Summary
Background
Von Willebrand factor (VWF) plays a key role in coagulation by tethering platelets to injured subendothelium through binding sites for collagen and platelet GPIb. Collagen binding assays (VWF:CB), however, are not part of the routine workup for von Willebrand disease (VWD).
Objectives
This study presents data on collagen binding for healthy controls and VWD subjects to compare three different collagens.
Patients/Methods
VWF antigen (VWF:Ag), VWF ristocetin cofactor activity, and VWF:CB with types I, III, and VI collagen were examined for samples obtained from the Zimmerman Program.
Results
Mean VWF:CB in healthy controls was similar and highly correlated for types I, III, and VI collagen. The mean VWF:CB/VWF:Ag ratios for types I, III, and VI collagen were 1.31, 1.19, and 1.21 respectively. In type 1 VWD subjects, VWF:CB was similar to VWF:Ag with mean VWF:CB/VWF:Ag ratios for types I, III, and VI collagen of 1.32, 1.08, and 1.1 respectively. For type 2A and 2B subjects, VWF:CB was uniformly low, with mean ratios of 0.62 and 0.7 for type I collagen, 0.38 and 0.4 for type III collagen, and 0.5 and 0.47 for type VI collagen.
Conclusions
Normal ranges for type I, III, and VI collagen are correlated, but higher values were obtained with type I collagen as compared to types III and VI. The low VWF:CB in type 2A and 2B subjects suggests that VWF:CB may also supplement analysis of multimer distribution. However, these results reflect only one set of assay conditions per collagen type and therefore may not be generalizable to all collagen assays.
Keywords: von Willebrand factor, von Willebrand disease, collagen
Introduction
Von Willebrand factor (VWF) has three main ligands through which it mediates its effects on hemostasis. It serves as a carrier protein for factor VIII (FVIII), and links platelets to injured subendothelium via individual binding sites for platelet glycoprotein Ib (GPIb) and for collagen. VWF binding to collagen types I and III has been localized to the VWF A1 and A3 domains [1]. VWF binds to type VI collagen via the A1 domain, while the A3 domain does not appear to play any role in this interaction [2]. The VWF collagen binding assays (VWF:CB) measure the interaction between VWF and various collagens in vitro [3,4].
Collagen binding assays are usually performed using type I or type III collagen, or a mixture of the two, to capture plasma VWF and determine quantitative binding. The original assay, reported by Brown and Bosak, used bovine type I collagen [3]. However, further studies have utilized type I or type III human collagen, and a combination of these collagens may in fact provide the greatest sensitivity for type 2 VWD [5]. Normal values of VWF:CB/VWF antigen (VWF:Ag) have been reported as greater than 0.6 to 0.7 [6,7]. VWF:CB assays, due to their sensitivity for the presence of the high molecular weight VWF multimers, can be utilized for distinguishing certain type 2 von Willebrand disease (VWD) variants [8].
VWD guidelines recently published by the NHLBI suggest that only subjects who have had abnormal initial screening results with VWF:Ag and VWF ristocetin cofactor activity (VWF:RCo) undergo testing with VWF:CB [9]. Other authors, however, advocate the use of VWF:CB as a supplement to the VWF:RCo (7]. The VWF:RCo assay measures VWF-platelet interactions via the GPIb receptor on the platelet surface, as induced by the antibiotic ristocetin, and therefore a VWF defect that is exclusive to the VWF-collagen axis could potentially be missed by omitting the VWF:CB assay. When collagen binding is measured, most commercial laboratories only evaluate types I and/or III collagen. Type VI collagen is also present in the subendothelium and can bind VWF at sites of injury [10]. No exploration of type VI collagen binding has been performed on VWD patients to date, prompting our study.
We report here on a series of healthy controls as well as VWD subjects enrolled in a large, multi-center US study of VWD, the Zimmerman Program for the Molecular and Clinical Biology of VWD. Our results establish a normal range for the VWF:CB with 3 different collagen types (types I, III, and VI) in this population and compare the results.
Methods
Patient population
Subjects were enrolled in the Zimmerman Program following informed consent as previously described [11]. This study was approved by the Institutional Review Boards at each of the participating institutions. Blood samples were also collected for VWF assays and DNA sequencing. Subjects were enrolled as healthy controls if they had no prior diagnosis of VWD or other bleeding disorders. Patients were enrolled if they had a pre-existing diagnosis of VWD. Immediate family members of VWD subjects were enrolled when available.
Clinical laboratory VWF assays
VWF:Ag, VWF:RCo, VWF:CB, and VWF multimer distribution were performed by the BloodCenter of Wisconsin clinical hemostasis reference laboratory on all samples. VWF:Ag was measured using an ELISA-based assay with monoclonal antibodies (AVW-1 and AVW-5, Blood Research Institute, Milwaukee, WI) for VWF capture and a polyclonal antibody for VWF detection (Dako, Carpinteria, CA). VWF:RCo was performed using the automated BCS system (Dade-Behring, Newark, DE) to measure platelet agglutination at a ristocetin concentration of 1 mg/ml. All values were referenced to an international plasma standard (SSC/ISTH standard lot #3). VWF multimer distribution was assessed using gel electrophoresis [12]. Blood group testing was performed using plasma samples analyzed for presence of isohemagglutinins. VWF propeptide was measured as previously described using monoclonal antibodies 293.2 and 239.3 for capture and biotinylated monoclonal antibodies 242.4 and 242.6 for detection (Blood Research Institute) [13].
Collagen binding assays
The VWF:CB ELISA performed in the clinical laboratory used human type III collagen at 1 μg/mL (Southern Biotech, Birmingham, AL) diluted in carbonate coating buffer (15 mM sodium carbonate, 35 mM sodium bicarbonate, 3 mM sodium azide, pH 9.5) on an Immulon Ib plate (Thermo Scientific, Rochester, NY) for VWF capture and a polyclonal antibody for VWF detection (Dako). Two dilutions were tested, in triplicate, for each test sample. Type I and type VI collagen binding assays were performed in the research laboratory. For type I collagen binding assays, Maxisorp (Nalge Nunc, Rochester, NY) ELISA plates were coated with type I human placental collagen at 5 μg/mL (Sigma, St. Louis, MO) diluted in carbonate coating buffer as above and incubated at 4° C overnight. Plasma samples diluted in phosphate-buffered saline were added to each well and incubated at room temperature for 1 hour. VWF bound to collagen was detected using a biotin-conjugated polyclonal antibody to VWF (Dako). Assays were repeated if dilutions were not within 10% of each other or if they were off the curve. Two dilutions were tested, in triplicate, for each test sample. Type VI collagen binding was assessed using the VWF:CBA ELISA for type VI collagen (Technoclone GmbH, Austria, distributed by Diapharma, West Chester, OH) following the manufacturer’s directions. One dilution, in duplicate, was performed for each sample as per the kit instructions, but if the value did not fall on the curve, repeat testing with additional dilutions was performed.
