Child (VL-10); MNI 21, 18, 9 |
|
| OFC | Risky: Adult > Adol. (Es-06); MNI −44, 14, −4 Social > Nonsocial: Adol. > Adult (Che-11); MNI −22, 47, −10 |
|||
| Amygdala | Favor: Adult > Adol. (E-05); MNI −26, −4, −14 | |||
| Insula | ||||
| ACC/mPFC | Risky: Adult > Adol. (Es-06); MNI ±2, 26, 30 Risky: Adol. > Adult (VL-10); MNI 12, 9, 27 Lower cost: Adol. > Adult (Chr-11); Tal −18, 46, −6 High penalty vs. low-penalty: Adult>Adol. (B-07); Tal 10, 21, 26 |
|||
| IFG | ||||
| LPFC | All: Adult > Adol. (Che-11); MNI −31, 5, 56 | |||
| Favor | favorable, such as reward, appetitive stimuli | |
| Unfav | unfavorable, such as punishment | |
| Passive Reward Tasks | ||
| G-08 | Guyer et al., 2008 | passive emotion task |
| VL-09 | Van Leijenhorst et al., 2009 | Slot Machine task |
| Instrumental-Reward Tasks | ||
| VL-06 | Van Leijenhorst et al., 2006 | Cake Gambling task |
| G-06 | Galvan et al., 2006 | Pirate task |
| B-04 | Bjork et al., 2004 | Monetary Incentive Delay |
| B-10 | Bjork et al., 2010 | Monetary Incentive Delay |
| Cho-11 | Cho et al., 2010 | Monetary Incentive Delay |
| Sm-11 | Smith et al., 2010 | continuous performance task |
| Ge-09 | Geier et al., 2009 | Antisaccade task |
| P-11 | Padmanabhan et al., 2011 | Antisaccade task |
| S-10 | Sommerville et al., 2011 | Go/No-go task |
| C-10 | Cohen et al., 2010 | Probabilistic Learning |
| Reward Decision-Making Tasks | ||
| Es-06 | Eshel et al., 2006 | Wheel of Fortune task |
| E-05 | Ernst et al., 2005 | Wheel of Fortune task |
| F-10 | Forbes et al., 2010 | Card Guessing Game |
| VL-10 | Van Leijenhorst et al., 2010 | Modified Cake Gambling task |
| Che-11 | Chein et al., 2011 | Stoplight task |
| Chr-11 | Christakou et al., 2011 | Hypothetical Delay Discounting task |
| B-07 | Bjork et al., 2007 | Chicken Game Task |
4.1. Passive exposure to incentive (positive/negative) stimuli
Among the simplest reward paradigms available are tasks that we have categorized as passive reward tasks, including such paradigms as Guyer and colleagues’ facial emotion task and Leijenhorst and colleagues’ slot machine task. First, Guyer and colleagues (2008) compared adolescents and adults on their behavioral and neural responses to a passive emotion task, which presented affect-laden stimuli in the form of happy, fearful, and neutral facial expressions. Although not examined from the perspective of reward, we include this study because happy faces can be seen as desirable stimuli that elicit approach behaviors, and thus can be conceptualized as rewarding (e.g., see supporting studies of the role of facial expression in motivated behavior: Aharon et al., 2001; Bayliss et al., 2009; Furl et al., 2012). Findings showed that the presentation of negatively valenced stimuli (fearful face), a potential index of response to punishment, resulted in greater amygdala activation (MNI 16, −4, −16; −20, −8, −6) among adolescents compared to adults. However, no significant age effect on neural activation was found in response to the presentation of appetitive stimuli (happy faces) versus neutral faces. Another example of a passive reward task, the Slot Machine task was used by Van Leijenhorst and colleagues (2009) (Figure 2a). This task passively presented probabilistic rewards, without any behavioral response required of the participants. This task provided a cue/anticipation stage and a feedback stage. In anticipation of a probabilistic reward, a negative linear relationship was found between activation in the right anterior insula (MNI 42, 12, −3) and age from early adolescence to adulthood. That is, insula activation in anticipation of a probabilistic reward (vs. no reward) was significant in children and adolescents but not in adults. No age-group differences in activation were found in the striatum during the anticipation stage. Conversely, the feedback stage showed greater activation of the striatum (MNI 12, 9, −15) to reward receipt in adolescents than adults or children, and greater activation of the orbitofrontal cortex (OFC; MNI −27, 48,−3) in adults than adolescents or children to reward omission.
Taken together (see Table 2b), these studies suggest that when probabilistic rewards are presented passively, the insula, within the avoidance module of the Triadic Model, seems to be more responsive in adolescents than in adults during reward anticipation. However, when evocative facial stimuli are passively presented, the amygdala is more highly reactive among adolescents than adults in response to fearful faces. The reward module (striatum and OFC), on the other hand, showed no age differences in response to evocative faces (regardless of valence) or in anticipation of passively receiving a probabilistic reward. With respect to the feedback stage of the passive probabilistic reward task, findings revealed different patterns of activation by age. That is, striatal activation was greater in adolescents than in children or adults in response to reward receipt, but OFC activation was greater in adults than in adolescents in response to non-reward receipt. Thus, two different types of aversive stimuli (fearful faces, reward omission) across the two passive reward tasks yielded different patterns of age-related neural activation in response to aversive stimuli or outcomes, with adolescents showing exaggerated amygdala activation to fearful faces, and adults showing enhanced OFC activation to reward omission. One possible interpretation is that OFC/vPFC modulates emotional reactivity in the context of disappointing/non-rewarding outcomes more strongly in adults than in adolescents, whereas the amygdala and striatum, in response to aversive and appetitive stimuli respectively, are not as moderated by control systems in adolescents relatively to adults, and, therefore, are more active among adolescents than adults. Overall, these patterns of age-related neural processing in the context of passive rewards are consistent with the Triadic Model theory that reward systems appear to be preferentially recruited relative to control systems among adolescents. In addition, the greater activation of the insula or amygdala in response to reward anticipation or fearful faces, respectively, challenges the idea of a dip in avoidance response in adolescents. However, the particular context of these findings, i.e., passive task, in addition to the dynamic (incentive) vs. static (unpleasant picture) stimuli, will be important to consider to reconcile findings of either greater reactivity or lower reactivity of the avoidance system in adolescence relative to younger or older age groups (e.g., Ernst et al., 2011).
