Abstract
The ability to remember to execute delayed intentions is referred to as prospective memory. Previous theoretical and empirical work has focused on isolating whether a particular prospective memory task is supported either by effortful monitoring processes or by cue-driven spontaneous processes. In the present work, we advance the Dynamic Multiprocess Framework, which contends that both monitoring and spontaneous retrieval may be utilized dynamically to support prospective remembering. To capture the dynamic interplay between monitoring and spontaneous retrieval we had participants perform many ongoing tasks and told them that their prospective memory cue may occur in any context. Following either a 20-min or a 12-hr retention interval, the prospective memory cues were presented infrequently across three separate ongoing tasks. The monitoring patterns (measured as ongoing task cost relative to a between-subjects control condition) were consistent and robust across the three contexts. There was no evidence for monitoring prior to the initial prospective memory cue; however, individuals who successfully spontaneously retrieved the prospective memory intention, thereby realizing that prospective memory cues could be expected within that context, subsequently monitored. These data support the Dynamic Multiprocess Framework, which contends that individuals will engage monitoring when prospective memory cues are expected, disengage monitoring when cues are not expected, and that when monitoring is disengaged, a probabilistic spontaneous retrieval mechanism can support prospective remembering.
Keywords: prospective memory, spontaneous retrieval, monitoring, intentions, cognitive control
1. Introduction
Each day humans form intentions, or prospective memories, that must be executed following a delay interval, such as remembering to take medication with breakfast. Since the development of a laboratory paradigm to study prospective memory (Einstein & McDaniel, 1990; Kvavilashvili, 1987), researchers have identified many factors that are associated with prospective memory successes and failures (Brandimonte, Einstein, & McDaniel, 1996; Kliegel, McDaniel, & Einstein, 2008). Yet the processes underlying successful prospective remembering are still being debated (Einstein & McDaniel, 2010; Smith, 2010).
There are at least two general cognitive processes that have been posited to support prospective memory retrieval: monitoring and spontaneous retrieval (McDaniel & Einstein, 2007). Monitoring refers to maintaining the prospective memory intention and searching the environment for cues that signal that the prospective memory action should be executed (for theories of monitoring see Guynn, 2003; Shallice & Burgess, 1996; Smith, 2003). Monitoring is dependent on the prefrontal cortex (e.g., Burgess, Quayle, & Frith, 2001) and working memory capacity (e.g., Brewer, Knight, Marsh, & Unsworth, 2010). Allocating attention toward monitoring results in fewer attentional resources being devoted to performing concomitant activities (i.e., the ongoing task), thereby leading to a performance cost (Marsh, Hicks, Cook, Hansen, & Pallos, 2003; Park, Hertzog, Kidder, Morrell, & Mayhorn, 1997; Smith, 2003).
Spontaneous retrieval processes can also support prospective memory (McDaniel & Einstein, 2007). Spontaneous retrieval is a probabilistic process that delivers an intention to consciousness in response to processing a retrieval cue (for elaborated views of spontaneous retrieval, see Lee & McDaniel, 2013; McDaniel, Guynn, Einstein, & Breneiser, 2004). In contrast to monitoring, spontaneous retrieval does not require preparatory activation of the prefrontal cortex (McDaniel, LaMontagne, Beck, Scullin, & Braver, in press), but instead has been linked to the hippocampus (Gordon, Shelton, Bugg, McDaniel, & Head, 2011; Moscovitch, 1994). Introspectively, spontaneous retrieval is experienced as an intention “popping” into mind (e.g., Meier, Zimmerman, & Perrig, 2006). Reflexive-automatic processes might underlie spontaneous retrieval (McDaniel et al., 2004), but spontaneous processes should not be equated with automatized prospective memory responding (Einstein, Smith, McDaniel, & Shaw, 1997; McDaniel & Scullin, 2010).
The Multiprocess Framework (McDaniel & Einstein, 2000) was developed to predict the variables that are associated with either spontaneous retrieval or monitoring processes (e.g., cue focality; Einstein & McDaniel, 2005). Since the proposal of this framework, numerous studies have attempted to isolate spontaneous retrieval and monitoring processes (for review, see McDaniel & Einstein, 2007). A provocative possibility that stems from observations of prospective memory in naturalistic settings (Grundgeiger, Sanderson, MacDougall, & Venkatesh, 2010; Kalpouzos, Eriksson, Sjölie, Molin, & Nyberg, 2010; Kvavilashvili & Fisher, 2007; Rose, Rendell, McDaniel, Aberle, & Kliegel, 2010; Sellen, Louie, Harris, & Wilkins, 1997) is that reliance on spontaneous retrieval and monitoring is a dynamic process within individuals. In the present work, we advance the Dynamic Multiprocess Framework (Figure 1) that spontaneous retrieval and monitoring may be interconnected processes that operate in a dynamic manner to support prospective remembering (Chen, Huang, & Yuan, 2010).
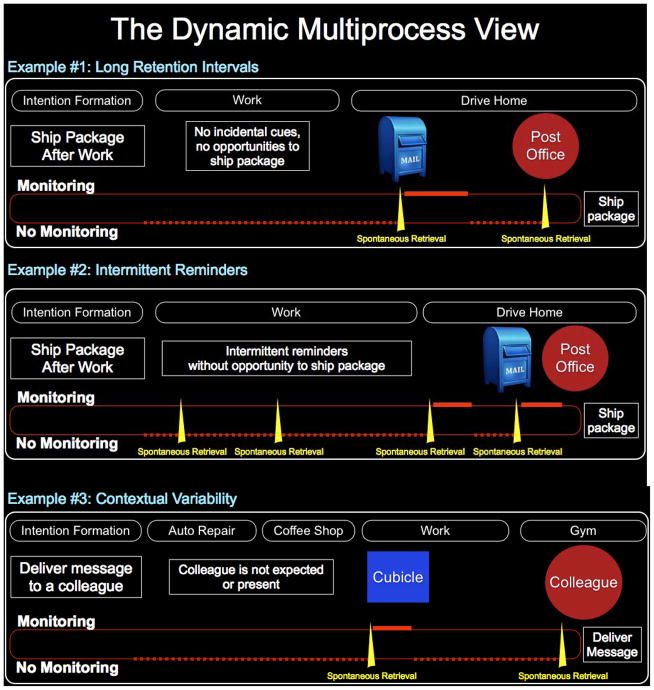
Figure 1. The Dynamic Multiprocess Framework.
Retrieval processes are shown to interact dynamically to support the prospective memory intentions of remembering to ship a package after work (Examples 1–2) and deliver a message to a colleague (Example 3). At the bottom of each example, monitoring is indicated by a bolded solid line, the absence of monitoring is indicated by a dashed line, and spontaneous retrievals are indicated by yellow spikes. In Example 1, there is a long retention interval that contains no incidental reminders or opportunities to ship the package, but during the drive home a mailbox spontaneously triggers memory of the intention to ship the package. The individual subsequently monitors for the post office, but then disengages the monitoring because there is not a post office nearby. Later encountering the post office spontaneously triggers retrieval of the intention to ship the package, and the individual completes that intention. In Example 2, intermittent reminders (e.g., cues related to the intention such as packing tape and shipping boxes) spontaneously trigger retrieval of the intention to ship the package but monitoring is not engaged because these retrievals occur while the individual is at work and does not have an opportunity to perform the intention (though it remains plausible that repeated retrievals may increases cue—intention associations and augment the probability of a later spontaneous retrieval; e.g., Ozgis, Rendell, & Henry, 2009; Svarras & NiedŸwieńska, 2011). When leaving work, the individual spontaneously retrieves the intention to ship the package and subsequently monitors, but no post office is encountered and therefore monitoring is disengaged. Later on the drive home, a mailbox spontaneously triggers retrieval of the intention and the individual monitors until a post office is shortly thereafter approached, and the package is shipped. Example 3 incorporates the idea of contextual variability (as present in the current study). An individual forms the intention to deliver a message to a colleague when seeing him that day. The individual does not monitor during periods of the day in which the colleague is not expected or present (coffee shop, auto repair shop), but spontaneously retrieves the intention when walking past the colleague’s cubicle. The individual then searches for the colleague in the workplace but does not see him and ceases to monitor. Later during the day, the individual goes to the gym and serendipitously encounters the colleague, which spontaneously triggers memory of the message.
2. Dynamic Interplay of Retrieval Processes in Prospective Memory
Our theoretical approach suggests that in prospective memory tasks there is often an interplay between spontaneous retrieval and monitoring that augments the functional value of these processes relative to what each alone enables (cf. Chen et al., 2010; Einstein et al., 1997; Gilbert, Hadjipavlou, & Raoelison, 2013). In naturalistic prospective memory tasks in which the delay intervals between intention formation and the opportunity to execute the intention are long (hours) and may even include sleep (days, weeks), it is very unlikely that individuals will or can sustain monitoring over that time interval. This assertion is suggested by several findings in the laboratory. First, even when the retention interval is on the order of minutes, monitoring may wax and wane across an ongoing task block (DeWitt, Hicks, Ball, & Knight, 2012; Einstein et al., 2005; Scullin, McDaniel, & Einstein, 2010; West & Craik, 1999). Second, when monitoring goes unreinforced (i.e., in the absence of target cues), participants often cease monitoring (Loft, Kearney, & Remington, 2008; McBride, Beckner, & Abney, 2011; Meier et al., 2006; Morgan, Weber, Rooney, Grant, & Woods, 2012; Scullin, McDaniel, Shelton, & Lee, 2010). Therefore, research has shown that monitoring is a process that is not necessarily continuously engaged.
