Abstract
Little is known on how the CNS would select its movement options when a person faces a novel or recurring perturbation of two opposing types (slip or trip) while walking. The purposes of this study were (1) to determine whether young adults’ adaptation to repeated slips would interfere with their recovery from a novel trip, and (2) to investigate the generalized strategies after they were exposed to a mixed training with both types of perturbation. Thirty-two young adults were assigned to either the training group, which first underwent repeated-slip training before encountering a novel, unannounced trip while walking, or to the control group, which only experienced the same novel, unannounced trip. The former group would then experience a mix of repeated trips and slips. The results indicated that prior adaptation to slips had only limited interference during the initial phase of trip recovery. In fact, the prior repeated-slip exposure had primed their reaction, which mitigated any error resulting from early interference. As a result, they did not have to take a longer compensatory step for trip recovery than did the controls. After the mixed training, subjects were able to converge effectively the motion state of their center of mass (in its position and velocity space) to a stable and generalized “middle ground” steady-state. Such movement strategies not only further strengthened their robust reactive control of stability, but also reduced the CNS’ overall reliance on accurate context prediction and on feedback correction of perturbation-induced movement error.
Keywords: stability, limb support, plasticity, interference, transfer
INTRODUCTION
Human locomotion is inherently unstable, whereby the challenges to both proactive and reactive control of stability compound in the presence of unexpected perturbation (e.g., slips or trips). Such perturbation often causes severe instability that can lead to an actual fall, when the CNS fails to respond properly. A vital functional plasticity of the CNS, is, therefore its ability to adapt to various postural threats. These adaptive improvements in the control of stability are postulated to occur via a recalibration of a generalizable internal representation of stability limits within the CNS. There is evidence to support generalization of such motor adaptation between different contexts (e.g., effectors, spatial constraints, or tasks) from the trained right to the untrained left limb, from training using low-friction platform to an untrained slip on oily floor, or from training in sit-to-stand-slip to a novel slip while walking (Bhatt and Pai, 2008, 2009; Wang et al., 2011). Because postural adaptation for successful recovery from slip was similar between these different contexts, it verifies the prediction of a common representation of stability which requires only fine tuning or modification (Yang et al., 2008)
What happens to this adaptive (and generalization) process when one suddenly encounters a diametrically opposite type of perturbation? It is known that adaptive responses for recovery from slips vs. trips are opposite in nature. Controlling horizontal position and velocity of the center-of-mass (COM) within stability limits is essential to balance recovery. The CNS learns to shift anteriorly the COM position and/or to increase its velocity with feed-forward and feedback mechanisms after repeated-slip exposure (Pai et al., 2010). Yet when facing a trip, the CNS must learn to posteriorly shift the COM position and/or to reduce its velocity (Wang et al., 2012). Evidence has shown that sensorimotor adaptation to perturbation that required opposing motor adjustments could in fact interfere with each other (Bock et al., 2001; Tong et al., 2002). Will interference (i.e., negative transfer) rather than generalization (i.e., positive transfer) occur when the stability limits require opposite postural adjustments?
Nevertheless, such training-induced vulnerability, if it exists, may also be quickly amended based on the CNS’ capability of recalibrating its internal representation of the stability limits against both forward and backward balance loss to optimize its response (Pai and Patton, 1997). Facilitation of positive transfer might be achieved by designing mixed practice protocols based on principles of motor learning, especially when opposing types of perturbation might still elicit some levels of shared control responses.
The purposes of this study were (1) to determine whether young adults’ adaptation to repeated slips would interfere with their recovery from a novel trip, and (2) to investigate the generalized strategies after they were exposed to a mixed training with both types of postural disturbance. We expected that the carryover from slip training could interfere with the recovery of the first unrehearsed trip. We nevertheless expected that after the mixed training, the CNS would display significant functional plasticity that could enable these subjects to maintain stability when facing the threats of both types of postural disturbance.
EXPERIMENTAL PROCEDURES
Subjects
Subjects were 32 healthy young adults (26 women; 6 men; aged 26 ± 4 years; height 171 ± 8 cm; mass 66 ± 12 kg); all right-leg dominant determined by self-report of the preferred leg for kicking a ball. Subjects’ informed consent was obtained after they were fully explained of the purposes and procedures, which were approved by the Institutional Review Board. None of the subjects had histories of neurological or musculoskeletal disorders which might have affected their balance control abilities. The subjects were randomly assigned to the training (N = 16, subjects underwent repeated-slip training before experiencing an unannounced trip) and control groups (N = 16, subjects experienced the same unannounced trip without any prior training).
Experimental setup
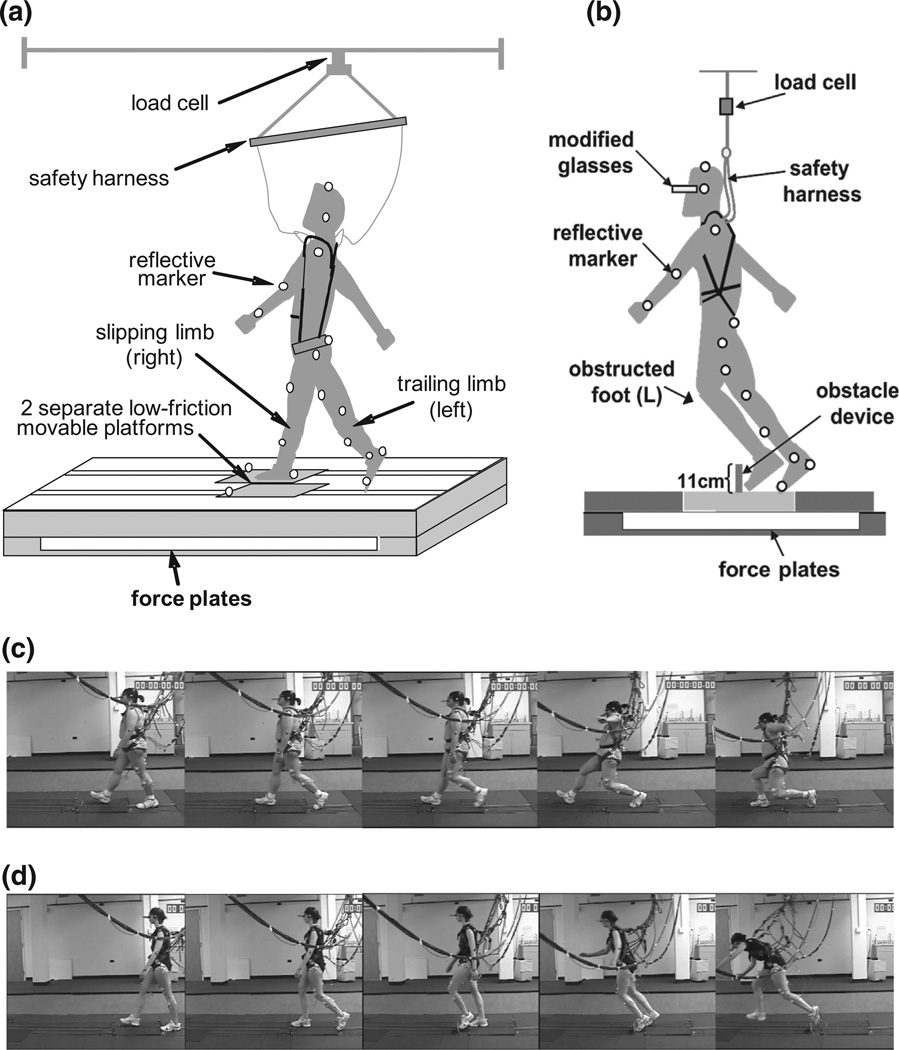
A slip was induced by a low-friction movable platform (65 × 30 × 0.6 cm, coefficient of friction <0.05), which was mounted on a frame with two rows of linear bearings. The frame was bolted onto two force plates (OR6-7-1000, AMTI, Newton, MA) to measure the ground reaction force. The platform was free to slide 150 cm forward when unlocked by a computer-controlled release mechanism. The platform peak velocities during slips ranged from 1.8 to 2.5 m/s at subjects’ self-selected walking speeds (Bhatt et al., 2005; Yang et al., 2009). The slip was induced by unlocking the moveable platform at touchdown of the slipping foot (i.e., right foot) during a slip trial (Fig. 1a, c). A trip was induced by an obstacle device, which consisted of a hinged aluminum plate 11-cm high, 27-cm wide and 0.5-cm thick (Fig. 1b). The plate lay flat under the lock by a pair of electromagnets during regular walking, and rose to an upright position upon a perturbed trial when the electromagnet pair was powered off, triggered by heel strike of the unperturbed limb (the response time for the plate to become upright was less than 150 ms). The trip was induced by obstructing the left limb during a trip trial (Fig. 1b, d). The electromagnets for the obstacle device and the low-friction platform were controlled by a computer program written in LabView (National Instruments Inc., Austin, TX). The moveable platforms and the obstacle device were embedded and camouflaged in a 7-m custom-designed walkway constructed using wooden platforms.
Fig. 1.
The diagrammatic representation of the experimental setup and video series for slip and trip. (a) A slip was induced by releasing a low-friction moveable platform free to slide 150-cm forward shortly after leading/slipping foot touchdown (i.e., right foot). The platform was mounted on a frame with two rows of linear bearings, and the frame was bolted onto two force plates to measure the ground reaction force. The movable platform was embedded in a 7-m walkway and made less noticeable to the subject by surrounding stationary decoy platforms. (b) A trip was induced by obstructing the subject’s left limb during mid-to-late swing phase using an obstacle device, which was triggered at right foot touchdown (RTD). The obstacle device consisted of an 11-cm tall plate, which was locked in a flat position by a pair of electromagnets during regular walking, and became upright to induce a trip when unlocked by powering off the electromagnets. A set of 28 light-reflective markers were placed on the subjects’ upper and lower extremities, torso, the movable platform, and the obstacle device. All subjects were required to wear a pair of modified glasses that blocked the lower half of the visual field, and a safety harness which was adjusted to prevent a fall to the ground. A load cell connected to the harness was used to measure the forces exerted on the harness. Video series were shown in typical (c) slip and (d) trip events.
Prior studies have used obstacle heights ranging from 2 to 15 cm to mimic trips encountered in the real life environment (Patla et al., 1991). Because an important concept of the perturbation-based training is to let an individual learn from falling, sufficient intensity of balance disturbance is required. An obstacle taller than 2 cm is supposed to produce a certain level of disturbance in gait, given that the minimum toe clearance is around 2 cm during gait (Patla et al., 1991). However, such intensity was insufficient to cause visible signs of balance disturbances. After several pilot tests, an 11-cm height of obstacle was eventually chosen in order to provide sufficient balance disturbances to induce effective learning. The rationale underlying inducing slip and trip perturbations on different sides were to keep the “same stance/support limb” at the time of perturbation. There is sufficient literature established on importance of the support limb in fall prevention (Pavol and Pai, 2007; Yang et al., 2009). The protocol was carefully designed to ensure that the support limb at the time of perturbation remained the same for both types of perturbations.