Notably, these two passive tasks differed on the probability of rewarding stimulus presentation and the type of reward presented (i.e., faces vs. monetary reward). These methodological differences are likely to underlie, at least in part, the discrepancies in findings. In addition, it is important to consider the limitations of passive reward paradigms for understanding real-world reward processing. That is, rewards are rarely obtained in the real-world in the absence of some kind of behavior on the part of the individual in order to access or receive the reward. Thus, more complex fMRI paradigms, such as those reviewed below, may tell us more about the neurodevelopmental processes driving reward-related motivated behavior over the course of development.
4.2. Instrumental-reward tasks
Most reward-related paradigms that have been used in developmental studies have been instrumental-reward tasks. These studies can be further classified into those concerned with the influence of incentive on cognitive processes involved in the instrumental test, and those which were not concerned with incentive-by-cognition interactions.
4.2.1. Studies without incentive-by-cognition analyses
In the Cake Gambling task (Van Leijenhorst et al., 2006), subjects (children and adults) were asked to select the “best”, or correct, option based on probability of winning points. The effects of age on neural activation in response to the Cake Gambling task were two-fold: (1) during the selection stage, the medial PFC/anterior cingulate cortex (ACC; MNI 0, 6, 20) was activated more in children than in adults for low vs. high probability trials. Of note, the dorsolateral PFC (DLPFC) and OFC were both more activated to low probability than high probability trials, but these activations were not influenced by age; and (2) during the feedback stage, the OFC (MNI 40, 46, −12) was more sensitive to negative than positive feedback in children than in adults. The greater recruitment of ACC in children, a region involved in conflict monitoring (van Veen and Carter, 2002) and anticipation of uncertain outcome (Volz et al., 2003) suggests that children are less efficient than adolescents or adults at processing uncertainty. The OFC result opposes the OFC finding described in the context of a passive reward task above, in which adults displayed greater OFC activation in response to reward omission on the slot machine task. Differences between these studies include the age of the pediatric sample (children vs. adolescents), the type of task (passive reward vs. instrumental-reward), and the stage of reward process being examined (cue appraisal vs. selection and feedback).
Another instrumental-reward task, the Pirate task, was developed as a delayed response task with two possible response options (Galvan et al., 2006). Subjects were asked to select the side of the screen where the Pirate just appeared, and they were rewarded for selecting the correct side. During the feedback stage of this task, adolescents showed greater reward-induced activation of the nucleus accumbens compared with children or adults, and lower OFC activation (peak coordinates not available) in response to correct trials compared with adults, but showed no significant differences from adults in analyses conducted across the whole trial. In this study, activation related to short-term spatial memory, such as within the DLPFC and parietal cortex (Corbetta et al., 2008; Geier et al., 2009a), was not examined.
Although not a reward task per se, the work by Somerville et al. (2010) is included here because it was interpreted by the authors along the framework of reward processes. This study examined the effects of positive “emotion” rather than positive reinforcement (i.e., reward), and employed a go/no-go paradigm using facial emotions as stimuli. In addition to reporting on reward-related activation, this study also addressed the capacity to inhibit responding to an appetitive stimulus (happy face) and focused on the right inferior frontal gyrus (Aron et al., 2003; Garavan et al., 1999). Findings revealed greater ventral striatal (Tal −4, 11, −9) activation, particularly to happy faces, in adolescents than either adults or children. A linear decrease in rIFG activation with age was also reported, but this correlation with age vanished after controlling for performance. This region was not modulated by the valence of the face (neutral vs. happy). Collectively, the findings of Van Leijenhorst et al. (2006), Galvan et al. (2006), and Somerville et al. (2010) support a stronger recruitment of striatal function relative to OFC function, in adolescents relative to adults.
In contrast to the above findings, three studies (Bjork et al., 2004; Bjork et al., 2010; Cho et al., 2012) used the Monetary Incentive Delay task (Figure 2b), which employs a reaction-time instrumental test paired with various magnitudes of gain or loss. In the first study, during the test preparation stage, the main finding was a lower ventral striatal activation (Tal −9, 10, 0; 11, 12, 0) in adolescents compared to adults when potential gains were at stake. During the feedback stage, both age groups similarly activated the PFC, nucleus accumbens, putamen, amygdala and hippocampus in response to gains. In response to non-losses vs. losses, both groups activated putamen (deactivation to losses), and only adults activated medial PFC (Tal 1, 53, −6). Overall, the group differences were significant only for the ventral striatum during the test preparation stage.
A more recent study by Cho, Fromm, and colleagues (Cho et al., 2012) replicated these striatal findings, although the putamen (MNI 26, −4, 12) was the region showing the largest difference between adolescents and adults during the preparation stage (gain cues vs. neutral cues). The motor cortex (MNI −38, −4, 56) was also more activated in adults than in adolescents, perhaps reflecting a stronger incentive effect on motor preparation in adults than in adolescents. The feedback stage was not examined in this study.