Participants may instead selectively engage monitoring when they enter into a context in which the prospective memory cue is expected. For example, Marsh, Hicks, and Cook (2006) found that when participants were told which context to expect a prospective memory cue, they monitored during that context, but not during a preceding context (see also Chen et al., 2010; Cook, Marsh, & Hicks, 2005; Knight et al., 2011; Scullin & Bugg, 2013). To selectively remember when to monitor, however, another process must be involved, and we suggest that one candidate is spontaneous retrieval prompted by either the onset of the particular context or some other environmental cue. In the present experiment, we focus on this sense of dynamic interplay between spontaneous processes and monitoring processes.
To make the dynamic interplay idea concrete (Figure 1), consider the common prospective memory task of having to remember to ship a package at the post office on the drive home from work. Upon forming this intention in the morning, an individual is not likely to monitor for the intention throughout her workday because of a lack of opportunities to actually ship the package (see Figure 1, Example 1). However, the event of getting into the car and beginning the trip home could stimulate retrieval of the intention to stop at the post office and ship the package (see Figure 1, Example 2). Upon retrieval of the intention in this context, the individual could begin monitoring for the post office on the way home. In sum, the Dynamic Multiprocess Framework suggests that prospective remembering might be viewed as an interplay of monitoring and a probabilistic spontaneous retrieval process. To examine this possibility, in the present experiment we modified the typical laboratory paradigm to better approximate some key features of naturalistic prospective memory tasks.
2.1. Incorporating Naturalistic Characteristics into a Laboratory Prospective Memory Task
Naturalistic and laboratory based prospective memory studies differ in several regards. In laboratory studies, participants are typically engaged in performing an ongoing task (Einstein & McDaniel, 1990), such as rating the pleasantness of faces. In addition to the ongoing task (e.g., pleasantness ratings), participants are instructed to remember to perform a designated action (e.g., press F1) in response to a target cue (e.g., a face with glasses), which is embedded within the ongoing task after some delay interval (Maylor, 1996). In laboratory paradigms the retention interval between forming the intention and the appearance of the prospective memory cue is nearly always 5 min or less, the prospective memory response has sometimes been required up to 100 times (frequency up to 10% of trials), and participants may be forced to withhold making an ongoing task response until after the prospective memory response (or vice versa; for further discussion, see Bisiacchi, Schiff, Ciccola, & Kliegel, 2009; Martin, Brown, & Hicks, 2011). By contrast, naturalistic intentions may be executed once or a few times (e.g., delivering news to a few colleagues) and with no limitations on response order. The retention interval may last several hours, days, or weeks (Ellis & Nimmo-Smith, 1993; Wilkins, 1979), and may include periods of sleep (Diekelmann, Wilhelm, Wagner, & Born, 2013; Scullin & McDaniel, 2010).
Another striking difference between most laboratory-based and naturalistic prospective memory tests is the treatment of ongoing task contexts. Laboratory tasks typically introduce participants to a single ongoing task context and instruct them that the prospective memory intention must be executed in that context (for exceptions, see Cook et al., 2005; Eren-Kanat, Ball, & Brewer, 2013; Kalpouzos et al., 2010; Kominsky, 2010; Maylor & Logie, 2010; Meier et al. 2006; Nowinski & Dismukes, 2005). Yet in the real world our contexts and ongoing activities change often (Marsh, Hicks, & Cook, 2008), and we may not know for certain the context in which the prospective memory intention needs to be executed. Moreover, as illustrated in Figure 1 (Example 3), even when we might predict the context in which an intention (e.g., delivering a colleague a message) is likely to be executed (e.g., the workplace), retrieval may not be so inflexible that it cannot occur in other contexts (e.g., encountering the colleague at the gym), as has been demonstrated by the intention interference literature (Brewer, Knight, Meeks, & Marsh, 2011; Cohen, Dixon, & Lindsay, 2005; Cohen, Kantner, Dixon, & Lindsay, 2011; Einstein et al., 2005; Knight et al., 2011; McDaniel & Scullin, 2010; Rummel, Einstein, & Rampey, 2012; Scullin, Einstein, & McDaniel, 2009; West, McNerney, & Travers, 2007; but cf. Schult & Steffens, 2013). We contend that the difference between laboratory and naturalistic tasks in the treatment of ongoing task context variability, intention frequency, and retention interval, may have obscured the laboratory investigation of the dynamic role of spontaneous processes and monitoring processes in the fulfillment of delayed intentions.
The major goal of the present paradigm was to create contextual variability and to include conditions with substantially increased retention intervals (including nocturnal sleep) than have been previously used in most laboratory paradigms. The paradigm, which is depicted in Figure 2, required participants to complete many ongoing tasks and we instructed participants that the prospective memory cues might occur during any point in the experiment. After approximately 20 minutes (single session condition) or 12 hours (two session condition), prospective memory cues were presented infrequently (~1% of trials) across three ongoing tasks and participants were allowed to make either the prospective memory response first or the ongoing task response first.
Figure 2. Experimental Procedure.
Participants first completed baseline tasks, then post-encoding tasks, and then the experimental tasks. Highlighted borders indicate that the prospective memory target cues were presented. An example of a task block that is divided into tertiles (red lines) is provided under the Experimental Tasks subheader. Participants in the two-session condition left the laboratory for approximately 12 hours after completing the syllable learning phase. The procedure was identical in the control condition except that the prospective memory intention was never encoded. This figure was modified from Scullin and McDaniel (2010).
3. Predictions
The present prospective memory paradigm (Figure 2) is informative because it introduces challenges for prospective memory retrieval processes that are similar to those faced in naturalistic tasks. For example, with a long retention interval that includes sleep one cannot monitor continuously (at least consciously) from intention formation until the target cue. Furthermore, because prospective memory cues may occur during any ongoing task context, participants cannot selectively limit monitoring to a particular context (cf. Marsh et al., 2006). To distinguish spontaneous retrieval and monitoring processes, the present experiment included a control group that never encoded the prospective memory task, and compared ongoing task responding between the prospective memory and control groups.
The monitoring-only view is that if participants are supporting prospective memory by monitoring for cues then ongoing task cost should be apparent prior to target cues (i.e., prior to correct prospective memory responses). On the other hand, the spontaneous-retrieval-only view is that if participants are biased to rely solely on spontaneous retrieval processes then ongoing task costs should not be observed prior to target cues. The Dynamic Multiprocess Framework (Figure 1) suggests a third, more complex possibility. Because it is unclear when to expect the prospective memory cue in the present paradigm, and continuous monitoring for infrequent/absent prospective memory cues would be wasteful of cognitive resources that could otherwise be devoted to performing ongoing activities, the interplay of retrieval processes will initially be characterized by a lack of monitoring. Therefore, prospective remembering to the first target cue should be supported (probabilistically) by spontaneous retrieval. However, once the target cue spontaneously triggered retrieval, and only if it did, the participant would have identified a context in which the prospective memory cue is occurring. At this point participants would be expected to engage monitoring for additional prospective memory cues (cf. Chen et al., 2010). This possibility builds on Marsh et al.’s (2006) argument that individuals set attention allocation policies at the outset of prospective memory blocks. However, an important distinction here is that under conditions of contextual variability and long retention intervals, the resetting of attention allocation may only occur following the initial spontaneous retrieval of an intention.
4. Method
4.1. Participants and Design
Washington University undergraduates (N=121) participated for partial class credit or monetary compensation. Seventy-three participants completed the study during a single evening (n=37) or morning (n=36) session, with some participants being randomly assigned to a prospective memory condition (n=48) and others to a control condition (n=25). In addition, forty-eight participants completed the prospective memory condition across two sessions that were split by an interval of nocturnal sleep (evening encoding and morning testing; n=24) or daytime wake (morning encoding and evening testing; n=24). As previously reported, there were no time-of-testing effects on the prospective memory or ongoing tasks, though there was a difference in the prospective memory hit rate between the nocturnal sleep and daytime wake groups (Scullin & McDaniel, 2010). Our previous report did not examine the potential dynamic interplay of spontaneous retrieval and monitoring processes, which is the focus of the present work.
4.2. Procedure
The procedure is depicted in Figure 2 (see also Scullin & McDaniel, 2010). As an overview, the first half of the experimental session (or the first experimental session for the two-session groups) included a working memory test, three decision-making tasks that did not include prospective memory cues, a prospective memory encoding phase, as well as a retrospective memory (syllable items) learning phase. The second half of the experimental session (or the second experimental session for the two-session groups) included a retrospective memory test, a working memory test, and several questionnaires. Following these tasks, participants performed three decision making (ongoing) tasks that included the prospective memory cues, as well as a third working memory test (symmetry span). Below we focus on the ongoing tasks component of the study.