Statistics
Data were log-transformed to obtain a normal distribution and Stata 11.1 (StataCorp LP, College Station, TX) was used for the analyses. Normal range was then defined as the geometric mean ± 1.96 times the standard deviation. The confidence interval was then back-transformed to the original data scale. Normality was tested using the Shapiro-Wilks and skewness-kurtosis tests. Comparisons between groups used the Mann-Whitney test and correlations were computed using the Spearman Rank correlation since some of the data were non-normal.
Results
VWF:CB in Zimmerman Program healthy controls
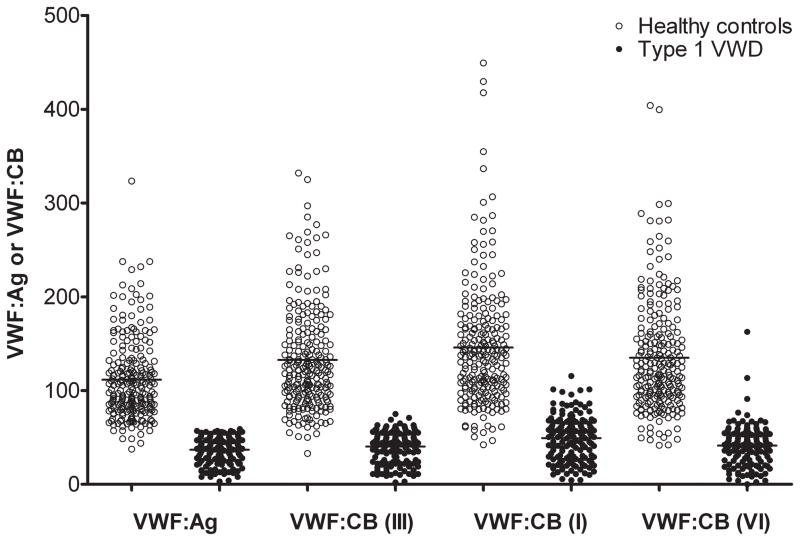
We first evaluated healthy controls to establish a normal range in our study population. There were 232 healthy control subjects enrolled in the Zimmerman Program who had VWF:Ag, VWF:RCo, and VWF:CB results from our clinical laboratory available for review, with 150 self-identifying as Caucasian and 66 as African American (with an additional 16 subjects self-identifying as other than Caucasian or African American). Statistical parameters are summarized in Table 1, first for all subjects and then by race. The mean VWF:Ag was 112 IU/dL and the mean VWF:RCo was 99 IU/dL. The mean VWF:CB with type III collagen, the type routinely used in our clinical laboratory, was 133 IU/dL, and the mean VWF:CB/VWF:Ag ratio was 1.19. No healthy control subject had a VWF:CB/VWF:Ag ratio less than 0.73 (figure 1). The normal range, calculated as ± 2 SD, for VWF:CB/VWF:Ag in our healthy control subjects was 0.87–1.50.
Table 1.
Summary of Zimmerman Program plasma VWF levels in healthy control subjects.
| All controls (n=232)
|
Caucasian (n=150)
|
African American (n=66)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean | Median | Normal range | Mean | Median | Normal range | Mean | Median | Normal range | |
| VWF:Ag | 112 | 102 | 52–210 | 108 | 97 | 53–192 | 127 | 118 | 64–313 |
| VWF:RCo | 99 | 93 | 46–190 | 99 | 99 | 46–185 | 103 | 93 | 45–210 |
| VWF:RCo/VWF:Ag ratio | 0.91 | 0.90 | 0.60–1.37 | 0.94 | 0.95 | 0.67–1.38 | 0.82 | 0.81 | 0.51–1.23 |
| FVIII | 107 | 102 | 54–177 | 103 | 99 | 53–176 | 123 | 121 | 67–187 |
| VWF:CB (type III) | 133 | 122 | 57–266 | 127 | 117 | 55–250 | 152 | 145 | 71–296 |
| VWF:CB/VWF:Ag ratio (type III) | 1.19 | 1.18 | 0.87–1.50 | 1.17 | 1.16 | 0.86–1.48 | 1.21 | 1.24 | 0.88–1.54 |
| VWF:CB (type I) | 146 | 137 | 61–297 | 138 | 131 | 61–268 | 174 | 153 | 45–210 |
| VWF:CB/VWF:Ag ratio (type I) | 1.31 | 1.29 | 0.86–1.92 | 1.29 | 1.27 | 0.89–1.80 | 1.38 | 1.39 | 0.77–1.99 |
| VWF:CB (type VI) | 135 | 121 | 55–280 | 130 | 118 | 53–268 | 154 | 135 | 64–313 |
| VWF:CB/VWF:Ag ratio (type VI) | 1.21 | 1.21 | 0.76–1.65 | 1.21 | 1.21 | 0.74–1.66 | 1.21 | 1.21 | 0.80–1.61 |
Data were log-transformed to obtain a normal distribution. Geometric means are given for VWF:Ag, VWF:RCo, FVIII, and VWF:CB and arithmetic means for the VWF:CB/VWF:Ag ratios. Normal range is the mean ± 2 SD. Data are given in IU/dL with the exception of type VI collagen binding, which was normalized to an international standard and is given in U/dL.
Figure 1. Comparison of VWF:Ag and VWF:CB for subjects enrolled in the Zimmerman Program.
This box plot shows the VWF:Ag and VWF:CB using human type III, type I, and type VI collagen as noted. All values are given in IU/dL except for CB (VI), which is in U/dL. Data are shown for healthy controls (open circles) and for type 1 VWD subjects (closed circles) whose enrollment laboratory VWF:Ag was < 60 IU/dL.
