Abstract
On retinal ganglion cells (RGCs) transmit light encoded information to the brain and receive excitatory input from On cone bipolar cells (CBPs). The synaptic CBP input onto On RGCs is mediated by AMPA-type glutamate receptors (AMPARs) that include both those lacking a GluA2 subunit, and are therefore permeable to Ca2+, and those that possess at least one GluA2 subunit and are Ca2+-impermeable. We have previously demonstrated in electrophysiological studies that periods of low synaptic activity, brought about by housing animals in darkness, enhances the proportion of GluA2-lacking AMPARs at the On CBP-On RGC synapse by mobilizing surface GluA2 containing receptors into a receptor pool that rapidly cycles in and out of the membrane. AMPAR cycling induction by reduced synaptic activity takes several hours. This delay suggests that changes in expression of proteins which regulate AMPAR trafficking may mediate the altered mobility of GluA2 AMPARs in RGCs. In this study, we test the hypothesis that AMPAR trafficking proteins couple synaptic activity to AMPAR cycling in RGCs. Immunocytochemical and biochemical analysis confirmed that darkness decreases surface GluA2 in RGCs and changed the expression levels of three proteins associated with GluA2 trafficking. GRIP was decreased, while PICK1 and Arc were increased. Knockdown of GRIP with siRNA elevated constitutive AMPAR cycling, mimicking effects of reduced synaptic activity, while knockdown of PICK1 and ARC blocked increases in constitutive GluA2 trafficking. Our results support a role for correlated, activity-driven changes in multiple AMPAR trafficking proteins that modulate GluA2 cycling which can in turn affect synaptic AMPAR composition in RGCs.
Keywords: AMPA receptor, retina, plasticity, PICK1, GRIP, Arc
Introduction
Activity-driven synaptic plasticity is widely expressed at synapses throughout the CNS, often through highly conserved mechanisms (Derkach et al., 2007; Malinow and Malenka, 2002). Such synaptic plasticity provides neurons with the ability to respond and adapt to changing environmental cues. While the retina exhibits multiple levels of adaptation that allow for visual responsiveness over a tremendous range of light intensities (Abraham and Bear, 1996; Demb, 2008; Laughlin, 1989), there has been little evidence that retinal neurons express forms of synaptic plasticity comparable to those at other central synapses. We recently have reported that On RGCs exhibit synaptic plasticity in response to chronic changes in ambient light levels. Similar to forms exhibited at a number of CNS synapses, RGC synaptic plasticity is expressed as a change in the composition of synaptic AMPA-type glutamate receptors (AMPARs) (Xia et al., 2006; Xia et al., 2007).
We previously reported a decrease in the proportion of surface GluA2-containing AMPARs in On RGCs of retina from mice housed in the darkness for > 8 hours compared to those from animals housed in light (Xia et al., 2007). As the GluA2 subunit confers calcium impermeability as well as decreased conductance and slowed kinetics on AMPARs (Baudry et al., 1980), this composition shift is predicted to greatly alter synaptic signaling. Slow, activity-mediated changes in AMPAR composition have been similarly reported in the barrel cortex and VTA (Bellone and Luscher, 2006; Clem and Barth, 2006). However, the mechanisms by which activity couples to these changes in AMPAR composition are largely unknown. In the retina we found that the activity- dependent shift in AMPAR composition was dependent on the initiation of constitutive cycling of GluA2-containing AMPARs in and out of the membrane surface (Xia et al., 2006) resulting in an increase in the proportion of GluA2- lacking AMPARs at the On CBP-On RGC synapse (Xia et al., 2007). The time course of the modulation of AMPAR trafficking suggests that protein translation may be critically involved in AMPAR plasticity in On ganglion cells.
Several proteins have been identified that could impact the constitutive endocytosis and exocytosis of GluA2-containing AMPARs. Arc/ARG 3.1, an immediate early gene, enhances the endocytosis of AMPARs in response to activity and is translationally regulated by activity (Chowdhury et al., 2006; Rial Verde et al., 2006; Shepherd et al., 2006). Glutamate Receptor Interacting Protein/AMPA Binding Proteins (GRIP/ABP) have been reported to have multiple effects on AMPAR trafficking: limiting endocytosis of bound GluA2 (Casimiro et al., 2011; Osten et al., 1998), increasing stabilization of GluA2 in intracellular pools (Braithwaite et al., 2002; DeSouza et al., 2002), and either reducing (Hanley and Henley, 2010) or enhancing (Mao et al., 2010; Thomas et al., 2012) activity-dependent AMPAR recycling. PICK1 competes with GRIP proteins for binding to the C-terminus of GluA2 (Chung et al., 2000). PICK1 also has been reported to be regulated by activity in hippocampal neurons (Narasimhan, 2007), enhance GluA2 endocytosis (Chung et al., 2003; Xia et al., 2000), reduce surface GluA2 expression (Sossa et al., 2006; Terashima et al., 2004), and maintain GluA2 in intracellular pools (Chung et al., 2003; Hill et al., 2004; Sossa et al., 2006). Like GRIP proteins, PICK1 either reduces (Lin and Huganir, 2007) or enhances (Gardner et al., 2005) the redelivery of GluA2 receptors to the membrane surface. While each of these proteins is able to modulate the trafficking of AMPARs, it is unknown whether activity dependent changes in the expression of one or more of these GluA2 regulatory proteins modulate the constitutive recycling of AMPARs.
Here, we have investigated molecular mechanisms by which physiological activity could couple to the enhanced cycling of GluA2-containing AMPARs and ultimately alter synaptic function in retinal ganglion cells (RGCs). Using immunocytochemistry and biochemical analysis we have observed a decrease in GRIP and an increase in both Arc and PICK1 expression after light deprivation, a stimulus previously shown to enhance constitutive GluA2 cycling (Xia et al., 2007). To determine whether changes in GRIP, Arc or PICK1 expression could contribute to altering constitutive GluA2 cycling in RGCs, we utilized siRNA mediated knockdown. The reduction of GRIP expression in the presence of synaptic activity elevated AMPAR cycling to levels observed in the absence of activity. Conversely, knockdown of Arc and PICK1 reduced the elevated GluA2 endocytosis observed in the absence of activity. Together our results indicate that GRIP, Arc and PICK1 regulate constitutive cycling of GluA2 in RGCs. Our results also indicate that changes in GRIP, Arc, and PICK1 expression by visual activity provide a mechanism by which ambient light can modulate synaptic function.