The three ongoing tasks that included prospective memory target cues during the second half of the experimental session were the living/nonliving task, lexical decision task, and semantic categorization task. In each of these three ongoing tasks participants were first given the task instructions. The living/nonliving task instructions were to determine whether a presented noun represented a living (e.g., dog) or nonliving (e.g., chair) object; the lexical decision task instructions were to determine whether a string of letters formed a word (e.g., kite) or nonword (e.g., itek); and, the semantic categorization task instructions were to determine whether one word was a member of a given category (e.g., SPORT hockey). Participants were instructed to make their responses as quickly and accurately as possible using keys marked Y and N on the number pad. Following the instructions for each task, the participants completed a 12-trial practice block that included speed and accuracy feedback, and then performed a 164-trial pre-experimental block. The living/nonliving, lexical decision, and semantic categorization pre-experimental blocks were used to establish a baseline measure of response times for each ongoing task in the absence of a prospective memory demand.
Once participants completed these baseline blocks, they encoded the prospective memory task. Except for the control group, participants were told that in addition to the different tasks they had been doing and would be doing there was a secondary interest in their memory for performing an action in the future. Participants were instructed to press the Q key if they ever saw the words table or horse during any point in the experiment. Furthermore, they were told to press the Q key when they remembered having seen their target word, even if that trial was no longer on the screen (i.e., there was no response order requirement). Participants were told that they would not be reminded of the target words or the target key and that their primary goal was to focus on whichever ongoing task they were performing. To check (or correct) their understanding of the prospective memory instructions they were required to write down the instructions.
Participants then completed the Symptom-Checklist 90 (Derogatis, 1977), responded to demographics and sleep habits questions, encoded a list of syllables, took a restroom and water break, completed the Morningness-Eveningness Questionnaire (Horne & Ostberg, 1976), free recalled the syllables, and performed the automated reading span task (Unsworth, Redick, Heitz, Broadway, & Engle, 2009). These tasks were conducted on the computer except for the free recall test and the Morningness-Eveningness Questionnaire, and they lasted approximately 20 minutes. The prospective memory and control conditions did not differ statistically on these tests and the results were discussed elsewhere (Scullin & McDaniel, 2010). In the two session condition, participants left the laboratory after encoding the list of syllables (i.e., at the point in which single-session condition participants took a restroom break) and returned to the laboratory approximately 12-hours later (following an interval of daytime wake or nocturnal sleep).
Participants next completed a second (experimental) block of the living/nonliving decision, lexical decision, and semantic categorization tasks. Stimuli appeared on the computer screen until a response was made and there was no response-stimulus interval to avoid possible “hidden” costs (Scullin, McDaniel, Shelton, & Lee, 2010). The first and last six trials of each 164-trial block were considered buffer trials, and when considering the remaining 152 trials, the target cues appeared on Trials 101 and 152. During each block, table and horse each appeared once, and table was presented first (Trial 101) during the living/nonliving and semantic categorization tasks. Two lists of filler items were constructed for each ongoing task and list order was counterbalanced across participants.
5. Results
5.1. Prospective Memory Performance
Prospective memory hits were operationally defined as Q presses on target trials or the following two trials. In the single session condition, proportion of prospective memory hits did not differ between the living/nonliving decision (M=.48), lexical decision (M=.45), and semantic categorization (M=.50) tasks (F<1). In the two session condition, prospective memory performance increased across tasks (MLiving/Nonliving=.22; MLexical Decision=.27; MSemantic Categorization=.34), F(2,94)=5.15, MSE=.037 (for discussion, see Scullin & McDaniel, 2010). In the present study, we observed good reliability for prospective memory performance (Cronbach’s = .89 and .93 in the single- and two-session conditions, respectively; cf. Kelemen, Weinberg, Alford, Mulvey, & Kaeochinda, 2006).
5.2. Ongoing Task Performance (Single Session Condition)
Because the control condition was completed in a single session we will first focus our analyses on the single session condition (collapsed across morning and evening groups), and then aim to replicate our findings in the two session condition (Section 5.3). We first confirmed that there were no baseline ongoing task performance differences between the prospective memory and control conditions during the ongoing task blocks that preceded the prospective memory encoding phase. There were no group differences in the proportion of correct ongoing task responses during the baseline living/nonliving (both Ms=.92, t<1), lexical decision (both Ms=.96; t<1), or semantic categorization (MProspective-Memory=.94, MControl=.95, t(71)=1.16) tasks. We next calculated mean response times on correct filler trials in each ongoing task block. Raw means are provided in Table 1. There were no significant group differences on mean response times during any of the baseline tasks (all ts<1).
Table 1.
Baseline (first block) and experimental (second block) response time means in milliseconds (standard deviations in parentheses) for the living/nonliving, lexical decision, and semantic categorization tasks across single and two-session conditions. Groups are split by whether a prospective memory response was observed (PM-Hit Subgroup) or not (PM-Miss Subgroup) for the first target event (Trial 101) during the corresponding experimental block.
| Baseline Living/Nonliving | Baseline Lexical Decision | Baseline Semantic Categorization | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Trials 1–50 | Trials 51–100 | Trials 102–151 | Trials 1–50 | Trials 51–100 | Trials 102–151 | Trials 1–50 | Trials 51–100 | Trials 102–151 | |
| Single Session Groups | |||||||||
| PM-Hit Subgroup | 865 (133) | 895 (151) | 858 (141) | 657 (87) | 656 (91) | 677 (88) | 1129 (174) | 1136 (173) | 1113 (229) |
| PM-Miss Subgroup | 887 (124) | 906 (161) | 906 (189) | 711 (112) | 705 (97) | 707 (118) | 1184 (203) | 1237 (213) | 1203 (200) |
| Control Group | 874 (193) | 936 (202) | 901 (171) | 677 (100) | 694 (83) | 685 (71) | 1159 (175) | 1171 (208) | 1197 (207) |
| Two Session Groups | |||||||||
| PM-Hit Subgroup | 928 (167) | 1025 (215) | 1003 (153) | 652 (64) | 668 (63) | 681 (95) | 1103 (104) | 1139 (157) | 1115 (154) |
| PM-Miss Subgroup | 870 (161) | 885 (153) | 857 (134) | 688 (124) | 676 (118) | 672 (113) | 1201 (233) | 1189 (236) | 1212 (258) |
| Experimental Living/Nonliving | Experimental Lexical Decision | Experimental Semantic Categorization | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Trials 1–50 | Trials 51–100 | Trials 102–151 | Trials 1–50 | Trials 51–100 | Trials 102–151 | Trials 1–50 | Trials 51–100 | Trials 102–151 | |
| Single Session Groups | |||||||||
| PM-Hit Subgroup | 790 (104) | 822 (146) | 884 (128) | 616 (68) | 648 (98) | 702 (98) | 1076 (183) | 1101 (162) | 1186 (182) |
| PM-Miss Subgroup | 793 (143) | 832 (156) | 830 (180) | 666 (113) | 687 (109) | 689 (104) | 1125 (220) | 1149 (209) | 1141 (207) |
| Control Group | 793 (157) | 822 (113) | 798 (109) | 616 (62) | 652 (78) | 646 (81) | 1062 (130) | 1127 (128) | 1113 (128) |
| Two Session Groups | |||||||||
| PM-Hit Subgroup | 883 (138) | 916 (111) | 1016 (153) | 650 (74) | 654 (68) | 723 (63) | 1061 (102) | 1115 (118) | 1203 (133) |
| PM-Miss Subgroup | 797 (156) | 811 (144) | 790 (175) | 634 (96) | 668 (119) | 655 (114) | 1095 (207) | 1144 (203) | 1132 (203) |
During the experimental blocks, there were no differences between the control group and prospective memory group in the proportion of correct ongoing task responses during the living/nonliving task (MProspective-Memory=.90, MControl=.91, t<1), the lexical decision task (MProspective-Memory=.95, MControl=.96, t<1), or the semantic categorization task (MProspective-Memory=.93, MControl=.94, t(71)=1.18). To evaluate the potential dynamics of spontaneous retrieval and monitoring processes we examined whether ongoing task cost was evident prior to the first target cue in each ongoing task or only following a prospective memory hit. We took the analytical approach of calculating mean response times across 50-trial tertiles within each ongoing task block. Specifically, we examined the first 50 post-buffer trials of the block (Trials 1–50), the 50 trials immediately preceding the first target cue (Trials 51–100), and the 50 trials interleaved between the first and second target cue (Trials 102–151). Our primary interest was in contrasting the control condition with participants who made a correct prospective memory response to the first target cue in a given block (hereafter, hit subgroup). Therefore, we conducted a series of 3 × 3 mixed ANCOVAs that included the within subjects variable of tertile and the between subjects variable of group (hit subgroup, miss subgroup, control). In all analyses we augmented statistical power to detect possible costs by covarying mean response times from the corresponding baseline block trials. The results, using baseline-adjusted means, are illustrated in Figure 3 (raw means are presented in Table 1).