We also examined VWF:CB with type I and type VI collagen. Since there is no international standard for type VI collagen binding, we used a value of 100 U/dL for the plasma standard. For type I collagen, the mean VWF:CB was 146 IU/dL, with a mean VWF:CB/VWF:Ag ratio of 1.31. For type VI collagen, the mean VWF:CB was 135 U/dL, with a mean VWF:CB/VWF:Ag ratio of 1.21. The correlation coefficient between VWF:CB with all three collagen types was high, with r=0.85 for type III vs type I, 0.83 for type III vs type VI, and 0.80 for type I vs type VI collagen (p<0.001 for all comparisons). VWF:CB assay results with type I collagen were, however, significantly increased compared to results seen with types III and VI collagen. Differences between VWF:CB with the various collagen sources were decreased at lower VWF concentrations; at VWF:Ag levels less than 100, there was no difference between type III and type I collagen binding. The difference between type I and type VI remained significant with p=0.03. Several subjects had an isolated decrease in binding exclusively to type VI collagen, and are the focus of further investigation.
Racial differences in VWF:Ag have previously been reported, with higher VWF:Ag and VWF:CB in African Americans compared to Caucasians [14]. We analyzed VWF:CB and VWF:CB/VWF:Ag ratio by race (table 1), and noted a significantly higher VWF:CB in the African American controls for all collagen types, with p<0.005 for type III collagen, p<0.001 for type I collagen, and p<0.02 for type VI collagen as compared to Caucasians. For type I collagen, there was also a statistical difference between African American and Caucasian controls, with p=0.024. However, no difference was noted in VWF:CB/VWF:Ag ratios by race for either type III or type VI collagen. Similarly, blood group differences may also account for VWF:CB variation. When analyzed by blood group, our data demonstrated the same trend as that seen by Favaloro’s group [15], with lower VWF:CB at 109 IU/dL for group O compared to 154 IU/dL for the non-O subjects. There was no difference in VWF:CB/VWF:Ag ratio in those with blood group O compared to non-blood group O subjects (mean of 1.17 vs. 1.20, p=NS).
VWF:CB in Zimmerman Program type 1 VWD subjects
We first examined all subjects enrolled in the Zimmerman Program who carried a pre-existing diagnosis of type 1 VWD at the time of study entrance. The values for the 263 subjects in this group are shown in table 2. The mean VWF:Ag was 60 IU/dL, the mean VWF:RCo was 58 IU/dL, and the mean VWF:CB with type III collagen was 65 IU/dL. These means were higher than expected because a number of subjects had VWF levels higher on study entry than were obtained at the time of original diagnosis.
Table 2.
Summary of Zimmerman Program plasma VWF levels in type 1 VWD subjects.
| All subjects (n=263) | VWF:Ag<60 (n=154) | VWF:Ag<30 (n=47) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean | Observed range | Mean | Observed range | Mean | Observed range | |
| VWF:Ag | 60 | 3–372 | 37 | 3–59 | 19 | 3–29 |
| VWF:RCo* | 58 | 5–306 | 36 | 5–68 | 20 | 5–36 |
| VWF:RCo/VWF:Ag ratio | 0.98 | <10–306 | 1.00 | 0.16–1.67 | 1.05 | 0.23–1.67 |
| FVIII | 69 | 1–204 | 56 | 1–99 | 20 | 1–88 |
| VWF:CB (type III) | 65 | 2–366 | 40 | 2–75 | 20 | 2–34 |
| VWF:CB/VWF:Ag ratio (type III) | 1.08 | 0.38–1.50 | 1.07 | 0.38–1.50 | 1.00 | 0.38–1.50 |
| VWF:CB (type I) | 78 | 4–370 | 49 | 4–116 | 22 | 4–45 |
| VWF:CB/VWF:Ag ratio (type I) | 1.32 | 0.48–2.42 | 1.30 | 0.62–2.31 | 1.15 | 0.62–1.61 |
| VWF:CB (type VI) | 67 | 0–393 | 41 | 0–163 | 20 | 2–40 |
| VWF:CB/VWF:Ag ratio (type VI) | 1.10 | 0–3.25 | 1.09 | 0–3.25 | 1.01 | 0.49–1.73 |
For nine subjects with a VWF:RCo <10 IU/dL, an arbitrary value of 5 IU/dL was assigned for calculation purposes. Geometric means are given for VWF:Ag, VWF:RCo, FVIII, and VWF:CB and arithmetic means for the VWF:CB/VWF:Ag ratios. Data are given in IU/dL with the exception of type VI collagen binding, which was normalized to an international standard and is given in U/dL.
We then restricted analysis to those with a diagnosis of type 1 VWD and VWF:Ag < 60 IU/dL, leaving a total of 154 subjects. The mean VWF:Ag in this subgroup was 37 IU/dL and the mean VWF:RCo was 36 IU/dL. Nine of the subjects with low VWF:Ag levels had VWF:RCo <10 IU/dL; for these subjects, a value of 5 IU/dL was arbitrarily used to calculate the mean VWF:RCo since exact values were not available. All of these subjects, however, met diagnostic criteria for type 1 VWD and none appeared to have a defect in collagen binding or platelet binding suggestive of type 2M VWD. The mean VWF:CB was 40 IU/dL with type III collagen, 49 IU/dL with type I collagen, and 41 U/dL with type VI collagen. Mean ratios, given in table 2, were similar to those seen for the healthy controls.
A cut-off value of VWF:Ag <30 IU/dL has been proposed for diagnosis of type 1 VWD [9]. When we restricted our analysis to only those subjects with a diagnosis of type 1 VWD and VWF:Ag <30 IU/dL, 47 subjects were available for analysis. The mean VWF:Ag for this group was 19 IU/dL. The mean VWF:CB/VWF:Ag ratio for type III collagen was 1.00, the mean ratio for type I collagen was 1.15, and the mean ratio for type VI collagen was 1.01 (table 2). One subject had an isolated defect in binding to type VI collagen and is addressed in a separate paper also under submission to this journal. These results suggest that decreased VWF:CB/VWF:Ag ratios may indicate a specific collagen binding defect, since low ratios were not typically present in our subjects with mildly decreased VWF:Ag levels.