All subjects were required to wear a pair of modified glasses, which blocked the lower half of the visual field throughout the test. The subjects were not able to directly see the platforms or the obstacle device at the start position. They could see straight ahead but not down. The subjects wore their own athletic shoes and a full-body safety harness, which was attached via shock-absorbing ropes to an overhead rail system through a load cell. Rope lengths were individually adjusted so their knees and arms could not touch the ground in case a fall occurred. The load cell measured the force exerted on the ropes.
Protocol
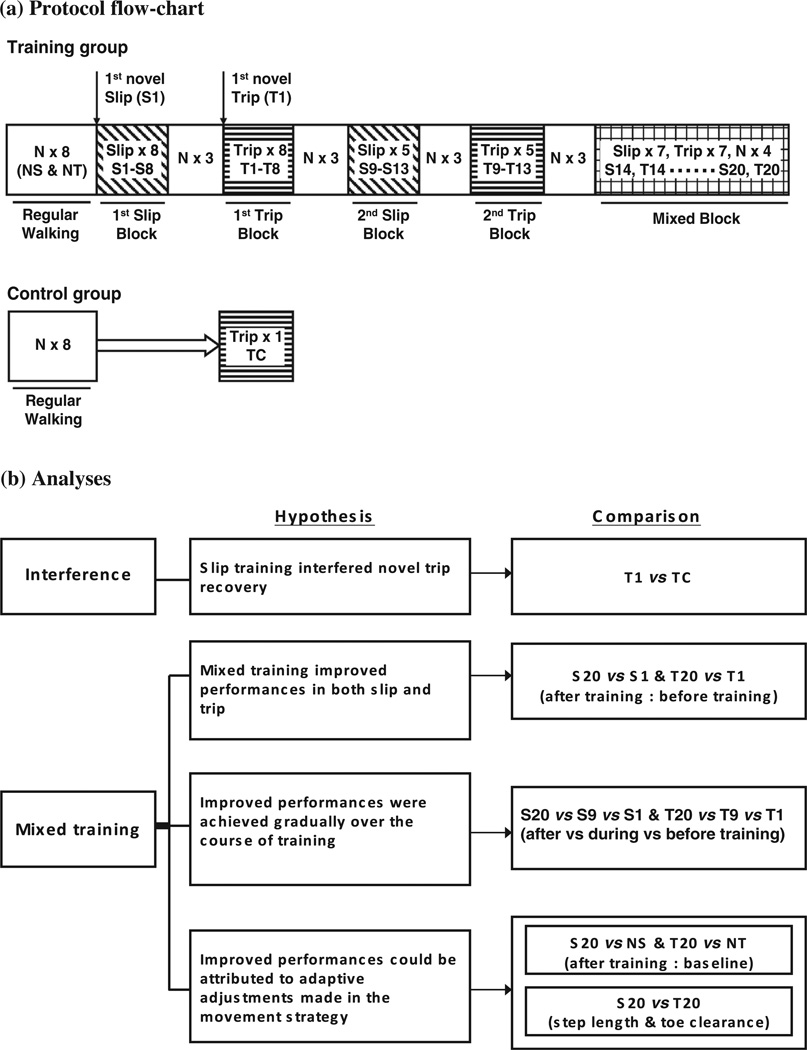
Subjects in the training group were instructed to walk at their normal pace on a 7-m walkway and were told that they might experience a slip or trip, without any specific trial or warning sign provided. They were told to recover their balance and continue walking in the case of a slip or trip. The subjects started with eight walking trials, which served as the baseline walking performance, and a slip was induced by releasing the movable platform at touchdown of the right foot without warning, followed by seven consecutive slips (the 1st slip block, S1–S8), and a block of three unperturbed trials (Fig. 2a). Afterwards, a trip was induced by releasing the obstacle device to obstruct the subject’s left foot during mid-to-late swing phase, in which a lowering strategy was likely to be implemented (Eng et al., 1994), followed by seven consecutive trips (the 1st trip block, T1–T8), and a block of three unperturbed trials (Fig. 2a). The subjects then again experienced another block of five repeated slips (the 2nd slip block, S9–S13) and three unperturbed trials, another block of five repeated trips (the 2nd trip block, T9–T13) and three unperturbed trials, followed by a mixed block of seven slips (S14–S20), seven trips (T14–T20), and four unperturbed trials interspersed (Fig. 2a). The exact trial order of the mixed block was as follows: S14 S15 T14 T15 N13 S16 T16 S17 T17 S18 T18 N14 S19 T19 N15 S20 N16 T20 (T = trip, S = slip, N = unperturbed trial. e.g., T20 = 20th trip trial).
Fig. 2.
Shown were testing protocols for (a) training and control groups, and (b) the hypotheses and analyses for evaluation of interference and mixed (interference) training effects. The protocol for the training group consisted of eight regular walking trials followed by a block of eight slips [including the first novel slip (S1) and another seven consecutive slips (S2–S8)], a block of three unperturbed trials (N), a block of eight trips [including the first novel trip (T1) and another seven consecutive trips (T2–T8)], another block of three unperturbed trials, a second block of five slips (S9–S13) followed by a block of three unperturbed trials, a second block of five trips (T9–T13) followed by a block of three unperturbed trials, and then a mixed block of seven slips (S14–S20), seven trips (T14–T20), and four unperturbed trials interspersed. NS and NT represented the last regular walking trial prior to the first novel perturbation (S1), and respectively served as the baseline walking performance for comparison with data from slip and trip trials. The protocol for the control group consisted of eight regular walking trials followed by an unannounced, novel trip (TC).
Subjects in the control group were given similar instructions where they were told that a trip could occur on any of the trials. The control group began with eight walking trials, which were followed by the first, novel trip (Fig. 2a). It should be noted that both the training and control groups were aware that they could experience a perturbation (i.e., slip or trip for the training group, and trip for the control group) in any of the upcoming trials. They had no knowledge of when, where and how the perturbations would occur, however, the probability of knowing the upcoming perturbation was equal and minimal in both groups.
Data collection
A set of 28 light-reflective markers were placed on bilateral upper and lower extremities, torso, the movable platform, and the obstacle device. The marker data were recorded by an 8-camera motion analysis system (Motion Analysis Corporation, Santa Rosa, CA) at 120 Hz. Marker displacement data were lowpass filtered at marker-specific cut-off frequencies (range 4.5–9 Hz) using fourth-order Butterworth filters. Force plate, harness load-cell data, and trigger-release onset signal were collected at 600 Hz and synchronized with motion data at the time of data collection.
Strategies for trip recovery
Strategies for recovery from a trip were classified as follows: (1) lowering-hit strategy: the obstructed limb was quickly lowered to the ground and the contralateral unobstructed limb (i.e., right limb) was used to execute recovery stepping (Eng et al., 1994), (2) elevating-hit strategy: the obstructed limb (i.e., left limb) was used to execute recovery stepping after obstacle-hit (Eng et al., 1994), and (3) elevating-cross strategy: the subjects were able to cross over the obstacle without hitting.
Pre- and post-slip/trip events
The instants of step liftoff and touchdown were identified from the vertical ground reaction forces and verified by the foot kinematic data. The following four events were obtained to represent pre- and post-slip/trip instants: (1) pre-slip instant was obtained at touchdown of the leading/slipping limb which was always right touchdown (RTD), (2) pre-trip instant was obtained at 30 ms prior to obstacle-hit/cross (hit/cross), and (3) post-slip instant was obtained at the instant immediately prior to recovery foot touchdown (RecTD), and (4) post-trip instant was obtained at RecTD.
The instant of obstacle-hit was defined as the time at the minimum acceleration of the toe marker of the obstructed foot (i.e., left foot) in the walking direction (Pijnappels et al., 2004). For regular walking and those trip trials where subjects did not hit the obstacle device (i.e., elevating-cross), the time of obstacle-hit was defined as the time when the left toe marker was right above the erect plate. RecTD during a slip was obtained at left foot touchdown after slip onset. RecTDs were obtained at right and left foot touchdown respectively for lowering and elevating strategy employed during trip trials. For regular walking trials, RecTD were obtained at left and right foot touchdown respectively for slip and trip trials for post-perturbation comparisons.
COM state stability
The COM position and velocity in the anteriorposterior direction were calculated using a 13-segment rigid body model (de Leva, 1996). The COM position (XCOM/BOS) was defined as the absolute COM position in anteroposterior direction relative to the rear of base-of-support (BOS) and normalized by foot length. The COM velocity (VCOM/BOS) was calculated from differentiation of COM position and normalized to , where g was the acceleration due to gravity and bh was the height of the subject. Stability during a slip was defined by the relative motion state (i.e., position and velocity) between the COM and the BOS, and was measured as the shortest distance between the instantaneous COM state and predicted feasible stability region (FSR) limits for backward balance loss under slip conditions (Pai and Iqbal, 1999; Yang et al., 2008). Greater backward stability values indicated greater stability against backward balance loss (Pai et al., 2003; Bhatt et al., 2005). Stability during a trip was measured as the shortest distance between the instantaneous COM state and predicted FSR limits for forward balance loss under non-slip conditions (Pai and Patton, 1997). Greater forward stability values indicated greater forward instability. Stability value of greater than 1 indicated less stability against forward balance loss (forward instability); stability value between 0 and 1 indicated that the COM state was within the FSR. Stability value of less than 0 indicated that the COM state fell below the FSR where backward balance loss was predicted to occur.