Materials and Methods
Retina cell culture preparation
Retinas were removed from P0 Sprague-Dawley rat pups and dissociated with papain (Worthington Biochemical Corp, Lakewood, NJ). Following mechanical trituration, cells were plated on Poly-L-Lysine coated coverslips. Cultures were maintained in DMEM (Cellgro, Mediatech Inc., Manassas VA) media supplemented with 5% Fetal Bovine Serum (Atlanta Biologicals, Lawrenceville, GA), Pen/Strep (Sigma/Aldrich, St Louis, MO) and serum extender (BD Biosciences, San Jose, CA). After 24 hours cells were fed with media containing 15mM KCl. At 72 h after plating, cells were treated with the antimitotics 5-fluoro-2-deoxyuridine (0.01 mg/ml) and uridine (0.026 mg/ml) for 24 h. Every second day, 50% of the culture medium was exchanged for fresh medium. To mimic high and low activity conditions, cultures were incubated either in 10 μM AMPA (+Activity) or CNQX (−Activity) for 12 hours prior to use in experiments. Drugs were washed out immediately prior to experiment.
Antibodies
Antibodies (Abs) used in the study included: GluA2 (immunocytochemistry, mouse monoclonal, Millipore, Billerica, MA) and rabbit polyclonal (Western blot, Millipore; Proteintech Group, Chicago, IL), GRIP1/2 (mouse monoclonal, BD Transduction Laboratories, San Jose, CA), Arc (rabbit polyclonal, Proteintech Group) and PICK1 (rabbit polyclonal, Thermo Scientific, Rockford, IL) PSD95 ( mouse monoclonal, Neuromab, Davis, CA), β-Actin (mouse monoclonal, Sigma-Aldrich, St. Louis, MO). Cy3, FITC and HRP-conjugated Abs raised in donkey (Jackson ImmunoResearch Laboratories, Inc West Grove, PA). Agonists and antagonists were purchased from Tocris Cookson (Ellisville, MO).
Measurement of constitutive GluA2 endocytosis
Live, cultured retinal neurons were labeled in conditioned growth media with GluA2 N-terminal antibody (10 μg/ml) for 30 minutes. After antibody removal, cells were returned to the incubator for various time periods as described in the Results. One slip was removed immediately to serve as a 0 minute control. At defined time points cells were briefly fixed in 4% paraformaldehyde. Following block in TBS, 2% BSA (30 min), surface AMPA receptors were labeled with a fluorescently conjugated secondary antibody (1:300; 1 hr). Neurons were washed with 1X TBS before mounting using Vectashield (Vector Laboratories, Burlingame, CA). Fluorescence intensity of GluA2 labeling was measured at each time point and normalized to values obtained from time=0 minute coverslips.
Image acquisition
Neurons were imaged with a Hamamatsu Orca ER camera mounted on an inverted Nikon fluorescent microscope with a 60X Plan Apo lens. Exposures were adjusted to ensure signals fell within the linear range of the camera. Images were analyzed using Metamorph software (Molecular Devices, Sunnyvale, CA). Background-subtracted images were thresholded to include signals more than 2-fold greater than the diffuse labeling in dendritic shafts. Integrated fluorescence signal intensity values were measured on dendrites, normalized to area, and graphed as a percent change from controls. For all experiments, except where noted, n values represent individual experiments in which 7–20 cells are imaged in each condition.
siRNA
Retina cultures, 8–11 DIV, were transfected with control siRNA or siRNAs against GRIP1 and 2 or Arc using lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to a procedure specified by the manufacturer. For GRIPsi, two sets of pre-designed siRNA sequences targeted to GRIP protein family coding sequences (GRIP1 exons 5 and 21; and GRIP2 exons 7 and 25, Ambion, Inc., Austin, TX). For Arc, one pre-designed siRNA sequence targeting exon 1 (Life Techonologies, Carlsbad, CA) and PICK1 was targeted by a single pre-designed sequence (Invitrogen Corp.). Following incubation for 2 hrs, the cells were washed in conditioned media and kept at 37°C. Neurons were treated with drugs as specified and immunocytochemically processed for labeling of surface GluA2 after 3 days.
Retina lysate preparation for Western Blot analysis
Sprague Dawely rats, P22–25, were housed in light or darkness for 12 hours prior to eye removal. All subsequent steps of retinal isolation were carried out in the light or dark to match housing conditions. Rats were anesthetized in isoflurane, and eyes were removed and immediately chilled to 4°C. Retinas were dissected in Ames media at 4°C and treated in Ames with hyaluronidase for 30 minutes at room temperature. Protein extracts were prepared by homogenization in Tris-HCl based lysis buffer (140mM NaCl, 50mM Tris-HCl, 0.1%Triton X-100, pH 7.6), and subject to centrifugation at 6000 rpm. Protein concentration of supernatants was determined by BCA assay. Proteins were separated by electrophoresis on 10% SDS-PAGE gels and transferred onto nitrocellulose membranes. Membranes were blocked in 5% milk or 5% BSA (for Proteintech GluA2). Blots were probed overnight with primary Ab, washed in TBS with 0.1% Tween20, and incubated with HRP conjugated secondary for 1 hour. Proteins were detected by enhanced chemiluminescence (ECL, Amersham Biosciences, UK).
Surface protein biotinylation
Following incubation in hyaluronidase as above, light or dark adapted retina were incubated in PBS with 2mg/ml EZ-link Sulfo NHS-LC biotin (Pierce/Thermo Scientific, Rockford, IL) for 2 hours 4°C. Unbound biotin was washed out with 4°C PBS. Protein extracts were prepared by homogenization in HEPES based lysis buffer (20mM HEPES, 150mM NaCl, 5mM EDTA, 0.1% SDS, 1% Tx-100). Lysates were spun at 6000 rpm, and the supernatant was retained and assayed for protein concentration. 700 μg of protein extract were incubated with 100ul High Capacity Streptavidin agarose Resin (Pierce/Thermo Scientific) overnight at 4°C. The protein-bound beads were washed in 3X in lysis buffer and pelleted by centrifugation for 2 min at 2000 rpm. The beads were resuspended in SDS sample buffer and the immune-complexes were eluted by boiling. Total and immunoprecipitated proteins were separated by electrophoresis on 10% SDS-PAGE gels and were transferred onto nitrocellulose membranes and probed with anti- GluA2 Ab (1:2,000, Proteintech Group).
Co-Immunprecipitation
Retina lysates (500–700 μg) were incubated with 5 μg of mouse monoclonal GluA2 antibody overnight at 4°C with constant shaking. The antibody-bound complexes were incubated with Protein A/G agarose (Thermo Scientific) for 2 hrs at 4°C. The protein-bound beads were washed in lysis buffer and pelleted by centrifugation for 2 min at 2000 rpm. The beads were resuspended in SDS sample buffer and the immune-complexes were eluted by boiling. Total and immunoprecipitated proteins were separated by electrophoresis on 10% SDS-PAGE gels and were transferred onto nitrocellulose membranes. The blots were first probed with a GluA2 polyclonal antibody (1:500), and then reprobed with a GRIP monoclonal antibody (1:1000).