VWF:CB in Zimmerman Program type 2 VWD subjects
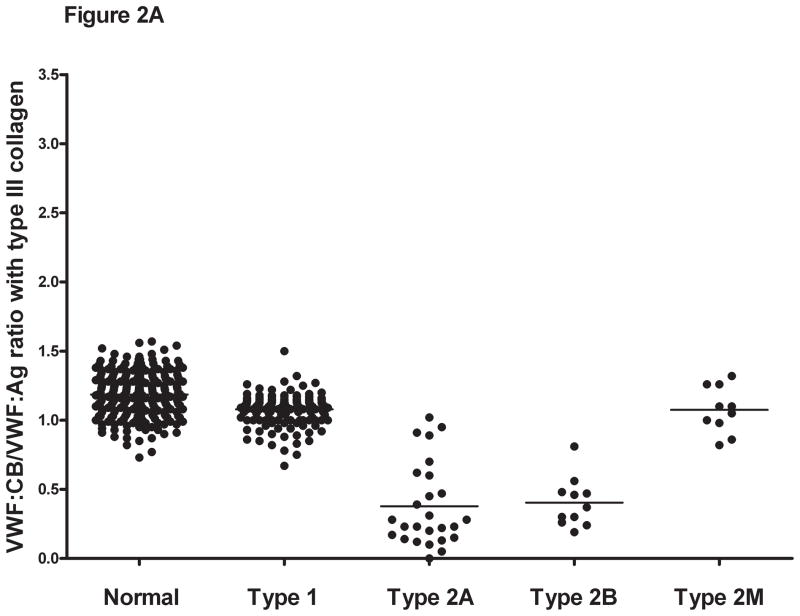
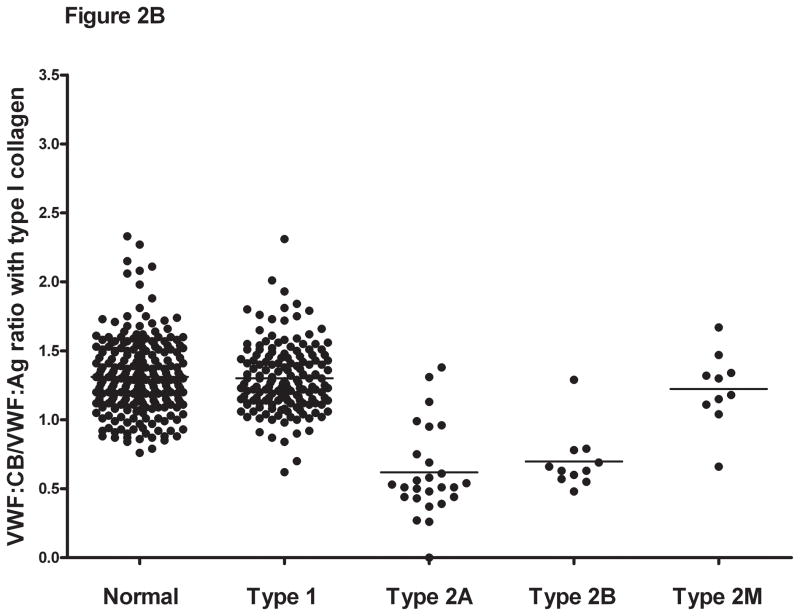
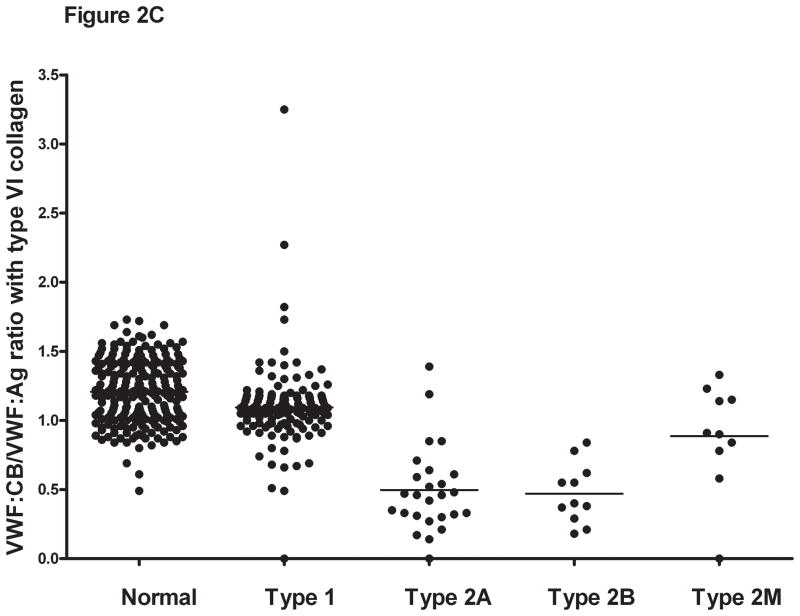
Collagen binding in the type 2 VWD subjects was compared for all three collagen types. For the type 2A and type 2B subjects, mean VWF:CB/VWF:Ag was approximately half of that seen for the healthy controls (table 3), with a mean ratio for type III collagen of 0.38 for type 2A and 0.4 for type 2B VWD. No significant difference was seen between the type 2A and type 2B subjects with any collagen type, suggesting that while VWF:CB is affected by loss of high molecular weight multimers, it does not discriminate between type 2A and type 2B VWD. Collagen binding was significantly decreased, however, compared to type 1 subjects and healthy controls (figure 2). Interestingly, although all the type 2A and type 2B subjects demonstrated loss of high molecular weight multimers, some did have VWF:CB levels overlapping with the normal range. This group does contain several novel VWF mutations, and it is possible that they represent type 1 or type 2M VWD rather than type 2A VWD. Future repeat testing will be needed in order to confirm the multimer distribution, and expression of the novel mutations will help clarify their classification.
Table 3.
Summary of Zimmerman Program plasma VWF levels in type 2 VWD subjects.
| Type 2A (n=26) | Type 2B (n=11) | Type 2M (n=10) | Type 2N (n=7) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean | Observed range | Mean | Observed range | Mean | Observed range | Mean | Observed range | |
| VWF:Ag | 30 | 8–86 | 31 | 17–52 | 35 | 10–88 | 85 | 51–135 |
| VWF:RCo* | 8 | <10–48 | 16 | <10–39 | 16 | <10–56 | 86 | 51–148 |
| VWF:RCo/VWF:Ag ratio* | 0.34 | 0–1 | 0.55 | 0–1.11 | 0.68 | 0–1.49 | 1.08 | 0.86–1.37 |
| FVIII | 45 | 19–92 | 37 | 19–55 | 63 | 27–145 | 28 | 7–55 |
| VWF:CB (type III) | 16 | 0–72 | 15 | 4–39 | 38 | 11–111 | 90 | 54–146 |
| VWF:CB/VWF:Ag ratio (type III) | 0.38 | 0–1.02 | 0.40 | 0.19–0.81 | 1.08 | 0.82–1.32 | 1.05 | 0.83–1.18 |
| VWF:CB (type I) | 23 | 0–104 | 22 | 11–37 | 45 | 11–130 | 133 | 71–387 |
| VWF:CB/VWF:Ag ratio (type I) | 0.62 | 0–1.38 | 0.70 | 0.48–1.29 | 1.22 | 0.66–1.67 | 1.47 | 0.79–2.87 |
| VWF:CB (type VI) | 20 | 0–110 | 17 | 3–37 | 33 | 0–100 | 89 | 61–128 |
| VWF:CB/VWF:Ag ratio (type VI) | 0.50 | 0–1.39 | 0.47 | 0.18–0.84 | 0.89 | 0–1.33 | 1.08 | 0.95–1.20 |
For 16 type 2A subjects, two type 2B subjects and one type 2M subject with VWF:RCo <10 IU/dL, an arbitrary value of 5 IU/dL was assigned for calculation purposes. Geometric means are given for VWF:Ag, VWF:RCo, FVIII, and VWF:CB and arithmetic means for the VWF:CB/VWF:Ag ratios. Data are given in IU/dL with the exception of type VI collagen binding, which was normalized to an international standard and is given in U/dL.
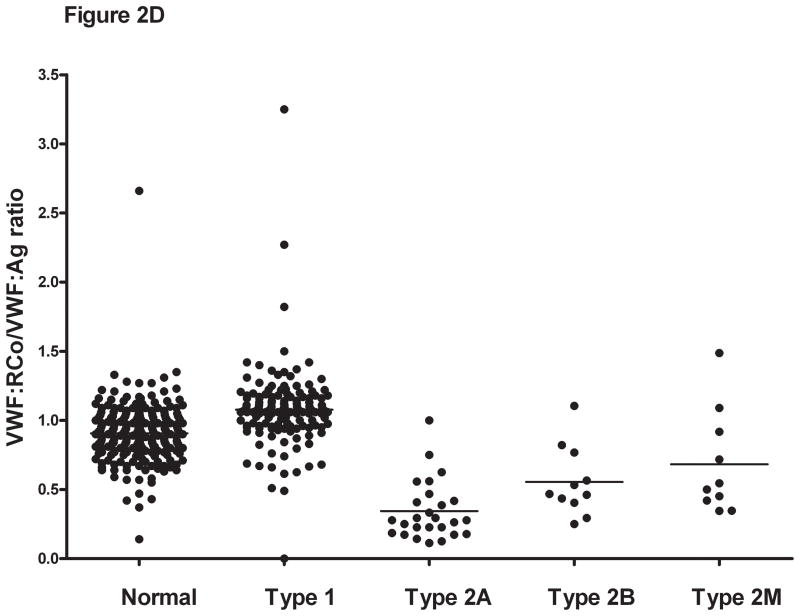
Figure 2. Comparison of VWF:CB/VWF:Ag ratios with types III, I, and VI collagen for healthy controls, type 1, and type 2 VWD subjects.
Panel A shows the VWF:CB/VWF:Ag ratios using type III collagen for healthy controls, type 1 VWD subjects with a VWF:Ag value <60 IU/dL at time of study enrollment, and type 2A, 2B, and 2M VWD subjects. Panel B shows the same subgroups using type I collagen. Panel C shows the same subgroups using type VI collagen. Panel D shows the VWF:RCo/VWF:Ag ratio for the same subgroups.
There was no clear pattern based on specific mutations. All subjects with R1597W had decreased VWF:RCo/VWF:Ag and VWF:CB/VWF:Ag ratios. One subject with G1180R had decreased VWF:CB/VWF:Ag ratios with all three collagen types, but another subject with that same mutation had a ratio of 0.62 with type III collagen but relatively normal ratios with type I and type VI collagen (0.96 and 0.85, respectively). One subject with C1458R had undetectable VWF:RCo, as well as undetectable binding to all the collagen types. This is not surprising, given the location of this mutation as the C-terminal partner in the A1 loop disulfide bond and its subsequent effect on multimerization. However, several other A1 loop mutations (M1304R and Y1349C) yielded relatively normal VWF:CB ratios, despite the decrease in VWF:RCo. Further characterization of those mutants is in progress as it is possible they do not represent true type 2A VWD and would instead be better classified as type 1 or type 2M.
For the type 2M subjects, mean VWF:CB/VWF:Ag was similar to that seen for the controls (table 3) with one exception. A subject enrolled as type 2M with historical VWF:Ag of 20 IU/dL and VWF:RCo of 8 IU/dL had binding to types I and III collagen at levels relatively equivalent to VWF:Ag, but undetectable binding with type VI collagen. This subject has a previously reported deletion of 33 base pairs in exon 28, leading to the deletion of amino acids 1392–1402 [16].
For the type 2N subjects, mean VWF:CB/VWF:Ag ratios were similar to that seen with the healthy controls, consistent with the normal to slightly decreased VWF:Ag in this subgroup (table 3).
VWF:CB in Zimmerman Program type 3 VWD subjects
We next evaluated VWF:CB in type 3 VWD subjects. As expected, all 18 subjects with a pre-existing diagnosis of type 3 VWD and undetectable VWF:Ag on their study sample had undetectable VWF:CB with type III collagen. In addition, binding to type I and type VI collagen was below the lower limit of detection of our assays, consistent with absent VWF protein in these type 3 subjects. There were several subjects with VWF:Ag >1 IU/dL who had received treatment with VWF containing concentrates immediately prior to having blood drawn; these subjects were excluded from this analysis.
Discussion
Diagnosis of VWD requires a constellation of findings, including a clinical history of bleeding, family history of symptoms or diagnosed VWD, and corroborative laboratory findings. Typical initial laboratory testing for VWD consists of VWF:Ag, VWF:RCo, FVIII activity, and VWF multimers. The utility of VWF:CB as part of this workup has been the subject of some debate. Some authors have argued for its inclusion in VWD workups as a supplement to the VWF:RCo, since VWF:CB displays much less variability, is sensitive to loss of high molecular weight multimers, and measures a function of VWF not assessed by the VWF:RCo [5,17]. Others would argue that VWF:CB is best utilized as an adjunct to the standard VWD screen, to help ascertain which type of VWD is present in subjects where the diagnosis is already suspected [9,18].
The type of collagen used is critically important, as there are significant differences between type I and type III collagen, particularly in relative sensitivity to loss of high molecular weight multimers [5]. Commercially available kits for measuring VWF:CB vary greatly in terms of collagen type and ability to identify abnormalities present in type 2A or 2B VWD [19]. In contrast to type I and type III collagen, type VI collagen appears to bind VWF through a site in the A1 domain [2,10,20]. Type VI collagen is present in the subendothelium and may be of physiologic relevance, particularly at low shear rates [10,21]. The relative contributions of VWF binding to type I, type III, and type VI collagen in vivo have yet to be determined. In our population, VWF:CB was higher with type I collagen than with types III or VI collagen. This likely does not represent an increased affinity of VWF to type I collagen but rather a difference in the standard that may ultimately require separate assigned type I and type III collagen binding values.
Few reports have extensively evaluated VWF:CB in healthy individuals. Blood group has been shown to affect VWF:CB, with lower VWF:CB (commensurate with the decrease in VWF:Ag) in healthy controls who were blood group O as compared to non-O [14,22]. Because both VWF:CB and VWF:Ag decrease in blood group O individuals, the ratio is not affected. This reduction is thought to be secondary to more rapid plasma clearance in blood group O compared to other blood groups [23]. Favaloro and colleagues examined 452 healthy donors and confirmed a decreased VWF:CB in those with blood group O, but did not see a difference based on sex or Rh status [15].
Several recent large studies have looked at VWF:CB in VWD subjects. A study of type 1 VWD subjects from the United Kingdom reported VWF:CB results although no official comparison was made to the VWF:RCo [24]. The European Molecular and Clinical Markers for the Diagnosis and Management of Type 1 VWD (MCMDM-1 VWD) study looked at VWF:CB in both index cases and affected family members, but none displayed a markedly lower VWF:CB as compared to the other assays of VWF [25]. Federici and colleagues examined type I and type III collagen binding in 27 healthy controls and a small number of type 1 and type 2 VWD subjects [6]. Their study, albeit small, showed better discrimination between type 1 and type 2A or 2B VWD with type I collagen. Favaloro has extensively reviewed this issue and concluded that collagen type was important, as was collagen preparation [5,7]. In addition, the VWF:CB can provide a rapid and sensitive assessment of high molecular weight multimers [17].
The type of collagen used, and the conditions under which it is utilized, however, are of utmost importance. The results presented above represent data from a single collagen source, using a single coating process. Purity of collagen sources may be an issue: we examined our collagen and found no contamination when monoclonal antibodies were used. A polyclonal antibody to type III collagen did minimally react with the type I and type VI collagen preparations, and a polyclonal antibody to type I collagen did minimally react with the type III collagen preparation. Recent work suggests that optimal collagen coating may occur with phosphate-buffered saline rather than carbonate coating buffer [26]. In addition, differences between coating at 4° C and room temperature have also been observed [26]. Differences between types I and III collagen seen here may therefore reflect assay conditions rather than a biological difference in VWF affinity.
Consensus guidelines suggest a diagnosis of type 1 VWD, with a proportional decrease in VWF:Ag, VWF:RCo, and/or VWF:CB, in patients with VWF:Ag < 30 IU/dL [9]. Those patients whose VWF:Ag level is > 30 IU/dL are less likely to have a genetic mutation in VWF [27]. The relationship between VWF level and bleeding symptoms is also unclear, as demonstrated by the MCMDM-1 VWD study where elevated bleeding scores were seen in both affected and unaffected family members [28]. Recent guidelines suggest using the terminology “low VWF” to refer to patients with VWF:Ag and VWF:RCo 30–50 IU/dL [9]. In this analysis, a cut-off of 60 IU/dL was chosen in order to include all subjects whose VWF levels might have been at the lower end of normal due to variability in the assay, but who might have true VWD.
The use of the VWF:CB/VWF:Ag ratio rather than the absolute VWF:CB may be helpful in diagnosing cases where a defect in collagen binding occurs. Previous studies have recommended a cut-off of 0.7 for the lower limit of normal with the VWF:CB/VWF:Ag ratio [6,7]. In the Zimmerman Program normal controls, no subject had a ratio less than 0.7 when using type III collagen. The normal range established in the Caucasian control subjects was 0.87–1.50. These data support a cut-off of 0.7 for the normal VWF:CB/VWF:Ag ratio and suggest that subjects with lower ratios be examined for specific genetic defects in the collagen binding domain. It should be noted, however, that this work examined only healthy controls and subjects with a diagnosis of type 1 VWD (and VWF:Ag ≤ 60 IU/dL). This range does not apply to those subjects with type 2 VWD and abnormal multimers.
Understanding the interplay between VWF and collagen is crucial for several reasons. Appropriate VWD diagnosis is required for optimal therapy and the VWF:CB provides a rapid and sensitive screen of VWF-collagen binding and of multimer distribution for examination of type 2 VWD variants. It may also be important to screen for defects in collagen binding that other VWF assays will fail to diagnose. VWF:CB is not routinely determined as part of the workup in patients with bleeding problems, but perhaps its inclusion in the diagnostic workup merits re-examination.
Acknowledgments
The authors would like to acknowledge the Zimmerman Program investigators, staff, and subjects who participated in this study. Invaluable assistance with collagen binding assays was provided by Patricia Morateck and Crystal Perry.
This work was supported by the National Institutes of Health K08 grant HL102260 (VF) and program project grant HL081588 (RRM). Support for VHF was also provided by a Mentored Research Award from the Hemophilia and Thrombosis Research Society and a Career Development Award from the National Hemophilia Foundation. Support for RRM was also provided through National Institutes of Health grants HL33721 and HL044612. This work was also supported in part by the Midwest Athletes Against Childhood Cancer and the Clinical & Translational Science Institute of Southeastern Wisconsin: NIH UL1RR031973.
The authors would also like to thank the numerous investigators involved with the Zimmerman Program for the Molecular and Clinical Biology of Von Willebrand Disease. Directors of the primary centers include T. Abshire and A. Dunn, Emory University School of Medicine, Atlanta, GA; J. Lusher, Wayne State University, Detroit, MI; D. Brown, University of Texas Health Science Center at Houston, Houston, TX; A. Shapiro, Indiana Hemophilia & Thrombosis Center, Indianapolis, IN; S. Lentz, University of Iowa, Iowa City, IA; J. Gill, Comprehensive Center for Bleeding Disorders, Milwaukee, WI; C. Leissinger, Tulane University Health Sciences Center, New Orleans, LA; M. Ragni, University of Pittsburgh, Pittsburgh, PA. In addition, numerous secondary centers contributed to subject recruitment: J. Hord, Akron Children’s Hospital, Akron, OH; M. Manco-Johnson, Mountain States Regional Hemophilia and Thrombosis Center, Aurora, CO; J. Strouse, Johns Hopkins Children’s Center, Baltimore, MD; A. Ma, University of North Carolina Chapel Hill, Chapel Hill, NC; L. Valentino, Rush University Medical Center, Chicago, IL; R. Gruppo, Cincinnati Children’s Hospital, Cincinnati, OH; B. Kerlin, Nationwide Children’s Hospital, Columbus, OH; R. Kulkarni, Michigan State University, East Lansing, MI; D. Green, Northwestern University, Evanston, IL; D. Mahoney, Baylor College of Medicine, Houston, TX; L. Mathias, Loma Linda University Medical Center, Loma Linda, CA; C. Diamond, University of Wisconsin Madison, Madison, WI; A. Neff, Vanderbilt University, Nashville, TN; D. DiMichele and P. Giardina, Weill Cornell Medical College, New York, NY; A. Cohen, Newark Beth Israel Medical Center, Newark, NJ; E. Werner, Children’s Hospital of the King’s Daughters, Norfolk, VA; A. Matsunaga, Children’s Hospital & Research Center Oakland, Oakland, CA; M. Tarantino, Comprehensive Bleeding Disorders Center, Peoria, IL; F. Shafer, Drexel University College of Medicine, Philadelphia, PA; B. Konkle and A. Cuker, University of Pennsylvania, Philadelphia, PA; P. Kouides, Rochester General Hospital, Rochester, NY; D. Stein, Toledo Children’s Hospital, Toledo, OH
Footnotes
Conflict of interest disclosures
JCG is a consultant for Archemix, Baxter, Bayer, and CSL Behring. RRM is a consultant for GTI Diagnostics, Baxter, Bayer, CSL Behring, and AstraZeneca. None of the other authors have conflicts of interest to disclose.
References
- 1.Pareti FI, Niiya K, McPherson JM, Ruggeri ZM. Isolation and characterization of two domains of human von Willebrand factor that interact with fibrillar collagen types I and III. J Biol Chem. 1987;262:13835–41. [PubMed] [Google Scholar]
- 2.Hoylaerts MF, Yamamoto H, Nuyts K, Vreys I, Deckmyn H, Vermylen J. von Willebrand factor binds to native collagen VI primarily via its A1 domain. Biochem J. 1997;324:185–191. doi: 10.1042/bj3240185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown JE, Bosak JO. An ELISA test for the binding of von Willebrand antigen to collagen. Thromb Res. 1986;43:303–11. doi: 10.1016/0049-3848(86)90150-7. [DOI] [PubMed] [Google Scholar]
- 4.Favaloro EJ. Von Willebrand factor collagen-binding (activity) assay in the diagnosis of von Willebrand disease: a 15-year journey. Semin Thromb Hemost. 2002;28:191–202. doi: 10.1055/s-2002-27821. [DOI] [PubMed] [Google Scholar]
- 5.Favaloro EJ. Collagen binding assay for von Willebrand factor (VWF:CBA): detection of von Willebrands Disease (VWD), and discrimination of VWD subtypes, depends on collagen source. Thromb Haemost. 2000;83:127–35. [PubMed] [Google Scholar]
- 6.Federici AB, Canciani MT, Forza I, Cozzi G. Ristocetin cofactor and collagen binding activities normalized to antigen levels for a rapid diagnosis of type 2 von Willebrand disease--single center comparison of four different assays. Thromb Haemost. 2000;84:1127–8. [PubMed] [Google Scholar]
- 7.Favaloro EJ. An update on the von Willebrand factor collagen binding assay: 21 years of age and beyond adolescence but not yet a mature adult. Semin Thromb Hemost. 2007;33:727–44. doi: 10.1055/s-2007-1000364. [DOI] [PubMed] [Google Scholar]
- 8.Budde U, Pieconka A, Will K, Schneppenheim R. Laboratory testing for von Willebrand disease: contribution of multimer analysis to diagnosis and classification. Semin Thromb Hemost. 2006;32:514–21. doi: 10.1055/s-2006-947866. [DOI] [PubMed] [Google Scholar]
- 9.Nichols WL, Hultin MB, James AH, Manco-Johnson MJ, Montgomery RR, Ortel TL, Rick ME, Sadler JE, Weinstein M, Yawn BP. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA) Haemophilia. 2008;14:171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 10.Rand JH, Patel ND, Schwartz E, Zhou SL, Potter BJ. 150-kD von Willebrand factor binding protein extracted from human vascular subendothelium is type VI collagen. J Clin Invest. 1991;88:253–9. doi: 10.1172/JCI115285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flood VH, Gill JC, Morateck PA, Christopherson PA, Friedman KD, Haberichter SL, Branchford BR, Hoffmann RG, Abshire TC, Di Paola JA, Hoots WK, Leissinger C, Lusher JM, Ragni MV, Shapiro AD, Montgomery RR. Common VWF exon 28 polymorphisms in African Americans affecting the VWF activity assay by ristocetin cofactor. Blood. 2010;116:280–6. doi: 10.1182/blood-2009-10-249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montgomery RR, Hathaway WE, Johnson J, Jacobson L, Muntean W. A variant of von Willebrand’s disease with abnormal expression of factor VIII procoagulant activity. Blood. 1982;60:201–7. [PubMed] [Google Scholar]
- 13.Haberichter SL, Castaman G, Budde U, Peake I, Goodeve A, Rodeghiero F, Federici AB, Batlle J, Meyer D, Mazurier C, Goudemand J, Eikenboom J, Schneppenheim R, Ingerslev J, Vorlova Z, Habart D, Holmberg L, Lethagen S, Pasi J, Hill FG, Montgomery RR. Identification of type 1 von Willebrand disease patients with reduced von Willebrand factor survival by assay of the VWF propeptide in the European study: molecular and clinical markers for the diagnosis and management of type 1 VWD (MCMDM-1VWD) Blood. 2008;111:4979–85. doi: 10.1182/blood-2007-09-110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller CH, Haff E, Platt SJ, Rawlins P, Drews CD, Dilley AB, Evatt B. Measurement of von Willebrand factor activity: relative effects of ABO blood type and race. J Thromb Haemost. 2003;1:2191–7. doi: 10.1046/j.1538-7836.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 15.Favaloro EJ, Soltani S, McDonald J, Grezchnik E, Easton L, Favaloro JW. Reassessment of ABO blood group, sex, and age on laboratory parameters used to diagnose von Willebrand disorder: potential influence on the diagnosis vs the potential association with risk of thrombosis. Am J Clin Pathol. 2005;124:910–7. [PubMed] [Google Scholar]
- 16.Mancuso DJ, Kroner PA, Christopherson PA, Vokac EA, Gill JC, Montgomery RR. Type 2M:Milwaukee-1 von Willebrand disease: an in-frame deletion in the Cys509-Cys695 loop of the von Willebrand factor A1 domain causes deficient binding of von Willebrand factor to platelets. Blood. 1996;88:2559–68. [PubMed] [Google Scholar]
- 17.Favaloro EJ. Toward a new paradigm for the identification and functional characterization of von Willebrand disease. Semin Thromb Hemost. 2009;35:60–75. doi: 10.1055/s-0029-1214149. [DOI] [PubMed] [Google Scholar]
- 18.Rodeghiero F, Castaman G, Tosetto A. How I treat von Willebrand disease. Blood. 2009;114:1158–65. doi: 10.1182/blood-2009-01-153296. [DOI] [PubMed] [Google Scholar]
- 19.Favaloro EJ. Evaluation of commercial von Willebrand factor collagen binding assays to assist the discrimination of types 1 and 2 von Willebrand disease. Thromb Haemost. 2010;104:1009–21. doi: 10.1160/TH10-06-0360. [DOI] [PubMed] [Google Scholar]
- 20.Denis C, Baruch D, Kielty CM, Ajzenberg N, Christophe O, Meyer D. Localization of von Willebrand factor binding domains to endothelial extracellular matrix and to type VI collagen. Arterioscler Thromb. 1993;13:398–406. doi: 10.1161/01.atv.13.3.398. [DOI] [PubMed] [Google Scholar]
- 21.Rand JH, Glanville RW, Wu XX, Ross JM, Zangari M, Gordon RE, Schwartz E, Potter BJ. The significance of subendothelial von Willebrand factor. Thromb Haemost. 1997;78:445–50. [PubMed] [Google Scholar]
- 22.Haley E, Babar N, Ritter C, Downes KA, Green D, Shurin S, Sarode R. Effect of ABO blood group on the collagen-binding assay for von Willebrand factor. Am J Hematol. 2002;71:229–31. doi: 10.1002/ajh.10238. [DOI] [PubMed] [Google Scholar]
- 23.Bowen DJ. An influence of ABO blood group on the rate of proteolysis of von Willebrand factor by ADAMTS13. J Thromb Haemost. 2003;1:33–40. doi: 10.1046/j.1538-7836.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 24.Cumming A, Grundy P, Keeney S, Lester W, Enayat S, Guilliatt A, Bowen D, Pasi J, Keeling D, Hill F, Bolton-Maggs PH, Hay C, Collins P UK Haemophilia Centre Doctors’ Organisation. An investigation of the von Willebrand factor genotype in UK patients diagnosed to have type 1 von Willebrand disease. Thromb Haemost. 2006;96:630–41. [PubMed] [Google Scholar]
- 25.Goodeve A, Eikenboom J, Castaman G, Rodeghiero F, Federici AB, Batlle J, Meyer D, Mazurier C, Goudemand J, Schneppenheim R, Budde U, Ingerslev J, Habart D, Vorlova Z, Holmberg L, Lethagen S, Pasi J, Hill F, Hashemi Soteh M, Baronciani L, Hallden C, Guilliatt A, Lester W, Peake I. Phenotype and genotype of a cohort of families historically diagnosed with type 1 von Willebrand disease in the European study, Molecular and Clinical Markers for the Diagnosis and Management of Type 1 von Willebrand Disease (MCMDM-1VWD) Blood. 2007;10:112–21. doi: 10.1182/blood-2006-05-020784. [DOI] [PubMed] [Google Scholar]
- 26.Mendelboum Raviv S, Szekeres-Csiki K, Jenei A, Nagy J, Shenkman B, Savion N, Harsfalvi J. Coating conditions matter to collagen matrix formation regarding von Willebrand factor and platelet binding. Thromb Res. 2012 doi: 10.1016/j.thromres.2011.09.030. in press. [DOI] [PubMed] [Google Scholar]
- 27.Eikenboom J, Van Marion V, Putter H, Goodeve A, Rodeghiero F, Castaman G, Federici AB, Batlle J, Meyer D, Mazurier C, Goudemand J, Schneppenheim R, Budde U, Ingerslev J, Vorlova Z, Habart D, Holmberg L, Lethagen S, Pasi J, Hill F, Peake I. Linkage analysis in families diagnosed with type 1 von Willebrand disease in the European study, molecular and clinical markers for the diagnosis and management of type 1 VWD. J Thromb Haemost. 2006;4:774–82. doi: 10.1111/j.1538-7836.2006.01823.x. [DOI] [PubMed] [Google Scholar]
- 28.Tosetto A, Rodeghiero F, Castaman G, Goodeve A, Federici AB, Batlle J, Meyer D, Fressinaud E, Mazurier C, Goudemand J, Eikenboom J, Schneppenheim R, Budde U, Ingerslev J, Vorlova Z, Habart D, Holmberg L, Lethagen S, Pasi J, Hill F, Peake I. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD) J Thromb Haemost. 2006;4:766–73. doi: 10.1111/j.1538-7836.2006.01847.x. [DOI] [PubMed] [Google Scholar]