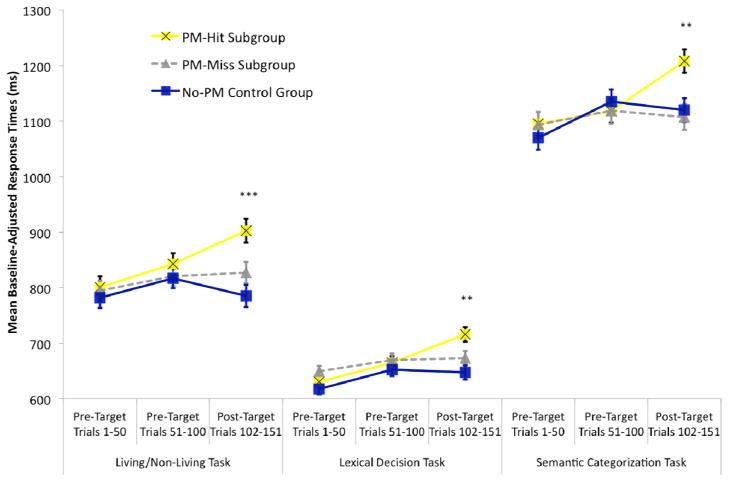
Figure 3. Single Session Conditions: Cost Across Segments of the Ongoing Tasks.
Mean response times in milliseconds (adjusted for corresponding baseline) in the single session condition. Results are presented across experimental ongoing task blocks in participants who made a prospective memory response on the first target cue (for the specified ongoing task; PM-Hit Subgroup) relative to those who did not (PM-Miss Subgroup) and control group participants. Response times are separated between the first 50 trials of the block, the 50 trials preceding the first target cue, and the 50 trials that followed the first target cue and preceded the second target cue. Error bars represent standard errors and asterisks indicate the significance of the group main effect for the given tertile (* indicates that p < .05, ** indicates that p < .01, *** indicates that p < .001, and all other unmarked contrasts were nonsignificant). Similar, but less pronounced patterns are observed after excluding the three trials following prospective memory responses (Section 5.4).
For the living/nonliving task, the 3 × 3 mixed ANCOVA resulted in a significant interaction, F(4,134)=5.24, MSE=3322.36, p<.001. The three groups did not differ prior to the first target cue on Trials 1–50 (F<1) and Trials 51–100 (F<1), but they did differ after the first target cue on Trials 102–151, F(2,69)=7.87, MSE=9717.47, p<.001. As illustrated in Figure 3, relative to the control group, there was significant ongoing task cost on Trials 102–151 in the hit subgroup, F(1,43)=15.32, MSE=8394.65, p<.001, but not the miss subgroup, F(1,49)=1.10, MSE=10028.61, p=.30. We confirmed that post-target response times were still significantly slower in the hit subgroup (MRaw=843 ms; MBaseline-Adjusted=853 ms) than the control group (MRaw=798 ms; MBaseline-Adjusted=789 ms) after removing the three trials following the prospective memory response (Meier & Rey-Mermet, 2012), F(1,43)=6.41, MSE=7375.12, p=.015. Thus, prospective remembering under conditions of contextual variability was associated with post-target cost but not with pre-target cost.
For the lexical decision task, the 3 × 3 mixed ANCOVA also demonstrated a significant group by tertile interaction, F(4,134)=4.36, MSE=1808.16, p=.002. Mean response times did not significantly differ across groups on pre-target Trials 1–50 (F(2,69)=1.72, MSE=2808.54, p=.19) or Trials 51–100 (F(2,69)=1.58, MSE=3672.01, p=.21), but there was a main effect of group for post-target Trials 102–151, F(2,69)=5.94, MSE=3975.54, p=.004. The main effect was observed because there was significant post-target cost in the hit subgroup, F(1,46)=11.60, MSE=4091.46, p=.001, but not in the miss subgroup, F(1,46)=2.50, MSE=3463.19, p=.12. Significant post-target cost was observed in the hit subgroup (MRaw=677 ms; MBaseline-Adjusted=680 ms) relative to the control group (MRaw=646 ms; MBaseline-Adjusted=643 ms) after excluding the three trials following a prospective memory response, F(1,46)=4.62, MSE=3622.11, p=.037.
Some research has suggested that in a lexical decision task that cost may be specific to word trials, and absent on nonword trials (Cohen, Jaudas, Hirschhorn, Sobin, & Gollwitzer, 2012). In our previous research in which participants can perform the prospective memory response either before or after making an ongoing response (no response order requirement) we have not observed this stimulus-specific effect (Scullin, McDaniel, Shelton, & Lee, 2010). A similar methodology (no response order requirement) was employed in the present study, and when re-computing the above ANCOVA with the additional within-subjects factor of trial type (word/nonword) we replicated the significant group by tertile interaction, F(4,128)=4.09, MSE=1423.74, p=.004, but did not observe any interactions with trial type (Fs<1). There were still no group main effects or interactions with trial type for pre-target Trials 1–50 or Trials 51–100 (largest F(2,68)=1.46, MSE=10776.17, p=.24, for the group main effect for Trials 51–100), but the group main effect for post-target Trials 102–151 was still significant, F(2,68)=6.00, MSE=8081.07, p=.004, and did not interact with trial type (F<1). When contrasting the control group with the hit subgroup for Trials 102–151, we observed significant cost for both word trials, F(1,46)=5.32, MSE=6413.06, p=.026, and nonword trials, F(1,46)=11.63, MSE=5308.67, p=.001 (contrasts between the control and miss subgroups were still nonsignificant, both ps>.10). Therefore, costs were stimulus-general in the hit subgroup.
The semantic categorization task results were consistent with the previous two ongoing task results. There was a significant group by tertile interaction, F(4,134)=6.64, MSE=3866.80, p<.001, reflecting that the groups differed on post-target trials (Trials 102–151), F(2,69)=6.26, MSE=15861.67, p=.003, but not pre-target trials (Trials 1–50: F(2,69)=1.12, MSE=11968.68, p=.33; Trials 51–100: F<1). Relative to the control group, the hit subgroup (but not the miss subgroup; F<1), responded significantly slower on post-target Trials 102–151, F(1,48)=8.63, MSE=16714.97, p=.005. Post-target cost in the hit subgroup (MRaw=1151 ms; MBaseline-Adjusted=1168 ms) relative to the control group (MRaw=1113 ms; MBaseline-Adjusted=1096 ms) was attenuated to marginally significant levels after excluding the three trials following the prospective memory response, F(1,48)=3.73, MSE=16914.49, p=.059.
Given the finding in each ongoing task block (Figure 1) that participants reset their attention allocation policy after spontaneously retrieving an intention, we were interested in whether such a reset benefited later prospective remembering. In most cases (76%), the hit subgroup participants (who, as a group, demonstrated post-target cost; i.e., reset their attention allocation policy) successfully responded to the second prospective memory cue in the living/nonliving (19 of 21), lexical decision (15 of 24), and semantic categorization (20 of 26) tasks. By contrast, for the miss subgroup participants (excluding any participant who never made a prospective memory response throughout the experiment), successful responding to the second prospective memory cue was only 43% (Fisher’s exact tests: living/nonliving: 6 of 12, p=.015; lexical decision: 4 of 9, p=.44; semantic categorization: 2 of 7, p=.027)(cf. Kelly, Hertzog, Hayes, & Smith, 2013; Maylor, 1996, recovery analyses). This difference does not simply reflect better activation of a particular target cue because the cue (horse or table) changed from the first to the second target. Finer grained analyses to more directly illuminate a possible relation between attentional-reset policies and responding to the second prospective memory cue were not viable; within the hit subgroup (first target), there was insufficient sample size and variability to compare response times during Trials 102–151 between those who made a correct prospective memory response to the second target cue and those who did not.
5.3. Ongoing Task Performance Costs (Two Session Condition)
We next investigated whether the same dynamic pattern of spontaneous retrieval and monitoring would be observed in the two session condition. We analyzed the data in the same manner as in the single session condition, and utilized the same control group as a comparison. The primary results are illustrated in Figure 4 (raw means in Table 1). For the baseline blocks, the prospective memory and control groups did not differ for response times on any ongoing task (all ts<1). For proportion of correct ongoing task responses, there were no group differences during the baseline living/nonliving (MProspective-Memory=.93, MControl=.92; t<1) or lexical decision (both Ms=.96; t<1) tasks, but there was a significant group difference for the baseline semantic categorization task (MProspective-Memory=.94, MControl=.95, t(71)=2.01, p=.048). Regardless of whether the latter finding is a Type I error or has some other meaning, ongoing task accuracy did not differ across groups in the experimental blocks of the living/nonliving task (both Ms=.91, t<1), the lexical decision task (both Ms=.96, t<1), or the semantic categorization task (MProspective-Memory=.93, MControl=.94, t(71)=1.19).