Data Analysis
Data was analyzed using two tailed Student’s t-tests. All error bars represent the standard error of the mean (SEM).
Results
Darkness decreases surface GluA2 levels in retinal neurons
We have previously shown that placing mice in darkness for > 8 hours causes a prolonged inactivation of the On pathway that initiates constitutive cycling of GluA2-containing AMPARs in RGCs (Xia et al., 2007). Placing mice in darkness for > 8 hours also causes a shift in the composition of On RGCs surface AMPARs from predominantly GluA2-containing to predominantly GluA2-lacking (Xia et al., 2006). These findings suggested that increased AMPAR cycling results in a net loss of GluA2-containing AMPARs from the membrane surface. We performed biochemical analysis of surface AMPAR levels in retinas of rats following exposure to either light or darkness for 12 hours to confirm that increased AMPAR cycling results in a net loss of membrane surface GluA2-containing AMPARs. Retinas were immediately incubated in biotin to label surface proteins. Surface proteins in biotinylated lysates were isolated by pull down with streptavidin coated beads and Western blots of surface and total proteins were probed for GluA2. Retinas from dark-housed animals showed a 32.6 ± 4.1 % decrease in surface GluA2 relative to light-housed retinas (Fig 1A, B, n=6, p < 0.01) while total GluA2 protein exhibited no significant changes (−15.4 ± 18.2%, n=6). Total expression of the synaptic protein PSD95 also exhibited no significant changes following activity modulation (−13.8 ± 9%, n=3).
Figure 1. Darkness decreases surface GluA2 levels in retinal neurons.
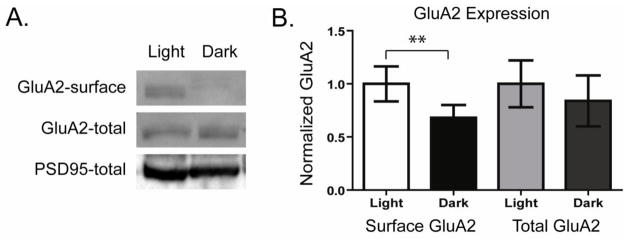
A. Surface proteins were labeled with biotin in retina lysates isolated from rats housed either in light (Light) or darkness (Dark) for the preceding 12 hours. Surface proteins isolated by avidin-bead precipitation were separated by SDS-PAGE. Western blots were probed to detect surface as well as total GluA2 AMPAR levels. β-Actin or PSD95 was probed to control for loading differences. B. Quantitation of experiments as in A. Surface and total GluA2 labeling intensities are normalized to mean values of the light exposed samples. Results show that light deprivation reduces surface but not total GluA2 levels in retinal neurons (n=6, ** p<0.01).
Darkness and reduced excitatory activity regulate the expression of AMPAR trafficking proteins in RGCs
The reduction in surface GluA2 in darkness occurs in parallel with and likely as the result of an increase in the cycling of GluA2 containing AMPARs (Xia et al., 2007). To determine how changes in ambient light levels could influence the constitutive trafficking of GluA2s, we investigated the expression levels of three proteins which have been linked to the endocytosis or exocytosis of GluA2s. Analysis of the effects of knockdown, overexpression, and inhibition PICK1 and GRIP binding to GluA2 has identified multiple roles for these proteins in different systems. GRIP, initially identified as stabilizing AMPARs at the membrane surface (Matsuda et al., 2000; Osten et al., 2000; Srivastava et al., 1998), has also been suggested to maintain AMPARs in intracellular pools and to enhance the surface delivery of receptors (Braithwaite et al., 2002; DeSouza et al., 2002; Mao et al., 2010; Thomas et al., 2012). PICK1 has been reported to enhance AMPAR endocytosis, and to enhance or retard redelivery of internalized receptors to the membrane surface (Chung et al., 2003; Gardner et al., 2005; Hill et al., 2004; Sossa et al., 2006; Xia et al., 2000). Arc, which is encoded by an immediate early gene, is regulated by activity and binds to endophilin 2 and 3 and enhances the endocytosis of AMPARs (Chowdhury et al., 2006; Rial Verde et al., 2006). Because of the important role of each these proteins in modulating the trafficking of AMPARs, we examined whether their expression levels were regulated by chronic light exposure in the retina. Protein levels were assayed in two ways. First, retina from animals housed in the light or darkness for 12 hours were isolated and prepared as lysates. Total protein levels were compared between the two activity conditions by Western blot analysis. In parallel studies, retinal cultures were exposed either to AMPA (10 μM, 12 hours, +Activity) or the AMPAR antagonist CNQX (10 μM, 12 hours, −Activity) to mimic changes in activity levels in the ON pathway in light or dark, respectively. Immunocytochemical analysis of protein labeling in the cultures provided a means to compare changes of protein expression specifically in RGCs with those measured in the intact retina.
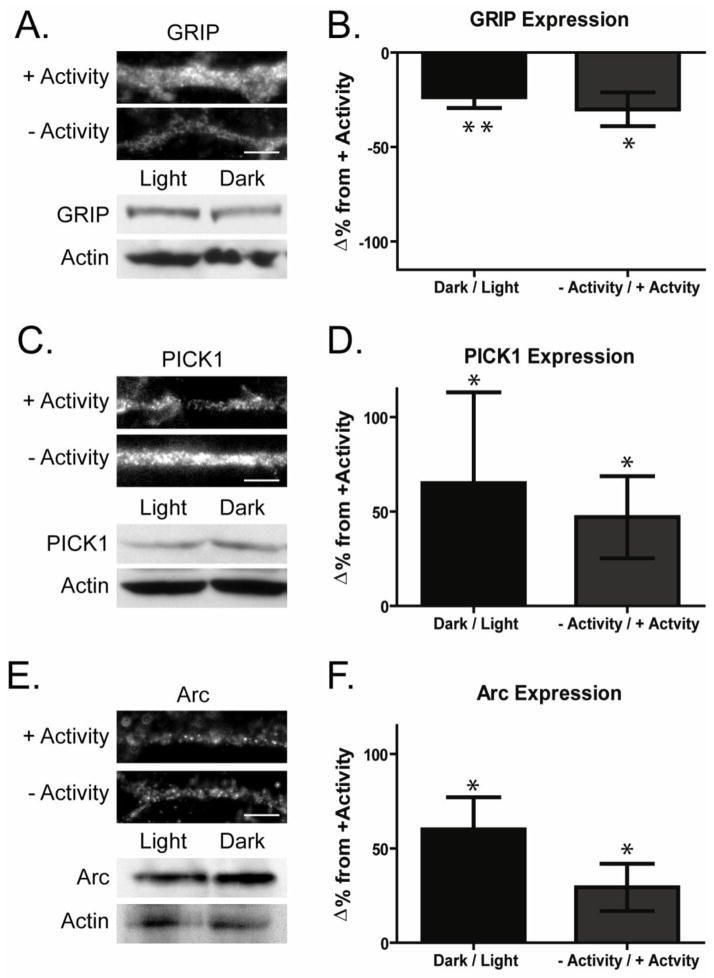
In light-deprived animals there was a significant decrease in the expression of GRIP proteins (Fig 2A,B −23.6 ± 4.0% change from light exposed animals, n=7, p< 0.05). This was paralleled by a decrease of dendritic GRIP immunolabeling in morphologically identified retina culture ganglion cells maintained for 12 hours in CNQX (−Activity −30.1 ± 8.6%, n=9, p<0.01). We have found this manipulation with CNQX to mimic the effects of dark housing on AMPAR cycling in RGCs (Xia, Carroll et al. 2006). In contrast, prolonged darkness increased the expression of PICK1 (Fig 2C,D 65.0 ± 48.0%, n=7, p<0.05) as well as Arc (Fig 2E,F 60.0 ± 17.0% increase, n=4, p<0.05). Comparably we found that dendritic labeling of PICK1 was increased in the absence of activity (Fig 2C,D 46.0 ± 21.8%, n=6, p<0.05) and Arc (Fig 2E,F 29.3 ± 12.5%, n=4, p<0.05) in cultured RGCs. We previously discussed that total GluA2 and PSD95 levels are not modified by activity. In contrast through these two complementary methods, our results indicate that the activity levels in RGCs regulate the dendritic expression levels of three proteins known to modulate the trafficking of GluA2s.