The third study by Bjork and colleagues (2010) was a replication of their first work with a larger sample and some modifications of the timing parameters to better dissociate the instrumental preparation stage from the feedback stage. The overall pattern of activation was replicated. The only significant group difference in this second study concerned the nucleus accumbens during test preparation: In response to both gain cues and loss cues, the nucleus accumbens activation was lower in adolescents than in adults. The group difference identified and replicated by Bjork and colleagues (2004; 2010) was contrary to previous studies that reported enhanced, rather than reduced, ventral striatal activation during the feedback stages of rewarded trials (Galvan et al., 2006; Van Leijenhorst et al., 2009). This contradictory finding may be accounted for by the fact that the reduced adolescent ventral striatum activation reported with the MID task occurred during the stage when participants were preparing to perform the task, whereas the findings of enhanced ventral striatum activation among adolescents occurred during the feedback stages of both passive reward and instrumental-reward tasks, when participants were informed of the reward receipt (consummatory stage). This may indicate that the process of working toward a reward may be less enticing for adolescents than for adults, in contrast to the actual receipt of a reward that may be more pleasurable for adolescents than for adults. Conceivably, the nature of the visual stimuli used in the MID task, which are black and white line drawings of shapes, could be particularly austere and unappealing to adolescents, compared to the stimuli employed in other tasks. This difference in the salience/valence of the stimuli themselves might contribute to the conflicting striatal responses across studies in adolescents compared to adults.
4.2.2. Studies with incentive-by-cognition analyses
Researchers have also examined incentive effects on various cognitive processes, including sustained-attention (Smith et al., 2011), inhibition (Geier et al., 2009b; Padmanabhan et al., 2011; Somerville and Casey, 2010), and learning (Cohen et al., 2010). Reward/incentives have been shown to improve perceptual, cognitive and motor performance as well as neural efficiency in the animal and human literature (Ding and Hikosaka, 2007; Pleger et al., 2008; Savine and Braver, 2010; Weil et al., 2010). However, little has been done to learn about how these reward-related performance enhancements evolve with age. The studies below can inform this question.
Smith et al. (2011) employed a continuous performance task (CPT) with 3 types of trials: non-targets, rewarded targets, and non-rewarded targets. Behaviorally, adolescents evidenced significantly slower responding to non-rewarded targets relative to adults. Adolescents also responded significantly faster to rewarded targets relative to non-rewarded targets, while no such difference was found among adults, suggesting that the effect of incentives on sustained attention was stronger in adolescents than adults. Regarding neural function, the comparison of neural activation in response to rewarded vs. non-rewarded targets revealed positive linear relationships of age with reward-induced activation in regions implicated in sustained attention (DLPFC, Tal 40, 44, 15; ventromedial OFC, Tal −29, 26, −2), but negative linear relationships between age and reward-induced activation in regions coding for visuospatial attention (putamen, Tal 29, −7, 4; posterior cingulate cortex, Tal −14, −37, 20; inferior temporal gyrus, Tal −14, −37, 20). This finding suggests that age modulated the effect of reward on the neural substrates of sustained attention and visuospatial attention, but in opposite ways. Interpreted in terms of neural engagement, adolescents compared to adults might show that reward is less effective at recruiting regions involved in sustained attention, but more effective at recruiting regions involved in visuospatial attention.
Geier et al. (2009b) examined the effects of reward on antisaccade eye movements, during the cue stage, saccade preparation (test preparation stage), and saccade execution (cognitive/motor stage). A time-course analysis over 18 seconds post-trial onset was used to assess neural responses during the three stages of cue, saccade preparation, and saccade execution. During the incentive cue, the ventral striatum was recruited more strongly in adults than in adolescents (Tal 14, 2, −7), during saccade preparation, it was recruited more strongly in adolescents than in adults (Tal 11, 8, −7), and the saccade execution (cognitive/motor stage) showed no group differences. The OFC showed a group difference only during saccade execution, with a stronger response to neutral trials in adolescents than adults (Tal −25, 44, −4). Regions typically recruited in antisaccade tasks tended to be less active among adolescents than adults in response to neutral trials, but showed no group differences to reward trials, suggesting that reward can push the engagement of oculomotor neural substrates to the adult level. In sum, this study showed a more responsive reward system in adolescents during preparation for action (here mostly inhibition) in reward trials. The other stages showed OFC group differences in the neutral trials, which disappeared when incentives were present, suggesting “normalization” by incentives of neural responses among adolescents to match those seen in adults.
In a later study (Padmanabhan et al., 2011), this research group added children to the comparison of adults with adolescents on a similar incentive antisaccade task. Main age-related findings emerged during the first half of the modeled response (8 × 1.5 s), showing that only adolescents, but not adults or children, manifested greater activation to reward relative to neutral trials in the ventral striatum. A similar age-effect was found in oculomotor control regions, including the intraparietal sulcus and putamen (Curtis and Connolly, 2008; Everling and Munoz, 2000). These findings support the theory of a peak during adolescence in neural response to reward, which in turn might boost the cognitive/motor function needed to perform correctly to obtain the reward. In addition, only adults showed a sensitivity to reward within the lateral OFC when modeling the entire trial. Finally, an age-related linear increase in control systems was suggested by the greater activation to neutral vs. reward trials in the cortical supplementary eye fields (SEF), and to both neutral and reward trials in the ACC in children relative to both adolescents and adults. A stronger activation in the youngest group suggests greater difficulty in performing the antisaccade task (Luna et al., 2001, 2004), or greater reliance on medial prefrontal structures.
From the perspective of the Triadic Model, these findings, along with those of Smith and colleagues (2011), suggest that providing rewards for engaging cognitive control functions (i.e., sustained attention and inhibition) may actually modify the typical developmental shift in the triadic equilibrium, such that adolescents are able to engage control system regions more effectively when they are rewarded for doing so, and in turn, reach adult levels of function.