Kinematic variables
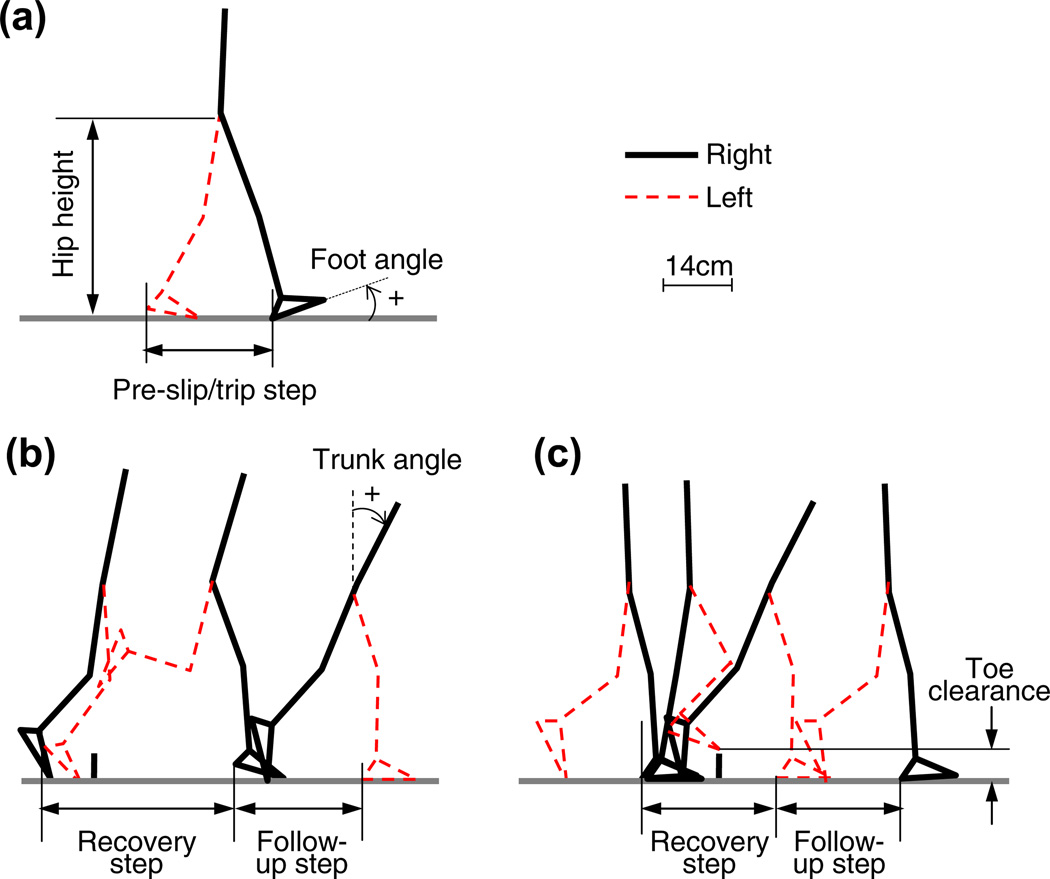
The following variables were obtained to analyze the contributing factors to adaptive changes during repeated-slip and -trip exposure (Pavol et al., 2001; Bhatt et al., 2006a,b): pre-slip/trip step length, post-trip recovery and follow-up step lengths, toe clearance, trunk angle, and hip height (as a measure to reflect limb support (Yang et al., 2009)). Pre-slip/trip step length was calculated as the horizontal distance measured from left heel to right heel during the stance phase of each leg prior to a slip/trip (Fig. 3a, b). Post-trip step length was the horizontal distance measured from one heel to the other during the stance phase of each leg after a trip. The same measurement was taken for the follow-up step length (i.e., the step taken after the recovery step). All step lengths were normalized by subjects’ height. Trunk angle was defined as the angle between the trunk segment and the vertical line (Fig. 3b). Toe clearance was measured as the vertical distance from the ground to the left toe (Fig. 3c). Hip height was assessed as the vertical distance from the ground to midpoint of bilateral hips and normalized by subjects’ height (Fig. 3a).
Fig. 3.
Demonstrations of the typical recordings of kinematic variables during slip and trip for (a) the hip height, pre-slip/trip step length, and the foot angle; (b) the recovery step length and the follow-up step length during trips with lowering strategy, and the trunk angle; and (c) the recovery step length and the follow-up step length during trips with elevating strategy, and the toe clearance.
The COM states (i.e., position and velocity) and stability were obtained at all four pre- and post-slip/trip events (RTD, hit/cross, RecTD for slip and trip). Toe clearance was obtained at hit/cross for trip trials, and at two-thirds of swing phase for slip trials. The maximum trunk extension for slip and maximum trunk flexion for trip, and minimum hip height during recovery (from RTD/(hit/cross) to RecTD) were calculated to further analyze reactive control of the trunk segment and limb support.
Behavioral outcomes
The outcome of a slip/trip was classified as a fall if the peak load cell force during the slip trial exceeded 30% body weight (Yang and Pai, 2011). A full recovery during a slip occurred when the moving average of load-cell force on the harness did not exceed 4.5% of body weight over any 1-s period after slip onset (Yang and Pai, 2011). Backward loss of balance during a slip occurred when subjects landed their contralateral limb posterior to the slipping heel. The trials where subjects landed their contralateral limb anterior to the slipping heel were classified as no loss of balance (Bhatt et al., 2006a,b). The outcome of a trip was classified as excessive instability with compensatory step if the step length of either the compensatory step or follow-up step exceeded 6 standard deviations of the step length averaged from six regular walking trials (SLbaseline) prior to the first trip (Wang et al., 2012). The outcome was classified as no loss of balance if the step length of both the compensatory and follow-up steps did not exceed six standard deviations of SLbaseline, and such an outcome was also true for those trials where subjects crossed over the obstacle device without hitting.
Statistics
To examine adaptation to slips, the Wilcoxon signed rank tests were performed to compare the outcomes of the first slip (S1) and the eighth slip (S8). Paired-t tests were performed to compare the following variables between S1 and S8: pre- and post-slip COM states (position and velocity) and stability, pre-slip step length, trunk extension, and hip height.
To examine interference to recovery following the first trip, the performance on the first trip of the training group (T1) was compared to that of the control group (TC, Fig. 2b), by comparing the following variables with independent t-tests: recovery step length, pre- and post-trip COM states (position and velocity) and stability (forward instability), pre-trip toe clearance, trunk flexion, hip height, trunk flexion, and hip height.
To analyze the effect of the mixed training on slips and trips, Cochran’s Q test with post hoc Wilcoxon signed-rank tests and one-way repeated measure analysis of variance (ANOVA) with post hoc planned paired-t tests, were applied. These were performed respectively to compare the slip/trip outcomes and variables related to slip/trip recovery (i.e., COM state, stability, step length, toe clearance, trunk angle, and hip height) on the following trials: NS/NT (regular walking performances during slip and trip), S1/T1 (before training), S9/T9 (during training), and S20/T20 (after training). Three hypotheses were tested by the following comparisons (Fig. 2b). First, to examine if the mixed training improved performances in slip and trip, performances on S20/T20 were compared to those on S1/T1. Second, to examine if improved performances were achieved gradually over the course of training, performances on S9/T9 were compared to those on S1/T1 and S20/T20. Last, to examine if improved performances could be attributed to adaptive adjustments made in the movement strategy, performances on S20/T20 were compared to those on NS/NT. In addition, pre-slip/trip step length and toe clearance were compared between S20 and T20. Additionally to confirm that adaptation to slip/trip perturbation training had occurred, planned trial-to-trial comparisons between consecutive slips (S1–S8) and trips (T1–T8) were performed using paired t-tests and Wilcoxon signed-rank respectively for stability (post-perturbation) and outcome. Similar planned comparisons were performed between trials N1 and NS, S1 and S8; and between trials N4 and NT, T1 and T8.
RESULTS
Recovery from first trip following slip training
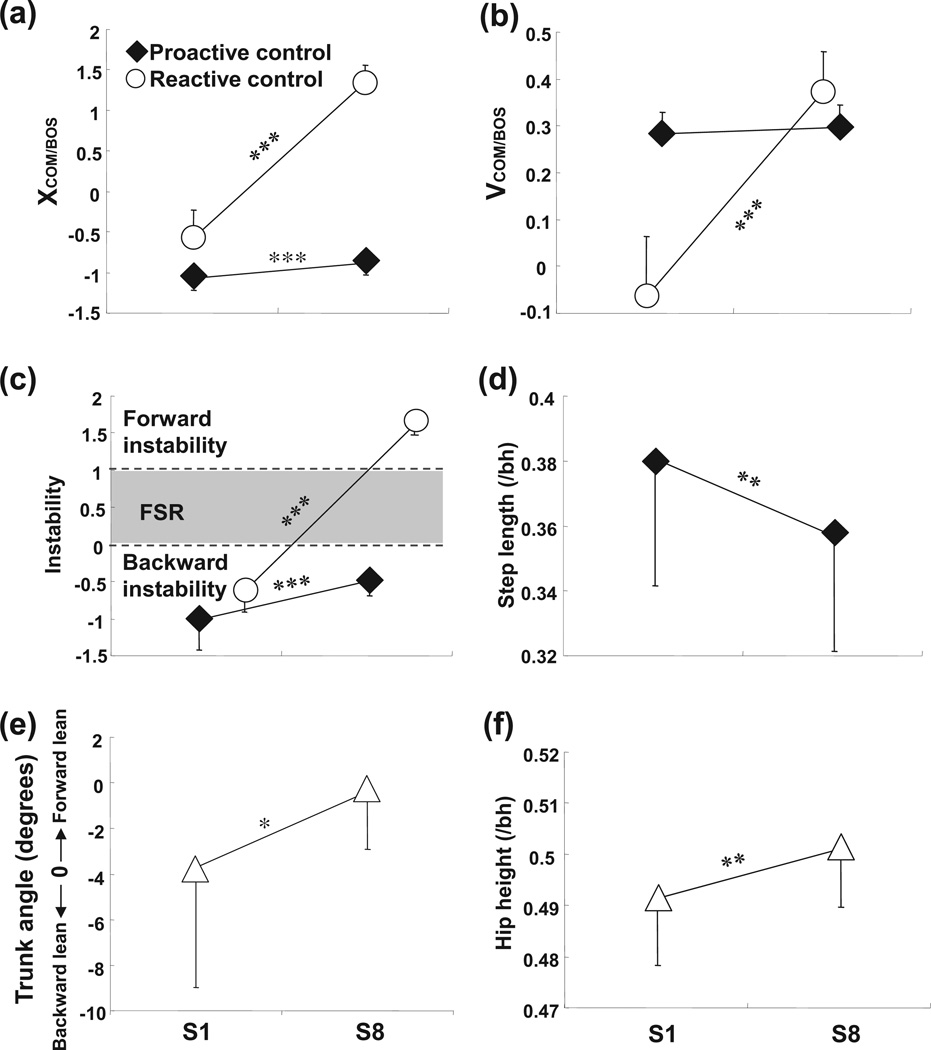
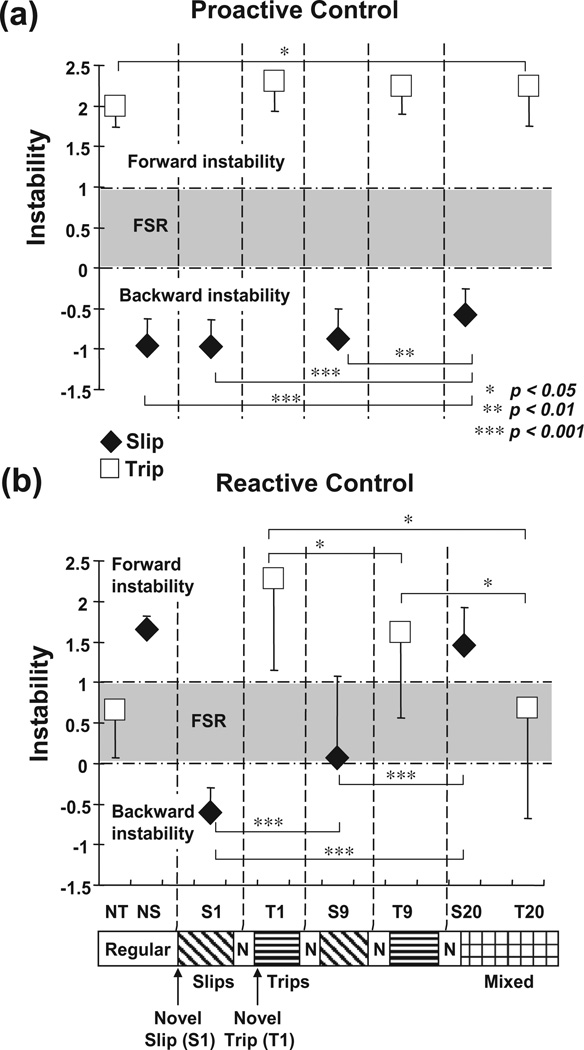
There were improvements in both proactive and reactive control of stability evidenced by significant increase in pre- and post-slip stability from S1 to S8 (both p < .001, Fig. 4c), greater pre- and post-slip forward COM position (both p < .001, Fig. 4a) and post-slip forward COM velocity (p < .001, Fig. 4b), a shorter step (p < .01, Fig. 4d), less trunk extension (p < .05, Fig. 4e), and greater hip height (p < .01, Fig. 4f). This was reflected in the improved slip outcomes. On the first novel slip (S1), all of the subjects (100%) had backward balance loss (one of them fell), but they were able to decrease such incidences to 0% on the eighth slip (S8, p < .001, Fig. 5a).