Figure 2. Activity regulates the expression levels of AMPAR regulatory proteins in retinal ganglion cells.
A,C,E. Expression levels of proteins that regulate GluA2 trafficking were analyzed in both intact retina and cultured retinal ganglion cells. Top panels, Images depict dendrites of cultured retinal ganglion cells maintained in the presence (+ Activity) or absence (−Activity) of synaptic activity for 12 hours and immunolabeled for GRIP (A), PICK1 (C) or Arc (E) (scale bar 5 μm). Bottom panels Lysates isolated from retina of rats maintained either in light (Light) or darkness (Dark) for the preceding 12 hours were probed by Western blot for GRIP (A), PICK1 (C) or Arc (E). B,D,F Graphs depict percent changes in levels of GRIP (B), PICK1 (D) or Arc (F) in retinas maintained in dark compared to those maintained in light (Western Blots) and cultures maintained in the absence compared to those maintained in the presence of activity (immunocytochemistry) (GRIP: WB n=7, *p=0.05, immuno n=9, ** p<0.01; PICK1: WB n=7, * p=0.05, immuno n=6, *p<0.05)(Arc: WB n=4,p<0.05; immuno n=4, * p<0.05). GRIP levels were reduced in retinal neurons in the absence of light or activity while PICK1 and Arc were both elevated.
Darkness reduces the interaction of GluA2 AMPARs with GRIP
We next compared the relationship between AMPA binding protein expression and activity dependent changes in constitutive AMPAR trafficking in RGCs. GRIP proteins exert their actions on GluA2 trafficking through direct interaction with the C terminal PDZ binding domain on the receptor (Dong et al., 1997; Srivastava and Ziff, 1999). As our data indicates that dark housing of rats reduces the expression of retinal GRIP proteins, we examined whether there was an accompanying reduction in GRIP binding to GluA2 supporting a functional consequence of altered protein expression. Interactions were measured by quantifying relative levels of GRIP co-immunoprecipitating with GluA2 receptors. In the dark housed retina there was a significant decrease in the amount of GRIP found in complex with GluA2 (Fig 3A,B 70.0 ± 8.8% decrease, n= 5, p<0.05).
Figure 3. Darkness reduces the interaction of GRIP with GluA2.
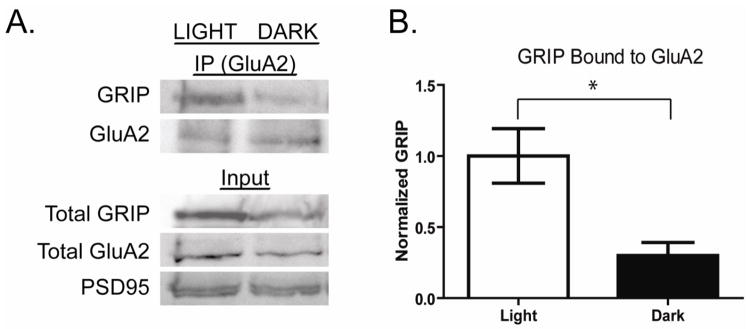
A. The interaction of GRIP with GluA2 was measured in retinal lysates from rats housed in light (Light) or darkness (Dark) for >8 hours. GluA2 and bound proteins were immunoprecipitated and separated by SDS PAGE. Western blots were probed for precipitated GluA2 and bound GRIP. Total lysates from which proteins were precipitated were also probed for GluR2 and PSD-95 levels. B. Quantitation of experiments as shown in A. GRIP-bound GluA2 intensities are normalized to mean values of the light exposed samples. The proportion of GRIP bound GluA2 decreases significantly in retina of animals maintained in darkness for 12 hours (Dark) compared to those housed in light (Light) (n=5, * p< 0.05).
Reductions in GRIP elevate constitutive GluA2 trafficking, mimicking effects of reduced activity
We have previously demonstrated that the extent of AMPAR cycling in retinal cultures can be estimated by immunocytochemically measuring the baseline rate of AMPAR endocytosis over time (Xia et al., 2007). As surface levels of receptors are fairly stable over the time course of the experiment, the net loss of receptors measured due to endocytosis must be balanced by equal new insertion. To measure endocytosis, we applied an antibody against an extracellular epitope of the GluA2 receptor to live retinal neurons. After saturated labeling, the excess antibody was removed and cells were incubated for various periods of time (0, 15, 30 and 45 minutes) allowing constitutive receptor endocytosis to occur. Cells were briefly fixed and the remaining surface expressed receptors were labeled with a fluorescent conjugated secondary antibody under non-permeabilizing conditions. Imaging of the remaining labeled surface receptors after different time points allows for an estimation of the rate and proportion of AMPARs undergoing endocytosis. We used this method to examine the constitutive AMPAR endocytosis in the presence and absence of activity. As previously reported we found that there was less cycling in the cultures in which activity was elevated prior to the cycling assay (+Activity, Fig 4A,B; Surface GluA2 remaining at 30 minutes: −Activity 27.9 ± 3.5% levels at time 0; +Activity 45.5 ± 5.6%, n=9, p<0.05).
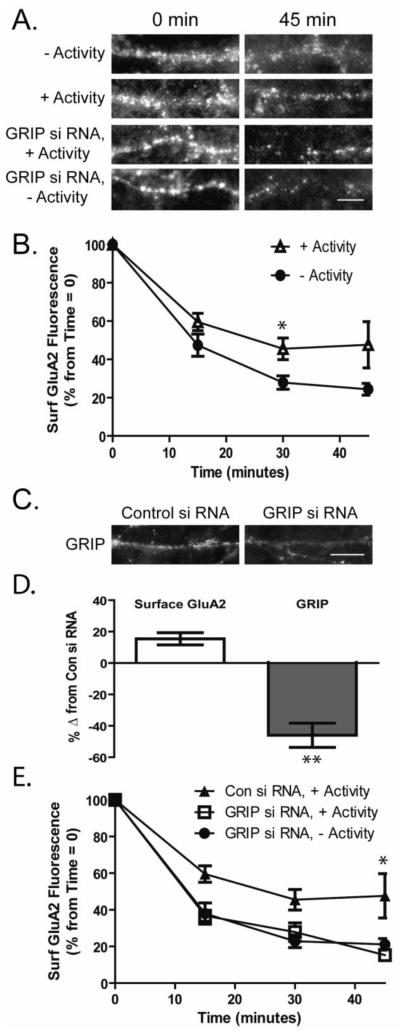
Figure 4. Knockdown of GRIP occludes the increased constitutive GluA2 cycling induced by the absence of activity.
A. Constitutive AMPAR endocytosis was measured in RGCs maintained for >8 hours in the presence or absence of synaptic activity (−Activity or +Activity). Surface GluA2 receptors were immunolabeled in live RGCs that were subsequently fixed at multiple time points. GluA2 endocytosis was also measured in RGCs 72 hours following knockdown of GRIP expression by siRNA. Endocytosis of GluA2 AMPARs was monitored in siRNA transfected (Control and GRIP) cells both maintained in the presence of activity, a condition under which GRIP levels are normally high, and in the absence of activity. Images depict remaining labeled surface GluA2 receptors at 0 and 45 minutes after initial labeling (scale bar = 5μm). B. Quantitation of constitutive GluA2 endocytosis in cultured retinal ganglion cells maintained for >8 hours in the absence or presence of synaptic activity (−Activity or +Activity). The reduction in surface GluA2 labeling is higher in the absence of activity at 30 minutes consistent with an increase in the cycling of the receptors (n=9, * p<.05). C. Images depict expression of GRIP following siRNA treatment (scale bar=10μm). D. GRIP and surface GluA2 levels were measured immunocytochemically in control and GRIP siRNA-treated neurons, 72 hours after infection. Knockdown was measured in cultures maintained for >8 hours in the presence of activity (+Activity), when GRIP levels are normally higher. While GRIP levels were significantly decreased in the siRNA treated cells (n=5, ** p= 0.01), there was little effect on surface GluA2 levels (n=6). E. Quantitation of constitutive AMPAR endocytosis in control and GRIP siRNA treated RGCs. Reduced GRIP expression in cultures maintained in activity (+Activity) resulted in enhanced AMPAR endocytosis comparable to that seen in the absence of activity (−Activity). Knockdown of GRIP had no further effect on AMPAR endocytosis in cultures maintained in the absence of activity, suggesting the effect on receptor trafficking is occluded (n=9, * p< 0.05).
Previous work suggests that GluA2 receptors bound to GRIP have limited mobility (Braithwaite et al., 2002; Osten et al., 2000; Srivastava et al., 1998). In hippocampus, knockdown of GRIP1/2 expression increased GluA2 constitutive cycling (Casimiro et al., 2011). We tested whether decreasing GRIP expression in ganglion cells would similarly increase constitutive GluA2 trafficking. As GRIP levels are elevated in the presence of activity, when GluA2 cycling is reduced, we compared the endocytosis of GluA2 in control and GRIP1/2 siRNA transfected neurons (72 hours) that were maintained in the presence of synaptic activity 12 hours prior to the experiments. Knockdown of GRIP by nearly 50% (Fig 4C,D) resulted in a non-significant change in surface GluA2 levels (Fig 4D 14.5 ± 10% n=7, p=.09). However, GRIP knockdown increased GluA2 endocytosis to levels comparable to that observed in the absence of activity. At 45 minutes surface GluA2 in the control siRNA treated neurons was reduced to 47.5 ± 12.1% of the initial surface GluA2 labeling at 0 minutes (Fig 4A,E). Surface GluA2 in the GRIP siRNA treated cells exhibited a greater decrease in surface GluA2 labeling at 45 minutes to 15.3 ± 7.1% of levels at time 0 (Fig 4A,E n=9, p<0.05). Blockade of activity in GRIP siRNA treated cells resulted in no further increase in endocytosis (Fig 4E, 21.2 ± 9.8 % surface GluA2 at 45 minutes compared to time 0 control) suggesting the effects of GRIP knockdown and activity on AMPAR cycling occlude each other.
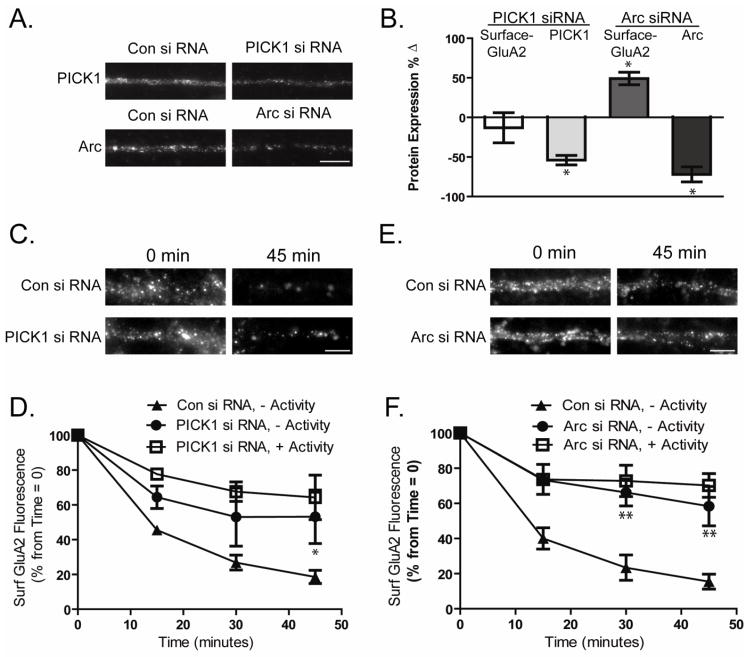
Reductions in PICK1 and ARC prevent increased GluA2 trafficking in the absence of activity
We performed similar analyses of constitutive AMPAR endocytosis in cells in which PICK1 or Arc expression were knocked down (Fig 5A,B). Effects of PICK1 or Arc knockdown were monitored in cells in which activity was blocked prior to the experiment, as this is the condition under which their expression is normally elevated. Seventy-two hours after introduction of PICK1 siRNA, there was no significant change in surface GluA2 levels at time 0 min (Fig 5B −13.1 ± 18.9% change from control siRNA treated cells, n=6). However, we found that GluA2 endocytosis over 45 minutes was significantly reduced (Fig 5C,D At 45 minutes: Control siRNA 18.56 ± 3.8% of time 0 control, PICK1 siRNA 53.2 ± 15.3%, n=6, P<0.05). In the presence of activity, PICK siRNA treated cells resulted in no further reduction in endocytosis (Fig 5D, PICK1 siRNA 64.32 ± 12.7 % surface GluA2 at 45 minutes compared to time 0 control) suggesting that the effects of PICK1 knockdown and reduced activity occlude each other in their modulation of AMPAR cycling.
Figure 5. PICK1 and Arc knockdown prevent increased AMPAR cycling of RGCs in the absence of activity.
A. Images depict expression of PICK1 or Arc following siRNA treatment (scale bar=10μm). B. Surface GluA2 levels were measured immunocytochemically in cultured RGCs 72 hours after transfection with PICK1 or Arc siRNA and compared to levels in control siRNA transfected cells. Knockdown of Arc but not PICK1 increased surface GluA2 levels (n>5, * p≤ 0.05). C–F. Cultured RGCs were transfected with siRNA against PICK1 or Arc. After 72 hours constitutive AMPAR endocytosis was monitored immunocytochemically in cultures maintained in the absence of synaptic activity (−Activity) a condition under which ARC and PICK1 are normally elevated. Effects of PICK1and Arc siRNA were also measured in the presence of activity (+ Activity). C,E. Images depict dendritic labeling of surface GluA2 in PICK1(C) or Arc(E) siRNA transfected RGCs at 0 and 45 minutes after initial immunolabeling (scale bar=5μm). D,F. Quantitation of surface GluA2 fluorescence in experiments as in B and D demonstrates that knockdown of PICK1 (n>5, *p<0.05) ( D) and Arc (n≥5, **p<0.01) (F) both prevent the increased constitutive GluA2 trafficking normally observed in RGCs in the absence or presence of synaptic activity (−Activity or +Activity) (see Fig 4B).
In cells exposed to Arc siRNA, we observed a significant increase in the surface levels of GluA2 AMPARs (Fig 5B 49.1 ± 7.9% of control siRNA treated cells, n=5, p<0.01). Similar to what we observed with PICK1 knockdown, GluA2 endocytosis was reduced to levels comparable to that seen in cells in the presence of activity (+Activity, Fig 5E,F At 45 minutes: Arc siRNA 58.41 ± 11.2% of time 0 control). In the presence of activity, ARC siRNA treated cells resulted in no further reduction in endocytosis (Fig 5F, Arc siRNA 70.19 ± 6.75 % surface GluA2 at 45 minutes compared to time 0 control). This finding suggest that Arc, like PICK1 and GRIP, contributes to the processes regulating AMPAR cycling in RGCs, and is a key factor in maintaining the reduction of surface GluA2s in retina of animals in darkness.
Discussion
We have shown that molecular manipulation of GRIP, PICK1 and Arc expression mimics the changes in constitutive GluA2 trafficking observed in response to chronically altered activity levels. Our findings support a model in which regulated changes in the protein expression of these AMPAR regulatory proteins coordinate to modulate the cycling of AMPARs.
We have previously reported that light deprivation for longer than eight hours elevates the cycling of GluA2-containing AMPARs in rodent RGCs which resulted in an increase in the proportion of calcium-permeable surface AMPARs (Xia et al., 2006; Xia et al., 2007). Parallel studies in cultured retinal neurons demonstrated that reductions in excitatory activity were responsible for the enhancement in AMPAR cycling (Xia et al., 2006). We examined the molecular events that contribute to the altered trafficking of RGC AMPARs linked to light-experience driven plasticity. As this change in trafficking develops over a period of hours, we examined whether darkness and reduced synaptic activation alter the expression of AMPARs or AMPAR regulatory proteins. We find that that while prolonged decreases in light exposure reduce the surface expression of GluA2 AMPARs in retinal cells, the total expression levels of these receptors does not change. An increase in surface GluA2 without an increase in total AMPARs indicates that altered trafficking mediates changes in surface receptor levels. In contrast, we find that the expression of three proteins that modulate GluA2 AMPAR trafficking are regulated by light deprivation: PICK1, Arc and GRIP. PICK1 and Arc are elevated while GRIP expression is decreased in light or activity-deprived retinal neurons in which AMPAR cycling is high. Furthermore, siRNA-mediated knockdown demonstrated that reductions in Arc and PICK1 block the elevated constitutive GluA2 endocytosis observed in activity-deprived RGCs. In contrast, knockdown of GRIP elevates AMPAR endocytosis even in the presence of activity. These results are consistent with a model in which changes in the expression of multiple AMPAR regulatory proteins coordinate to regulate AMPAR trafficking and consequently surface AMPAR composition in response to chronic changes in activity in RGCs.
A shift in AMPAR composition also has been reported in response to chronic changes in activity levels in cortical and hippocampal neurons (Goel et al., 2006; Narasimhan, 2007; Sutton et al., 2006; Thiagarajan et al., 2005). Similar to our findings, these studies found an increase in the proportion of surface GluA2-lacking receptors with decreased activity levels. However, while we have found a key role for the removal of GluA2-containing receptors in the regulation of AMPAR composition (Xia et al., 2007), previous studies have predominantly reported increased insertion and sometimes synthesis of GluA2-lacking AMPARs as the means of modulating this composition switch. Our data highlight a role for regulation of constitutive GluA2 receptor trafficking in reducing the levels of GluA2-containing AMPARs at the membrane surface. Our data do not preclude a role for GluA2-lacking AMPARs being actively inserted with light deprivation. In fact our previous study suggested that the increased GluA2 cycling was not accompanied by a significant decrease in response size (Xia et al., 2006), it is likely that there is at least a partial replacement of internalized AMPARs with GluA2-lacking receptors. In cortical and hippocampal neurons, reduced activity may also enhance constitutive endocytosis, and perhaps recycling of GluA2-containing AMPARs enabling or working in concert with GluA2-lacking AMPAR insertion to modulate synaptic receptor composition.
Our studies focused the role of three proteins GRIP, PICK1, and Arc in the regulation of AMPAR cycling. Expression of each of these proteins was found to be altered in response to changes in activity levels in the retina, and each was found to play an important role in modulating the constitutive trafficking of GluA2 AMPARs (Table 1). The function of GRIP proteins is clearly multifaceted and likely influenced by both GRIP isoforms. While early studies suggested that GRIP may act to stabilize AMPARs at the membrane surface, later findings have pointed to a role for GRIP in maintaining AMPARs in intracellular pools and in mediating the delivery of AMPARs to the membrane surface through interaction with the exocyst complex (Mao et al., 2010). An additional study describes an isoform specific contribution of GRIP in AMPAR trafficking with GRIP1a enhancing the recycling of internalized AMPARs to the membrane surface, while GRIP1b promotes intracellular stabilization of AMPARs following endocytosis (Hanley and Henley, 2010). Thus the relative expression of GRIP isforms in a given cell likely dictates the overall impact of these protein on AMPAR trafficking. While multiple forms of GRIP1 are found in retina, the specific expression of GRIP1 and 2 in ganglion cells is unknown. The GRIP antibody used in our studies recognizes all GRIP1 and 2 isoforms and the siRNA does not distinguish GRIP1a and 1b. However, our results suggest that in RGCs the net effect of GRIP proteins is to predominantly reduce GluA2 recycling and elevate intracellular retention of GluA2 AMPARs. We have observed a similar role for GRIP proteins in hippocampal neurons, where knockdown of GRIP expression enhanced constitutive AMPAR endocytosis (Casimiro et al., 2011).
Table 1. Relationship between activity, GluA2 trafficking and AMPAR regulatory protein expression in the retina.
Table summarizes results demonstrating the effects of activity on AMPAR regulatory protein expression (top) and AMPAR regulatory protein expression and GluA2 trafficking (bottom) in the retina. Up and down arrows indicate an increase and decrease in protein expression or extent of GluA2 trafficking, respectively.
| Effect of Activity on Protein Expression | ||
|---|---|---|
| Dark/Light | −Activity/+Activity | |
| Surface GluA2 | ⬇ | |
| GRIP expression | ⬇ | ⬇ |
| PICK1 expression | ⬆ | ⬆ |
| Arc expression | ⬆ | ⬆ |
| GRIP bound GluA2 | ⬇ | |
| Constitutive Endocytosis Following siRNA | ||
|---|---|---|
| −Activity | +Activity | |
| No siRNA | ⬆ | ⬇ |
| GRIP siRNA | ⚌ | ⬆ |
| PICK1 siRNA | ⬇ | ⚌ |
| Arc siRNA | ⬇ | ⚌ |
Like GRIP, PICK1 has been reported to have several functions including facilitating the endocytosis, intracellular retention, or synaptic delivery of GluA2 AMPARs. Our results show that knockdown of PICK1 in activity-deprived RGCs resulted in a decrease in constitutive GluA2 endocytosis. This decrease in the constitutive trafficking of GluA2 appears most consistent with PICK1 primarily playing a role in enhancing AMPAR endocytosis itself in RGCs. In hippocampal neurons, reduced activity (TTX) has been found to decrease levels of PICK1(Narasimhan, 2007), while we find that activity blockade has the opposite effect on PICK1 levels in RGCs. However in both cases conditions which elevate PICK1 levels also reduce surface GluA2 AMPARs (Terashima et al., 2004). Interestingly in a number brain areas including the VTA following cocaine exposure, the barrel cortex in response to activity from a spared whisker, and in hippocampal neurons following glycine stimulation, surface GluA2-lacking receptor levels are enhanced in a PICK1-dependent mechanism (Bellone and Luscher, 2006; Lynch, 2004; Narasimhan, 2007). These results suggest that changes of GluA2-containing receptor trafficking via a PICK1 dependent mechanism may be a common feature of plasticity expressed by a switch in AMPAR composition.
Arc is an immediate early gene modulated by neuronal activity and coupled to the regulation of surface AMPARs. While several studies have reported a positive correlation of activity levels and Arc expression (Shepherd et al., 2006; Steward et al., 1998; Zhao et al., 2004), we find that Arc expression is increased in the absence of activity in RGCs. Arc, through its interaction with endophilin has been suggested to modulate the endocytosis of AMPARs (Chowdhury et al., 2006), and its expression has been linked to elevated endocytosis of receptors, consistent with its effect on AMPAR endocytosis in RGCs. Arc has been indirectly linked to the regulation of GluA1 AMPARs as Arc’s down-regulation or genetic knockout results in elevated levels of GluA2-lacking receptors at the membrane surface (Chowdhury et al., 2006; Nijholt et al., 2004; Shepherd et al., 2006; Zhao et al., 2004). However, studies have also found Arc to modulate levels of GluA2-containing receptors (Chowdhury et al., 2006; Rial Verde et al., 2006). Our results support a role for Arc in modulating the endocytosis of GluA2-containing AMPARs in RGCs. As Arc and its binding partner endophilin do not directly interact with AMPARs, Arc may not exhibit a preference for any particular subtype but rather positions endocytic machinery which then induces the endocytosis of available receptors (Hill et al., 2004). In this scenario, other factors may control which population of receptors may be available for enhanced endocytosis. In the hippocampus and visual cortex, inactivity is associated with the enhanced surface expression of GluA1 AMPARs which may be somewhat unstable and subject to removal by Arc when activity levels are restored. In contrast, in the retina, changes in the expression of GRIP and or PICK1 may provide a key for the targeting of GluA2-containing AMPARs for endocytosis, while GluA2-lacking AMPARs appear to be quite stable (Xia et al., 2007). Similar to other studies we find that knockdown of Arc increased the basal levels of surface AMPARs in RGCs, further supporting Arc’s role in the enhanced endocytosis of AMPARs affecting the surface expression levels of AMPARs.
In our study we find that while knockdown of both GRIP and PICK1 significantly altered GluA2 cycling, loss of these two proteins did not significantly change the surface levels of GluA2, at least as detected immunocytochemically. Only with Arc knockdown did we observe significant changes in detected surface AMPARs. This suggests the process of altering constitutive GluA2 trafficking alone is not sufficient to regulate surface AMPAR levels. It is possible that the immunofluorescent detection was not sensitive enough in retina neurons to detect changes in surface GluA2 which we have detected electrophysiologically (Xia et al., 2007) and biochemically. More likely multiple processes together with altered AMPAR cycling levels contribute to changes in surface AMPARs. An increase in the overall AMPA endocytosis and reinsertion will not necessarily result in a loss of surface or more specifically synaptic GluA2s. However, altered trafficking levels together with mechanisms that maintain GluA2s away from the membrane surface or synapse may be necessary.
Alternatively, changes in surface GluA2 receptors may require changes in both GluA2 and GluA1 receptor trafficking. Increased GluA2 turnover and endocytosis may permit the entry of GluA1 receptors into the synapses which then competes with and reduces overall synaptic GluA2 levels. Thus, while changes in activity could trigger mechanisms that induce both GluA2 cycling and GluA1 insertion, knockdown of GluA2 regulatory proteins might alter trafficking of that subtype with no effect on GluA1 resulting in no net impact on surface receptor levels. Future experiments aimed at elucidating the role of reinsertion for both GluA1 and GluA2 would be necessary to fully understand the means by which activity can induce subunit switch of AMPARs in RGCs.
Our data suggest that in the retina, changes in the expression of multiple AMPAR regulatory proteins can modulate the constitutive trafficking of AMPARs following prolonged changes in activity levels. While our findings which utilize siRNA knockdown support a critical role for PICK1 and Arc in maintaining high levels of constitutive GluA2 endocytosis, our results investigating the role of GRIP1 suggest a more complex interplay between proteins. Knockdown of GRIP is able to enhance GluA2 endocytosis with no increases in PICK1 or Arc expression. One interpretation of this result is that these AMPAR regulatory proteins act in an equilibrium determined by the relative expression of each to drive the basal level of receptor constitutive cycling, with GRIP reducing AMPAR cycling and both PICK1 and Arc favoring constitutive cycling. With the loss of GRIP by siRNA knockdown, the existing levels of PICK1 and Arc become sufficient to mediate the enhanced endocytosis of AMPARs.
It is clear that the constitutive cycling of AMPARs can play a critical role in modulating synaptic function (Casimiro et al., 2011; Park et al., 2004; Xia et al., 2007). Consequently regulation of this dynamic process can serve as an important form of synaptic plasticity or metaplasticity. Here we find that activity-dependent modulation of the expression of multiple proteins regulate AMPAR cycling which can play a key role in altering the composition of synaptic AMPARs in the retina. As it has become clear that similar activity driven switches in AMPAR subunit composition are an important form of plasticity throughout the CNS, it will be of significant interest to determine how similar activity regulated changes in AMPAR cycling shape synaptic function throughout the brain.
Acknowledgments
We would like to thank Kevin Fisher for expert help in manuscript and figure preparation. This work was supported by National Institutes of Health Grant EY017428 (SN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Baudry M, Oliver M, Creager R, Wieraszko A, Lynch G. Increase in glutamate receptors following repetitive electrical stimulation in hippocampal slices. Life Sci. 1980;27:325–330. doi: 10.1016/0024-3205(80)90200-3. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nature neuroscience. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Xia H, Malenka RC. Differential roles for NSF and GRIP/ABP in AMPA receptor cycling. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7096–7101. doi: 10.1073/pnas.102156099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro TM, Sossa KG, Uzunova G, Beattie JB, Marsden KC, Carroll RC. mGluR and NMDAR activation internalize distinct populations of AMPARs. Molecular and cellular neurosciences. 2011;48:161–170. doi: 10.1016/j.mcn.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49:663–670. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Demb JB. Functional circuitry of visual adaptation in the retina. The Journal of physiology. 2008;586:4377–4384. doi: 10.1113/jphysiol.2008.156638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nature reviews Neuroscience. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- DeSouza S, Fu J, States BA, Ziff EB. Differential palmitoylation directs the AMPA receptor-binding protein ABP to spines or to intracellular clusters. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:3493–3503. doi: 10.1523/JNEUROSCI.22-09-03493.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Goel A, Jiang B, Xu LW, Song LH, Kirkwood A, Lee HK. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nature neuroscience. 2006;9:1001–1003. doi: 10.1038/nn1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley LJ, Henley JM. Differential roles of GRIP1a and GRIP1b in AMPA receptor trafficking. Neuroscience letters. 2010;485:167–172. doi: 10.1016/j.neulet.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Froc DJ, Fox CJ, Gorzalka BB, Christie BR. Prolonged cannabinoid treatment results in spatial working memory deficits and impaired long-term potentiation in the CA1 region of the hippocampus in vivo. Eur J Neurosci. 2004;20:859–863. doi: 10.1111/j.1460-9568.2004.03522.x. [DOI] [PubMed] [Google Scholar]
- Laughlin SB. The role of sensory adaptation in the retina. The Journal of experimental biology. 1989;146:39–62. doi: 10.1242/jeb.146.1.39. [DOI] [PubMed] [Google Scholar]
- Lin DT, Huganir RL. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:13903–13908. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mao L, Takamiya K, Thomas G, Lin DT, Huganir RL. GRIP1 and 2 regulate activity-dependent AMPA receptor recycling via exocyst complex interactions. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19038–19043. doi: 10.1073/pnas.1013494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. Embo J. 2000;19:2765–2774. doi: 10.1093/emboj/19.12.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan K. Unraveling the mechanisms of Angelman Syndrome. Nature neuroscience. 2007;10:275. doi: 10.1038/nn0307-275. [DOI] [PubMed] [Google Scholar]
- Nijholt I, Farchi N, Kye M, Sklan EH, Shoham S, Verbeure B, Owen D, Hochner B, Spiess J, Soreq H, et al. Stress-induced alternative splicing of acetylcholinesterase results in enhanced fear memory and long-term potentiation. Mol Psychiatry. 2004;9:174–183. doi: 10.1038/sj.mp.4001446. [DOI] [PubMed] [Google Scholar]
- Osten P, Khatri L, Perez JL, Kohr G, Giese G, Daly C, Schulz TW, Wensky A, Lee LM, Ziff EB. Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron. 2000;27:313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]
- Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, et al. The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron. 1998;21:99–110. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossa KG, Court BL, Carroll RC. NMDA receptors mediate calcium-dependent, bidirectional changes in dendritic PICK1 clustering. Molecular and cellular neurosciences. 2006;31:574–585. doi: 10.1016/j.mcn.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, et al. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Ziff EB. ABP: a novel AMPA receptor binding protein. Ann N Y Acad Sci. 1999;868:561–564. doi: 10.1111/j.1749-6632.1999.tb11329.x. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Terashima A, Cotton L, Dev KK, Meyer G, Zaman S, Duprat F, Henley JM, Collingridge GL, Isaac JT. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:5381–5390. doi: 10.1523/JNEUROSCI.4378-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Hayashi T, Chiu SL, Chen CM, Huganir RL. Palmitoylation by DHHC5/8 targets GRIP1 to dendritic endosomes to regulate AMPA-R trafficking. Neuron. 2012;73:482–496. doi: 10.1016/j.neuron.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Xia Y, Carroll RC, Nawy S. State-dependent AMPA receptor trafficking in the mammalian retina. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5028–5036. doi: 10.1523/JNEUROSCI.0169-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Nawy S, Carroll RC. Activity-dependent synaptic plasticity in retinal ganglion cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12221–12229. doi: 10.1523/JNEUROSCI.2086-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Xu H, Xu X. Effects of naloxone on the long-term potentiation of EPSPs from the pathway of Schaffer collateral to CA1 region of hippocampus in aged rats with declined memory. Brain research. 2004;996:111–116. doi: 10.1016/j.brainres.2003.10.017. [DOI] [PubMed] [Google Scholar]