The last instrumental-reward task by Cohen et al. (2010) involved probabilistic learning and probed learning through repetition. With increasing experience, subjects learned which of two options was most frequently correct (and rewarded). Since this task did not involve risk-taking or the formation of a preference, but examined the effect of reward on a cognitive task (learning), we decided to include it in the instrumental-reward task section rather than in the category of decision-making tasks, although we recognize that this choice is debatable. In this task, participants were shown pairs of abstract stimuli, and were asked to classify them into two categories (i.e., Eastern or Northern). Feedback as to whether their response was correct was given after each trial. Two types of stimuli were presented: predictable (associated at a rate of 83% for a given category) and random (associated at a rate of 50% for each category). There were also two levels of reward, including high ($0.25) and low ($0.05) values. During the feedback stage, activation in response to positive prediction errors (unexpected gain) peaked in the striatum (MNI 14, 16, 4) and angular gyrus (MNI 60, −44, 28) in adolescents relative to adults or children. In addition, decision value (i.e., the value assigned to each potential choice (Kahneman and Tversky, 1979)) during the selection stage was associated with a linear decrease in inferior medial PFC activation with age (MNI 0, 50, −8). Of note, behaviorally, the estimated learning rate did not differ across age-groups. Thus, whereas learning did not differ between adolescents and adults, medial PFC was recruited more efficiently as age increased.
Taken together (see Table 2b), these instrumental-reward studies showed that striatal activation was lower in adolescents during the cue stage in one task (Bjork et al., 2004; Bjork et al., 2010; Geier et al., 2009b), and during the test preparation stage of another task (Bjork et al., 2004; Bjork et al., 2010; Cho et al., 2012). The distinction between these two early stages of reward processing (cue appraisal and test preparation, see Figure 2), which has not been explicitly noted in the past (but see Bjork et al., 2012; Bjork et al., 2008), may be quite important, based on the differential relative reliance on striatal function by adolescents and adults across the two stages. However, during test preparation in tasks requiring inhibitory responses/internally generated action (e.g., antisaccade as in Geier et al., 2009b), or a manual response (e.g., Somerville et al., 2010), striatal activation during reward trials was greater in adolescents than in adults. This was also the case when the test consisted of the learning of values (Cohen et al., 2010). During the actual cognitive/motor stage, prefrontal cortical regions (IFG and DLPFC) were more active in youths than in adults on non-rewarded trials (Somerville and Casey, 2010; Van Leijenhorst et al., 2006). Similarly, the cognitive/motor stage (antisaccade here (Geier et al., 2009b)) during a neutral trial (not rewarded) was accompanied with greater OFC activation in adolescents than in adults; however, this group difference disappeared on reward trials. Smith and colleagues (2011), on the other hand, reported greater DLPFC activation with age on rewarded versus non-rewarded target trials. Analysis of the trials as a whole revealed greater striatal activation in positive trials in adolescents than in adults, but lower OFC activation in children than in adults, with the adolescents not differing from the adults (Galvan et al., 2006).
In summary, during the cue stage of the trial, adolescents activate the striatum less than adults, but during the test preparation stage (i.e., preparation for inhibitory action, or learning-retrieval), adolescents activate striatum more than adults, particularly in reward-trials. However, when the action consists of time-sensitive response, adolescents activate striatum less than adults (Bjork et al., 2004; Bjork et al., 2010). More work is needed to validate these observations. One strategy to reconcile these discrepancies would be to design a paradigm including different types of instrumental actions that challenge different perceptual/cognitive/motor functions, such as working memory, spatial attention, timed response, or inhibition, while keeping reward conditions constant. Studies have started to carefully parse out temporally the early stages of reward processes (Bjork et al., 2012; Bjork et al., 2008). The use of other methodologies, such as magnetoencephalography (Apitz and Bunzeck, 2012; Doñamayor et al., 2012) could provide a more refined temporal maps of activation patterns across stages of reward behavior. Finally, during positive feedback stage, stronger striatal activation (Cohen et al., 2010), and during negative feedback, weaker OFC activation (Van Leijenhorst et al., 2006), emerged in adolescents compared to adults. Of interest, these instrumental-reward tasks do not seem to modulate amygdala or insula differentially as a function of age.
Overall, findings are in line with hypotheses derived from the Triadic Model such that adolescents tend to display enhanced reward system functioning relative to adults; however, this pattern of enhanced functioning is dependent on the specific reward stage that is being examined, the type of instrumental test (e.g., inhibition vs. timed performance) and perhaps the salience of the stimuli being used. Adolescents also tend to display reduced control system functioning relative to adults, but this lesser reliance on the control system can be reversed when adolescents are rewarded specifically for engaging these control functions.
4.3. Decision-making tasks
Six decision-making paradigms have been examined in different age-groups. The WOF task required participants to select one of two options that varied by magnitude and probability of monetary gains. Three stages were modeled, including selection (which encompassed cue appraisal, preference formation, and cognitive/motor response; see Figure 2c), anticipation, and feedback. Only selection and feedback were analyzed. During the selection stage, regions of the OFC/ventrolateral PFC (MNI −44, 14, −4) and ACC (MNI ±2, 26, 30) were significantly more engaged in adults than in adolescents, when selecting the most risky (most uncertain but potentially most lucrative) option (Eshel et al., 2007). Other regions, such as ventral striatum, amygdala, and DLPFC, were also activated by this contrast, but in a similar way for both age groups (Ernst et al., 2005). In contrast, the feedback stage was accompanied by significant age-differences in activation of the ventral striatum and amygdala. The amygdala (MNI −26, −4, −14) was more activated in adults than adolescents in response to gain vs. no-gain outcomes (greater deactivation to no-gain). In contrast, the nucleus accumbens (MNI −16, 20, −4), within the ventral striatum, was more activated in adolescents compared to adults (higher activation to gain). Taken together, these findings support a hypersensitivity of the reward neural system during adolescence and a reduction of conflict-related ACC engagement in adolescents relative to adults in a task of reward-related decision-making.
In the modified Cake Gambling task, participants were asked to select from two options, each with a fixed reward probability of 33% and 66% (Van Leijenhorst et al., 2010). Trials differed on the potential gain associated with the 33% (risky) option (2, 4, 6 or 8 Euros), while the 66% (safe) option always provided a potential 1 euro gain. The selection (also including cue-appraisal) and feedback stages were analyzed separately. During the selection stage of risky (33% gain probability) high incentive vs. low incentive options, a linear decrease with age was found in dorsal ACC (MNI 12, 9, 27) and central opercular postcentral gyrus (MNI 51, −6, 21) activation. In contrast to the previous WOF study, adolescents showed stronger activation compared to adults or children in the medial ventral PFC/subcallosal cortex (MNI −9, 27, −12). During the feedback stage in the gain condition, no linear associations of brain activation with age were found, but a region of significantly greater activation in adolescents relative to adults or children was detected in the right caudate nucleus (MNI 21, 18, 9). The authors concluded that the reduction of reward-related activation in the ACC with age indexed maturation of cognitive control regions, whereas the previous work (Eshel et al., 2007) argued that the more mature PFC was more readily activated in adults than in adolescents. Only with additional studies will these controversies be understood. For example, contrasting sets of trials with invariant probabilities but varying magnitudes (Van Leijenhorst et al., 2010) with sets of trials in which both magnitudes and probabilities vary (Eshel et al., 2007) could clarify the differences in PFC activation patterns between adolescents and adults, and relate these patterns to specific parameters of magnitude and probability in the two different types of trials. Such a strategy could validate these two studies, and start providing clues regarding the opposite findings of the mPFC recruitment as a function of task context. In addition, in line with previous work, the peak activation of the medial ventral PFC and striatum seen in adolescents was attributed to the unique sensitivity of adolescents to positive incentives.
The Stoplight task is a simulated driving game that was performed by adolescents, as well as young and older adults (Chein et al., 2011). Participants were asked to decide whether to risk a collision by driving through an orange light and save time, or to stop and be safe. Completion of the task in a timely fashion was rewarded by a monetary incentive. In addition, the task was performed in two contextual conditions, a social context in which two age-matched peers supported the participant, and a non-social context in which participants completed the task alone. The adolescents were the only group to take more risks in the social than the non-social context. Risky and safe selections were collapsed together, and were compared to an implicit baseline. Adults showed greater activation than adolescents during the selection stage in the lateral PFC (MNI −31, 5, 56), inferior parietal cortex (MNI −52, −37, 41), and fusiform gyrus (−52, −55, −19). No regions were more activated in adolescents than in adults in the selection vs. baseline contrast. However, in the comparison of social and nonsocial conditions, adolescents activated the ventral striatum (MNI 9, 12, −8) and OFC (MNI −22, 47, −10) more strongly than adults. This study illustrates the critical role of context, particularly social context, in neurodevelopmental correlates of motivated behavior.
A hypothetical discount task (Christakou et al., 2011) assessed the subjective cost associated with a delay in reward attainment as reflected in type of selection. Performance data showed that this subjective cost of delay was higher in adolescents than in adults. The neuroimaging data of the selection stage found that this cost was associated with higher activation of the ventral striatum (Tal −7, 26, −13), putamen/thalamus (Tal −22, −15, 4), and superior parietal lobule (Tal 25, −63, 53) in adolescents when selecting immediate reward, and temporal regions during delayed choices. The only region showing increasing activation with age, and with decreased cost (i.e., larger delays), was the ventromedial PFC (Tal −18, 46, −6). In addition, connectivity analyses showed strengthening of functional links between ventromedial PFC and ventral striatum with age during selection of immediate options. The authors concluded that ventromedial PFC was progressively more able to incorporate information about the delay-dependent value of future rewards.
The Card Guessing Game (Forbes et al., 2010b) was similar to a coin-toss game, with 50% chance of guessing the winning option. Here, participants were asked to guess whether a card, presented on its back, is higher or lower than 5 (possible values 1–9). The design of this task allowed for separate examination of reward anticipation (after the preferred option was selected, but before results were displayed) and response to feedback. Pre/early pubertal (mean age = 11.4 years) vs. mid-late pubertal (mean age = 12.2 years) vs. adults (mean age = 45.5 years) were compared on the [reward-anticipation vs. baseline] and [win-feedback vs. baseline] contrasts. Significant group differences were present only in the feedback stage, and not in the anticipation stage. Mid/late pubertal adolescents were compared to pre/early pubertal adolescents after controlling for age and sex. The mid/late pubertal group evidenced a hypoactive caudate nucleus (MNI 10, −5, 15), but hyperactive medial PFC (BA 32, MNI 10, 23, 28) in response to a win-feedback. The adult response lay between the responses of the two adolescent groups, and did not differ from either group. These findings might suggest a unique effect of puberty on reward processes, although a full dissociation of the effects of age from puberty remains challenging. Furthermore these findings are difficult to reconcile with the findings of other reward decision-making studies because of the substantial older mean age of the adults and the absence of other data in the literature on pubertal effects.
The Game of Chicken probed conflict monitoring associated with decision-making, pitting potential greater rewards against potential greater losses (Bjork et al., 2007). This task echoes the BART task (Lejuez et al., 2003), in which participants have to decide when to stop a trial in order to avoid a penalty. The Game of Chicken featured four conditions: control motor, reward without penalty, reward with low penalty, and reward with high penalty. Reaction time to the cue announcing the trial type was slower in adolescents than adults. However, latency to stop the trials did not differ between groups. Group differences emerged only in the low-penalty (risky) vs. no-penalty (not risky) contrast, for which adolescents showed no differences in brain activation, while the adults activated a number of regions, including posterior medial PFC and striatum, greater in response to risky relative to non-risky conditions. Significant group differences were restricted to the medial PFC (Tal 10, 21, 26).
Collectively, these studies suggest that during a reward decision-making task (see Table 2b), the early stage of cue-appraisal/selection is associated with greater striatal activation in adolescents than adults. However, discrepant findings in OFC and mPFC (e.g., greater activation in adults than adolescents in risky vs. non-risky decisions, and greater activation in adolescents than in adults in more social and less costly decisions) did emerge. More work is warranted to resolve these differences. Discrepancies may be due to the various types of cognitive processes that are engaged in the different tasks, some at the service of receiving the reward, some independent, and some possibly interfering with getting the reward. These distinct relationships between reward and cognitive processes could be manipulated in future tasks, providing ways to better understand neural mechanisms underlying the coding of motivated behavior, particularly across development. During the feedback stage, favorable outcomes were associated with greater striatal activation in adolescents than adults, but greater activation in the amygdala in adults than adolescents. However, here again, discrepancies emerged when pubertal status was examined (Forbes et al., 2010b). This review of reward decision-making task studies, more than ever, underscores the need to replicate findings in large studies and to carefully isolate and test specific task-related parameters that may be driving discrepancies across studies.
5. Conclusion
Overall, findings across all the reward-related tasks reveal mixed results that cannot be attributed solely to the type of task. The scarcity and heterogeneity of studies make it difficult to clearly map the role of specific neural mechanisms onto the developmental changes in reward-related behaviors that are seen during adolescence. The theoretical construct underlying the triadic model, which is used here as an organizational tool, falls short in explaining all the discrepancies among studies. However, we lack knowledge in the multiple ways that different variables, including subject characteristics, experimental factors, and environmental contexts, can influence the neural systems underlying reward-related behavior. In addition, most studies do not systematically evaluate the three systems of the triadic model separately and in interaction, including measures of functional connectivity. For example, the aversion/avoidance network is seldom reported in these studies, suggesting that developmental functional changes of the amygdala network are not easily captured in the context of a reward task. Paradigms could be developed to specifically engage the avoidance system, and introduce a reward context. Furthermore, it would be helpful to examine tasks more systematically as a function of the neural regions that they uniquely engage. This categorization would help develop a compilation of tasks that could be used to probe specific regions that are sensitive to age. To go a step further, the identification of a priori regions to be examined could be based not only on known local function from prior basic neuroscience and functional neuroimaging studies, but also on intrinsic connectivity. Such methodology is expected to be developed in the near future providing more valid ways to delineate regions of interest based on their unique functional connections, for example providing clear distinction between ventral striatum, dorsal striatum or extended amygdala. Such strategies could also decrease variability in findings across studies by ensuring that the same functional regions are being examined.
We showed that the modulation of the balance across the Triadic Model is not straightforward to interpret for a number of reasons. First, the use of different tasks that probe different component processes and brain functions prevent a step-by-step logical testing of reward function, or more generally, motivated behavior. However, these studies now provide a large basis for engaging in such systematic analysis. Second, each module of the triadic system is not systematically assessed separately as well as in relation with one another across extant studies. To test the Triadic Model, study designs should orthogonalize the experimental manipulations of the approach, avoidance, and control systems. A third glowing gap is the quasi-absence of functional connectivity studies during reward-related processes. The field is still very young, yet studies of resting state connectivity across development are starting to emerge (Fair et al., 2009; Stevens et al., 2009), providing important starting points for investigations of functionally constrained studies.
Research on the effects of individual difference factors, including sex, and pubertal status, and psychopathology on the developmental trajectories of reward systems is also greatly needed. Sex-related differences are noted in structural brain maturation (e.g. Lenroot et al., 2007; Pangelinan et al., 2011; Sowell et al., 2007). Studying typical, in addition to atypical, development is critical not only to illuminate the neural mechanisms underlying adolescent behavior, particularly the behaviors that put adolescents at risk, but also to understand patterns of neural functioning that are either risk factors for the development of psychiatric disorders or mediate symptoms of psychopathology. A number of studies have already emerged, examining reward systems in different pathologies, such as depression (Forbes et al., 2010a), anxiety and risk for anxiety disorders (Guyer et al., 2012), substance use (Peters et al., 2011), and ADHD (Rubia et al., 2009) in adolescence. To fully interpret these studies, it is imperative to map the typical trajectories of neural system development spanning childhood, adolescence, and adulthood, to which neural mechanisms across clinical samples may be compared.
For completeness, we would like to mention that a number of reward tasks have been assessed only in pediatric samples (e.g., Bar-Haim et al., 2009b; Casey et al., 2008; Crone and van der Molen, 2004; Crowley et al., 2009; Forbes et al., 2010b; Guyer et al., 2006; May et al., 2004; Rubia et al., 2007a). These studies suggest that the neural pathways involved in reward-related processes in youth are similar to those in adults. However, only a direct comparison of different age-groups can address age effects on the neural substrates of behavior.
General issues related to fMRI paradigms are worth mentioning, and need to be considered when interpreting functional neuroimaging findings. One is the significance of group differences in neural activation when performance does not differ between groups, or in contrast, when neural activation does not differ but performance differs between groups. The first case is more common than the second case, and it is generally interpreted as a reflection of the lower sensitivity of behavior relative to neural function. Alternative explanations include the fact that behavior is tested in a constrained environment and may not represent behavior in real life.
The second issue is the interpretation of activation (or deactivation) of the fMRI signal (see Durston et al., 2006; Poldrack, 2010). Activation does not differentiate between the firing of inhibitory vs. excitatory neurons and the implication of an excitatory role vs. inhibitory role requires the use of qualifying variables, such as behavior or functional connectivity. In addition, it is also not clear whether less activation represents more efficient function that engages less neural resources, or whether it represents a deficit that prevents appropriate neural recruitment. Similarly, greater activation in younger age groups has been interpreted as “compensation” or inefficiency for the maturational lag, as a greater functional reliance on the given structure, or as the deployment of more effort. Here again, performance and clinical correlates can uniquely provide interpretative clues.
A third issue is the difficulty in separating the fMRI BOLD response among the different stages of a trial, particularly when these stages are contiguous with no sufficient temporal and psychological separation. For example, a number of tasks do not clearly separate anticipation from feedback, and the neural response to feedback is affected by the BOLD signal to anticipation, given that similar neural structures are recruited in both stages. Careful task design is crucial to dissociate these two stages (Bjork et al., 2010), but often comes at the expense of longer trial durations, and subsequent restriction in the number of repeats of a given condition.