Fig. 4.
Comparison of group means (±standard deviation [SD]) of the following variables between the first slip (S1) and the eighth slip (S8) for the training group: pre- and post-slip (a) COM position, (b) COM velocity, and (c) stability, pre-slip (d) step length and (e) foot angle, and (f) post-slip hip height. The COM position (XCOM/BOS) was defined as the absolute COM position in anteroposterior direction relative to the rear BOS and normalized by foot length. The COM velocity (VCOM/BOS) was calculated from differentiation of COM position and normalized to . Stability was defined as the shortest distance between the instantaneous COM state (i.e., position and velocity) and predicted FSR limits for backward balance loss under slip conditions. Stability value between 0 and 1 indicated that the COM state was within the FSR. Stability value of less than 0 indicated that the COM state fell outside of the FSR where backward balance loss was predicted. Pre-slip step length was calculated as the horizontal distance measured from the most posterior position of the left heel marker during the stance phase of the left foot to the most posterior position of the right heel marker during the stance phase of the right foot. Foot angle was defined as the angle between foot segment and the horizontal; a smaller angle indicates a more flat-footed landing. Trunk angle was defined as the angle between the trunk segment with the vertical line (+: extension; −: flexion). Hip height was calculated as the vertical distance from the ground to midpoint of bilateral hips and normalized by subjects’ height (/bh). *p < 0.05; **p < 0.01; ***p < 0.001.
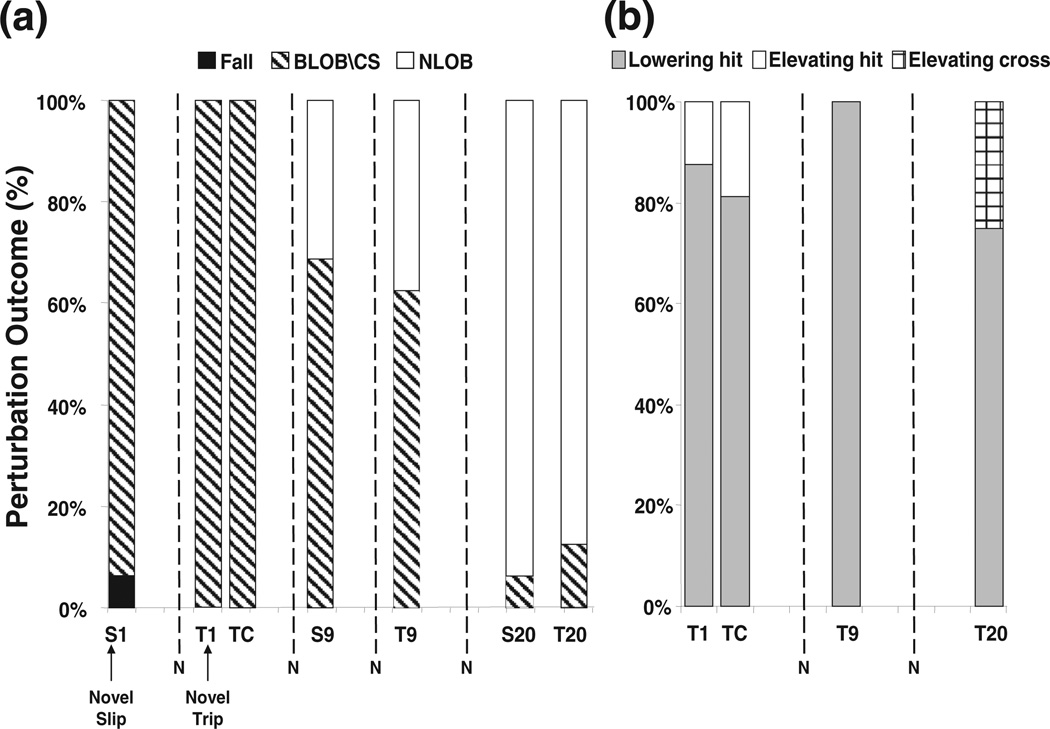
Fig. 5.
Shown are (a) the outcomes for selected trials of slips (S1, S9, and S20) and trips (T1, T9, and T20) for the training group, and the outcome of the first novel trip (TC) for the control group. A decrease in the percentage of falls (filled) and backward balance loss (BLOB) or compensatory stepping (CS) (hatched lines) was associated with an increase in the percentage of no loss of balance (NLOB) (unfilled) for the training group. All of the subjects in both the training and control groups had to take compensatory step to recover their balance on the first novel trip (T1 & TC). (b) Also shown were percentage changes in the strategy employed for recovery from selected trials of trips (T1, T9, and T20) for the training group and the first novel trip (TC) for the control group. Lowering-hit strategy (filled): the obstructed foot was rapidly lowered to the ground and the contralateral foot took the compensatory step after obstacle-hit. Elevating-hit strategy (unfilled): the obstructed foot took compensatory step after obstacle-hit. Elevating-cross strategy (cross lines): the subjects could cross over the obstacle without hitting. Overall, over 75% of the subjects in both training and control groups used lowering strategy to recover from a trip.
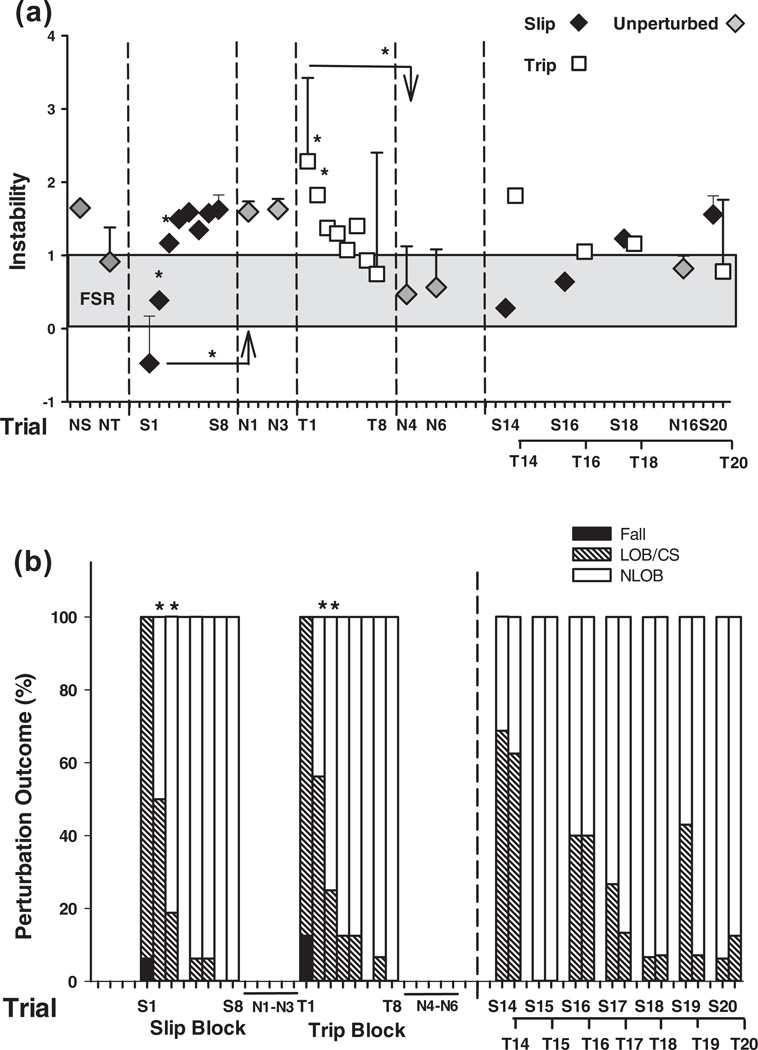
Note that subjects demonstrated a trial-to-trial increase in reactive stability from the first through the 8th trials (Fig. 6a) such that there was a significant difference in stability from NS to S1, S1 to S2, S2 to S3 (p < 0.05 for all comparisons) with a plateau effect (no significant changes) in the latter trials (S4–S8, p > 0.05). Stability on the first unperturbed trial was significantly greater than the first slip trial (S1 vs. N1, p < 0.05, Fig. 6a) but not different than that on S8 (p > 0.05). The trial-to-trial changes in slip outcome paralleled the changes in stability with significant decreases in backward balance loss from trial S1 to S2 and S2 to S3 with no change thereafter (p < 0.05) (Fig. 6b).
Fig. 6.
Shown are (a) group means (±SD) of adaptive changes in post-slip/trip stability (reactive control) and (b) outcomes on selected trials which include the first block of slips (S1 through S8) and trips (T1 through T8) for the training group. Stability during a slip (backward stability) was measured as the shortest distance between the instantaneous COM state and predicted FSR limits for backward loss of balance under slip conditions. Also represented (a) are stability values on selected slip and trip trials from the mixed block (14, 16, 18 and 20) and (b) the percentage outcome on all trials of the mixed block. Stability during a trip (forward instability) was measured as the shortest distance between the instantaneous COM state and predicted FSR limits for forward loss of balance under non-slip conditions. Stability value of greater than 1 indicated less stability against forward balance loss; stability value between 0 and 1 indicated that the COM state was within the FSR. Stability value of less than 0 indicated that the COM state fell outside of the FSR where backward balance loss was predicted. Also shown are unperturbed trials after the slip and trip blocks (N1–N3 and N4–N6 trials) and the last unperturbed trial of the entire protocol (N16). NS represented the data for the regular walking trial prior to the first novel perturbation (i.e., S1). NT represented the data for the regular walking trial prior to S1. Both NS and NT were obtained at left foot touchdown. (b) **p < 0.05; **p < 0.01; **p < 0.001.
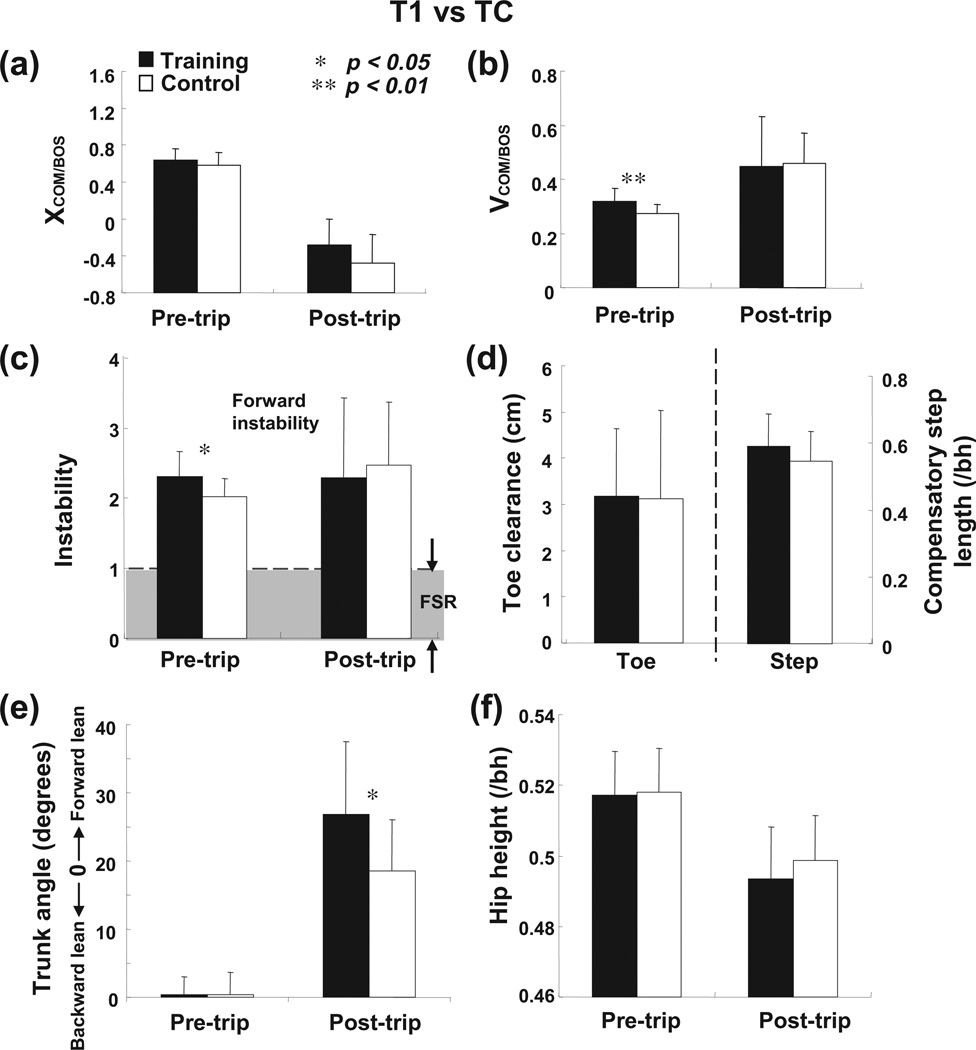
The training and control groups had similar outcomes that none of the subjects fell on the first novel trip, although all of them had to take compensatory step to restore stability (T1 and TC in Fig. 5a). All of the subjects hit the obstacle on the first trip, and 88% and 81% respectively for the training and control groups used lowering strategy to recover from the trip (Fig. 5b). In comparison to the control group, the training group demonstrated significantly greater pre-trip forward instability (p < .05, Fig. 7c) with greater pre-trip forward COM velocity during mid-stance immediately prior to obstacle contact (p < .01, Fig. 7b). Such between-group differences diminished thereafter. Although the training group had greater post-trip trunk flexion (p < .05, Fig. 7e), there was no difference in post-trip forward instability (p = .889, Fig. 7c), in toe clearance (p = .914, Fig. 7d) or in hip height (p = .833 and p = .297, respectively, Fig. 7f). As a result, the training group did not have to take a longer compensatory step than the control group did to overcome the instability (p = .100, Fig. 7d).
Fig. 7.
Comparison of group means (±SD) of the following variables between the first novel trip from the training (T1) and control (TC) groups: pre-and post-trip (a) COM position, (b) COM velocity, and (c) forward instability, (d) toe clearance and compensatory step length, pre- and post-trip (e) trunk angle and (f) hip height. The COM position (XCOM/BOS) was defined as the absolute COM position in anteroposterior direction relative to the rear BOS and normalized by foot length. The COM velocity (VCOM/BOS) was calculated from differentiation of COM position and normalized to . Stability was measured as the shortest distance between the instantaneous COM state and predicted FSR limits for forward loss of balance under non-slip conditions. Stability value of greater than 1 indicated less stability against forward balance loss; stability value between 0 and 1 indicated that the COM state was within the FSR. The toe clearance was measured as the vertical distance from the ground to the left toe. Trunk angle was defined as the angle between the trunk segment with the vertical line (+: extension; −: flexion). Hip height was calculated as the vertical distance from the ground to midpoint of bilateral hips and normalized by subjects’ height (/bh). *p < 0.05; **p < 0.01.
Effect of mixed (slip-trip) training
Significant trial main effects were identified for the outcomes for slips (Q(2, 16) = 23.333, p < .001) and trips (Q(5, 16) = 21.143, p < .001, Fig. 5a), pre-and post-slip backward stability (F(3, 45) = 12.304 and F(3, 45) = 62.505, respectively, both p < .001), pre- and post-trip forward instability (F(3, 45) = 4.416, and F(3, 45) = 11.272, respectively, both p < .05, Fig. 10), and all other variables including pre-slip/trip and post-slip/trip COM positions and velocities (Figs. 8 and 9), step length, toe clearance, trunk angle, and hip height (all p < .05) (Fig. 11). Over the course of the mixed training, performances during both slip and trip gradually improved and stabilized. The incidences of backward balance loss during slip and taking compensatory step during the trip gradually decreased from 100% on both S1 and T1 to 69% and 63% respectively for S9 and T9 (both p < .05, Fig. 5a), and then to 6% and 13% respectively for S20 and T20 (both p < .01, Fig. 5a). This was associated with substantial improvements first in reactive responses during mid-training (S9/T9) and later in both proactive and reactive adjustments by the end of training (S20/T20).
Fig. 10.
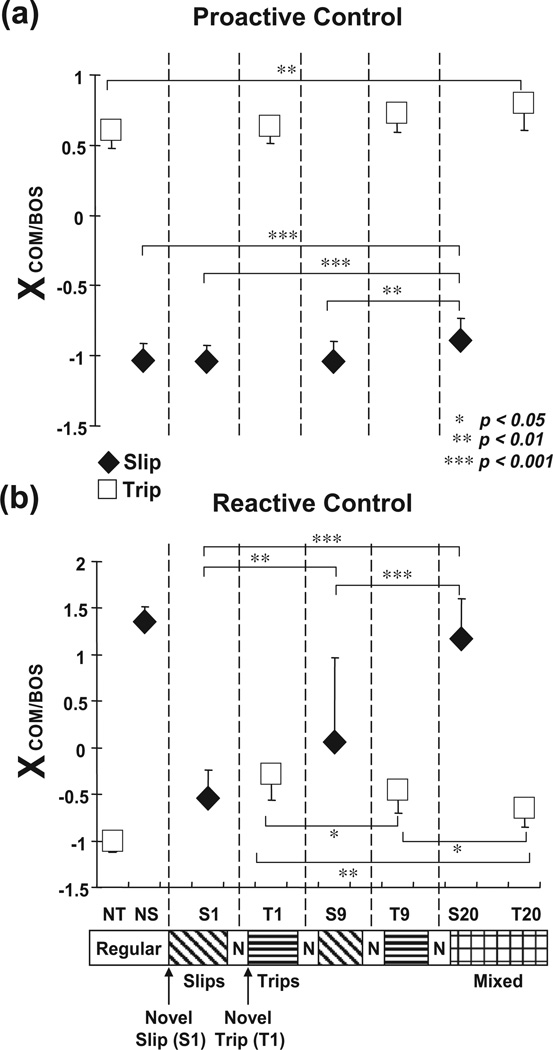
Shown are group means (±SD) of adaptive changes in (a) pre-slip/trip stability and (b) post-slip/trip stability on selected trials of slips (S1, S9, S20) and trips (T1, T9, T20) for the training group. Stability during a slip (backward stability) was measured as the shortest distance between the instantaneous COM state and predicted FSR limits for backward loss of balance under slip conditions. Stability during a trip (forward instability) was measured as the shortest distance between the instantaneous COM state and predicted FSR limits for forward loss of balance under non-slip conditions. Stability value of greater than 1 indicated less stability against forward balance loss; stability value between 0 and 1 indicated that the COM state was within the FSR. Stability value of less than 0 indicated that the COM state fell outside of the FSR where backward balance loss was predicted. NS represented the data for the regular walking trial prior to the first novel perturbation (i.e., S1), and its pre- and post-slip data were obtained respectively at leading/ slipping foot (i.e., right foot) and left foot touchdown. NT represented the data for the regular walking trial prior to S1, and its pre- and post-trip data were obtained respectively at 30 ms prior to the time when the left toe marker was right above the erect plate and left foot touchdown. *p < 0.05; **p < 0.01; ***p < 0.001.
Fig. 8.
Shown are group means (±SD) of adaptive changes in (a) pre-slip/trip COM position and (b) post-slip/trip COM position on selected trials of slips (S1, S9, S20) and trips (T1, T9, T20) for the training group. The COM position (XCOM/BOS) was defined as the absolute COM position in anteroposterior direction relative to the rear BOS and normalized by foot length. NS represented the data for the regular walking trial prior to the first novel perturbation (i.e., S1), and its pre- and post-slip data were obtained respectively at leading/ slipping foot (i.e., right foot) and left foot touchdown. NT represented the data for the regular walking trial prior to S1, and its pre- and post-trip data were obtained respectively at 30 ms prior to the time when the left toe marker was right above the erect plate and left foot touchdown. *p < 0.05; **p < 0.01; ***p < 0.001.
Fig. 9.
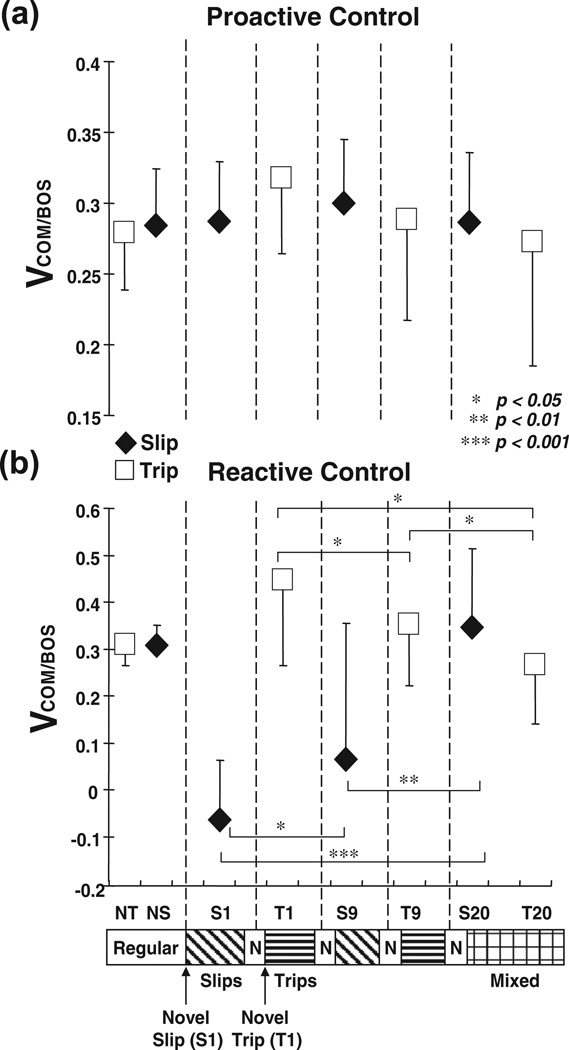
Shown are group means (±SD) of adaptive changes in (a) pre-slip/trip COM velocity and (b) post-slip/trip COM velocity on selected trials of slips (S1, S9, S20) and trips (T1, T9, T20) for the training group. The COM velocity (VCOM/BOS) was calculated from differentiation of the COM position and normalized to . NS represented the data for the regular walking trial prior to the first novel perturbation (i.e., S1), and its pre- and post-slip data were obtained respectively at leading/slipping foot (i.e., right foot) and left foot touchdown. NT represented the data for the regular walking trial prior to S1, and its pre- and post-trip data were obtained respectively at 30 ms prior to the time when the left toe marker was right above the erect plate and left foot touchdown. *p < 0.05; **p < 0.01; ***p < 0.001.
Fig. 11.
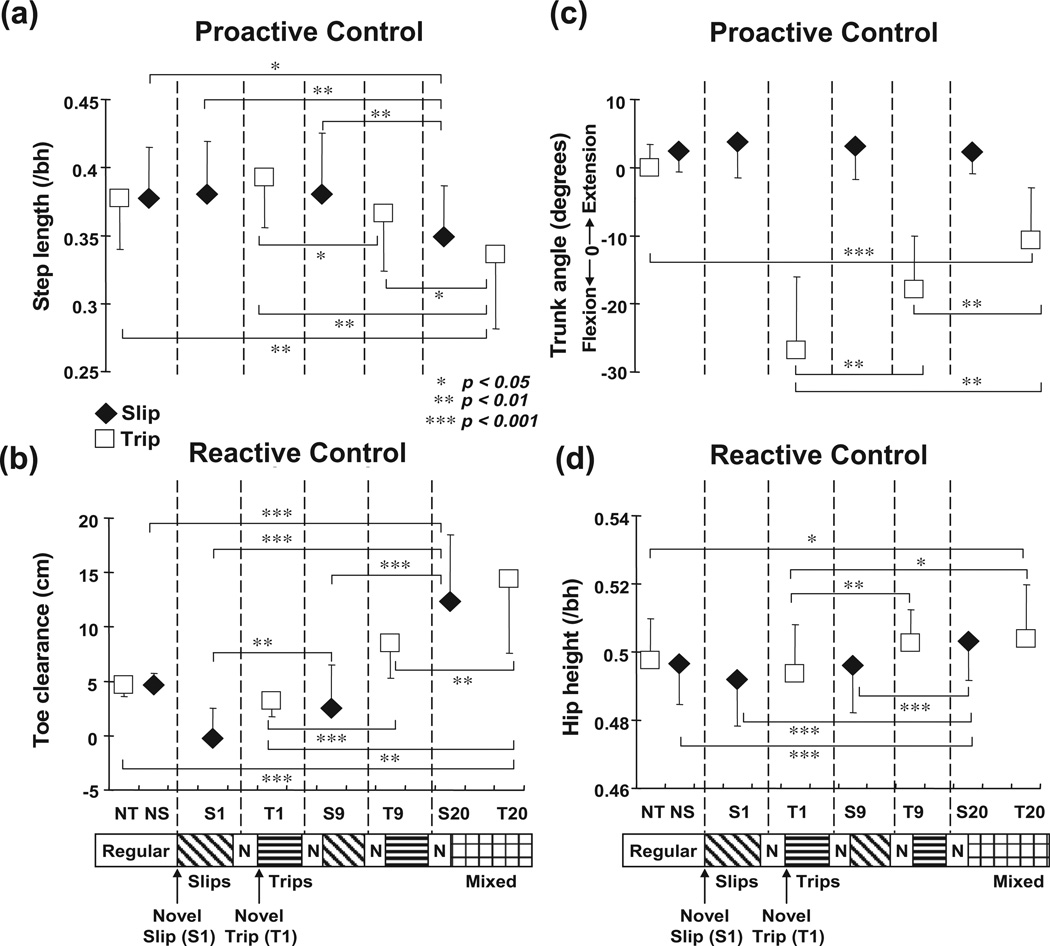
Shown are group means (±SD) of adaptive changes in (a) pre-slip/trip step length, (b) pre-slip/trip toe clearance, (c) post-slip/trip trunk angle, and (d) post-slip/trip hip height on selected trials of slips (S1, S9, and S20) and trips (T1, T9, and T20) for the training group. Pre-slip/trip step length was calculated as the horizontal distance measured from the most posterior position of the left heel marker during the stance phase of the left foot to the most posterior position of the right heel marker during the stance phase of the right foot prior to a slip/trip. Toe clearance was measured as the vertical distance from the ground to the left toe. Trunk angle was defined as the angle between the trunk segment with the vertical line (+: extension; −: flexion). Hip height was calculated as the vertical distance from the ground to midpoint of bilateral hips and normalized by subjects’ height (/bh). NS represented the data for the regular walking trial prior to the first novel perturbation (i.e., S1), and its pre- and post-slip data were obtained respectively at leading/slipping foot (i.e., right foot) and left foot touchdown. NT represented the data for the regular walking trial prior to S1, and its pre- and post-trip data were obtained respectively at 30 ms prior to the time when the left toe marker was right above the erect plate and left foot touchdown. *p < 0.05; **p < 0.01; ***p < 0.001.
A consistent trend emerged during slip portion of the mixed training. There were no differences in pre-slip stability (p = .101, Fig. 10a), step length (p = .958, Fig. 11a), trunk extension (p = .661, Fig. 11c), and hip height (p = .149, Fig. 11d) between S9 and S1. Greater post-slip stability (p < .01, Fig. 11b) together with greater post-slip forward COM position and velocity (p = .003 and p = .043, respectively, Figs. 8b and 9b) were identified on S9 in comparison to those on S1. By the end of training both pre- and post-slip stabilities on S20 were greater than those on S9 and S1 (all p < .01, Fig. 10a, b). In comparison to S9 and S1, S20 had greater pre- and post-slip forward COM position and velocity (all p < .01, Figs. 8a, b and 9a, b), shorter step (both p < .01, Fig. 9a), and greater hip height (both p < .001, Fig. 11d).
A consistent trend also emerged during trip portion of the mixed training. Pre-trip forward instability was not different between T9 and T1 (p = .522, Fig. 10a), but pre-trip toe clearance was greater on T9 in comparison to that on T1 (p < .001, Fig. 11b). Post-trip forward instability decreased (p = .032, Fig. 10b), less post-trip forward COM position and velocity (p = .023 and p = .026, respectively, Figs. 8b and 9b), reduced trunk flexion (p = .001, Fig. 11c), and greater hip height (p = .003, Fig. 11d) were identified on T9 in comparison to those on T1. Stability adjustments on T20 were most evident in post-trip stability. Post-trip forward instability on T20 was significantly lower than those on T9 and T1 (both p < .05, Fig. 11b). In comparison to T9 and T1, T20 had less post-trip forward COM position and velocity (all p < .05, Figs. 8b and 9b), less trunk flexion (both p < .01, Fig. 9c), and greater hip height (T20–T1, p < .05, Fig. 11d). T20 also had greater pre-trip toe clearance in comparison to those on T9 and T1 (both p < .01, Fig. 11b).
It must be noted that the two blocks of trip training were sufficient to induce adaptive training effects. Similar to the slip adaptation noted, there was a significant trial-to-trial change in stability from the baseline walking trial (NT) to T1, T1 to T2 and T2 to T3 (p < 0.05 for all trials, Fig. 6a). There was a plateau in adaptation reached from trials T4 through T8 (trial-to-trial differences, p > 0.05). Although there was no significant difference in forward instability between the unperturbed trial after the first trip block, N4, and the last trip training trial, T8 (N4 vs. T8, p > 0.05), it was significantly lower than on the first trip T1 (p < 0.01) and that on the regular unperturbed trial prior to trip training, NT (p < 0.05, Fig. 6a). There was no significant difference in stability between N6, the 3rd, unperturbed trial after the first trip training block and the last unperturbed trial, N16 (p > 0.05) which occurred after the mixed training. The trial-to-trial changes in trip outcome paralleled the changes in stability with significant decreases in compensatory steps from trial T1 to T2, T2 to T3 (p < 0.05) with no change thereafter (p > 0.05). Note that the resulting adaptation in reactive stability did not result from an alteration in gait velocity. There was no difference in gait velocity on the first unperturbed trial post slip training (N1, 1.23 ± 0.18 m/s) and post trip training (N4, 1.20 ± 0.19 m/s) and the last unperturbed trial post mixed training (N16, 1.16 ± 0.23) compared to the baseline natural walking trials (1.17 ± 0.17 m/s, p > 0.05 for all comparisons).