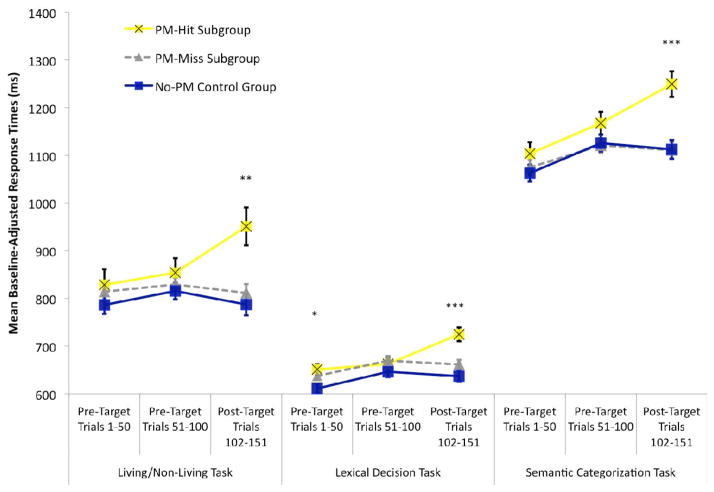
Figure 4. Two Session Condition: Cost Across Segments of the Ongoing Tasks.
Mean response times in milliseconds (adjusted for corresponding baseline) in the two-session condition. Results are presented across experimental ongoing task blocks in participants who made a prospective memory response on the first target cue (for the specified ongoing task; PM-Hit Subgroup) relative to those who did not (PM-Miss Subgroup) and control group participants. Response times are separated between the first 50 trials of the block, the 50 trials preceding the first target cue, and the 50 trials that followed the first target cue and preceded the second target cue. Error bars represent standard errors and asterisks indicate the significance of the group main effect for the given tertile (* indicates that p < .05, ** indicates that p < .01, *** indicates that p < .001, and all other unmarked contrasts were nonsignificant). Similar, but less pronounced patterns are observed after excluding the three trials following prospective memory responses (Section 5.4).
To test for the dynamic interplay of retrieval processes in the experimental living/nonliving task, we conducted a 3 × 3 mixed ANCOVA that included tertile (Trials 1–50, 51–100, 102–151) and group (hit subgroup, miss subgroup, control) and controlled for baseline response times. Similar to the single session condition, there was a significant tertile by group interaction, F(4,134)=4.87, MSE=3638.27, p=.001. As illustrated in Figure 4, the three groups were statistically similar on Trials 1–50 (F<1) and Trials 51–100 (F<1), but they were statistically different on Trials 102–151, F(2,69)=5.26, MSE=15047.19, p=.007. Relative to the control group, there was not significant ongoing task cost on Trials 102–151 in the miss subgroup (F<1), but there was significant cost in the hit subgroup, F(1,31)=18.55, MSE=10225.89, p<.001, even after removing the three trials following the prospective memory response, F(1,31)=9.72, MSE=9095.36, p=.004 (PM hit subgroup: MRaw=956 ms, MBaseline-Adjusted=928 ms; Control group: MRaw=798 ms, MBaseline-Adjusted=808 ms). This pattern (Figure 4) replicates the dynamic interplay of spontaneous retrieval and monitoring processes observed in the single session conditions (Figure 3).
For the lexical decision task, the 3 × 3 mixed ANCOVA also produced a significant group by tertile interaction, F(4,134)=5.33, MSE=1406.76, p<.001. Figure 4 illustrates that the groups displayed significantly different response times on Trials 1–50, F(2,69)=3.22, MSE=3402.62, p=.046, but this cost mostly dissipated prior to the first target cue, as evidenced by no significant group difference on Trials 51–100 (F(2,69)=1.97, MSE=3493.35, p=.15). There was a significant main effect of group for Trials 102–151, F(2,69)=9.85, MSE=3192.33, p<.001, because there was significant post-target cost in the hit subgroup, F(1,37)=16.64, MSE=3573.89, p<.001, even after removing the three trials following the prospective memory response, F(1,37)=7.08, MSE=3712.48, p=.01 (PM hit subgroup: MRaw= 696 ms, MBaseline-Adjusted=698 ms; Control group: MRaw=646 ms, MBaseline-Adjusted=645 ms) . Post-target cost was not significant in the miss subgroup, F(1,55)=2.45, MSE=2587.47, p=.12. We also confirmed in the hit subgroup that significant post-target cost occurred for both word trials, F(1,37)=9.87, MSE=6093.49, p=.003, and non-word trials, F(1,37)=17.00, MSE=3596.47, p<.001 (there were also no interactions with trial type when re-computing the above 3 × 3 ANCOVA, ps>.10; cf. Cohen et al., 2012).
The 3 × 3 mixed ANCOVA on semantic categorization response times also yielded a significant group by tertile interaction, F(4,134)=4.30, MSE=4054.53, p=.003. There was not a group main effect for pre-target Trials 1–50 or Trials 51–100 (both Fs<1), but there was a significant main effect of group for post-target Trials 102–151, F(2,69)=9.95, MSE=10399.31, p<.001. There was no evidence for post-target cost in the miss subgroup (F<1). In the hit subgroup, there was evidence for significant post-target cost, F(1,37)=18.06, MSE=8390.85, p<.001, even after removing the three trials following the prospective memory response, F(1,37)=10.83, MSE=7609.01, p=.002 (PM hit subgroup: MRaw=1168 ms, MBaseline-Adjusted=1194 ms; Control group: MRaw=1113 ms, MBaseline-Adjusted=1098 ms).
As in the single session condition, there was too small of a sample size within the hit-subgroups to compare response times on Trials 102–151 depending on who successfully responded to the second prospective memory cue. However, in most instances (82%), the hit subgroup which displayed significant post-target cost in each block (i.e., reset attention allocation policy), successfully responded to the second prospective memory cue in the living/nonliving task (8/9), lexical decision task (10/15), and semantic categorization task (14/15). In contrast, the miss subgroup (excluding any participant who never made a prospective memory response throughout the experiment), which did not display post-target cost, showed reduced responding (38%) to the second prospective memory cue (Fisher’s exact tests: living/nonliving: 4 of 12, p=.024; lexical decision: 1 of 6, p=.064; semantic categorization: 4 of 6, p=.18).
5.4. Combined Conditions Analyses
The primary focus of our response time cost analyses, determined a priori, was to include all post-target trials so as to avoid eliminating conceptually important trials (e.g., trials on which the attentional shift is occurring) and because we did not know the timeline of the attentional shift (if found). There is no recognized standard for eliminating trials following prospective memory responses (often no trials are removed), however, to be thorough, we conducted post-hoc cost analyses eliminating the three trials following prospective memory hits1 (for justification for using three trials, see Meier & Rey-Mermet, 2012). As reported in Sections 5.2 and 5.3, post-target cost was still significant in the hit subgroup in 5 of 6 analyses, but we observed that the 3 (group) × 3 (tertile) interactions reported above typically became nonsignificant (ps ranged from .09 to .19, except in the single session, category decision task, F(4,134)=2.98, MSE=3956.27, p=.021). Inadequate statistical power to detect interactions may have precluded these interactions from reaching significance (observed power ranged from .47 to .59). In line with this possibility, when we collapsed across the single session and two session conditions to increase power (observed power ranged from .70 to .87), the 3 (group) × 3 (tertile) interactions were significant for the living/nonliving task, F(4,230)=2.45, MSE=3401.01, p=.047, lexical decision task, F(4,230)=2.90, MSE=1552.33, p=.023, and semantic categorization task, F(4,230)=3.57, MSE=4177.09, p=.008.
We also examined whether the hit subgroups (collapsed across conditions), who were more likely to reset their attention allocation policy, were also more likely to successfully respond to the second prospective memory cue than the miss subgroups. With the larger sample sizes we conducted chi square tests rather than Fisher exact tests. The hit subgroup was more likely to successfully respond to the second prospective memory cue during the living/nonliving task, χ2(1)=14.44, p<.001, lexical decision task, χ2(1)=4.15, p=.04, and semantic categorization task, χ2(1)=6.95, p<.01
6. Discussion
The primary interest in this study was to examine the possible interplay of prospective memory processes. The findings were highly consistent across three ongoing task contexts: Participants who successfully remembered to perform the prospective memory intention monitored following, but not prior to, encountering the initial prospective memory cue. These results were inconsistent with monitoring-only views that either sustained preparatory monitoring (Smith, 2003) or prolonged retrieval modes (Guynn, 2003) are required for intentions to be retrieved. Such monitoring-only theories were initially reasonable considering the extensive literature demonstrating ongoing task cost during brief ongoing tasks in which the prospective memory cue could easily be anticipated (see Smith, Hunt, McVay, & McConnell, 2007, for review). However, the present results clearly converge with the notion that, in the absence of cost-inducing monitoring or retrieval modes, a cue that is linked to an intention can spontaneously trigger intention retrieval (Brandimonte, Ferrante, Feresin, & Delbello, 2001; Brewer et al., 2010; Cohen, Jaudas, & Gollwitzer, 2008; Cona, Bisiacchi, & Moscovitch, 2013; Einstein et al., 2005; Fink, 2013; Harrison & Einstein, 2010; Knight et al., 2011; Kvavilashvili & Fisher, 2007; Loft & Humphreys, 2012; McBride et al., 2011; McDaniel et al., 2004; Meier, von Wartburg, Matter, Reber, & Rothen, 2011; Morita, 2006; Penningroth, Graf, & Gray, 2012; Rummel & Meiser, in press; Scullin, McDaniel, Shelton, & Lee, 2010).
