Abstract
Two tyrosine kinases, Src64 and Tec29, regulate the growth of actin rich-ring canals in the Drosophila ovary. We have shown previously that Src64 directs the localization of Tec29 to ring canals, but the mechanism underlying this process was unknown. Here, we show that Tec29 localizes to ring canals via its Src homology 3 (SH3) and Src homology 2 (SH2) domains. Tec29 activity is required for its own ring canal localization, suggesting that a phosphotyrosine ligand for the SH2 domain is generated by Tec29 itself. Src64 regulates this process by phosphorylating Y677 within the kinase domain of Tec29, an event required for Tec29 activation. We also show that the pleckstrin homology (PH) domain of Tec29 has dual functions in mediating Src64 regulation. In the absence of Src64, the PH domain prevents Tec29 ring canal localization. In the presence of Src64, it enhances membrane targeting of Tec29 by a PI(3,4,5)P3-mediated mechanism. In the absence of its PH domain, Tec29 constitutively localizes to ring canals, but still requires Src64 for full activation.
Keywords: PH domain, ring canals, SH2 domain, SH3 domain, tyrosine kinase
Introduction
Modulation of the actin cytoskeleton is essential to cell shape change and cell movement. Misregulation of these fundamental processes can contribute to cellular transformation. Members of the Src family of nonreceptor tyrosine kinases (SFKs) have been shown to regulate the stability and remodeling of actin structures by both loss-of-function (Boyce et al, 1992) and gain-of-function (Boschek et al, 1981) studies. As a result of of their potential roles in oncogenesis, signaling by SFKs has been carefully examined, and a number of cellular substrates have been identified (Martin, 2001). Despite these efforts, the pathways and mechanisms utilized by SFKs in vivo are still not well understood. This is due, at least in part, to a high degree of redundancy of SFKs in mammalian systems (Brown and Cooper, 1996; Thomas and Brugge, 1997).
The fruit fly Drosophila melanogaster has two SFKs: Src64 and Src42 (Simon et al, 1985; Takahashi et al, 1996). Mutations in Src64 lead to a reduction in female fertility (Dodson et al, 1998). In wild-type ovaries, a germline cystoblast undergoes four rounds of mitosis, thus generating a 16-germ cell cyst. These four rounds of cell divisions are characterized by incomplete cytokinesis, and actin-rich structures called ring canals form on the arrested cleavage furrows. Ring canals are stabilized by proteins such as HTS-RC and kelch, and become intercellular bridges that connect all 16 germ cells (Robinson and Cooley, 1997). Interestingly, phosphotyrosine-containing proteins appear on ring canals at an early stage during their formation, and persist throughout oogenesis (Cooley, 1998). We have shown that, compared to wild-type ring canals, Src64 mutant rings are smaller and lack the characteristic phosphotyrosine content, suggesting that Src64 activity is required for the growth of ring canals (Dodson et al, 1998). Recently, ring canal protein kelch was shown to be phosphorylated in an Src64-dependent manner (Kelso et al, 2002). This phosphorylation event affects kelch binding to F-actin in vitro, and is thought to play a role in regulating actin crosslinking and ring canal growth.
A second tyrosine kinase, Tec29, was also demonstrated to be a key downstream effector of Src64 during ring canal growth (Guarnieri et al, 1998; Roulier et al, 1998). Mutations in Tec29 enhance the ring canal size defect seen in animals with reduced Src64 expression. Src64 and Tec29 mutants also share similar phenotypes: both have small ring canals that lack phosphotyrosine content. Importantly, we showed that Tec29 protein is localized to ring canals in an Src64-dependent manner, implying that Src64 activity leads to the recruitment of Tec29 to ring canals, which may then mediate the function of Src64 by phosphorylating target proteins on ring canals. Therefore, elucidating the mechanism by which Tec29 localizes to ring canals, and demonstrating how Src64 regulates this localization, are essential for understanding how Src64 and Tec29 coordinate to regulate actin networks in Drosophila.
Tec29 belongs to the Tec family of tyrosine kinases (TFKs), which also include mammalian Btk, Itk, Tec, Etk/Bmx and Txk (Qiu and Kung, 2000; Smith et al, 2001). Mutations in Btk cause X-linked agammaglobulinemia (XLA) in humans and X-linked immunodeficiency (Xid) in mice, each characterized by the arrest of B-cell development at an early stage and the lack of mature B cells in adults (Satterthwaite and Witte, 2000; Maas and Hendriks, 2001). Interestingly, recent studies in these systems have demonstrated that, similar to Tec29, mammalian TFKs also play important roles in cytoskeletal regulation. For instance, activation of β-integrins following T-cell stimulation requires Itk, as the expression of a dominant-negative Itk specifically blocked that process (Woods et al, 2001). Also, Etk/Bmx and focal adhesion kinase (FAK) associate with each other in vivo, an interaction that is required for Etk activation, as well as for host cell migration (Chen et al, 2001). TFKs have also been shown to bind to Vav and WASP, both of which are involved in actin polymerization (Machide et al, 1995; Bunnell et al, 1996). However, it is not clear how the activity and localization of TFKs are regulated in this capacity, nor is it known whether the lack of actin regulation in the absence of TFK genes contributes to the failure in lymphocyte development.
To better understand the signaling components and pathways employed by Src64 and Tec29 to regulate the growth of actin-rich ring canals, we have undertaken an analysis of the molecular mechanism by which Src64 regulates Tec29 localization and activation. Our results reveal a novel aspect of the pleckstrin homology (PH) domain function, and identify the Src homology 3 (SH3) and Src homology 2 (SH2) domains as essential regions for directly mediating the localization of Tec29 to ring canals. Furthermore, we show that Src64-dependent phosphorylation stimulates Tec29's activity, which, in turn, is essential for the localization and function of Tec29 at ring canals.
Results
Ring canal localization of type 2 Tec29, the major ovarian form, is regulated by Src64
Most TFKs share a primary structure that consists of a PH domain at the amino terminus, followed by a Tec homology (TH) domain, an SH3, an SH2 and a protein tyrosine kinase domain. The PH domain is the most distinguishing feature of the TFK family when compared to other tyrosine kinases. Due to alternative splicing, two types of proteins are encoded by the Tec29 gene in Drosophila (Figure 1B): type 1 Tec29 lacks a PH domain, whereas type 2 Tec29 contains a PH domain and is thus more similar to canonical TFKs (Baba et al, 1999). The two types of Tec29 proteins also have minor differences at the beginning of their TH domains, but are otherwise identical.
Figure 1.
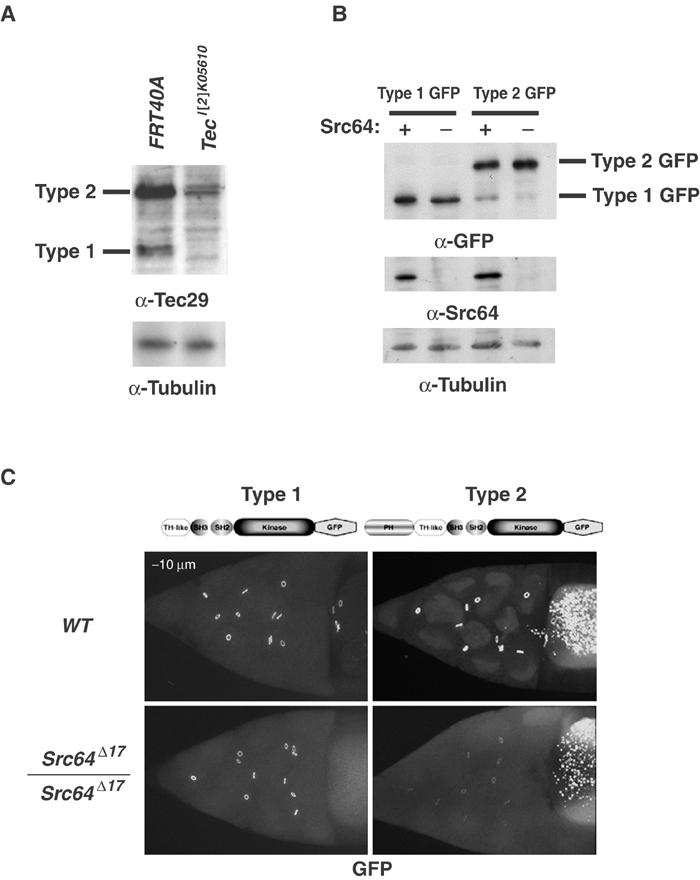
Src64 regulates the localization of type 2, but not type 1 Tec29, to ring canals. (A) Type 2 Tec29 is the predominant form expressed in female germ line. Ovary lysates from FRT40A control and Tec29l(2)K05610 germline mutants were assayed for the amount of type 1 and type 2 expression by Western blotting and probing with an anti-Tec29 antibody. Total cellular protein (50 μg) was loaded in each lane. See Materials and methods for details. (B) Expression of GFP-tagged type 1 and type 2 Tec29 is not affected by Src64. Ovary lysates from wild-type or Src64Δ17 homozygous mutants carrying one copy of a type 1 Tec29–GFP or type 2 Tec29–GFP transgene were assayed by immunoblotting. (C) Ring canal localization of GFP-tagged type 1 (left) or type 2 (right) Tec29 in a wild-type (upper) or Src64Δ17 (lower) background was visualized. All images were taken under the same confocal imaging conditions. The genotypes of the flies in (A) from left to right are (1) hsFLP/+; [OVOD2La], [OVOD2Lb], FRT40A/FRT40A, (2) hsFLP/+; [OVOD2La], [OVOD2Lb], FRT40A/ Tecl(2)k05610, FRT40A. The genotypes of the flies in (C) are [Tec29 type 1/2-GFP]/+ (upper panels) and [Tec29 type 1/2-GFP]/+, Src64Δ17/Src64Δ17 (lower panels).
To study the localization of Tec29 to ring canals, it was necessary to determine the relative expression of type 1 and type 2 Tec29 in germline-derived nurse cells. We first analyzed their expression in whole ovaries by immunoblotting with an antibody that recognizes both forms. Although both types of Tec29 are found in wild-type ovaries, type 2 protein is expressed at much higher levels than type 1 protein (Figure 1A, first lane). As somatically derived follicle cells may also contribute to this expression pattern, we generated ovaries whose germ cells are mutant for Tec29, using a loss-of-function allele Tec29l(2)k05610 and the FLP-DFS technique (Chou and Perrimon, 1996). Expression of both types of protein in these ovaries is greatly reduced, indicating that germline expression contributes to the majority of the Tec29 protein found in ovaries (Figure 1A, second lane). Therefore, type 2 Tec29 is the predominant form expressed in nurse cells, and type 1 Tec29 is expressed at a low level.
We have previously shown that Src64 promotes the localization of endogenous Tec29 to ring canals, as determined by immunostaining with an antibody that recognized both forms of Tec29 (Guarnieri et al, 1998). To investigate whether the localization of both types of Tec29 is regulated by Src64, we tagged each at the carboxyl terminus with GFP and examined their localization in wild-type and Src64Δ17 mutant ovaries. Each tagged protein can rescue Tec29l(2)k05610 mutant phenotypes, indicating that either form can substitute for endogenous Tec29 function when overexpressed (data not shown and Figure 5A). Expression of the transgenes was not affected by the presence or absence of Src64 (Figure 1B). In wild-type egg chambers, both proteins localized to ring canals equally well (Figure 1C, upper panels). In Src64 mutant egg chambers, ring canal localization of type 2 Tec29 was dramatically reduced. Surprisingly, type 1 localization was not affected by the absence of Src64 (Figure 1C, lower panels). Given that type 1 Tec29 only constitutes a small fraction of the total endogenous Tec29 protein in germ cells (Figure 1A), it is most likely below the detection limit of anti-Tec29 immunostaining in our initial experiment (Guarnieri et al, 1998). We conclude that the localization of type 2, but not type 1 Tec29, is regulated by Src64. Therefore, the PH domain is necessary to mediate Src64 regulation, and its presence can inhibit type 2 Tec29 ring canal localization in the absence of Src64. This is in contrast to the function of PH domains of other TFKs, which have only been shown to play a positive role in mediating host protein localization to membranes (Varnai et al, 1999; Nore et al, 2000).
Figure 5.
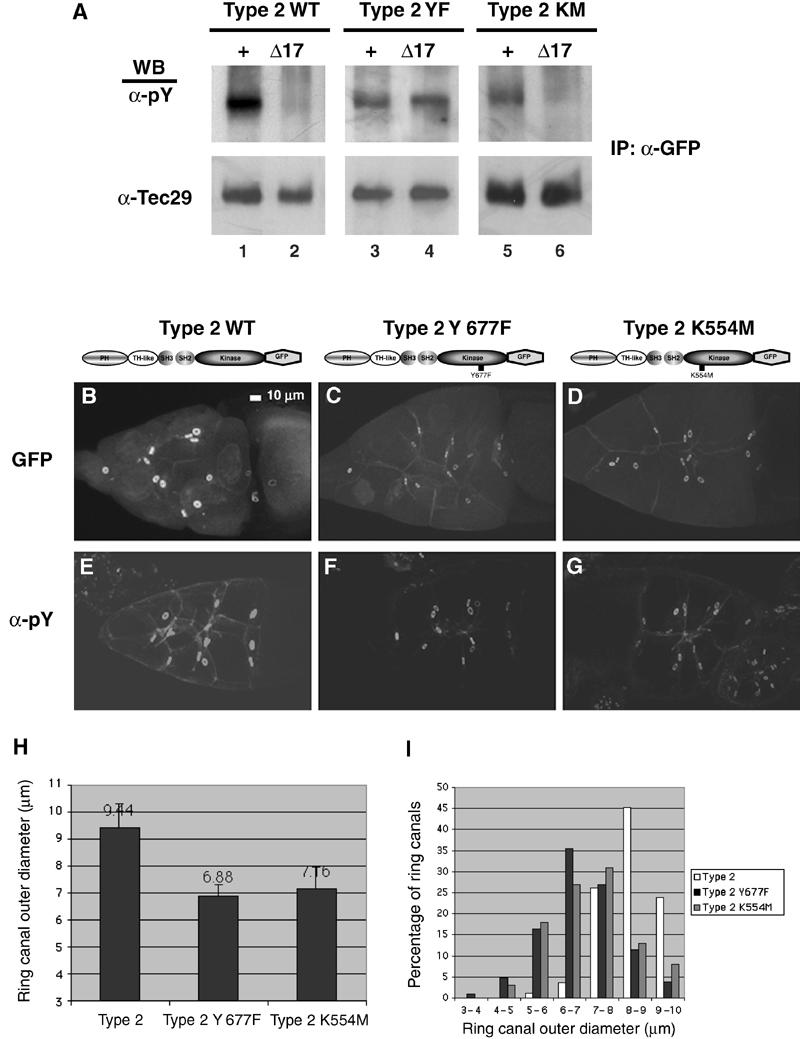
Y677 is required for Tec29 function in vivo. (A) Src64-dependent tyrosine phosphorylation in vivo at Y677. GFP-tagged wild-type (WT) and Y677F (YF), K554M (KM) type 2 proteins were immunoprecipitated from ovary lysates and probed for phosphotyrosine (upper panel), and then stripped and reprobed for Tec29 (lower panel). (B–G) Y677 and Tec29 kinase activity is required for its function at ring canals. Full-length type 2 Tec29 (B, E), type 2 Y677F (C, F) or type 2 K554M (D, G) that are tagged with GFP are examined for their ability to rescue Tecl(2)k05610 mutant phenotypes at ring canals. (B–D) GFP fluorescence. (E–G) Antiphosphotyrosine staining. The genotypes are as follows: (B, E) hsFLP/+; [OVOD 2La], [OVOD2Lb], FRT40A/ Tecl(2)k05610, FRT40A; [Tec29type2GFP]/+; (C, F) hsFLP/+; [OVOD 2La], [OVOD2Lb], FRT40A/ Tecl(2)k05610, FRT40A; [Tec29type2Y677FGFP]/+; (D, G) hsFLP/+; [OVOD 2La], [OVOD 2Lb], FRT40A/ Tecl(2)k05610, FRT40A; [Tec29type2K554MGFP]/+. (H) Average sizes of ring canals in Tecl(2)k05610 germline clones expressing wild-type or mutant type 2 proteins. At least 10 stage-10A egg chambers were measured for each genotype. (I) Size distribution of ring canals in (H).
SH3+SH2 domains are sufficient to direct ring canal localization
To map the minimal region with Tec29 that is sufficient for ring canal localization in a wild-type background, we constructed a series of GFP-tagged truncation mutants (Figure 2A). The corresponding proteins were expressed in Drosophila ovaries under the Tub67C promoter, which confers high levels of expression (Guarnieri et al, 1998). Localization of these proteins to ring canals in a wild-type background was visualized in vivo by GFP fluorescence. We have shown that GFP-tagged full-length type 1 and type 2 Tec29 localized to ring canals (Figure 1B). A truncated Tec29 lacking the kinase domain (ΔKinase) localized to ring canals to the same extent as full-length proteins (Figures 2A and 3A), indicating that the TH, SH3 or the SH2 domain might mediate Tec29 localization. Importantly, a construct containing only the SH3 and SH2 domains is sufficient to direct ring canal localization in a wild-type ovary (Figure 2B). The PH, TH or the kinase domains by themselves were unable to localize to ring canals, confirming that the Tec29 localizes to ring canals through its SH3 and SH2 domains (Figure 2A and data not shown).
Figure 2.
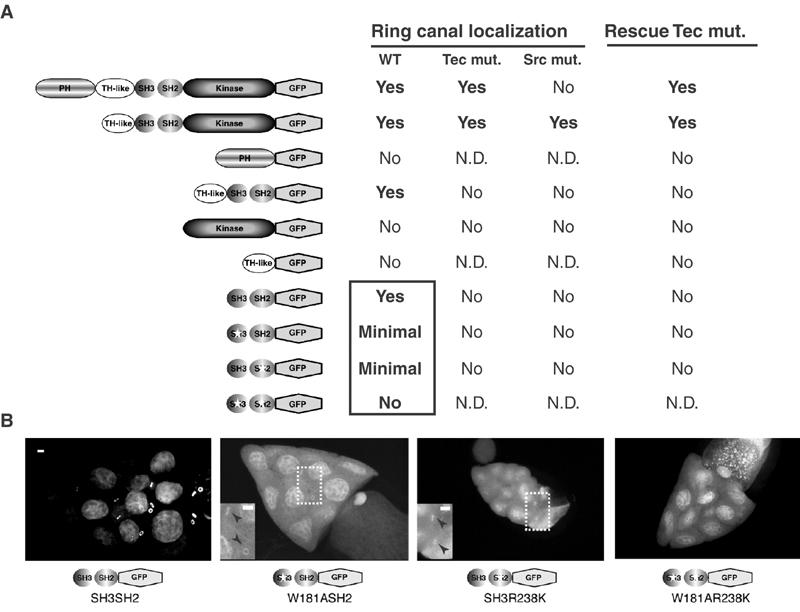
Tec29 localizes to ring canals via its SH3 and SH2 domains. (A) Schematic diagram of constructs and summary of results. For deletion mutants that failed to localize to ring canals, at least two insertions with high expression level were tested. N.D.: not determined. Boxed results: data shown in Figure 4B. The TH-like+SH3+SH2 construct is also called ΔKinase. ‘Rescue of Tec mut.' refers to the rescue of ring canal phenotypes associated with Tec29l(2)k05610. (B) Ring canal localization requires both SH3 and SH2 domains. Wild-type SH3+SH2, SH3+SH2 containing W379A or R436K mutations, or SH3+SH2 containing the W379AR436K double mutation were examined for ring canal localization in a wild-type background by GFP fluorescence. The brightness and contrast on the three images on the right were increased to visualize any minimal localization. Insets in the middle panels: magnified view of the region inside the dotted box to show ring canal localization (arrowheads). The intense nuclear localization is also seen with GFP alone. Scale bar, 10 μm.
Figure 3.
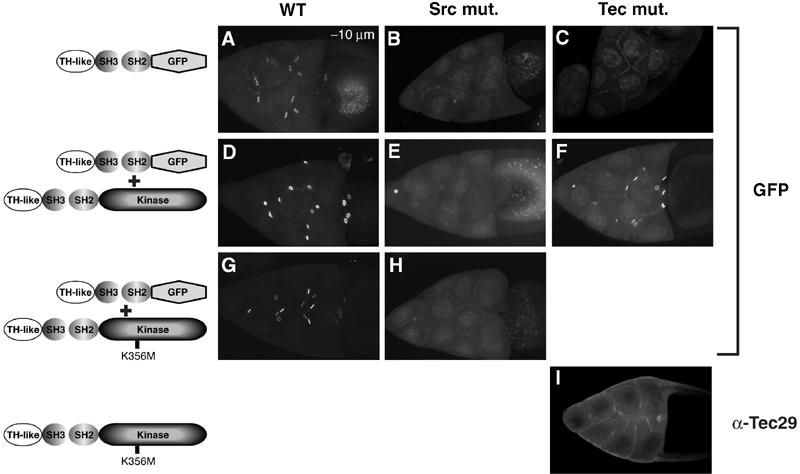
When activated by Src64, Tec29 can generate docking sites for itself. (A–H) ΔKinase localization to ring canals was used as a reporter of available Tec29 docking sites in various backgrounds. (A) wild-type; (B) Tecl(2)k05610 mutant; (C) Src64Δ17 mutant; (D) wild-type background with coexpression of a type 1 transgene; (E) Src64Δ17 mutant background with a type 1 transgene; (F) Tecl(2)k05610 mutant background with a type 1 transgene; (G) wild-type background with coexpression of a kinase-inactive (K356M) type 1 transgene; (H) Src64Δ17 mutant background with coexpression of a kinase-inactive (K356M) type 1 transgene. The same ΔKinase transgene on the second chromosome was used for all except (C) and (F), in which a third chromosome transgene insert that expresses comparable levels of Δkinase was used. (I) Kinase-inactive type 1 Tec29 failed to localize to ring canals in a Tecl(2)k05610 mutant background.
To assess whether the SH3 or SH2 domain was responsible for ring canal localization of the minimal region SH3SH2–GFP, we introduced point mutations at key conserved residues within each domain. A tryptophan (W379, type 2 amino-acid number) and an arginine (R436) residue have been shown to be required for the function of SH3 and SH2 domains, respectively (Moran et al, 1990; Noble et al, 1993). Disruption of either the SH3 domain (W379A) or the SH2 domain (R436K) function in the context of the SH3SH2–GFP fusion protein caused a drastic reduction in ring canal localization, although low levels of localization were still detectable (Figure 2B, insets). When both mutations were introduced, however, the SH3+SH2 double mutant protein failed to localize to ring canals. Therefore, while either the SH3 or the SH2 domain contains residual ability to direct ring canal localization, both domains together are required to achieve maximal localization. These results represent the first demonstration of a role for the SH3 and SH2 domains in directing subcellular localization of TFKs.
Tec29 activity is required for its localization to ring canals
The SH3 and SH2 domains are well-documented protein–protein interaction modules. The requirement for the SH2 domain in ring canal localization suggests that Tec29 docking sites contain phosphotyrosine residues (Anderson et al, 1990; Moran et al, 1990). As the localization of type 1 Tec29 is Src64 independent, phosphotyrosine residues within another ring canal protein, rather than Src64 itself, are likely to serve as Tec29 docking sites. Loss of either Src64 or Tec29 results in the loss of phosphotyrosine content on ring canals; therefore, either kinase might phosphorylate substrates on ring canals and generate ligands for Tec29's SH2 domain. To test which kinase is responsible, we used ΔKinase (TH+SH3+SH2) as a reporter for the presence of Tec29 ring canal docking sites. Src64 localization is independent of Tec29 (Guarnieri et al, 1998; Roulier et al, 1998). Therefore, if Src64 is responsible for generating Tec29's docking sites, one would expect ΔKinase to localize to ring canals in a Tec29 mutant egg chamber. However, we observed that ΔKinase failed to localize to ring canals in a Tec29 mutant egg chamber (compare Figure 3A and C), indicating that endogenous Tec29 is required to provide docking sites for ΔKinase, and suggesting that Src64 plays an indirect role by stimulating Tec29 (Figure 3B). Expression of a type 1 Tec29 transgene in Tec29 mutant rescued ΔKinase localization, thereby confirming our hypothesis (Figure 3F).
To further demonstrate the importance of Tec29 activity in its localization, we examined the effect of increasing Tec29 expression on ΔKinase localization to ring canals. Overexpression of a full-length type 1 Tec29 protein enhanced ΔKinase localization in a wild-type background (Figure 3D), while expression of a mutant form of Tec29 protein that lacks kinase activity (K356M) failed to do the same (Figure 3G). Taken together, these results strongly suggest that Tec29 kinase activity is directly involved in generating ring canal binding sites for other undocked Tec29 protein molecules. A prediction of this argument is that in the absence of endogenous Tec29, kinase-inactive Tec29 expressed from a transgene, similar to ΔKinase, would not localize to ring canals. We observed that this was indeed the case (Figure 3I).
Earlier we showed that type 1 Tec29 was able to localize to ring canals independently of Src64 (Figure 1). This suggests that type 1 Tec29 is somewhat activated in the absence of Src64 and can generate its own binding sites on ring canals. However, ring-canal-localized type 1 Tec29 may still need Src64 for full activation. As the activity of type 1 Tec29 is reflected in the number of additional docking sites for ΔKinase it can generate (Figure 3A, D and G), we examined the ability of type 1 Tec29 to enhance ΔKinase localization in a Src64Δ17 mutant egg chamber. In this egg chamber, type 1 Tec29 is localized to ring canals (Figure 1B). However, it did not enhance ΔKinase ring canal localization (Figure 3E), suggesting that Src64 is necessary to further stimulate and fully activate ring-canal-localized type 1 Tec29. As expected, a kinase-inactive type 1 Tec29 also failed to rescue ΔKinase localization in an Src64Δ17 mutant egg chamber (Figure 3H). We concluded that fully active Tec29 is necessary for generating ring canal ligands for its own SH2 domain, as well as for other cytoplasmic Tec29 proteins to be targeted to ring canals.
Tec29 is phosphorylated in vivo in an Src64-dependent manner
TFKs contain a highly conserved tyrosine residue within their kinase domains. This tyrosine resides in a region known as the ‘activation loop' in all tyrosine kinases, including SFKs (Brown and Cooper, 1996). Phosphorylation of this residue allows access of ATP and substrate to the active site, and is crucial to kinase activation. Therefore, a possible mechanism for the activation of Tec29 by Src64 was that Src64 might phosphorylate Tec29 at this conserved tyrosine residue. We began testing this possibility by first asking whether Src64 kinase activity was required for its function. The Src64Δ17 mutation leads to a small ring canal phenotype, as well as a dramatic reduction in ring canal localization of endogenous Tec29 (Guarnieri et al, 1998), phenotypes that are fully rescued by a wild-type Src64 transgene (Dodson et al, 1998 and data not shown). Expression of catalytically inactive Src64 failed to rescue either defect (Figure 4A–F). In fact, its expression resulted in a dominant-negative effect on ring canal growth, leading to a further reduction of the average ring canal size from 8.43 μm in Src64Δ17 mutants to 6.87 μm in Src64Δ17 mutants carrying the transgene (Figure 4G). The distribution of ring canals by size confirmed this result (Figure 4H). Therefore, we concluded that Src64 kinase activity is required for the localization of Tec29 and for ring canal growth.
Figure 4.
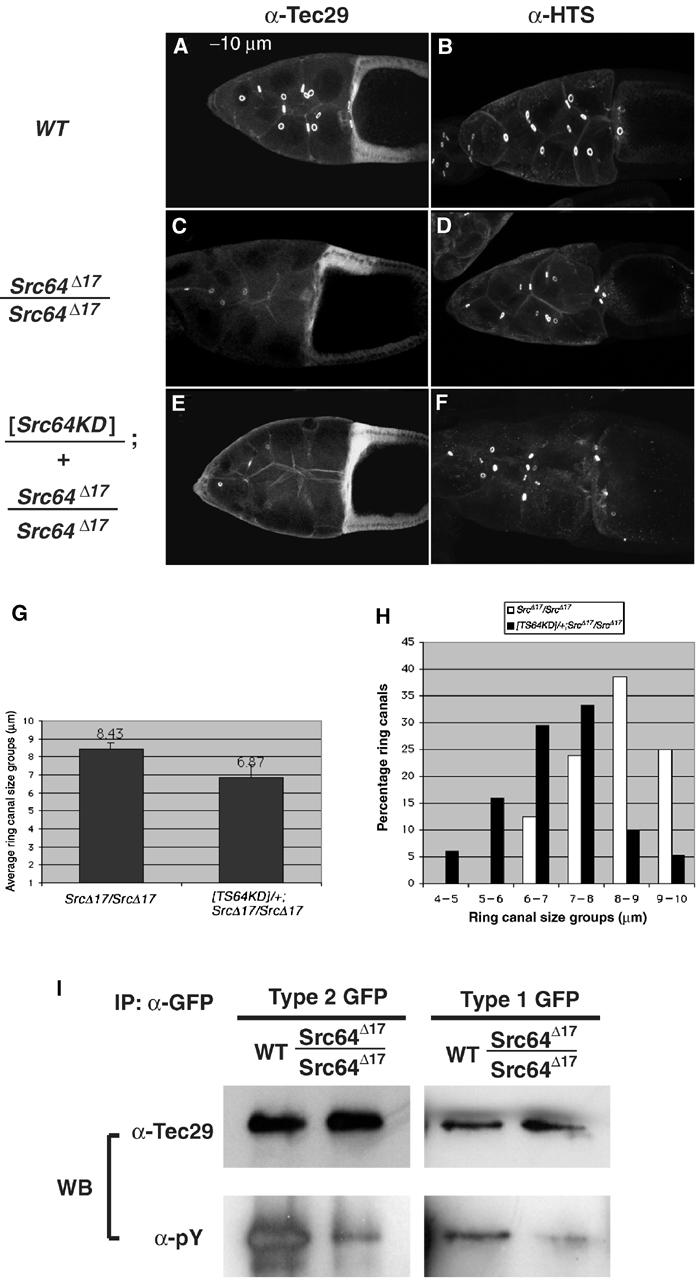
Both types of Tec29 are phosphorylated in an Src64-dependent manner. (A–F) Src64 kinase activity is required for Tec29 localization and ring canal growth. (A, C, E) Immunostain with anti-Tec29. (B, D, F) Immunostain with anti-HTS. HTS is the Drosophila homolog of adducin and a ring canal component (Yue and Spradling, 1992). (G) Average ring canal size of Src64Δ17/Src64Δ17 and [Src64KD]/+; Src64Δ17/Src64Δ17 ovaries. Images of 10 stage-10A egg chambers stained with anti-HTS (such as the ones in (D) and (E)) were taken and ring canals sizes were measured according to Materials and methods. (H) Size distribution of ring canals in (G). (I) Both types of Tec29 are phosphorylated in an Src64-dependent manner. GFP-tagged type 1 (left panels) or type 2 (right panels) Tec29 was immunoprecipitated from a wild-type or Src64Δ17/Src64Δ17 background and analyzed by Western blotting and probing with anti-pY (bottom panels), then stripped and reprobed with anti-Tec29 antibodies (top panels).
To examine whether Tec29 is phosphorylated in vivo, GFP-tagged type 1 or type 2 protein was immunoprecipitated from ovary lysates and analyzed by immunoblotting with antiphosphotyrosine and anti-Tec29 antibodies (Figure 4I). Both proteins are phosphorylated in a wild-type background (Figure 4I, left lanes in each panel). To determine whether Tec29 phosphorylation was Src64 dependent, we performed a similar experiment in a Src64Δ17 mutant background, and observed that phosphorylation of both proteins was diminished (right lanes in each panel). Thus, type 1 and type 2 Tec29 are phosphorylated in an Src64-dependent manner in vivo.
Y677, a conserved Src phosphorylation site, is required for Tec29 function in vivo
Our results above are consistent with a model in which Src64 activates both types of Tec29 by direct phosphorylation. However, it is also possible that Src64 might also activate Tec29 by other means, and the phosphorylated Tec29 we detected was a result of Tec29's own kinase activity. To test whether this was the case, we examined the phosphotyrosine profile of a kinase-inactive form of Tec29 (K554M). Inactivation of Tec29 reduced its phosphorylation in a wild-type background, indicating that Tec29's own activity only accounts for part of the phosphotyrosine signal we detected (Figure 5A, lanes 1 and 5). To test whether the remaining phosphotyrosine signal was Src64 dependent, the K554M mutant protein was expressed in a Src64Δ17 ovary. We observed that K554M phosphorylation is further reduced to background level (Figure 5A, lane 6). Therefore, Tec29 is either directly phosphorylated by Src64 or by a kinase that is activated by Src64.
As mentioned above, the conserved tyrosine within the activation loop of type 2 Tec29, Y677, represents the best candidate site for Src64-dependent phosphorylation. To test the role of Y677, we examined the phosphorylation of the type 2 Y677F mutant protein. As shown in Figure 5A, in wild-type ovaries, this mutant protein has reduced tyrosine phosphorylation compared to wild-type Tec29, indicating that Y677 is normally phosphorylated (lanes 1 and 3). Importantly, this low level of phosphorylation remained unchanged when type 2 Y677F protein was expressed in a Src64Δ17 background (lanes 3 and 4). Therefore, Y677 is required to mediate the effect of Src64 on Tec29 phosphorylation, and is most likely the site of Src64 phosphorylation. Taken together, these results strongly suggest that Src64 activates Tec29 in vivo by phosphorylating Y677. Activated Tec29, in turn, autophosphorylates at additional tyrosine residues as well as target sites on ring canals, which leads to its localization and the recruitment of additional Tec29 protein.
To examine the in vivo significance of Y677 and kinase activity to Tec29's role in regulating ring canal morphogenesis, we expressed type 2 Y677F and type 2 K554M proteins in ovaries lacking endogenous Tec29, and tested their ability to localize to ring canals and substitute for endogenous Tec29. In Tec29l(2)k05610 mutant ovaries, type 2 Y677F has minimal localization to ring canals compared to wild-type protein (Figure 5B and C). Importantly, it failed to rescue the loss of phosphotyrosine content on ring canals (Figure 5E and F) in Tec29l(2)k05610 mutants. The kinase-inactive Tec29 (type 2 K554M) behaved similarly (Figure 5D and G). Moreover, both Y677 and Tec29 kinase activity are also required for ring canal growth. Expression of a wild-type type 2 Tec29 transgene in Tec29l(2)k05610 mutant ovaries rescued the ring canal size defect, resulting in an average ring canal diameter of 9.49 μm (Figure 5H). In contrast, the average ring canal diameter from Tec29l(2)k05610 mutant ovaries expressing the type 2 Y677F protein is 6.88 μm, which is similar to ring canal sizes from Tec29l(2)k05610 mutants (Guarnieri et al, 1998; Figure 5H; also see Materials and methods). The distribution of ring canals by size again confirmed this result (Figure 5I). The kinase-inactive type 2 K554M protein also failed to rescue ring canal size defects associated with Tec29l(2)k05610 mutants (Figure 5H and I). Taken together, these experiments provide in vivo evidence that the Src64 phosphorylation site Y677, as well as Tec29's own kinase activity, is required for Tec29 to localize to ring canals and regulate their growth.
DPTEN antagonizes the localization of type 2 Tec29
Our results indicate that the PH domain of type 2 Tec29 mediates one of the effects of Src64 by preventing ring canal localization of type 2 Tec29 in the absence of Src64 (Figure 1). However, the PH domain may also play additional positive roles during Src64-dependent activation of type 2 Tec29. For instance, PH domains of other TFKs have been shown to have in vitro specificity for phosphatidylinositol-3,4,5-triphosphate (PtdIns(3,4,5)P3, or PIP3), a lipid molecule on cell membranes generated by phosphatidylinositol 3-kinase (PI 3-K) (Salim et al, 1996; Rameh et al, 1997). As SFKs can activate PI 3-K (Pleiman et al, 1994), it is possible that Src64 activity can indirectly lead to an increase in PIP3 levels. This would result in the recruitment of more type 2 Tec29 molecules via its PH domain to the cortical membrane, where Src64 resides.
Membrane PIP3 level is determined by the balance between PI 3-K and the tumor suppressor protein PTEN, a dual-specificity phosphatase (Toker and Cantley, 1997; Vanhaesebroeck et al, 2001). We coexpressed the Drosophila homolog of PTEN (DPTEN) with GFP-tagged type 1 or type 2 Tec29 in Tec29l(2)k05610 mutant ovaries (Goberdhan et al, 1999). The ability of type 1 Tec29 to localize to ring canals and rescue the small ring canal phenotype of Tec29l(2)k05610 was unaffected by DPTEN expression (Figure 6). In contrast, reduction of PIP3 level by DPTEN overexpression blocked the localization of type 2 Tec29 and prevented the rescue of Tec29l(2)k05610 mutant phenotypes. The average ring canal diameter in stage-10A egg chambers coexpressing a type 2 Tec29 transgene and DPTEN is 7.59±0.40 μm, significantly smaller than those expressing only the type 2 transgene (9.44±0.88 μm) (Figure 6A and B). The size distribution of stage-10A ring canals within these four groups of egg chambers showed similar results (Figure 6C). As DPTEN can also act as a protein phosphatase, we conducted a similar experiment in which GFP-tagged type 1 or type 2 Tec29 was coexpressed with a dominant-negative form of PI-3K, Dp110D954A (Leevers et al, 1996). Very similar effects on type 2 Tec29 localization and function were observed by coexpressing Dp110D954A (data not shown), confirming that opposing activities of PI 3-K and DPTEN regulate PIP3 levels, which in turn influence Tec29 ring canal localization.
Figure 6.
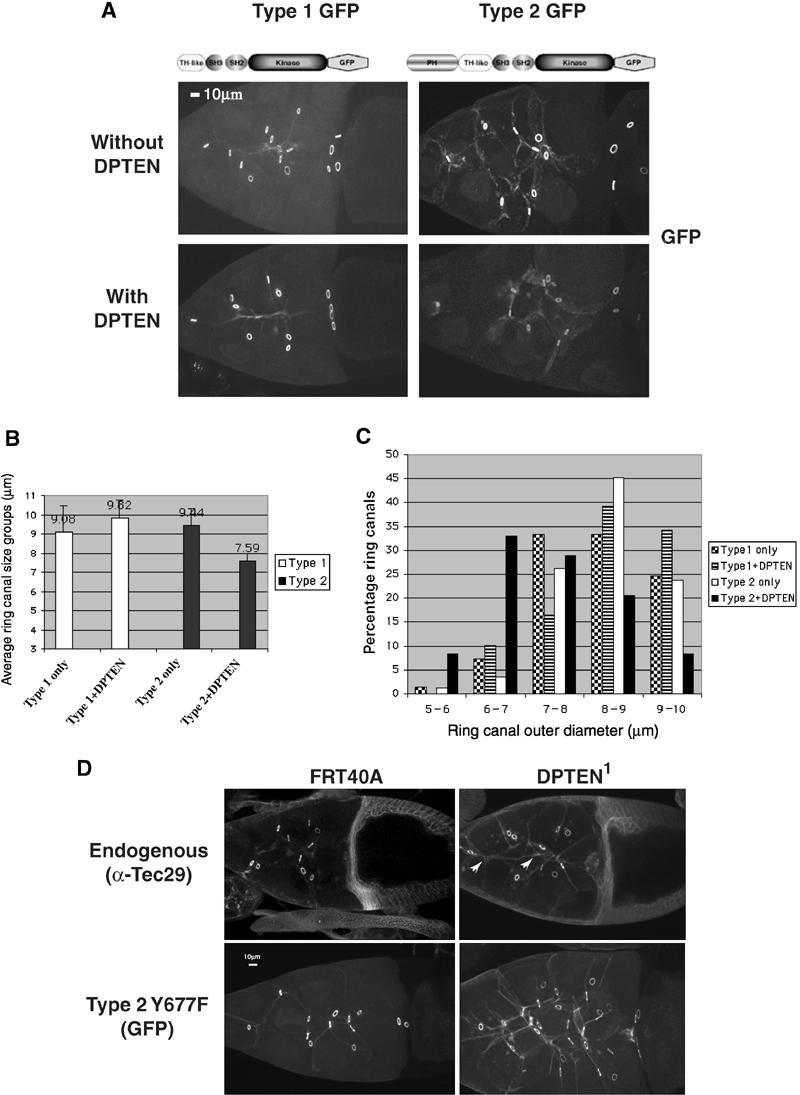
PI(3,4,5)P3-mediated membrane targeting of type 2 Tec29 is necessary, but not sufficient for ring canal localization. (A) DPTEN expression reduces the localization of type 2, but not type 1, Tec29 to ring canals in a Tecl(2)k05610 mutant background. The genotypes of the animals are as follows: for type 1, hsFLP/+; Tecl(2)k05610, FRT40A/[OVOD 2La], [OVOD 2Lb], FRT40A; [type1Tec29GFP]/TM2 and hsFLP/+; Tecl(2)k05610, FRT40A/[OVOD 2La], [OVOD 2Lb], FRT40A; [type1Tec29GFP]/[DPTEN]; and for type 2, hsFLP/+; Tecl(2)k05610, FRT40A/[OVOD 2La], [OVOD 2Lb], FRT40A; [type2Tec29GFP]/TM2 and hsFLP/+; Tecl(2)k05610, FRT40A/[OVOD 2La], [OVOD 2Lb], FRT40A; [type2Tec29GFP]/[DPTEN]. (B) DPTEN expression interferes with the ability of type 2 Tec29 to rescue the ring canal size defect in Tecl(2)k05610 mutants. At least 10 stage-10A egg chambers were imaged for each genotype in (A), and ring canal sizes were measured and processed according to Materials and methods. (C) Size distribution of ring canals. (D) Deficiency in DPTEN enhances type 2 Tec29 membrane localization independently of phosphorylation by Src64. (Top) Mutation in DPTEN enhances membrane localization of endogenous type 2 Tec29 (arrowheads) but does not affect ring canal localization. (Bottom) Membrane localization of type 2 Y677F is clearly enhanced by DPTEN mutation. Type 2 Y677F localized to ring canals in both egg chambers because there was endogenous Tec29 to provide docking sites. More than 15 ring canals and nurse cells are visible in the right panel. This could be due to an effect DPTEN1 has on growth, or a nonspecific effect, because control FRT40A germline clones occasionally exhibit a similar phenotype.
To determine whether raising PIP3 level is sufficient to enhance ring canal localization of type 2 Tec29, we compared endogenous Tec29 localization in a DPTEN mutant egg chamber to that in a control egg chamber (Figure 6D, top panels). As the majority of endogenous Tec29 is the type 2 form, our observation would likely reflect its behavior. Mutation in DPTEN enhanced the membrane localization of endogenous Tec29 (arrowheads). Interestingly, localization of Tec29 to ring canals is not affected. Therefore, localization of type 2 Tec29 seems to contain two discrete stages: localization to the cortical membrane and localization to ring canals. PIP3 level regulates membrane localization, and is required for ring canal localization. Increasing PIP3 level alone, however, is not sufficient to drive ring canal localization of type 2 Tec29, which is likely limited by activation by Src64 (Figure 5). To confirm this model and to test whether Src64-dependent activation of Tec29 also participates in membrane localization, we examined the localization of type 2 Y677F in wild-type and DPTEN1 mutant egg chambers. Similar to endogenous Tec29, the localization of type 2 Y677F to membrane was dramatically increased in a DPTEN1 mutant egg chamber, showing that Src64-dependent phosphorylation is not required for membrane localization (Figure 6D, bottom panels). As expected, ring canal localization of type 2 Y677F was not affected by the DPTEN1 mutation.
Discussion
In this report, we investigated the mechanisms by which Tec29 localizes to ring canals and demonstrated how this process is regulated by upstream activators in vivo. Our results show that the SH3 and SH2 protein–protein interaction domains of Tec29 are necessary and sufficient for ring canal localization. Localization of a truncated protein that contains these two domains (ΔKinase), however, is dependent on endogenous Tec29 activity. A likely reason for this result is that endogenous Tec29 activity can generate ring canal binding sites for the SH2 domain of ΔKinase. The fact that Tec29 activity is directly correlated with phosphotyrosine contents on ring canals (Figure 5) is consistent with this model. Alternatively, endogenous Tec29 may phosphorylate ΔKinase, thus allowing it to bind to an SH2-domain-containing protein on the ring canal. A tyrosine residue within the SH3 domain of Btk has been shown to be a major autophosphorylation site (Rawlings et al, 1996). When the corresponding tyrosine was mutated in Tec29, however, the mutant protein localized to ring canals and fully rescued all defects associated with Tec29 mutants (data not shown), indicating that this residue is not important for the function of Tec29 on ring canals. In addition, we detected no phosphotyrosine content within the ΔKinase protein by immunoblotting with an antiphosphotyrosine antibody (data not shown). Therefore, the most likely scenario is that Tec29 phosphorylates a ring canal protein, thus generating binding sites for its own SH2 domain.
As Src64 protein is localized to nurse cell cortical membrane, as well as ring canals (Dodson et al, 1998), it is interesting to consider why localization of Tec29, a process regulated by Src64, is directed to ring canals. One possible explanation is that Src64 activates Tec29 everywhere on the membrane, but the substrate (and therefore binding partner) of Tec29 is only present on ring canals. Alternatively, Tec29's substrate may be present on membranes and ring canals, but Src64 may only be activated at ring canals to ensure local activation of Tec29. A third possibility is that activation of Src64 and Tec29's substrates are both restricted to ring canals. Our data that overexpressed type 1 Tec29 localizes to ring canals in the absence of Src64 are consistent with its substrates being on ring canals only (Figure 1C). We therefore propose a model for how type 2 Tec29 may localize to ring canals (Figure 7). Interactions between the PH domain and PIP3 can target type 2 Tec29 to the cortical membrane, thus allowing it to be phosphorylated and activated by Src64. Once activated, the Tec29 protein that is localized to the membrane region adjacent to ring canals might access and phosphorylate substrate proteins, and bind to them via its SH3 and SH2 domains. In addition, ring-canal-localized Tec29 can phosphorylate additional substrate proteins, thus generating ring canal binding sites and directly recruit undocked Tec29 protein from the cytoplasm through a positive feedback mechanism (Figure 7).
Figure 7.
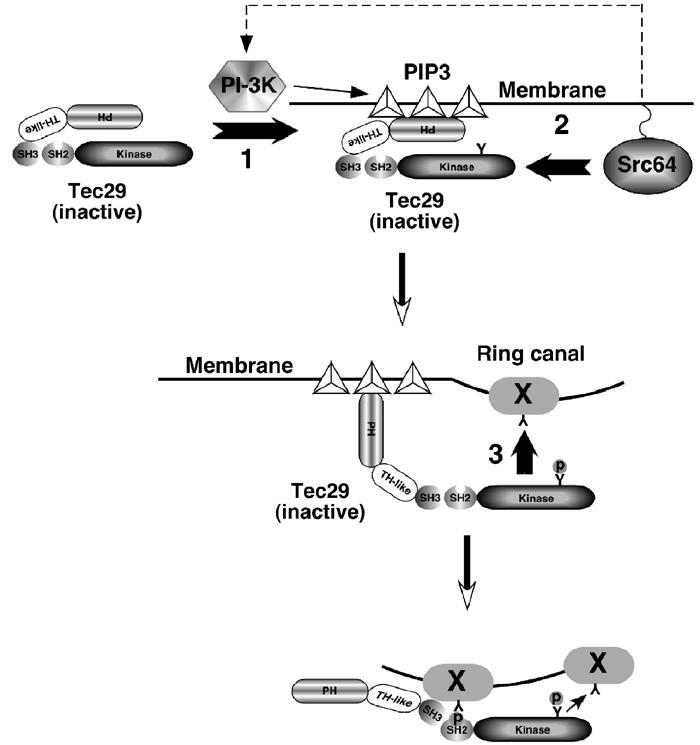
Schematic diagram of a model for the activation and ring canal localization of type 2 Tec29. ‘1', Type 2 Tec29 is targeted to the membrane by PIP3, which is generated by PI 3-K. As SFKs have been known to activate PI-3K in other systems (Pleiman et al, 1994), Src64 can potentially enhance this first step (dotted arrow). ‘2', Tec29 is phosphorylated on Y677 by Src64, which resides on the membrane. This phosphorylation activates Tec29. ‘3', Activated type 2 Tec29 phosphorylates target protein(s) and localizes to ring canals via its SH2 and SH3 domains. In addition, activated Tec29 can generate docking sites for more Tec29 molecules, creating a positive feedback mechanism for localization. X: Tec29's ring-canal-binding partner.
Our results suggest that Tec29's binding partner on the ring canal is one of its substrates. This substrate may be a scaffolding protein, on which a signaling complex can assemble, or it may be an effector that is directly involved in the formation and rearrangement of actin networks on ring canals. Several proteins that interact with either the SH3 (hnRNP-K, Vav, WASp, etc) or the SH2 domain (BLNK, BRDG-1, SLP-76) of other TFKs have been identified (Smith et al, 2001). Although none of these proteins has been shown to be a direct substrate of TFKs, their Drosophila homologs remain attractive candidates for downstream effectors of Tec29. For example, the SCAR protein, which has functions similar to WASp in promoting actin polymerization, has been shown to localize to actin-rich structures during Drosophila development. SCAR mutant ring canals have abnormal morphology and exhibit growth defects (Zallen et al, 2002). Analysis of the phosphorylation of these and other candidate proteins, including kelch, in response to Tec29, their mutant phenotypes in the ovary and the functional consequences of their potential association with Tec29 may lead to the identification of Tec29's ring canal binding partner.
TFKs are the only group of nonreceptor protein tyrosine kinases that contain PH domains. The existence of Tec29 splicing variants that differ in this domain provided an opportunity to analyze the in vivo significance of its function and how it affects Tec29 localization. Our results show that the PH domain can accentuate Src64 regulation of Tec29 by inhibiting Src64-independent ring canal localization of Tec29. Interestingly, a recent study showed that truncation of the PH domain increased the basal activity of Btk in vitro, but eliminated PIP3-dependent regulation (Saito et al, 2001). This suggests that an inhibitory function by the PH domain may be more universal among other TFKs, perhaps to confer a more stringent regulatory mechanism by activators such as SFKs. However, these results also raised questions as to the biological significance of type 1 Tec29. The fact that type 1 Tec29 lacks a PH domain dictates that it can be regulated by Src64 but not PIP3. This may be important in other tissues and developmental processes, where temporal and spatial coordination of multiple signaling pathways may be critical. Interestingly, Tec29 has been shown to be involved in dorsal closure and male genital formation (Baba et al, 1999; Tateno et al, 2000). Type 1 or type 2 Tec29-specific expression in the CNS has also been described (Baba et al, 1999). Further experiments will be necessary to address the physiological importance of type 1 and type 2 Tec29 proteins in these tissues.
Materials and methods
Fly strains
w1118 is used for all P-element-mediated transformation experiments. Tecl(2)k05610/Cyo was obtained from the BDGP. Src64Δ17 is maintained as homozygotes (Dodson et al, 1998). ficP/Cyo was obtained from D Yamamoto (1999). DPTEN1,FRT40A/Cyo was provided by C Wilson (Goberdhan et al, 1999). To generate germline mutant clones, the FLP/DFS method was used and the necessary strains were obtained from Bloomington Stock Center. All genotypes are in w1118 background unless noted.
Antibodies and cDNAs
Monoclonal anti-Tec29 antibody (clone I19) was provided by S Wadsworth (Vincent et al, 1989). Monoclonal anti-HTS antibody was provided by L Cooley. Monoclonal anti-GFP antibody (clone 3E6) from Molecular Probes, Inc. (A-11120) was used for all immunoprecipitation experiments.
Type 2 Tec29 cDNA was provided by D Yamamoto, DPTEN cDNA was provided by C Wilson, and Dp110D954A cDNA was provided by S Leevers.
Transgenic flies
All full-length and mutant versions of Tec29 were fused to GFP as follows: an mGFP6 cDNA (Andrea Brand) was PCR amplified and cloned into SpeI–NotI-digested D277Matg (Tub67C promoter; Dodson et al, 1998), generating D277–GFPc. Tec29 cDNAs were then amplified with 5′ oligos that contain a BglII site in the overhang, and 3′ oligos that contain a SpeI site in the overhang. All oligos contain 18–20 nt sequences to Tec29 cDNA. The PCR fragments were digested with BglII and SpeI and cloned into BglII–SpeI-digested D277–GFPc. The collection of deletion mutants used in Figure 4 corresponds to Tec29 (type 2 a.a. number unless indicated) peptide sequence as follows: PH (1–231), TH-like (1–145, type 1 a.a. number), SH3 (344–409), SH2 (410–501), Kinase (502–788). The ΔKinase construct is TH-like+SH3+SH2 in type 1. For all full-length wild-type and mutant type 2 constructs, the 5′ PCR primer has a BclI instead of BglII site in the overhang. These PCR products were digested with BclI and SpeI, and ligated into BglII–SpeI-digested D277–GFPc. The point mutations W379A, R436K, K554M and Y677F were generated by oligonucleotide-directed mutagenesis. Catalytically inactive Src64 has a K312M mutation and is under the regulation of the Tub67C promoter (S Dodson, unpublished results). DPTEN and Dp110D954A cDNAs were myc-tagged and expressed from D277Matg.
Germline transformation was carried out as previously described in w1118 hosts (Rubin and Spradling, 1982; Spradling and Rubin, 1982). Transformants were selected by orange eye color conferred by the P[w+] in D277Matg. A total of 3–20 independent insertions were identified and maintained for each transgene. When necessary, more insertions were generated by mobilizing an established P element with Δ2–3 transposase and selecting for flies with darker eye colors.
GFP imaging and ring canal size measurement
Ovaries expressing GFP-tagged proteins were dissected into cold 1 × PBS and carefully separated into individual egg chambers using fine-tip forceps. For a quick assessment, egg chambers in PBS are mounted on a glass slide, and GFP fluorescence can be directly visualized under a Nikon Eclipse E600 fluorescence microscope using an FITC filter set. For imaging purposes, dissected egg chambers were fixed in 1 × PBS containing 4% paraformaldehyde for 15 min, washed three times in 1 × PBS, mounted in Vectashield (Vector Laboratories, Inc.) and sealed with nail polish. Anti-Tec29, anti-HTS and antiphosphotyrosine staining was carried out as described in Guarnieri et al (1998). All images were collected using a Bio-Rad MRC 1024 confocal laser scanning microscope and Lasersharp software (Bio-Rad Inc.). Within each figure where comparisons of fluorescent intensities were made, the images were taken under the same confocal imaging conditions.
To measure ring canal size, a whole Z-stack is projected using NIH image with a ‘CLSM' macro. The resulting image is calibrated, and each ring canal is measured by drawing a line across the outer diameter of the ring. The length of the line is then measured by the software. The rest of the statistical processing and analysis is conducted as described previously (Guarnieri et al, 1998). We found that the internal scale factor in the CoMOS software used to generate our previous data was not accurate. As a result, the actual ring canal diameters should be approximately 1.34-fold the numbers described (Guarnieri et al, 1998). The ring canal measurement in this report is generated using the correct scale factor.
Ovary lysates and immunoprecipitation
In all, 50 3- to 5-day-old females are aged for 24–48 h in a freshly yeasted vial with 50 males at 25°C (if females are unhealthy and have small ovaries, we used more). Ovaries are then dissected into cold 1 × PBS, washed once and homogenized in 300 μl IP buffer: 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 0.5% NP-40 (or IGEPAL CA 630 from Sigma), protease inhibitors cocktail (Sigma P2714, 1:100) and phosphatase inhibitors cocktail (Sigma P5726, 1:100). The debris was cleared by centrifugation in a microfuge at 14 000 rpm for 10 min at 4°C. The lipid layer on top was removed with a pipette, and then the supernatant was transferred to a fresh vial. A small aliquot was used to measure protein concentration by BCA assay (Pierce 23223 and 23224) and the rest of the lysate was stored at −80°C.
For immunoprecipitation, anti-GFP antibody (3E6) was coupled to protein A–agarose gel (Bio-Rad 153-6153) using DMP (Pierce) as per the manufacturer's suggestions. Ovary lysates were then added to beads and IP buffer was added to bring the final volume to 300 μl. Fresh protease and phosphatase inhibitor cocktails were added, incubated at 4°C for 2 h with rotation, and spun at low speed to pellet beads. The supernatant was saved. The beads were washed 4 × with 0.5 ml cold IP buffer for 10 min each. They were then boiled in 40 μl 1 × SDS sample buffer and centrifuged. The supernatants were loaded on an 8% SDS gel and separated by electrophoresis. Proteins were transferred onto PVDF membrane by Western blotting and probed with antibodies. For this purpose, we used anti-pY (4G10, Upstate Biotechnology) at 1:2000 and anti-Tec29 (I19) at 1:500. ECL plus detection system (Invitrogen) was used to detect signals.
Acknowledgments
We thank Byron Miyazawa for helping with the generation of transgenic lines. Steve Dodson generated the kinase-inactive Src64 construct and transgenic line (Figure 4). We thank Alana O'Reilly and Michelle Arbeitman for critical reading of the manuscript. This work is supported by NIH grant GM60629 to MAS.
References
- Anderson D, Koch CA, Grey L, Ellis C, Moran MF, Pawson T (1990) Binding of SH2 domains of phospholipase C gamma 1, GAP, and Src to activated growth factor receptors. Science 250: 979–982 [DOI] [PubMed] [Google Scholar]
- Baba K, Takeshita A, Majima K, Ueda R, Kondo S, Juni N, Yamamoto D (1999) The Drosophila Bruton's tyrosine kinase (Btk) homolog is required for adult survival and male genital formation. Mol Cell Biol 19: 4405–4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschek CB, Jockusch BM, Friis RR, Back R, Grundmann E, Bauer H (1981) Early changes in the distribution and organization of microfilament proteins during cell transformation. Cell 24: 175–184 [DOI] [PubMed] [Google Scholar]
- Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR (1992) Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest 90: 1622–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Cooper JA (1996) Regulation, substrates and functions of src. Biochim Biophys Acta 1287: 121–149 [DOI] [PubMed] [Google Scholar]
- Bunnell SC, Henry PA, Kolluri R, Kirchhausen T, Rickles RJ, Berg LJ (1996) Identification of Itk/Tsk Src homology 3 domain ligands. J Biol Chem 271: 25646–25656 [DOI] [PubMed] [Google Scholar]
- Chen R, Kim O, Li M, Xiong X, Guan JL, Kung HJ, Chen H, Shimizu Y, Qiu Y (2001) Regulation of the PH-domain-containing tyrosine kinase Etk by focal adhesion kinase through the FERM domain. Nat Cell Biol 3: 439–444 [DOI] [PubMed] [Google Scholar]
- Chou TB, Perrimon N (1996) The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144: 1673–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley L (1998) Drosophila ring canal growth requires Src and Tec kinases. Cell 93: 913–915 [DOI] [PubMed] [Google Scholar]
- Dodson GS, Guarnieri DJ, Simon MA (1998) Src64 is required for ovarian ring canal morphogenesis during Drosophila oogenesis. Development 125: 2883–2892 [DOI] [PubMed] [Google Scholar]
- Goberdhan DC, Paricio N, Goodman EC, Mlodzik M, Wilson C (1999) Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes Dev 13: 3244–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri DJ, Dodson GS, Simon MA (1998) SRC64 regulates the localization of a Tec-family kinase required for Drosophila ring canal growth. Mol Cell 1: 831–840 [DOI] [PubMed] [Google Scholar]
- Kelso RJ, Hudson AM, Cooley L (2002) Drosophila Kelch regulates actin organization via Src64-dependent tyrosine phosphorylation. J Cell Biol 156: 703–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers SJ, Weinkove D, MacDougall LK, Hafen E, Waterfield MD (1996) The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J 15: 6584–6594 [PMC free article] [PubMed] [Google Scholar]
- Maas A, Hendriks RW (2001) Role of Bruton's tyrosine kinase in B cell development. Dev Immunol 8: 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machide M, Mano H, Todokoro K (1995) Interleukin 3 and erythropoietin induce association of Vav with Tec kinase through Tec homology domain. Oncogene 11: 619–625 [PubMed] [Google Scholar]
- Martin GS (2001) The hunting of the Src. Nat Rev Mol Cell Biol 2: 467–475 [DOI] [PubMed] [Google Scholar]
- Moran MF, Koch CA, Anderson D, Ellis C, England L, Martin GS, Pawson T (1990) Src homology region 2 domains direct protein–protein interactions in signal transduction. Proc Natl Acad Sci USA 87: 8622–8626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble ME, