Abstract
OBJECTIVE
White matter hyperintensity (WMH) confers increased mortality risk in patients with cardiovascular diseases. However, little is known about differences in survival times among adults 65 years and older who have WMH and live in the community. To characterize the factors that may reduce mortality risk in the presence of WMH, measures of race, sex, ApoE4, neuroimaging, cardiometabolic, physiological and psychosocial characteristics were examined, with a particular focus on information processing as measured by the Digit Symbol Substitution Test(DSST).
METHODS
Cox-proportional models were used to estimate mortality risks in a cohort of 3513 adults (74.8years, 58%women, 84%white) with WMH (0–9 points), DSST (0–90 points), risk factor assessment in 1992–94 and data on mortality and incident stroke to 2009 (median follow-up [range]:14.2[0.5–18.1]years).
RESULTS
WMH predicted a 48% greater mortality risk (age-adjusted hazard ratio (HR)[95% confidence interval(CI)] for WMH>3 points=1.48[1.35–1.62]). This association was attenuated after adjustment for DSST (HR[CI]: 1.38[1.27–1.51]) or lacunar infarcts (HR[CI]: 1.37[1.25,1.50]) but not after adjustment for other factors. The interaction between DSST and WMH was significant (p=0.011). In fully adjusted models stratified by WMH>3, participants with DSST>median had a 34% lower mortality risk among those with WMH>3 (n=532/1217) and a 28% lower mortality risk among those with WMH<3 (n=1364/2296), compared to participants with DSST<median (HR[95%CI]: 0.66[0.55,0.81] and 0.72[0.62,0.83], respectively).
CONCLUSION
WMH is associated with increased long-term mortality risk in community-dwelling adults aged 65 and older. The increased risk is attenuated for those with higher DSST. Assessment of cognitive function with DSST may improve risk stratification of individuals with WMH.
Keywords: mortality, information processing, white matter hyperintensity
1. Introduction
White matter hyperintensity (WMH) in the brain is associated with greater risk of functional decline and death in patients with cardiovascular diseases (1–4). However, much less is known about the association between WMH and mortality risk in adults 65 and older living in the community. Initial population-based studies of relatively small cohorts of adults indicate that higher WMH is related to higher mortality risk over 5 to 10 years of follow-up (4,5). Yet, for reasons that are still unclear, the presence of WMH does not entirely explain the variance of mortality risk, and some adults with WMH survive much longer than others.
Although several health-related factors influence mortality risk in adults aged 65 and older, the particular characteristics that are potentially associated with reduced mortality risks in community-dwelling adults with WMH are not well understood. For example, prior studies, including ours, have shown that slower information processing as quantified using the Digit symbol Substitution Test (DSST), is associated with higher mortality risk and also with WMH (13–19). However, these prior studies did not have concurrent measures of WMH and DSST and/or have mostly relied on short follow-up time. At present, it is unknown whether higher DSST performance in adults with WMH would attenuate or modify their increased mortality risk over a long period of time.
In this study, characteristics that may attenuate or modify the higher mortality risk in the presence of WMH are investigated in a large cohort of adults aged 65 and older for a long period of time. In addition to well established risk factors for mortality, including biological make-up, neuroimaging, cardiometabolic, physiological and psychosocial characteristics, (2,7–12) the contribution DSST was examined.
Identifying the characteristics that may attenuate or modify the increased mortality risk in adults with WMH may have practical and mechanistic implications. Such characteristics could aid in identifying, among individuals with WMH, those more likely to live longer and who may benefit more from interventions to promote health (6). Identification of such characteristics may also help develop a model and explain the mechanisms underlying the lower mortality risk of some elderly with WMH.
2. Methods
The Cardiovascular Health Study is a population-based study of risk factors for cardiovascular disease in 5,888 adults aged 65 and above (20). In 1992–94, 3,660 participants underwent magnetic resonance imaging (MRI) of the brain(21). Participants received yearly in-person visits from 1992–94 through 1998–1999 and on-going semiannual telephone interviews twice per year for new diagnoses, hospitalizations, procedures, and disability through 2009 at all sites. Health characteristics were identified based on prior knowledge(2, 7–12) and included: demographic and genetic characteristics, cardiometabolic diseases, psychosocial/lifestyle factors; and markers of subclinical risk(2).
2.1 Sample Selection
Among the 3660 participants with brain MRI in 1992–94, risk estimates of mortality through 2009 were computed in 3513 participants who had both DSST and white matter hyperintensity (WMH) data available in 1992–1993. All participants provided written informed consent. The University of Pittsburgh Institutional Review Board approved the protocol.
2.2 Variables of Interest
All measures were obtained by centrally-trained and certified staff. Quality assurance and control protocols were regularly implemented(20).
2.2.1 Digit symbol substitution test (DSST)
DSST was obtained concurrently with the brain MRI in 1992–94 according to a protocol previously described(18); DSST score was our metric for information processing. Briefly, the DSST is a pencil-and-paper test of psychomotor performance(22) in which participants are given a key-grid of numbers and matching symbols and a test section with numbers and empty boxes. The test consists of filling as many empty boxes as possible with a symbol matching each number. The completion time is 90 seconds, and the score is the number of correct number-symbol matches. The strategy to solve the DSST consists of sequential encoding and retrieval of numbers and matching symbols. Short-term memory, perceptual organization, visuomotor coordination, and selective attention are important factors that determine the final score. This test has high test-retest reliability(23). The DSST was recoded into a dichotomous variable using a cut-off of 38 points, which was the median value from the parent cohort of 5,888 participants that also predicted mortality (18).
2.2.2 White Matter Hyperintensity
Standardized sagittal T1-weighted spin-echo images, axial spin density/T2 weighted, and T1-weighted images were acquired in 1992–94 using a 1.5T Signa scanner (GE Medical Systems) with high performance gradients (4 G/cm and 150 T/m-s) (24). A volumetric Spoiled Gradient Recalled Acquisition (SPGR) sequence with parameters optimized for maximal contrast among gray matter, white matter, and cerebrospinal fluid was acquired in the coronal plane (TE/TR = 5/25, flip angle = 40 degrees, NEX = 1, slice thickness = 5 mm/0 mm inter-slice gap). Scanned data were interpreted at a central MRI Reading Center by a neuroradiologist trained in a standardized protocol according to an atlas of predefined visual standards (21, 25) (24)‥ WMH was quantified as presence of signal abnormalities/hyperintensities from the white matter of the periventricular and subcortical regions(21) on standardized sagittal axial spin-density/T2-weighted images. The burden of WHM thus identified was graded from 0 (lowest) to 9 (highest) points and classified as “high” (3 points or higher) or “none/minimal” (0, 1, or 2 points) based on a cut-off between grades 2 and 3 identified in previous studies conducted in the parent CHS cohort of 3660 participants (10, 26).
2.2.3 Other neuroimaging markers
Brain atrophy was quantified as ventricular enlargement, graded on a scale from 0 (lowest) to 9 (highest). (24). Brain infarcts were identified as lesions of 3 mm or larger without vascular distributions which were hyper-intense on both spin-density and T2-weighted sequences (27, 28) (24). Most of the infarcts thus identified are lacunar (single or multiple subcortical brain infarcts of 3–20mm), with only a small proportion being cortical brain infarcts of any size or brain infarcts greater than 20mm located anywhere in the brain. Moreover, those with at least 1 lacunar infarct largely overlap with those with these other types of infarcts.
WMH and brain infarcts were also computed using brain MRIs obtained in 1997–99 in those eligible and interested in a brain MRI (n=2079 of 3513). These measures were considered as covariates in the analyses.
2.2.4 Mortality
Mortality outcomes included all-cause mortality ascertained via adjudicated events occurring through June 30, 2009. To enhance the complete ascertainment of events, data from Health Care Finance Administration records were also obtained for missing hospitalizations(29).
2.2.5 Markers of demographic and genetic characteristics
Age, race and gender were collected at study entry. APOE ε 4 alleles were assessed from blood samples collected in 1997–99 (30).
2.2.6 Markers of cardiometabolic diseases and conditions
Hypertension was defined as self-report of a previous diagnosis confirmed by antihypertensive medication use, or having a current systolic blood pressure of ≥140 mmHg or a diastolic blood pressure ≥ 90 mmHg. Persons were considered diabetic if they had a validated medical diagnosis of diabetes or a fasting glucose level ≥ 126 mg/dl. History of myocardial infarction and congestive heart failure were assessed from self-report and medical records. A composite score of clinical cardiovascular disease presence/absence was also computed based on: baseline history of myocardial infarction, congestive heart failure, atrial fibrillation, presence of a pacemaker, history of coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty, history of angina or use of nitroglycerin, history of claudication or peripheral vascular surgery or history of stroke, transient ischemic attack, or carotid artery surgery(29). A composite measure of subclinical cardiovascular conditions was defined as having any one of the following: major electrocardiogram abnormalities based on the Minnesota Code, a ratio of ankle-arm/systolic blood pressure ≤0.9, percent stenosis of internal carotid artery based on ultrasound >25% or intimal-medial thickness of the internal or common carotid artery >80th percentile of the CHS distribution, positive Rose angina, or claudication without clinical history of angina.
2.2.7 Subclinical/Physiological markers
Pulse pressure was computed as the difference between systolic and diastolic blood pressure. Glucose and blood lipids were measured from fasting blood samples. Cystatin C was quantified from frozen samples collected at the baseline visit using a BNII nephelometer (Siemens, Deerfield, IL), and values were used as measures of kidney integrity (31). Left ventricular mass was quantified using 2-dimensionally directed M-mode images from parasternal short-axis recordings and used as a measure of cardiac integrity (32). Interleukin-6 and C-reactive protein were measured from blood samples collected in 1997–99, using previously published protocols(33). Medication use to control levels of lipids, blood pressure, and glucose were also obtained from medical records.
2.2.8 Psychosocial/lifestyle markers
Years of education, income, alcohol (number of drink/day) and smoking history (ever/never) were obtained by self-report in 1992–94. Physical activity was obtained from self-reported regular leisure-time physical activity level (kcal) from the last 12 months. Global cognitive function was assessed using the 100-points modified Mini-Mental State Exam (3MSE) score. Mood was assessed using the Center for Epidemiologic Studies Depression Scale (CES-D).
2.3 Statistical Analysis
Differences in population characteristics between those excluded and included and between the groups of interest (e.g. based on WMH and DSST cut-offs) were tested using analysis of covariance, partial correlation, and chi-square tests when appropriate. Due to skewness of the distributions, physical activity, glucose, left ventricular mass, Cystatin C, IL-6, and CRP were log-transformed. Between-group comparisons of HDL, pulse pressure, and glucose were adjusted for lipid medication use, hypertensive medications, and insulin use, respectively. Because the total number of comparisons was relatively high (25 comparisons for each between-group comparison), statistical significance was examined after applying a Sidak correction factor (34) of P=0.002. Participants who died or were lost to follow-up were censored at the time of death or date of last information in 2009. Kaplan-Meier analysis was used to examine survival (i.e., time till mortality). Log-rank Mantel-Cox tests were used to examine group differences. Crude incidence rates of events were computed per 1,000 person-years. Cox proportional hazard models were constructed to investigate the multivariate relationship of covariates and outcomes, provided that the assumption of proportional hazards was met. (35).
Age-adjusted models predicting risk of mortality were generated in the cohort of 3513 participants with WMH (as dichotomous and also as ordinal 0–9) as the main independent variable and adjusted for the population characteristics’ measures and also for incident stroke, incident brain infarcts and incident WMH at the second MRI. Although the characteristics examined for each domain (Section 2.2) were selected a priori for their known association with mortality, we noted that the variables in the domains “subclinical/physiological” and “lifestyle/psychosocial” were highly correlated with each other (p<0.001). To address colinearity and over-adjustment, each of these variables entered the model one at a time within that block and changes in the coefficient of the main independent variable were examined for each model. For simplicity, we report models adjusted for demographics and for the following measures: Model 1: ventricular grade and brain infarcts for the domain “neuroimaging markers”; Model 2: prevalence of cardiovascular diseases for the domain “cardiometabolic diseases” (all other measures in this domain are included in this summary measure); Model 3: prevalence of subclinical vascular conditions for the domain “subclinical/physiological markers” (the other measures in this domain were not included because they were associated with subclinical vascular conditions at p<0.0001); Model 4: education for the domain “lifestyle/psychosocial factors” (the other measures in this domain were associated with education at p<0.0001). Model 5: a final model adjusted for all measures.
To identify the characteristics that would attenuate or modify the mortality risk related to WMH, each measure was added to age-adjusted models one at a time to evaluate the change in the coefficient of WMH predicting mortality after adding that measure to the model. In a separate model with WMH and DSST predicting mortality, the interaction term of WMH by DSST was tested to estimate whether the mortality risk related to high WMH varies for different levels of DSST.
All models were also repeated for those with and without high WMH (WMH>3). Sensitivity analyses in which participants with brain infarcts, history of stroke, and those with incident stroke during follow-up are excluded were also conducted.
All analyses were performed using SAS version 9.1.3 (SAS Institute, Inc. Cary, NC).
3. Results
Comparisons between the 3513 participants with WMH and DSST and the parent cohort of 3660 participants with brain MRI from the four sites were not significant and have been previously published (36). By the end of 2009 (median follow-up 14.23 years, range: 0.5- 18.1 years), 2110 (60%) of the 3513 participants had died, consistent with event rates obtained for shorter follow-up in prior studies of the parent cohort (18, 37, 38).
In this cohort of 3513 participants, the group with high WMH (WMH>3) had a less favorable risk factor profile at study entry (Table 1), as compared to the group with none/minimal WMH (WMH<3). Differences were statistically significant at p <0.05 for all measures, with the exception of congestive heart failure, diabetes and smoking (Table 1). The correlation between WMH and DSST was statistically significant (rho=−0. 23, p<0.0001).
Table 1.
Characteristics of in 3513 participants stratified by WMH > 3 in 1992–1994
| Number |
Total |
Group WMH≥3 |
Group 2 WMH<3 |
Age-adjusted Comparisons * |
|
|---|---|---|---|---|---|
| 3513 | 1217 | 2296 | |||
| Biological make-up | Age, years , Mean (SD) | 74.76 (5.10) | 76.37(5.38) | 73.90 (4.72) | <.0001 |
| Race, white, N (%) | 2962 (84.70) | 1941 (84.83) |
1021 (84.45) | 0.062 | |
| Gender , female, N (%) | 2046 (58.24) | 731 (60.07) | 1315 (57.27) |
0.009 | |
| APOe4 allele, N (%) | 792 (24.80) | 292 (25.96) | 500 (24.18) | 0.028 | |
| Other MRI markers | Ventricular grade≥4, N (%) |
681 (19.41) | 356 (29.28) | 325 (14.17) | <.0001 |
| Brain infarcts, N (%) | 1072 (30.52) | 567 (46.59) | 505 (21.99) | <.0001 | |
| Presence of cardio-metabolic diseases | Cardiovascular diseases, N (%) |
292 (26.44) | 543 (23.65) | 386 (31.72) | 0.001 |
| Stroke, N (%) | 151 (4.56) | 76 (6.90) | 75 (3.40) | <.0001 | |
| Myocardial infarction, N (%) |
301 (9.10) | 118 (10.72) | 183 (8.29) | 0.066 | |
| Diabetes , N (%) | 493 (14.13) | 180 (14.88) | 313 (13.74) | 0.182 | |
| Congestive heart failure, N (%) |
149 (4.50) | 87 (3.94) | 62 (5.63) | 0.106 | |
| Hypertension, N (%) | 1389 (40.02) | 565 (47.20) | 824 (36.24) | <.0001 | |
| Sub-clinical/ physiological markers | Subclinical vascular conditions, N (%) |
2362 (69.65) | 882 (75.51) | 1480 (66.58) |
0.002 |
| HDL2, dl/L, Mean (SD) | 53.42 (14.43) | 53.48 (14.19) |
53.39 (14.55) |
0.004 | |
| Glucose1,4, dl/l, Mean (SD) |
107.53 (34.97) |
107.53 (31.73) |
107.52 (36.55) |
<.0001 | |
| Pulse pressure3, mmHg, Mean (SD) |
63.95 (17.60) | 67.18 (18.36) |
62.25 (16.95) |
<.0001 | |
| LV heart mass1, cm3 Mean (SD) |
152.26 (31.31) |
152.17 (31.57) |
152.31 (31.17) |
<.0001 | |
| Cystatin C1, mg/dl, Mean (SD) |
1.10 (0.29) | 1.14 (0.29) | 1.09 (0.29) | 0.006 | |
| IL-61, mg/dl, Mean (SD) | 2.04 (1.80) | 2.23 (2.11) | 1.95 (1.62) | <.0001 | |
| C-Reactive protein 1 mg/dl, Mean (SD) |
5.04 (8.67) | 5.22 (8.66) | 4.95 (8.68) | <.0001 | |
| Psycho-social/ Lifestyle | Physical activity, Kcal1 Mean (SD) |
1467.22 (1739.11) |
1312.37 (1697.53) |
1549.23 (1755.58) |
<.0001 |
| Income < $25,000, N (%) |
1929 (58.38) | 721 (63.64) | 1208 (55.64) |
<.0001 | |
| Education>HS, N (%) | 1657 (47.25) | 551 (45.39) | 1106 (48.23) |
0.070 | |
| Smoking , current, N (%) | 323 (9.45) | 100 (8.44) | 223 (9.99) | 0.094 | |
| Alcohol consumption, N (%) |
2.14 (5.33) | 2.13 (5.64) | 2.15 (5.16) | <.0001 | |
| 3MS, points , Mean (SD) | 91.17 (8.20) | 89.44 (9.79) | 92.08 (7.06) |
<.0001 | |
| CES-D, points, Mean (SD) |
5.15 (4.75) | 5.54 (5.04) | 4.94 (4.58) | <.0001 |
ANOVA for continuous variables, chi-square for ordinal variables; age comparisons were not age-adjusted.
Comparisons based on log-transformed values
comparisons adjusted for lipid medication use
comparisons for glucose adjusted for insulin use
comparisons adjusted for antihypertensive medications.
Apoe4 = Apolipoprotein E4; CRP = C-reactive protein; DSST = Digit Symbol Substitution Test; HDL = high-density lipoprotein cholesterol; HS = high school; IL-6 = interleukin-6; LV = left ventricular; 3MS = Mini-Mental Status Examination; MRI = magnetic resonance imaging; SD = standard deviation; WMH = white matter hyperintensities.
In this cohort of 3513 participants, high WMH was associated with greater risk of mortality from 1992 to 2009 (unadjusted HR [95%CI]: 1.818 [1.667, 1.983]) independent of demographics, ventricular grade, brain infarcts, prevalence of cardiovascular, prevalence of subclinical conditions, and education: 1.324 [1.204, 1.457]). This is consistent with previous reports of the Cardiovascular Health Study based on shorter follow-up times (14). WMH coded as 0–9 was also associated with mortality in the full cohort and in the group with WMH>3 (HR [95% CI]: 1.118 [1.084, 1.153], p<0.0001 and 1.067 [1.009, 1.129], p = 0.020, respectively, for each point of WMH, adjusted for demographics, ventricular grade, brain infarcts, prevalence of cardiovascular, prevalence of subclinical conditions, and education). Of note, higher DSST was associated with lower mortality risk (age-adjusted HR: 0.975 [0.972, 0.979], p<0.0001 for each point of DSST and 0.554[0.507,0.606], p<0.0001 for DSST>38).
In age-adjusted models with WMH and each participants’ characteristic added one by one (Table 2), the association between high WMH and greater mortality risk was attenuated by more than 10% after adjustment for DSST or for brain infarcts but not after adjustment for the other risk factors (Table 2).
Table 2.
Age-adjusted Hazard Ratio and 95% confidence intervals (CI) of mortality from study entry in 1992–94 to 2009 for participants with WMH> 3 as compared to WMH < 3 (referent) in 1992–1994. N=3513
| Hazard Ratio and 95% CI of WMH>3 as compared to WMH<3 (referent) |
|||
|---|---|---|---|
| Age-adjusted | 1.481 | (1.354, 1.618) | |
| Measures entering the above age-adjusted model one at a time: | |||
| Digit Symbol Substitution Test, points | 1.384 | (1.265, 1.514) | |
| Biological make-up |
Race, white | 1.462 | (1.337, 1.599) |
| Gender , female | 1.526 | (1.395, 1.668) | |
| APOe4 allele | 1.497 | (1.363, 1.643) | |
| Other MRI markers |
Ventricular grade≥4 | 1.425 | (1.303, 1.559) |
| Brain infarcts, presence | 1.373 | (1.252, 1.504) | |
| Presence of cardio-metabolic diseases |
Cardiovascular diseases | 1.461 | (1.336, 1.596) |
| Stroke | 1.457 | (1.327, 1.600) | |
| Myocardial infarction | 1.479 | (1.348, 1.624) | |
| Diabetes | 1.488 | (1.361, 1.627) | |
| Congestive heart failure | 1.478 | (1.347, 1.623) | |
| Hypertension | 1.454 | (1.328, 1.591) | |
| Sub-clinical/ physiological markers | Subclinical vascular conditions | 1.461 | (1.334, 1.600) |
| HDL2, dl/L | 1.491 | (1.361, 1.634) | |
| Glucose1,4, dl/l | 1.490 | (1.359, 1.633) | |
| Pulse pressure3, mmHg | 1.468 | (1.341, 1.605) | |
| LV heart mass1, cm3 | 1.481 | (1.352, 1.622) | |
| Cystatin C1, mg/dl | 1.511 | (1.380, 1.655) | |
| IL-61, mg/dl | 1.467 | (1.335, 1.611) | |
| C-Reactive protein 1, mg/dl | 1.455 | (1.330, 1.592) | |
| Psycho-social/ Lifestyle | Physical activity, Kcal1 | 1.462 | (1.328, 1.610) |
| Income < $25,000 | 1.445 | (1.318, 1.584) | |
| Education>HS | 1.475 | (1.350, 1.613) | |
| Smoking , current | 1.468 | (1.341, 1.606) | |
| 3MS, points | 1.440 | (1.317,1.575) | |
| CES-D, points | 1.476 | (1.351, 1.614) | |
log-transformed values
adjusted for lipid medication use
adjusted for insulin use
adjusted for antihypertensive medications
Apoe4 = Apolipoprotein E4; CRP = C-reactive protein; HDL = high-density lipoprotein cholesterol; HS = high school; IL-6 = interleukin-6; LV = left ventricular; 3MS = Mini-Mental Status Examination; MRI = magnetic resonance imaging; SD = standard deviation; WMH = white matter hyperintensities.
DSST also modified the association of WMH>3 with mortality in multivariable models adjusted for demographics, ventricular grade, brain infarcts, prevalence of cardiovascular and of subclinical conditions and education (Table 3). In these multivariable models, both WMH and DSST were associated with mortality independent of each other and of other factors (Table 3). The point estimates of the hazard ratios were similar and the confidence intervals were overlapping when WMH and DSST entered the model using other coding (e.g. WMH as a 0–9 score, and DSST as >38), after adjustment for incident stroke, incident infarcts and incident WMH, and also after exclusion of participants with brain infarcts, history of stroke, and those with incident stroke during follow-up (not shown).
Table 3.
Hazard Ratios and 95% Confidence Intervals for WMH>3 points predicting Total Mortality From study entry in 1992–94 to 2009 in 3513 participants, before and after adjustment for DSST
| Hazard Ratios (95% confidence interval) | |||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| WMH> 3 points |
1.497* 1.366,1.641) |
1.339* (1.217, 1.473) |
1.326* (1.205, 1.459) |
1.324* (1.204, 1.457) |
1.346* (1.201, 1.508) |
| WMH< 3 points |
1.410* 1.286,1.547) |
1.277* (1.160, 1.405) |
1.268* (1.152, 1.395) |
1.266* (1.151, 1.394) |
1.312* (1.171, 1.471) |
| DSST, points | 0.978* (0.974,0.981) |
0.978* (0.975,0.982) |
0.980* (0.976,0.983) |
0.979* (0 .975,0.983) |
0.988* (0 .981,0.990) |
WMH= White Matter Hyperintensities; DSST = Digit Symbol Substitution Test; Model 1: adjusted for age, race, gender; Model 2: further adjusted for ventricular grade, infarcts; Model 3: further adjusted for cardiovascular diseases, subclinical conditions. Model 4: further adjusted for education. Model 5: further adjusted for diabetes, congestive heart failure, stroke, Cystatin-C, IL-6, left ventricular mass, pulse pressure, income.
significant at p<0.0001
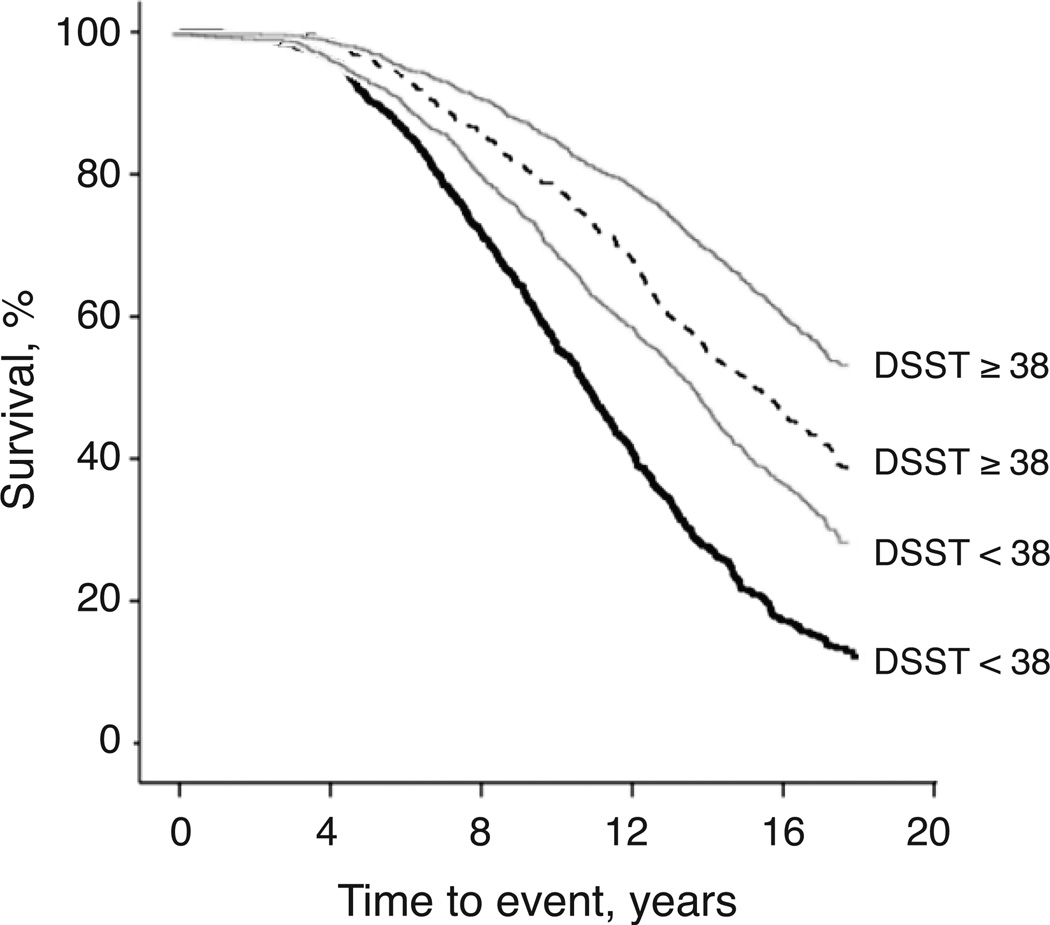
In a separate model of mortality risk with WMH and DSST, the interaction of WMH by DSST was statistically significant (Odds Ratio, [95% CI]: 1.002 [1.001, 1.004], p= 0.011). In subsequent analyses of mortality stratified by WMH>3 (Table 4), those with DSST>38 had lower mortality rates as compared to those with DSST<38 in both strata (log-rank tests statistically significant at P<0.001 for all); of note, the mortality rate of participants with high WMH and DSST>38 was similar to the mortality rate of those with WMH<3. The association of DSST>38 with reduced mortality risk among those with WMH>3 was independent of f demographics, ventricular grade, brain infarcts, cardiovascular disease, subclinical conditions and education (Table 5). These associations were similar to, albeit less strong than, those observed for participants with WMH<3 (Table 5). Kaplan-Meier estimates (Figure 1) illustrated that trends of death rates were significantly slower for those with DSST>38 as compared to those with DSST<38. This trend was similar for both groups of WMH severity, although mortality rates remained higher over time for those with WMH>3 as compared to those with WMH<3.
Table 4.
Event Rates for Total Mortality From 1992 to 2009 for 3513 participants grouped by severity of white matter hyperintensities >3 and stratified by presence/absence of DSST > 38
| Included (N) | No. of Events (%) | Events per 1,000 Person-Years (95% Confidence intervals) |
|
|---|---|---|---|
| WMH> 3 | |||
| Total | 1217 | 896 (74) | 62.67 (56.29, 69.81) |
| DSST>38 | 532 | 320 (60) | 48.92 ( 40.78 , 58.72) |
| DSST<38 | 685 | 576 (84) | 75.63 ( 65.80 , 87.10) |
| WMH< 3 | |||
| Total | 2296 | 1214 (53) | 45.88 (41.75,50.42) |
| DSST>38 | 1364 | 602 (44) | 36.96 (32.06, 42.61) |
| DSST<38 | 932 | 612 (66) | 57.15 (50.22,65.07) |
WMH= White Matter Hyperintensities; DSST = Digit Symbol Substitution Test
Table 5.
Hazard Ratios of presence/absence of DSST > 38 predicting Total Mortality From 1992 to 2009 for a cohort of 3513 adults aged 65 and above, stratified by presence of white matter hyperintensities >3. All are statistically significant at p<0.0001
| Hazard Ratios (95% confidence interval) | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| WMH> 3 | ||||
|
DSST> 38 (yes/no) |
0.53 (0.46, 0.62) | 0.54 (0.46, 0.63) | 0.55 (0.47, 0.64) | 0.57 (0.49, 0.67) |
| WMH< 3 | ||||
|
DSST> 38 (yes/no) |
0.59 (0.52, 0.66) | 0.63(0.56, 0.72) |
0.64 (0.56, 0.73) |
0.67 (0.59, 0.76) |
WMH= White Matter Hyperintensities; DSST = Digit Symbol Substitution Test; Model 1: age-adjusted; Model 2: adjusted for age, race, gender; Model 3: further adjusted for ventricular grade, infarcts. Model 4: further adjusted for cardiovascular diseases, subclinical conditions and education.
Figure 1.
Kaplan-Meier plots of survival event rates for participants with (black lines) and without WMH>3 (gray lines), stratified by presence (solid lines) or absence (dashed lines) of DSST > 38. Follow-up period was from 1992–2009.
Hazard ratios of DSST predicting mortality among those with WMH>3 also remained substantially unchanged after exclusion of participants with infarcts, history of stroke or incident stroke during follow-up (HR [95%CI] adjusted for age, race, gender, ventricular grade prevalence of cardiovascular diseases and conditions, IL-6, and Cystatin-C= 0.55 [0.42, 0.73], p<0.0001 for DSST>38 and 0.98 [0.97, 0.99], p<0.0001 for each point of DSST). The association between DSST and mortality among those with WMH>3 also remained independent of other variables related to DSST (list in Supplementary Table 1): age, race, income, education, 3MSE scores, CRP levels, WMH burden, hypertension, subclinical vascular conditions, pulse pressures, IL-6 levels.
4. Discussion
In this large cohort of well-characterized adults aged 65 and above, WMH predicts long-term mortality risk and this increased risk is attenuated for adults with higher DSST. This attenuation was not explained by any of the factors associated with DSST, or by the burden of overt clinical vascular diseases, genetic or subclinical vascular factors. Among adults with WMH, those who also maintain higher DSST performance had half the risk of dying as compared to those with lower DSST performance.
These findings extend prior work and prepare new lines of inquiry in several ways.
First, our study provides long-term mortality risk estimates associated to WMH in adults living in the community. Prior studies estimating the association of WMH with mortality have mostly relied on follow-up times of 5 years and focused on patient populations. Among the few pioneer works examining community dwelling adults (4), we are aware of only two examining a follow-up time longer than 5 years (39, 40). However, neither of these quantified the characteristics that could attenuate the increased mortality risk related to high WMH and one examined a very selected and small sample size free from stroke and vascular events (39).
Second, the finding that DSST attenuates the association of WMH with mortality highlights the importance of studying the association between neural systems and mortality. DSST performance is thought to reflect information processing, as mediated by neural networks in frontal systems. (41–44) A prior functional neuroimaging study (45) indicates that maintenance of higher DSST performance in the presence of WMH is related to additional activation of parietal regions and this pattern may reflect neural plasticity processes. Specifically, individuals with overall WMH, who nonetheless have higher DSST, might have a more favorable WMH spatial distribution and/or a lower burden of micro-structural abnormalities in normal appearing parenchyma, which may spare tracts critical for DSST performance and thus permit neural activation supporting DSST performance. In light of the findings hereby presented, maintaining DSST, or other neurocognitive functions, in the presence of WMH (possibly through compensatory neural activation patterns in the face of age-related changes in morphology) may be a marker of long-term survival through mechanisms that need to be identified. For example, such compensatory neural activation patterns may reflect greater brain health at the micro-structural and/or neurovascular level and/or greater vascular health in other systems and organs. The present study could not answer these questions because it relied on visual estimation of overall burden of WMH and it did not have longitudinal measures of micro-structure or functional MRI. To investigate the mechanisms linking this apparent ‘brain plasticity’ and longevity, neuroimaging studies of adults with WMH would need to first quantify and compare the spatial distribution and temporal evolution of WMH and of micro-structural tissue changes in those who maintain cognitive performance (in DSST or other neurocognitive tests) and in those who do not, and then quantify the association of these patterns with future mortality risks and with prior long term exposure to risk factors and with presence of health-related conditions. The identification of prior exposure to risk factors presence of specific health-related conditions can then help design tailored interventions to promote health.
Third, our work also tested alternative explanations of these main findings in that analyses controlled for factors that differed between those with and without high DSST. Of note, among the multiple health-related factors known to influence mortality risk, only DSST and infarcts appeared to attenuate the higher mortality risk in the presence of WMH. Other participants’ characteristics and conditions, including hypertension, stroke or blood pressure, did not substantially attenuate the risk of mortality associated with high WMH. It is possible that longitudinal trajectories of risk factors, rather than one measurement at one point in time, could have explained these associations. A more gradual worsening of the risk factors of pulse pressure, IL-6, and CRP, as opposed to a rapid abrupt worsening, may slow down the accrual of brain abnormalities and thus maintain function. For example, a more favorable inflammatory state might facilitate the ability of the brain to compensate for overall burden of WMH (46). This study did not have retrospective measures of these risk factors, and therefore could not address this question.
Strengths of this study include the comprehensive assessment of health-related factors, including behavioral, imaging, and other physiologic data and functional measures over a long period of time in a large and diverse cohort. One possible limitation of this study is the use of population-specific cut-offs for DSST and WMH. Because of these cut-offs, results may not be generalizable. However, the associations remained significant when using continuous measures of DSST. Another factor that limits generalizability of these results is that this cohort was well functioning, with 65% of participants having little or no WMH by the beginning of the observation time in 1992–94. Furthermore, our statistical adjustments for single measures of confounders may have left residual confounding. In addition, a one-time score of DSST at baseline was applied for the group classifications, and DSST was the only measure of information processing available. Future studies to characterize other aspects of neurocognitive function in adults aged 65 and above with higher WMH who seem to be resilient to mortality are needed.
Supplementary Material
Acknowledgments
A full list of CHS principal investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
This research was supported by NHLBI contracts HHSN268201200036C, N01-HC-85239, N01-HC-85079 through N01-HC-85086 (N01-HC-85082 Kuller); N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133 and NHLBI grant HL080295, with additional contribution from NINDS. Additional support was provided through AG-023629 (Newman), AG-15928 (Kuller), AG-20098 (Lopez), AG-027058, and K23 AG028966 (Rosano) from the NIA.
Acronyms used
- WMH
white matter hyperintensity
- DSST
digit symbol substitution test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No author reports a conflict of interest.
References
- 1.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr., Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- 2.Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci Med Sci. 2004;59:818–826. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- 3.Conijn MM, Kloppenborg RP, Algra A, Mali WP, Kappelle LJ, Vincken KL, van der Graaf Y, Geerlings MI. Cerebral small vessel disease and risk of death, ischemic stroke, and cardiac complications in patients with atherosclerotic disease: the Second Manifestations of ARTerial disease-Magnetic Resonance (SMART-MR) study. Stroke. 2011;42:3105–3109. doi: 10.1161/STROKEAHA.110.594853. [DOI] [PubMed] [Google Scholar]
- 4.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longstreth WT, Jr., Arnold AM, Kuller LH, Bernick C, Lefkowitz DS, Beauchamp NJ, Jr., Manolio TA. Progression of magnetic resonance imaging-defined brain vascular disease predicts vascular events in elderly: the Cardiovascular Health Study. Stroke. 2011;42:2970–2. doi: 10.1161/STROKEAHA.111.622977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manton KG, Corder L, Stallard E. Chronic disability trends in elderly United States populations: 1982–1994. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2593–2598. doi: 10.1073/pnas.94.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soreca I, Rosano C, Jennings JR, Sheu LK, Kuller LH, Matthews KA, Aizenstein HJ, Gianaros PJ. Gain in adiposity across 15 years is associated with reduced gray matter volume in healthy women. Psychosom Med. 2009;71:485–490. doi: 10.1097/PSY.0b013e3181a5429d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiseman RM, Saxby BK, Burton EJ, Barber R, Ford GA, O'Brien JT. Hippocampal atrophy, whole brain volume, and white matter lesions in older hypertensive subjects. Neurology. 2004;63:1892–1897. doi: 10.1212/01.wnl.0000144280.59178.78. [DOI] [PubMed] [Google Scholar]
- 9.den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, Koudstaal PJ, Breteler MM. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology. 2005;64:263–267. doi: 10.1212/01.WNL.0000149641.55751.2E. [DOI] [PubMed] [Google Scholar]
- 10.Dufouil C, de Kersaint-Gilly A, Besancon V, Levy C, Auffray E, Brunnereau L, Alperovitch A, Tzourio C. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology. 2001;56:921–926. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- 11.Jongen C, Biessels GJ. Structural brain imaging in diabetes: a methodological perspective. Eur J Pharmacol. 2008;585:208–218. doi: 10.1016/j.ejphar.2007.11.085. [DOI] [PubMed] [Google Scholar]
- 12.Brands AM, Biessels GJ, Kappelle LJ, de Haan EH, de Valk HW, Algra A, Kessels RP. Cognitive functioning and brain MRI in patients with type 1 and type 2 diabetes mellitus: a comparative study. Dement Geriatr Cogn Disord. 2007;23:343–350. doi: 10.1159/000100980. [DOI] [PubMed] [Google Scholar]
- 13.Anstey KJ, Luszcz MA, Giles LC, Andrews GR. Demographic, health, cognitive, and sensory variables as predictors of mortality in very old adults. Psychol Aging. 2001;16:3–11. doi: 10.1037/0882-7974.16.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Grigsby J, Kaye K, Baxter J, Shetterly SM, Hamman RF. Executive cognitive abilities and functional status among community-dwelling older persons in the San Luis Valley Health and Aging Study. J Am Geriatr Soc. 1998;46:590–596. doi: 10.1111/j.1532-5415.1998.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 15.Pavlik VN, de Moraes SA, Szklo M, Knopman DS, Mosley TH, Jr., Hyman DJ. Relation between cognitive function and mortality in middle-aged adults: the atherosclerosis risk in communities study. Am J Epidemiol. 2003;157:327–334. doi: 10.1093/aje/kwf209. [DOI] [PubMed] [Google Scholar]
- 16.Schupf N, Tang MX, Albert SM, Costa R, Andrews H, Lee JH, Mayeux R. Decline in cognitive and functional skills increases mortality risk in nondemented elderly. Neurology. 2005;65:1218–1226. doi: 10.1212/01.wnl.0000180970.07386.cb. [DOI] [PubMed] [Google Scholar]
- 17.Swan GE, Carmelli D, LaRue A. Performance on the digit symbol substitution test and 5-year mortality in the Western Collaborative Group Study. Am J Epidemiol. 1995;141:32–40. doi: 10.1093/oxfordjournals.aje.a117342. [DOI] [PubMed] [Google Scholar]
- 18.Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56:1618–1625. doi: 10.1111/j.1532-5415.2008.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swindell WR, Cummings SR, Sanders JL, Caserotti P, Rosano C, Satterfield S, Strotmeyer ES, Harris TB, Simonsick EM, Cawthon PM. Data mining identifies digit symbol substitution test score and serum cystatin C as dominant predictors of mortality in older men and women. Rejuvenation Res. 2012;15:405–413. doi: 10.1089/rej.2011.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 21.Yue NC, Arnold AM, Longstreth WT, Jr., Elster AD, Jungreis CA, O'Leary DH, Poirier VC, Bryan RN. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the cardiovascular health study. Radiology. 1997;202:33–39. doi: 10.1148/radiology.202.1.8988189. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler Adult Intelligence Scale- revised San Antonio Psychological Corporation. 1981 [Google Scholar]
- 23.Matarazzo JD, Herman DO. Base rate data for the WAIS-R: test-retest stability and VIQ-PIQ differences. J Clin Neuropsychol. 1984;6:351–366. doi: 10.1080/01688638408401227. [DOI] [PubMed] [Google Scholar]
- 24.Bryan RN, Manolio TA, Schertz LD, Jungreis C, Poirier VC, Elster AD, Kronmal RA. A method for using MR to evaluate the effects of cardiovascular disease on the brain: the cardiovascular health study. AJNR Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 25.Longstreth WT, Jr., Arnold AM, Beauchamp NJ, Jr., Manolio TA, Lefkowitz D, Jungreis C, Hirsch CH, O'Leary DH, Furberg CD. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 26.Kuller LH, Shemanski L, Manolio T, Haan M, Fried L, Bryan N, Burke GL, Tracy R, Bhadelia R. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke. 1998;29:388–398. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- 27.Longstreth WT, Jr., Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, Jr., O'Leary D, Carr J, Furberg CD. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 28.Bryan RN, Wells SW, Miller TJ, Elster AD, Jungreis CA, Poirier VC, Lind BK, Manolio TA. Infarctlike lesions in the brain: prevalence and anatomic characteristics at MR imaging of the elderly--data from the Cardiovascular Health Study. Radiology. 1997;202:47–54. doi: 10.1148/radiology.202.1.8988191. [DOI] [PubMed] [Google Scholar]
- 29.Ives DG, Fitzpatrick AL, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Annals of Epidemiolology. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 30.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 31.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 32.Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith VE, Tabatznik B, Siscovick D, Lobodzinski S, Anton-Culver H, Manolio TA. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 33.Keller C, Katz R, Sarnak MJ, Fried LF, Kestenbaum B, Cushman M, Shlipak MG CHS study. Inflammatory biomarkers and decline in kidney function in the elderly: the Cardiovascular Health Study. Nephrol Dial Transplant. 2010;25:119–124. doi: 10.1093/ndt/gfp429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ludbrook I. Multiple comparison procedures updated. Clin Exp Pharmacol PhysioI. 1998;25 doi: 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 35.Wei LJ. The accelerated failure time model: a useful alternative to the Cox regression model in survival analysis. Stat Med. 1992;11:1871–1879. doi: 10.1002/sim.4780111409. [DOI] [PubMed] [Google Scholar]
- 36.Rosano C, Longstreth WT, Jr., Boudreau R, Taylor CA, Du Y, Kuller LH, Newman AB. High blood pressure accelerates gait slowing in well-functioning older adults over 18-years of follow-up. J Am Geriatr Soc. 2011;59:390–397. doi: 10.1111/j.1532-5415.2010.03282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosano C, Brach J, Longstreth WT, Jr., Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26:52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 38.Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 39.Kerber KA, Whitman GT, Brown DL, Baloh RW. Increased risk of death in community-dwelling older people with white matter hyperintensities on MRI. J Neurol Sci. 2006;250:33–38. doi: 10.1016/j.jns.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 40.Kuller LH, Arnold AM, Longstreth WT, Jr., Manolio TA, O'Leary DH, Burke GL, Fried LP, Newman AB. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28:1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Venkatraman VK, Aizenstein H, Guralnik J, Newman AB, Glynn NW, Taylor C, Studenski S, Launer L, Pahor M, Williamson J, Rosano C. Executive control function, brain activation and white matter hyperintensities in older adults. NeuroImage. 2010;49:3436–3442. doi: 10.1016/j.neuroimage.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosano C, Venkatraman VK, Guralnik J, Newman AB, Glynn NW, Launer L, Taylor CA, Williamson J, Studenski S, Pahor M, Aizenstein H. Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. J Gerontol A Biol Sci Med Sci. 2009;65:639–647. doi: 10.1093/gerona/glq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosano C, Aizenstein H, Cochran J, Saxton J, De Kosky S, Newman AB, Kuller LH, Lopez OL, Carter CS. Functional neuroimaging indicators of successful executive control in the oldest old.Epub 2005 Oct 12. Neuroimage. 2005;28:881–889. doi: 10.1016/j.neuroimage.2005.05.059. [DOI] [PubMed] [Google Scholar]
- 44.Rosano C, Aizenstein HJ, Cochran JL, Saxton JA, De Kosky ST, Newman AB, Kuller LH, Lopez OL, Carter CS. Event-related functional magnetic resonance imaging investigation of executive control in very old individuals with mild cognitive impairment. Biol Psychiatry. 2005;57:761–767. doi: 10.1016/j.biopsych.2004.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkatraman V, Aizenstein H, Guralnik J, Newman AB, Glynn NW, Taylor C, Studenski S, Launer L, Pahor M, Williamson J, Rosano C. Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage. 2010;49:3436–3442. doi: 10.1016/j.neuroimage.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.