By the end of training, a compromised solution appeared to emerge against these two types of perturbation. In comparison to regular walking prior to the mixed training (NS), S20 had greater pre-slip stability (p < .001, Fig. 10a), accompanied by greater pre-slip forward COM position (p < .01, Fig. 8a) and shorter step (p < .05, Fig. 11a). S20 had similar post-slip stability to NS (p = .106, Fig. 10b), but demonstrated greater post-slip hip height (p < .001, Fig. 9d). In comparison to the performances during regular walking (NT), T20 had slightly greater pre-trip forward instability (p < .05, Fig. 10a), pre-trip forward COM position (p < .01, Fig. 8a), and post-trip trunk flexion (p < .001, Fig. 11c). However, T20 had similar post-trip stability to NT (p = .933, Fig. 10b), anddemonstrated greater pre-trip toe clearance (p < .001, Fig. 11b) and post-trip hip height (p < .05, Fig. 11d). Finally, gradual decrease in pre-slip/trip step length (factor associated mostly with slip adaptation) and increase in toe clearance (factor associated mostly with trip adaptation) were observed during the entire training (Fig. 11a, b). There were no differences in pre-slip/trip step length and toe clearance between S20 and T20 (both p = .194 and p = .110, respectively, Fig. 11a, b), indicating that those adjustments were made regardless of the type of perturbation.
DISCUSSION
This study was the first to investigate how slip training might interfere with the recovery from a sudden unrehearsed trip, and whether the generalized strategy that could eventually emerge after a mixed (slip-trip) training when facing the uncertainty of these two types of diametrically opposing postural disturbance. The results indicated that prior adaptation to slips in fact only had very limited interference during trip recovery, in part because of the success of the robust reactive responses primed following repeated-slip exposure. Further, the mixed training enabled these subjects to improve their control of stability and find a “middle ground” that satisfied, two opposing sets of task objectives for successful recovery.
Recovery from the novel trip following slip training
Consistent with previous findings, subjects were able to improve proactive and reactive control of COM stability, and reactive control of limb support to reduce the incidence of balance loss and falls after exposure to repeated slips (Bhatt et al., 2006a,b). Adaptive improvements in COM stability mainly resulted from an anterior shift of the COM position caused by a shorter preslip step length at slipping foot touchdown and a significant increase in post-slip forward COM velocity prior to RecTD (Bhatt et al., 2006a,b). While these adjustments improved these people’s COM stability against backward balance loss, they also increased their vulnerability against forward instability. Stability against a trip was mainly achieved by posterior shift of the COM position and/or reduce forward COM velocity (Wang et al., 2012). Viewed in this way, one would expect interference rather than positive generalization taking place during trip recovery following slip training, based on previous findings of motor interference (Bock et al., 2001; Tong et al., 2002). Indeed, adaptive adjustments acquired from prior slip training predisposed these subjects to an elevated level of instability related to forward falling (caused by greater forward COM velocity in mid-stance prior to the trip onset).
Rather unexpectedly, repeated-slip training had also induced robust reactive control of stability that was sufficient to mitigate any initial interference. This was exhibited by a lack of difference in recovery outcomes (rather than a worsened outcome) between the groups. The training group was able to amend the early pre-trip instability following the trip onset (Fig. 7c), such that there was no difference in post-trip stability and hence subjects in the training group did not have to take a longer recovery step than the controls to compensate for the early instability and to regain balance (Fig. 7d).
Although limited interference was also demonstrated by the weaker control of forward trunk rotation after trip onset in the training group (significantly greater trunk flexion) than the controls, such interference did not affect the training group’s ability to produce sufficient limb support for preventing a fall (no difference in hip height between groups). While one might argue that the limited interference found in the training group could be attributed to their anticipation of the perturbation, this might not be the case given that both the training and control groups were given identical information pertaining to the upcoming trip prior to the test. Rather, the difference should be attributed to factors other than the anticipatory status, such as the CNS’s flexibility in the control of stability, especially in its ability to scale up reactive correction of any earlier error from interference.
Such flexibility may result from the multiple degrees-of-freedom (DOF) afforded in gait. As in single- or dual-degrees-of-freedom task of reaching movement of the upper limb, perturbation from an opposing force field environment indeed can produce rather prominent interfering effects (Shadmehr and Brashers-Krug, 1997). Adjustments made for recovery from gait-slip or gait-trip can be many, whereby a person can quickly accommodate to such a new context by altering leading limb’s landing angle, foot clearance, step length, or step velocity, just to name a few, in addition to altering the trailing (recovery) limb’s push-off mechanics and the control of trunk orientation after repeated slip training (Bhatt et al., 2011; Wang et al., 2012). These adjustments appear to be amendable when the context is altered again. The present study provides an example on how the CNS can quickly take advantage of such flexibility afforded by movement with multiple DOF within merely a few hundred milliseconds to reactively overcome and compensate for the early interference that was still significant and detectable in several aspects.
Effect of mixed (slip-trip) training
Any interference that could exist clearly diminished following repeated-trip exposure. Over the course of the mixed training, performances in both slip and trip improved progressively, whereby the CNS appears to have adopted a general movement strategy that could accommodate both slip and trip by adaptively adjusting both proactive and reactive control through trial-and-error practice. These subjects preferred shorter steps (resulting in more anteriorly positioned COM to better resist backward falling) and greater toe clearance (reducing the likelihood of obstacle contact to avoid a trip) (Figs. 8a and 11b). In addition, a common reactive strategy emerged during training to improve limb support (Fig. 11d), suggesting that the CNS was able to take advantage of similarity in control of limb support in the vertical direction — a common element shared by the two opposing perturbations.
In terms of feed-forward control, the CNS might have had at least two options to deal with uncertainties in the present study. It could rely solely on its context prediction based on the immediate experience to counter each individual type of perturbation (Scheidt et al., 2001; Witney et al., 2001). For example, based on a slip that has just occurred, if the CNS anticipates another slip to occur, its movement strategy would be reflected by a significant anterior shift of the XCOM/BOS or a substantial reduction in step length, etc. Errors in such predictive control (if a trip instead of a slip occurred) could force it to increase its reliance on reactive control. Alternatively, it can reduce the reliance on predictive control by opting for a generalized “middle-ground” strategy (Takahashi et al., 2001). This latter strategy may not represent the best or most economical response to each individual perturbation. Yet, it could reduce the CNS’ reliance on the accuracy of prediction, and hence could reduce the potentially injurious penalty arising from the mistakes of context prediction. This appears to be the option the CNS chose.
With repeated practice, a compromise between competing needs began to emerge with pre-programed “middle-ground” strategy consisting of a shortened step length and an increased toe clearance. A shortened step length which led to more anteriorly positioned COM, was the adaptive strategy specific to slip adaptation (Marigold and Patla, 2002; Bhatt et al., 2006a,b). Such an adaptive strategy in fact does not help trip recovery. Instead, it worsens the extent of forward instability caused by the trip due to greater amount of COM forward displacement (Wang et al., 2012). An elevated swing can assist toe clearance to avoid a contact with the obstacle and an ensuing trip. Walking constantly with a higher than normal toe clearance is unnecessary because it would require additional energy consumption, and such strategy is certainly unnecessary during slip recovery. If the CNS adopts a strategy solely for either a slip or a trip, shortened step length and elevated toe clearance should not have appeared together.
Noticeably, the “middle-ground” strategy adopted in the proactive (feed-forward) control was accompanied by improved reactive control of stability and limb support – two key variables which have been demonstrated to contribute significantly to fall-risk (Pavol and Pai, 2007; Yang et al., 2009). Such findings have been reported in our previous studies examining slip alone, where a steady-state movement pattern emerged and was reflected in the COM state stability control (Bhatt et al., 2006a). This adaptation could be considered as a generalized motor strategy for falls reduction. This could also explain why a significant forward trunk leaning did not alter the recovery outcome on the first, novel trip in the training group. It must be noted that the trial order in the mixed block was not completely random, such that the slip trial always preceded the trip trial. Thus there is a possibility of an order effect. If this were the case then the trip trials would show significantly better performance than the preceding slip trials, both for outcome and stability, especially for the later perturbation trials (T18 through T20). However, our results do not indicate this. Performance on both S20 and T20 was very similar, with no significant difference in perturbation outcome between the two trials (6% vs. 13% balance loss) (Fig. 6b). Further there was no difference in pre-slip stability and step length between S20 and T20, indicating that trial effect did not affect these changes (Fig. 11a, b).
As reported in previous work (Wang et al., 2012), adaptation to repeated trips induced by an obstacle in fact consisted of two components: one was to directly adjust the relationship between the COM and BOS to maintain the stability, and the other was to avoid the contact of the obstacle by elevating the foot clearance. Both strategies are effective in restoring balance and they might even be somewhat interlinked or coupled, as one might have been assumed. Choices also exist in adaptation to repeated slips, in which subjects could increase stability against backward falling by a forward shift of their COM relative to BOS, by simply increasing the COM velocity relative to the BOS, or by a combination of the two. The strategy selection was an implicit process by the CNS, and it could depend on multiple factors such as subjects’ perception of threat, personality of the individual (risk taking vs. conservational approach, etc.) as well as physical, functional or anatomical constraints. Again, multiple DOF movement in walking can afford these choices.
The findings of the present study demonstrated the adaptation process within the CNS through proactive spatial parameterization of step length and foot clearance and improved reactive control of COM state stability and vertical limb support for negotiating two opposing types of postural disturbance. The locus of control responsible for such adaptation is still far from certain. While spinal cord circuitries have the plasticity and memory required for storing adaptive responses (Frigon and Rossignol, 2008; Rossignol et al., 2011), cortical and subcortical structures may be the storage sites for locomotor-balance adaptations to complex and challenging perturbations (Lawrence and Kuypers, 1968a,b; Kably and Drew, 1998; Prentice and Drew, 2001). In particular, cerebellum may have a role in the acquisition and storage of locomotor adaptations (Morton and Bastian, 2004, 2006), as suggested through cerebellar-thalamo-cortical pathway within such spatial domain (Vasudevan et al., 2011). The sensory consequences of the perturbation received by the cerebellum via the spinocerebellar tracts could be processed and transmitted to the cortex via the midbrain (Valle et al., 2000; Mori et al., 2004a,b). Descending commands could then be communicated to the central pattern generators in this adaptive process (Mori et al., 1978, 1982; Mori, 1987; Matsuyama et al., 2004).
In summary, while repeated-slip training indeed produced early destabilizing interference upon a sudden occurrence of an unannounced and unrehearsed trip, it also provided the benefit of priming these young adults’ reactive control, which was sufficient to mitigate the movement error from early interference. The subsequent mixed-slip-and-trip training did not lead to the emergence of two opposing movement patterns, which would require a rather accurate context prediction. Instead, subjects were able to develop a compromised yet generalized movement strategy by adaptively adjusting both proactive and reactive control of COM stability and reactive control of limb support.