6.1. The Dynamic Multiprocess Framework
Even though the results consistently demonstrated that pre-target cost was not required for successful prospective memory performance, we also observed that cost consistently followed successful prospective remembering. This pattern augments Marsh et al.’s (2006) finding that participants may monitor once they have determined that they are performing an ongoing activity in which the prospective memory intention also needs to be performed, and Cohen et al.’s (2012, Experiment 3) finding that when cost is already present prior to a target cue that cost may then increase following the target cue. The theoretical nuance between these views and the Dynamic Multiprocess Framework (Figure 1) elaborated in the present work is that attention allocation policies need not be set at the outset of new ongoing activities nor does some level of monitoring need to already be present before target cues to observe post-target increases in monitoring; instead, individuals might spontaneously retrieve an intention, then reset their attention allocation policy to monitor for that intention, but can also flexibly disengage monitoring (e.g., if the prospective memory cue is not soon encountered). One reviewer raised the possibility that the increased response times, rather than reflecting preparatory monitoring processes (e.g., Smith, 2003) could instead reflect more cautious responding (cf. Boywitt & Rummel, 2012; Horn, Bayen, & Smith, 2011) due to remembering that there is a second task to be performed. Such a strategic process would be consistent with Guynn’s (2003) idea of engaging a retrieval mode when the prospective memory intention is expected, and Bugg, McDaniel, and Einstein’s (2013) idea that several cognitive control processes may fall under the broad umbrella of “attention allocation processes” (see also Brewer, 2011). Regardless of whether the increased response times indicated preparatory monitoring processes, an engaged retrieval mode, or some other attention-demanding process, our results demonstrated that such effortful, strategic processes can work dynamically with cue-driven spontaneous retrieval processes.
Given the observation that individuals successfully relied on relatively effortless spontaneous retrieval processes, one might pose the question why an individual would then engage effortful monitoring following execution of the prospective memory action. First, prospective remembering was imperfect when supported by spontaneous retrieval alone (highest mean was 50% during a given ongoing task) and prospective memory responses appeared more likely (ranging from 62% to 93%) after participants adjusted their resource allocation policy. Though caution is warranted because we were unable to conduct fine-grained analyses of post-target response times in the hit subgroups across who did and did not successfully respond to the second target cue (due to insufficient variability and sample size), the findings are consistent with the notion that engaging monitoring or other attention-demanding processes can improve prospective remembering (e.g., Albiński, Sedek, & Kliegel, 2012; Loft & Remington, 2013; Smith, 2003; West, Krompinger, & Bowry, 2005).
Second, monitoring might produce stronger benefits when an intention needs to be repeated after a brief interval (e.g., air traffic controllers might have to re-route multiple airlines in response to weather patterns; Loft, Finnerty, & Remington, 2011) or when one cannot execute an intention immediately following retrieval. For example, a common prospective memory intention includes remembering to deliver a message to a colleague, and seeing the colleague might spontaneously trigger retrieval of the message to deliver. But, if the colleague’s phone rings before the message can be given, one must delay delivering the intention. It would then become advantageous to maintain the intention in mind and monitor for the colleague’s phone call to end (cf. the delay-execute paradigm; e.g., Einstein, McDaniel, Williford, Pagan, & Dismukes, 2003; Evans & Beran, 2012). Under conditions of long retention intervals, such as those employed in the present study and often encountered in naturalistic prospective memory situations, such strategic deployment of monitoring without the help of spontaneous retrieval appears to be highly challenging if not improbable.
Third, in other naturalistic contexts, the prospective memory cue may not itself be present, but a related cue might spontaneously trigger retrieval (Figure 1). For example, Kvavilashvili and Fisher (2007) found that, when individuals were given the naturalistic prospective memory task of phoning the experimenter later in the week, incidental cues such as hearing a phone ring spontaneously triggered memory of the intention to phone the experimenter. In the present study, it is possible that noticing the target cue table caused participants to search for the other target cue horse (or vice versa). In each of the above cases, the view of the Dynamic Multiprocess Framework (Figure 1) is that monitoring will be engaged when the prospective memory cue is expected, disengaged when it is not expected, and that a probabilistic spontaneous retrieval process may support prospective remembering during intervals in which monitoring is disengaged.
6.2. Conclusions and Translational Implications
The ability to successfully remember to execute delayed intentions is not only important for successfully performing everyday demands, but also has implications for healthcare (Dieckmann, Reddersen, Wehner, & Rall, 2006), workplace performance (Dismukes, 2008), and the search for missing/wanted persons (Lampinen, Arnal, & Hicks, 2009). Because continuously maintaining intentions in mind can improve prospective memory performance but is costly to ongoing task performance, the Dynamic Multiprocess Framework proposes that individuals will dynamically deploy attention-demanding processes (e.g., monitoring) and spontaneous retrieval processes. Spontaneous processes that respond to environmental cues seem to be the driving force for recognizing prospective memory contexts (and prompting subsequent monitoring) under conditions of long retention intervals and contextual variability; therefore, prospective memory strategies in such contexts might target the spontaneous retrieval process. Specifically encoding an environmental cue (Gollwitzer, 1999) and/or context (Marsh et al., 2008) in which the prospective memory intention needs to be executed can increase later prospective remembering via bolstering spontaneous retrieval and stimulating monitoring for detecting the precise opportunity, or subsequent opportunities, to execute the intention.
Highlights.
Prospective memory can be supported by monitoring or spontaneous retrieval
Monitoring and spontaneous retrieval are dynamically interconnected processes
Engaging monitoring is dictated by the expectation of prospective memory cues
Incorporating contextual variability benefits the study of prospective memory
Acknowledgments
National Institutes of Health Grant F32AG041543 and a Cottrell Fellowship partially supported M.K.S. We are appreciative of Michael Clerkin for his assistance with collecting data, Zachary Shipstead and Randy Engle’s laboratory for sharing the automated working memory tasks, and Elizabeth Maylor, Joana Lourenço, Tyler Harrison, and Gil Einstein for their comments on an earlier version of this paper.