Finally, we hope that this review will help researchers better map the next questions of reward systems/motivated behavior across adolescence and bridge the many gaps revealed herein. Of course, longitudinal studies are the gold standard for studying changes with age. Indeed the longitudinal design permits researchers to assess continuities and discontinuities of development within the same individuals over time, using a within-subjects design which provides more statistical power than between-subjects designs. Cross-over studies can only reveal differences between age groups and are less successful at delineating the process of development. However, a major drawback of longitudinal studies is their cost difficulty, as well as potential confounds (e.g., familiarity with instruments repeated over time, attrition, cohort effects). Despite these costs and challenges, the demonstration of functional neural changes across adolescence in the coding of motivated behavior fully justifies investment in such studies.
Research Highlights.
Literature on neurodevelopment of reward processing is marked by inconsistencies
Disparate findings may be due to heterogeneity of reward paradigms across studies
The Triadic Model is a framework to inform the neurodevelopment of reward processing
Three different task types allow for manipulation of specific reward components
Task parameters may influence neurodevelopmental correlates of reward processing
Footnotes
The opinions and assertions contained in this paper are the private views of the authors and are not construed as official or as reflecting the views of the NIMH or the Department of Health and Human Services.
Contributor Information
Jessica M. Richards, Email: jessic1@umd.edu.
Rista C. Plate, Email: rplate@wisc.edu.
Monique Ernst, Email: ernstm@mail.nih.gov.
References
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Apitz T, Bunzeck N. Reward modulates the neural dynamics of early visual category processing. Neuroimage. 2012;63:1614–1622. doi: 10.1016/j.neuroimage.2012.08.046. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Adolescent storm and stress, reconsidered. Am Psychol. 1999;54:317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, Perez-Edgar K, Pine DS, Ernst M. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol Sci. 2009a;20:1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, Perez-Edgar K, Pine DS, Ernst M. Neural Correlates of Reward Processing in Adolescents With a History of Inhibited Temperament. Psychological Science. 2009b;20:1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss AP, Griffiths D, Tipper SP. Predictive gaze cues affect face evaluations: The effect of facial emotion. Eur J Cogn Psychol. 2009;21:1072–1084. doi: 10.1080/09541440802553490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knuton B, Fong GW, GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-Elicited Brain Activation in Adolescents: Similarities and Differences from Young Adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: Comparing motivational neurocircuitry recruitment using fMRI. PlosONE. 2010;5:e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Mesolimbic recruitment by nondrug rewards in detoxified alcoholics: effort anticipation, reward anticipation, and reward delivery. Hum Brain Mapp. 2012;33:2174–2188. doi: 10.1002/hbm.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J Neurosci. 2007;27:4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008;42:1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The Role of Puberty in the Developing Adolescent Brain. Hum Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JDE. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carlisi C, Pavletic N, Ernst M. New perspectives on neural systems models of adolescent behavior: Functional brain connectivity. Neuropsyciatrie de l’enfance et de l’adolescence (in English) in press. [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the Adolescent Brain and Behavior: Implications for Substance Use Disorders. J Am Acad Child Adolesc Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. J Cogn Neurosci. 2007;19:69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- Cho YT, Fromm S, Guyer AE, Detloff A, Pine DS, Fudge JL, Ernst M. Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage. 2012;66C:508–521. doi: 10.1016/j.neuroimage.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. NeuroImage. 2011;54:1344–1354. doi: 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nature Neuroscience. 2010;13:669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012a;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social affective engagement and goal flexibility. Nat Rev Neurosci. 2012b;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Crone EA, van der Molen MW. Developmental changes in real life decision making: performance on a gambling task previously shown to depend on the ventromedial prefrontal cortex. Dev Neuropsychol. 2004;25:251–279. doi: 10.1207/s15326942dn2503_2. [DOI] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Crutcher C, Bailey CA, Lejuez CW, Mayes LC. Risk-taking and the feedback negativity response to loss among at-risk adolescents. Dev Neurosci. 2009;31:137–148. doi: 10.1159/000207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, Connolly JD. Saccade preparation signals in the human frontal and parietal cortices. J Neurophysiol. 2008;99:133–145. doi: 10.1152/jn.00899.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Ding L, Hikosaka O. Temporal development of asymmetric reward-induced bias in macaques. J Neurophysiol. 2007;97:57–61. doi: 10.1152/jn.00902.2006. [DOI] [PubMed] [Google Scholar]
- Doñamayor N, Schoenfeld MA, Münte TF. Magneto- and electroencephalographic manifestations of reward anticipation and delivery. Neuroimage. 2012;62:17–29. doi: 10.1016/j.neuroimage.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR, Barch DM, Petersen SE, Schlaggar BL. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Daniele T, Frantz K. New perspectives on adolescent motivated behavior: Attention and conditioning. Dev Cogn Neurosci. 2011;1:377–389. doi: 10.1016/j.dcn.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience & Biobehavioral Reviews. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson E, Jazbec S, McClure E, Monk C, Leibenluft E, Blair J, Pine D. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol Biochem Behav. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Ernst M, Spear LP. Reward Systems. In: de Haan M, Gunnar MR, editors. Handbook of developmental social neuroscience. Guilford Press; New York: 2009. p. 558. [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL, Dahl RE. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn Affect Behav Ne. 2010a;10:107–118. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Mayles DL, Tarr JA, Sciarrillo SR, Dahl RE. Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Adolesc Psychiatry. 2010b;49:162–172. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furl N, Gallagher S, Averbeck BB. A Selective Emotional Decision-Making Bias Elicited by Facial Expressions. Plos One. 2012:7. doi: 10.1371/journal.pone.0033461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front Hum Neurosci. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacol Biochem Behav. 2009;93:212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Garver K, Terwilliger R, Luna B. Development of working memory maintenance. J Neurophysiol. 2009a;101:84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in Reward Processing and Its Influence on Inhibitory Control in Adolescence. Cereb Cortex. 2009b;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, Fox NA, Pine DS, Ernst M. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry. 2012;169:205–212. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin M, Roberson-Nay R, Monk CS, Bjork JM, Henderson HA, Pine DS, Fox NA, Ernst M. Striatal Functional Alteration in Adolescents Characterized by Early Childhood Behavioral Inhibition. J Neurosci. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Mandell D, Mueller SC, Dahl RE, Pine DS, Ernst M. Inhibitory control in anxious and healthy adolescents is modulated by incentive and incidental affective stimuli. Journal of Child Psychology and Psychiatry. 2009a;50:1550–1558. doi: 10.1111/j.1469-7610.2009.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Pine DS, Ernst M. The influence of context valence in the neural coding of monetary outcomes. Neuroimage. 2009b;48:249–257. doi: 10.1016/j.neuroimage.2009.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Knoepfle DT, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J Neurosci. 2010;30:583–590. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-Control in Decision-Making Involves Modulation of the vmPFC Valuation System. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Benson BE, Plate RC, Guyer AE, Detloff AM, Pine DS, Leibenluft E, Ernst M. Developmental effects of decision-making on sensitivity to reward: an fMRI study. Dev Cogn Neurosci. 2012;2:437–447. doi: 10.1016/j.dcn.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biol Psychiatry. 2005;58:632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect Theory: An analysis of decision under risk. Exonometrica. 1979;47:263–291. [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Larson RW, Moneta G, Richards MH, Wilson S. Continuity, stability, and change in daily emotional experience across adolescence. Child Dev. 2002;73:1151–1165. doi: 10.1111/1467-8624.00464. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lejuez C, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J Adolescence. 2003;26:475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeholm M, Tricomi E, O’Doherty JP, Balleine BW. Neural correlates of instrumental contingency learning: differential effects of action-reward conjunction and disjunction. J Neurosci. 2011;31:2474–2480. doi: 10.1523/JNEUROSCI.3354-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M. Adolescent brain development: current themes and future directions. Introduction to the special issue. Brain Cogn. 2010;72:1–5. doi: 10.1016/j.bandc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A, Geier CF, Ordaz SJ, Teslovich T, Luna B. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Dev Cogn Neurosci. 2011;1:517–529. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangelinan MM, Zhang G, VanMeter JW, Clark JE, Hatfield BD, Haufler AJ. Beyond age and gender: Relationships between cortical and subcortical brain volume and cognitive-motor abilities in school-age children. NeuroImage. 2011;54:3093–3100. doi: 10.1016/j.neuroimage.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, Conrod PJ, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Martinot JL, Paus T, Poline JB, Robbins TW, Rietschel M, Smolka M, Strohle A, Struve M, Loth E, Schumann G, Buchel C. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn Sci. 2012;16:322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger B, Blankenburg F, Ruff CC, Driver J, Dolan RJ. Reward facilitates tactile judgments and modulates hemodynamic responses in human primary somatosensory cortex. J Neurosci. 2008;28:8161–8168. doi: 10.1523/JNEUROSCI.1093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Interpreting developmental changes in neuroimaging signals. Hum Brain Mapp. 2010;31:872–878. doi: 10.1002/hbm.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Rubia K, Hyde Z, Halari R, Giampietro V, Smith A. Effects of age and sex on developmental neural networks of visual spatial attention allocation. NeuroImage. 2010;51:817–827. doi: 10.1016/j.neuroimage.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A, Taylor E. Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child Neuropsychol. 2007a;13:276–304. doi: 10.1080/09297040600770761. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007b;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savine AC, Braver TS. Motivated cognitive control: reward incentives modulate preparatory neural activity during task-switching. J Neurosci. 2010;30:10294–10305. doi: 10.1523/JNEUROSCI.2052-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schultz W, Tremblay L, Hollerman JR. Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology. 1998;37:421–429. doi: 10.1016/s0028-3908(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: links to depressive symptoms and problem behavior. Child Dev. 2003;74:1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Smith AB, Halari R, Giampetro V, Brammer M, Rubia K. Developmental effects of reward on sustained attention networks. Neuroimage. 2011;56:1693–1704. doi: 10.1016/j.neuroimage.2011.01.072. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu DR, Zhu HT, Thompson PM, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebral Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1:392–400. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence - New perspectives from brain and behavioral science. Curr Dir Psychol Sci. 2007;16:55–59. [Google Scholar]
- Steinberg L. A Social Neuroscience Perspective on Adolescent Risk-Taking. Dev Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymula A, Rosenberg Belmaker LA, Roy AK, Ruderman L, Manson K, Glimcher PW, Levy I. Adolescents’ risk-taking behavior is driven by tolerance to ambiguity. Proc Natl Acad Sci U S A. 2012;16:17135–40. doi: 10.1073/pnas.1207144109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Crone E, Bunge S. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44:2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision-making: Neurocognitive development of reward and control regions. NeuroImage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, Crone EA. What Motivates the Adolescent? Brain Regions Mediating Reward Sensitivity across Adolescence. Cerebral Cortex. 2009;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY. Predicting events of varying probability: uncertainty investigated by fMRI. Neuroimage. 2003;19:271–280. doi: 10.1016/s1053-8119(03)00122-8. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil RS, Furl N, Ruff CC, Symmonds M, Flandin G, Dolan RJ, Driver J, Rees G. Rewarding feedback after correct visual discriminations has both general and specific influences on visual cortex. J Neurophysiol. 2010;104:1746–1757. doi: 10.1152/jn.00870.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SM, Mermelstein RJ, Hankin BL, Hedeker D, Flay BR. Longitudinal Patterns of Daily Affect and Global Mood During Adolescence. J Res Adolesc. 2007;17:587–600. doi: 10.1111/j.1532-7795.2007.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]