Acknowledgements
The authors wish to thank Daniel Phelps, Natalie Menick, Hyosub Kim, and Noman Khan for assistance with data collection. This work was funded by NIH RO1-AG029616.
Abbreviations
- BOS
base-of-support
- COM
center-of-mass
- DOF
degrees-of-freedom
- FSR
feasible stability region
- RecTD
recovery foot touchdown
- RTD
right touchdown
- SD
standard deviation
Footnotes
CONFLICT OF INTEREST
None.
REFERENCES
- Bhatt T, Pai YC. Immediate and latent interlimb transfer of gait stability adaptation following repeated exposure to slips. J Mot Behav. 2008;40:380–390. doi: 10.3200/JMBR.40.5.380-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt T, Pai YC. Generalization of gait adaptation for fall prevention: from moveable platform to slippery floor. J Neurophysiol. 2009;101:948–957. doi: 10.1152/jn.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt T, Wening JD, Pai Y-C. Influence of gait speed on stability: recover from anterior slips and compensatory stepping. Gait Posture. 2005;21:146–156. doi: 10.1016/j.gaitpost.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Bhatt T, Wening JD, Pai YC. Adaptive control of gait stability in reducing slip-related backward loss of balance. Exp Brain Res. 2006a;170:61–73. doi: 10.1007/s00221-005-0189-5. [DOI] [PubMed] [Google Scholar]
- Bhatt T, Wang E, Pai YC. Retention of adaptive control over varying intervals: prevention of slip-induced backward balance loss during gait. J Neurophysiol. 2006b;95:2913–2922. doi: 10.1152/jn.01211.2005. [DOI] [PubMed] [Google Scholar]
- Bhatt T, Espy D, Yang F, Pai YC. Dynamic gait stability, clinical correlates, and prognosis of falls among community-dwelling older adults. Arch Phys Med Rehabil. 2011;92:799–805. doi: 10.1016/j.apmr.2010.12.032. [DOI] [PubMed] [Google Scholar]
- Bock O, Schneider S, Bloomberg J. Conditions for interference versus facilitation during sequential sensorimotor adaptation. Exp Brain Res. 2001;138:359–365. doi: 10.1007/s002210100704. [DOI] [PubMed] [Google Scholar]
- de Leva P. Adjustments to Zatsiorsky-Seluyanov’s segment inertia parameters. J Biomech. 1996;29:1223–1230. doi: 10.1016/0021-9290(95)00178-6. [DOI] [PubMed] [Google Scholar]
- Eng JJ, Winter DA, Patla AE. Strategies for recovery from a trip in early and late swing during human walking. Exp Brain Res. 1994;102:339–349. doi: 10.1007/BF00227520. [DOI] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Locomotor and reflex adaptation after partial denervation of ankle extensors in chronic spinal cats. J Neurophysiol. 2008;100:1513–1522. doi: 10.1152/jn.90321.2008. [DOI] [PubMed] [Google Scholar]
- Kably B, Drew T. Corticoreticular pathways in the cat. II. Discharge activity of neurons in area 4 during voluntary gait modifications. J Neurophysiol. 1998;80:406–424. doi: 10.1152/jn.1998.80.1.406. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968a;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968b;91:15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- Marigold DS, Patla AE. Strategies for dynamic stability during locomotion on a slippery surface: effects of prior experience and knowledge. J Neurophysiol. 2002;88:339–353. doi: 10.1152/jn.00691.2001. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Nakajima K, Mori F, Aoki M, Mori S. Lumbar commissural interneurons with reticulospinal inputs in the cat: morphology and discharge patterns during fictive locomotion. J Comp Neurol. 2004;474:546–561. doi: 10.1002/cne.20131. [DOI] [PubMed] [Google Scholar]
- Mori S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog Neurobiol. 1987;28:161–195. doi: 10.1016/0301-0082(87)90010-4. [DOI] [PubMed] [Google Scholar]
- Mori S, Nishimura H, Kurakami C, Yamamura T, Aoki M. Controlled locomotion in the mesencephalic cat: distribution of facilitatory and inhibitory regions within pontine tegmentum. J Neurophysiol. 1978;41:1580–1591. doi: 10.1152/jn.1978.41.6.1580. [DOI] [PubMed] [Google Scholar]
- Mori S, Kawahara K, Sakamoto T, Aoki M, Tomiyama T. Setting and resetting of level of postural muscle tone in decerebrate cat by stimulation of brain stem. J Neurophysiol. 1982;48:737–748. doi: 10.1152/jn.1982.48.3.737. [DOI] [PubMed] [Google Scholar]
- Mori F, Nakajima K, Tachibana A, Takasu C, Mori M, Tsujimoto T, Tsukada H, Mori S. Reactive and anticipatory control of posture and bipedal locomotion in a nonhuman primate. Prog Brain Res. 2004a;143:191–198. doi: 10.1016/S0079-6123(03)43019-7. [DOI] [PubMed] [Google Scholar]
- Mori S, Nakajima K, Mori F, Matsuyama K. Integration of multiple motor segments for the elaboration of locomotion: role of the fastigial nucleus of the cerebellum. Prog Brain Res. 2004b;143:341–351. doi: 10.1016/S0079-6123(03)43033-1. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Prism adaptation during walking generalizes to reaching and requires the cerebellum. J Neurophysiol. 2004;92:2497–2509. doi: 10.1152/jn.00129.2004. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai Y, Iqbal K. Simulated movement termination for balance recovery: can movement strategies be sought to maintain stability in the presence of slipping or forced sliding? J Biomech. 1999;32:779–786. doi: 10.1016/s0021-9290(99)00074-3. [DOI] [PubMed] [Google Scholar]
- Pai YC, Patton J. Center of mass velocity-position predictions for balance control. J Biomech. 1997;30:347–354. doi: 10.1016/s0021-9290(96)00165-0. [DOI] [PubMed] [Google Scholar]
- Pai YC, Wening JD, Runtz EF, Iqbal K, Pavol MJ. Role of feedforward control of movement stability in reducing slip-related balance loss and falls among older adults. J Neurophysiol. 2003;90:755–762. doi: 10.1152/jn.01118.2002. [DOI] [PubMed] [Google Scholar]
- Pai YC, Bhatt T, Wang E, Espy D, Pavol MJ. Inoculation against falls: rapid adaptation by young and older adults to slips during daily activities. Arch Phys Med Rehabil. 2010;91:452–459. doi: 10.1016/j.apmr.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patla AE, Prentice SD, et al. Visual control of locomotion: strategies for changing direction and for going over obstacles. J Exp Psychol Hum Percept Perform. 1991;17:603–634. doi: 10.1037//0096-1523.17.3.603. [DOI] [PubMed] [Google Scholar]
- Pavol MJ, Pai Y-C. Deficient limb support is a major contributor to age-differences in falling. J Biomech. 2007;40:1318–1325. doi: 10.1016/j.jbiomech.2006.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavol MJ, Owings TM, Foley KT, Grabiner MD. Mechanisms leading to a fall from an induced trip in healthy older adults. J Gerontol A Biol Sci Med Sci. 2001;56:M428–M437. doi: 10.1093/gerona/56.7.m428. [DOI] [PubMed] [Google Scholar]
- Pijnappels M, Bobbert MF, van Dieen JH. Contribution of the support limb in control of angular momentum after tripping. J Biomech. 2004;37:1811–1818. doi: 10.1016/j.jbiomech.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Prentice SD, Drew T. Contributions of the reticulospinal system to the postural adjustments occurring during voluntary gait modifications. J Neurophysiol. 2001;85:679–698. doi: 10.1152/jn.2001.85.2.679. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Frigon A, Barriere G, Martinez M, Barthelemy D, Bouyer L, Belanger M, Provencher J, Chau C, Brustein E, Barbeau H, Giroux N, Marcoux J, Langlet C, Alluin O. Chapter 16 – spinal plasticity in the recovery of locomotion. Prog Brain Res. 2011;188:229–241. doi: 10.1016/B978-0-444-53825-3.00021-8. [DOI] [PubMed] [Google Scholar]
- Scheidt RA, Dingwell JB, Mussa-Ivaldi FA. Learning to move amid uncertainty. J Neurophysiol. 2001;86:971–985. doi: 10.1152/jn.2001.86.2.971. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Brashers-Krug T. Functional stages in the formation of human long-term motor memory. J Neurosci. 1997;17:409–419. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi CD, Scheidt RA, Reinkensmeyer DJ. Impedance control and internal model formation when reaching in a randomly varying dynamical environment. J Neurophysiol. 2001;86:1047–1051. doi: 10.1152/jn.2001.86.2.1047. [DOI] [PubMed] [Google Scholar]
- Tong C, Wolpert DM, Flanagan JR. Kinematics and dynamics are not represented independently in motor working memory: evidence from an interference study. J Neurosci. 2002;22:1108–1113. doi: 10.1523/JNEUROSCI.22-03-01108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle MS, Bosco G, Poppele R. Information processing in the spinocerebellar system. Neuroreport. 2000;11:4075–4079. doi: 10.1097/00001756-200012180-00033. [DOI] [PubMed] [Google Scholar]
- Vasudevan EV, Torres-Oviedo G, Morton SM, Yang JF, Bastian AJ. Younger is not always better: development of locomotor adaptation from childhood to adulthood. J Neurosci. 2011;31:3055–3065. doi: 10.1523/JNEUROSCI.5781-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TY, Bhatt T, Yang F, Pai YC. Generalization of motor adaptation to repeated-slip perturbation across tasks. Neuroscience. 2011;180:85–95. doi: 10.1016/j.neuroscience.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TY, Bhatt T, Yang F, Pai YC. Adaptive control reduces trip-induced forward gait instability among young adults. J Biomech. 2012;45:1169–1175. doi: 10.1016/j.jbiomech.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witney AG, Vetter P, Wolpert DM. The influence of previous experience on predictive motor control. Neuroreport. 2001;12:649–653. doi: 10.1097/00001756-200103260-00007. [DOI] [PubMed] [Google Scholar]
- Yang F, Pai YC. Automatic recognition of falls in gait-slip training: harness load cell based criteria. J Biomech. 2011;44:2243–2249. doi: 10.1016/j.jbiomech.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Anderson FC, Pai YC. Predicted threshold against backward balance loss following a slip in gait. J Biomech. 2008;41:1823–1831. doi: 10.1016/j.jbiomech.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Bhatt T, Pai YC. Role of stability and limb support in recovery against a fall following a novel slip induced in different daily activities. J Biomech. 2009;42:1903–1908. doi: 10.1016/j.jbiomech.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]