Footnotes
We thank Elizabeth Maylor for this suggestion.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albiński R, Sedek G, Kliegel M. Differences in target monitoring in a prospective memory task. Journal of Cognitive Psychology. 2012;24:916–928. [Google Scholar]
- Bisiacchi PS, Schiff S, Ciccola A, Kliegel M. The role of dual-task and task- switch in prospective memory: Behavioural data and neural correlates. Neuropsychologia. 2009;47:1362–1373. doi: 10.1016/j.neuropsychologia.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Boywitt CD, Rummel J. A diffusion model analysis of task interference effects in prospective memory. Memory & Cognition. 2012;40:70–82. doi: 10.3758/s13421-011-0128-6. [DOI] [PubMed] [Google Scholar]
- Brandimonte ME, Einstein GO, McDaniel MA. Prospective memory: Theory and applications. Lawrence Erlbaum Associates Publishers; 1996. [Google Scholar]
- Brandimonte MA, Ferrante D, Feresin C, Delbello R. Dissociating prospective memory from vigilance processes. Psicológica. 2001;22:97–113. [Google Scholar]
- Brewer GA. Analyzing response time distributions: Methodological and theoretical suggestions for prospective memory researchers. Journal of Psychology. 2011;219:117–124. [Google Scholar]
- Brewer GA, Knight JB, Marsh RL, Unsworth N. Individual differences in event-based prospective memory: Evidence for multiple processes supporting cue detection. Memory & Cognition. 2010;38:304–311. doi: 10.3758/MC.38.3.304. [DOI] [PubMed] [Google Scholar]
- Brewer GA, Knight J, Meeks JT, Marsh RL. On the role of imagery in event-based prospective memory. Consciousness and Cognition. 2011;20:901–907. doi: 10.1016/j.concog.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Bugg JM, McDaniel MA, Einstein GO. Event-based prospective remembering: An integration of prospective memory and cognitive control theories. In: Reisberg D, editor. The Oxford Handbook of Cognitive Psychology. Oxford University Press; 2013. pp. 267–283. [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Chen YZ, Huang XT, Yuan H. Processing mechanisms underlying a mixed prospective memory. Acta Psychologica Sinica. 2010;42:1040–1049. [Google Scholar]
- Cohen AL, Dixon RA, Lindsay DS. The intention interference effect and aging: Similar magnitude of effects for young and old adults. Applied Cognitive Psychology. 2005;19:1177–1197. [Google Scholar]
- Cohen AL, Jaudas A, Gollwitzer PM. Number of cues influence the cost of remembering to remember. Memory & Cognition. 2008;36:149–156. doi: 10.3758/mc.36.1.149. [DOI] [PubMed] [Google Scholar]
- Cohen AL, Jaudas A, Hirschhorn E, Sobin Y, Gollwitzer PM. The specificity of prospective memory costs. Memory. 2012;20:848–864. doi: 10.1080/09658211.2012.710637. [DOI] [PubMed] [Google Scholar]
- Cohen AL, Kantner J, Dixon RA, Lindsay DS. The intention interference effect: The difficulty of ignoring what you intend to do. Experimental Psychology. 2011;58:425–433. doi: 10.1027/1618-3169/a000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona G, Bisiacchi PS, Moscovitch M. The effects of focal and nonfocal cues on the neural correlates of prospective memory: Insights from ERPs. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht116. [DOI] [PubMed] [Google Scholar]
- Cook GI, Marsh RL, Hicks JL. Associating a time-based prospective memory task with an expected context can improve or impair intention completion. Applied Cognitive Psychology. 2005;19:345–360. [Google Scholar]
- Derogatis LR. SCL-90: Administration, scoring, and procedure manual - I. Baltimore: Johns Hopkins; 1977. [Google Scholar]
- DeWitt MR, Hicks JL, Ball BH, Knight JB. Encountering items previously paired with prospective memory target events can serve to reactivate intentions. Journal of Cognitive Psychology. 2012;24:981–990. [Google Scholar]
- Dieckmann P, Reddersen S, Wehner T, Rall M. Prospective memory failures as an unexplored threat to patient safety: Results from a pilot study using patient simulators to investigate the missed execution of intentions. Ergonomics. 2006;49:526–543. doi: 10.1080/00140130600568782. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Wilhelm I, Wagner U, Born J. Sleep to implement an intention. Sleep. 2013;36:149–153. doi: 10.5665/sleep.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismukes RK. Prospective memory in aviation and everyday settings. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory: Cognitive, neuroscience, developmental, and applied perspectives. New York: Taylor & Francis Group/Lawrence Erlbaum Associates; 2008. pp. 411–431. [Google Scholar]
- Einstein GO, McDaniel MA. Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:717–726. doi: 10.1037//0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Prospective memory: Multiple retrieval processes. Current Directions in Psychological Science. 2005;14:286–290. [Google Scholar]
- Einstein GO, McDaniel MA. Prospective memory and what costs do not reveal about retrieval processes: A commentary on Smith, Hunt, McVay, and McConnell (2007) Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:1082–1088. doi: 10.1037/a0019184. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, Breneiser J. Multiple processes in prospective memory retrieval: Factors determining monitoring versus spontaneous retrieval. Journal of Experimental Psychology: General. 2005;134:327–342. doi: 10.1037/0096-3445.134.3.327. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Williford CL, Pagan JL, Dismukes RK. Forgetting of intentions in demanding situations is rapid. Journal of Experimental Psychology: Applied. 2003;9:147–162. doi: 10.1037/1076-898X.9.3.147. [DOI] [PubMed] [Google Scholar]
- Einstein GO, Smith RE, McDaniel MA, Shaw P. Aging and prospective memory: The influence of increased task demands at encoding and retrieval. Psychology and Aging. 1997;12:479–488. doi: 10.1037//0882-7974.12.3.479. [DOI] [PubMed] [Google Scholar]
- Ellis JA, Nimmo-Smith I. Recollecting naturally-occurring intentions: A study of cognitive and affective factors. Memory. 1993;1:107–126. doi: 10.1080/09658219308258227. [DOI] [PubMed] [Google Scholar]
- Eren-Kanat S, Ball BH, Brewer GA. IFARM: A model of intention formation and retrieval. Poster presented at the Context and Episodic Memory Symposium; Philadelphia, Pennsylvania. 2013. [Google Scholar]
- Evans TA, Beran MJ. Monkeys exhibit prospective memory in a computerized task. Cognition. 2012;125:131–140. doi: 10.1016/j.cognition.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink NL. Unpublished dissertation thesis. Clemson University; 2013. Picture superiority effect in prospective memory: Examining the influence of age and attention load. [Google Scholar]
- Gilbert SJ, Hadjipavlou N, Raoelison M. Automaticity and control in prospective memory: A computational model. PLoS One. 2013;8:e59852. doi: 10.1371/journal.pone.0059852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollwitzer PM. Implementation intentions: Strong effects of simple plans. American Psychologist. 1999;54:493–503. [Google Scholar]
- Gordon BA, Shelton JT, Bugg JM, McDaniel MA, Head D. Structural correlates of prospective memory. Neuropsychologia. 2011;49:3795–3800. doi: 10.1016/j.neuropsychologia.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundgeiger T, Sanderson P, MacDougall HG, Venkatesh B. Interruption management in the intensive care unit: Predicting resumption times and assessing distributed support. Journal of Experimental Psychology: Applied. 2010;16:317–334. doi: 10.1037/a0021912. [DOI] [PubMed] [Google Scholar]
- Guynn MJ. A two-process model of strategic monitoring in event-based prospective memory: Activation/retrieval mode and checking. International Journal of Psychology. 2003;38:245–256. [Google Scholar]
- Harrison TL, Einstein GO. Prospective memory: Are preparatory attentional processes necessary for a single focal cue? Memory & Cognition. 2010;38:860–867. doi: 10.3758/MC.38.7.860. [DOI] [PubMed] [Google Scholar]
- Horn SS, Bayen UJ, Smith RE. What can the diffusion model tell us about prospective memory? Canadian Journal of Experimental Psychology. 2011;65:69–75. doi: 10.1037/a0022808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness- eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- Kalpouzos G, Eriksson J, Sjölie D, Molin J, Nyberg L. Neurocognitive systems related to real-world prospective memory. PloS One. 2010;5:e13304. doi: 10.1371/journal.pone.0013304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen WL, Weinberg WB, Alford HS, Mulvey EK, Kaeochinda KF. Improving the reliability of event-based laboratory tests of prospective memory. Psychonomic Bulletin & Review. 2006;13:1028–1032. doi: 10.3758/bf03213920. [DOI] [PubMed] [Google Scholar]
- Kelly AJ, Hertzog C, Hayes MG, Smith AD. The effects of age and focality on delay-execute prospective memory. Aging, Neuropsychology, and Cognition. 2013;20:101–124. doi: 10.1080/13825585.2012.691152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory: Cognitive, neuroscience, developmental, and applied perspectives. New York: Taylor & Francis Group/Lawrence Erlbaum Associates; 2008. [Google Scholar]
- Knight JB, Meeks JT, Marsh RL, Cook GI, Brewer GA, Hicks JL. An observation on the spontaneous noticing of prospective memory event-based cues. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2011;37:298–307. doi: 10.1037/a0021969. [DOI] [PubMed] [Google Scholar]
- Kominsky TK. Unpublished dissertation thesis. Oklahoma State University; 2010. The effects of context expectation on older and younger adults’ prospective memory performance. [DOI] [PubMed] [Google Scholar]
- Kvavilashvili L. Remembering intention as a distinct form of memory. British Journal of Psychology. 1987;78:507–518. [Google Scholar]
- Kvavilashvili L, Fisher L. Is time-based prospective remembering mediated by self- initiated rehearsals? Role of incidental cues, ongoing activity, age, and motivation. Journal of Experimental Psychology: General. 2007;136:112–132. doi: 10.1037/0096-3445.136.1.112. [DOI] [PubMed] [Google Scholar]
- Lampinen JM, Arnal JD, Hicks JL. Prospective person memory. In: Kelley M, editor. Applied memory. New York, NY: Nova Science; 2009. pp. 167–184. [Google Scholar]
- Lee JH, McDaniel MA. Discrepancy-plus-search processes in prospective memory retrieval. Memory & Cognition. 2013;41:443–451. doi: 10.3758/s13421-012-0273-6. [DOI] [PubMed] [Google Scholar]
- Loft S, Finnerty D, Remington RW. Using spatial context to support prospective memory in simulated air traffic control. Human Factors. 2011;53:662–671. doi: 10.1177/0018720811421783. [DOI] [PubMed] [Google Scholar]
- Loft S, Humphreys MS. Enhanced recognition of words previously presented in a task with nonfocal prospective memory requirements. Psychonomic Bulletin & Review. 2012;19:1142–1147. doi: 10.3758/s13423-012-0303-1. [DOI] [PubMed] [Google Scholar]
- Loft S, Kearney R, Remington R. Is task interference in event-based prospective memory dependent on cue presentation? Memory & Cognition. 2008;36:139–148. doi: 10.3758/mc.36.1.139. [DOI] [PubMed] [Google Scholar]
- Loft S, Remington RW. Wait a second: Brief delays in responding reduce focality effects in event-based prospective memory. Quarterly Journal of Experimental Psychology. 2013 doi: 10.1080/17470218.2012.750677. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL, Cook GI. Task interference from prospective memories covaries with contextual associations of fulfilling them. Memory & Cognition. 2006;34:1037–1045. doi: 10.3758/bf03193250. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL, Cook GI. On beginning to understand the role of context in prospective memory. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory: Cognitive, neuroscience, developmental, and applied perspectives. New York: Taylor & Francis Group/Lawrence Erlbaum Associates; 2008. pp. 77–100. [Google Scholar]
- Marsh RL, Hicks JL, Cook GI, Hansen JS, Pallos AL. Interference to ongoing activities covaries with the characteristics of an event-based intention. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:861–870. doi: 10.1037/0278-7393.29.5.861. [DOI] [PubMed] [Google Scholar]
- Martin BA, Brown NL, Hicks JL. Ongoing task delays affect prospective memory more powerfully than filler task delays. Canadian Journal of Experimental Psychology. 2011;65:48–56. doi: 10.1037/a0022872. [DOI] [PubMed] [Google Scholar]
- Maylor EA. Age-related impairment in an event-based prospective memory task. Psychology and Aging. 1996;11:74–78. doi: 10.1037//0882-7974.11.1.74. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Logie RH. A large-scale comparison of prospective and retrospective memory development from childhood to middle age. Quarterly Journal of Experimental Psychology. 2010;63:442–451. doi: 10.1080/17470210903469872. [DOI] [PubMed] [Google Scholar]
- McBride DM, Beckner JK, Abney DH. Effects of delay of prospective memory cues in an ongoing task on prospective memory task performance. Memory & Cognition. 2011;39:1222–1231. doi: 10.3758/s13421-011-0105-0. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology. 2000;14:S127–S144. [Google Scholar]
- McDaniel MA, Einstein GO. Prospective memory: An overview and synthesis of an emerging field. Thousand Oaks, CA: Sage; 2007. [Google Scholar]
- McDaniel MA, Guynn MJ, Einstein GO, Breneiser J. Cue-focused and reflexive-associative processes in prospective memory retrieval. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2004;30:605–614. doi: 10.1037/0278-7393.30.3.605. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, LaMontagne P, Beck SM, Scullin MK, Braver TS. Dissociable neural routes to successful prospective memory. Psychological Science. doi: 10.1177/0956797613481233. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Scullin MK. Implementation intention encoding does not automatize prospective memory responding. Memory & Cognition. 2010;38:221–232. doi: 10.3758/MC.38.2.221. [DOI] [PubMed] [Google Scholar]
- Meier B, Rey-Mermet A. Beyond monitoring: After-effects of responding to prospective memory targets. Consciousness and Cognition. 2012;21:1644–1653. doi: 10.1016/j.concog.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Meier B, von Wartburg P, Matter S, Reber R, Rothen N. Performance predictions improve prospective memory and influence retrieval experience. Canadian Journal of Experimental Psychology. 2011;65:12–18. doi: 10.1037/a0022784. [DOI] [PubMed] [Google Scholar]
- Meier B, Zimmermann TD, Perrig WJ. Retrieval experience in prospective memory: Strategic monitoring and spontaneous retrieval. Memory. 2006;14:872–889. doi: 10.1080/09658210600783774. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Weber E, Rooney AS, Grant I, Woods SP The HIV Neurobehavioral Research Program (HNRP) Group. Longer ongoing task delay intervals exacerbate prospective memory deficits in HIV-associated neurocognitive disorders (HAND) Journal of Clinical and Experimental Neuropsychology. 2012;34:416–427. doi: 10.1080/13803395.2012.654764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T. Reminders supporting spontaneous remembering in prospective memory tasks. Japanese Psychological Research. 2006;48:34–39. [Google Scholar]
- Moscovitch M. Memory and working with memory: Evaluation of a component process model and comparisons with other models. In: Schacter DL, Tulving E, editors. Memory systems. Cambridge: MIT/Bradford Press; 1994. pp. 269–310. [Google Scholar]
- Nowinski JL, Dismukes RK. Effects of ongoing task context and target typicality on prospective memory performance: The importance of associative cueing. Memory. 2005;13:649–657. doi: 10.1080/09658210444000313. [DOI] [PubMed] [Google Scholar]
- Ozgis S, Rendell PG, Henry JD. Spaced retrieval significantly improves prospective memory performance of cognitively impaired older adults. Gerontology. 2009;55:229–232. doi: 10.1159/000163446. [DOI] [PubMed] [Google Scholar]
- Park DC, Hertzog C, Kidder DP, Morrell RW, Mayhorn CB. Effect of age on event-based and time-based prospective memory. Psychology and Aging. 1997;12:314–327. doi: 10.1037//0882-7974.12.2.314. [DOI] [PubMed] [Google Scholar]
- Penningroth SL, Graf P, Gray JM. The effect of a working memory load on the intention-superiority effect: Examining three features of automaticity. Applied Cognitive Psychology. 2012;26:441–450. [Google Scholar]
- Rose NS, Rendell PG, McDaniel MA, Aberle I, Kliegel M. Age and individual differences in prospective memory during a “Virtual Week”: The roles of working memory, vigilance, task regularity, and cue focality. Psychology and Aging. 2010;25:595–605. doi: 10.1037/a0019771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel J, Einstein GO, Rampey H. Implementation-intention encoding in a prospective memory task enhances spontaneous retrieval of intentions. Memory. 2012;20:803–817. doi: 10.1080/09658211.2012.707214. [DOI] [PubMed] [Google Scholar]
- Rummel J, Meiser T. The role of metacognition in prospective memory: Anticipated task demands influence attention allocation strategies. Consciousness and Cognition. doi: 10.1016/j.concog.2013.06.006. (in press) [DOI] [PubMed] [Google Scholar]
- Schult JC, Steffens MC. Tuned for the future: Intentions are only accessible when a retrieval opportunity is near. Memory & Cognition. 2013 doi: 10.3758/s13421-013-0337-2. [DOI] [PubMed] [Google Scholar]
- Scullin MK, Bugg JM. Failing to forget: Prospective memory commission errors can result from spontaneous retrieval and impaired executive control. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2013;39:965–971. doi: 10.1037/a0029198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, Einstein GO, McDaniel MA. Evidence for spontaneous retrieval of suspended but not finished prospective memories. Memory & Cognition. 2009;37:425–433. doi: 10.3758/MC.37.4.425. [DOI] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA. Remembering to execute a goal: Sleep on it! Psychological Science. 2010;21:1028–1035. doi: 10.1177/0956797610373373. [DOI] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA, Einstein GO. Control of cost in prospective memory: Evidence for spontaneous retrieval processes. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:190–203. doi: 10.1037/a0017732. [DOI] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA, Shelton JT, Lee JH. Focal/nonfocal cue effects in prospective memory: Monitoring difficulty or different retrieval processes? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:736–749. doi: 10.1037/a0018971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellen AJ, Louie G, Harris JE, Wilkins AJ. What brings intentions to mind? An in situ study of prospective memory. Memory. 1997;5:483–507. doi: 10.1080/741941433. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess P. The domain of supervisory processes and temporal organization of behaviour. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1996;351:1405–1412. doi: 10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Smith RE. The cost of remembering to remember in event-based prospective memory: Investigating the capacity demands of delayed intention performance. Journal of Experimental Psychology: Learning Memory & Cognition. 2003;29:347–361. doi: 10.1037/0278-7393.29.3.347. [DOI] [PubMed] [Google Scholar]
- Smith RE, Hunt RR, McVay JC, McConnell MD. The cost of event-based prospective memory: Salient target events. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33:734–746. doi: 10.1037/0278-7393.33.4.734. [DOI] [PubMed] [Google Scholar]
- Smith RE. What costs do reveal and moving beyond the cost debate: Reply to Einstein and McDaniel (2010) Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:1089–1095. doi: 10.1037/a0019183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarras K, NiedŸwieńska A. The role of rehearsals in self-generated prospective memory tasks. International Journal of Psychology. 2011;46:346–353. doi: 10.1080/00207594.2011.565342. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Redick TS, Heitz RP, Broadway JM, Engle RW. Complex working memory span tasks and higher order cognition: A latent-variable analysis of the relationship between processing and storage. Memory. 2009;17:635–654. doi: 10.1080/09658210902998047. [DOI] [PubMed] [Google Scholar]
- West R, Craik FIM. Age-related decline in prospective memory: The roles of cue accessibility and cue sensitivity. Psychology and Aging. 1999;14:264–272. doi: 10.1037//0882-7974.14.2.264. [DOI] [PubMed] [Google Scholar]
- West R, Krompinger J, Bowry R. Disruptions of preparatory attention contribute to failures of prospective memory. Psychological Bulletin & Review. 2005;12:502–507. doi: 10.3758/bf03193795. [DOI] [PubMed] [Google Scholar]
- West R, McNerney MW, Travers S. Gone but not forgotten: The effects of cancelled intentions on the neural correlates of prospective memory. International Journal of Psychophysiology. 2007;64:215–225. doi: 10.1016/j.ijpsycho.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ. Remembering to remember. Paper presented at a colloquium of the Department of Experimental Psychology; Cambridge University, Cambridge, UK. 1979. [Google Scholar]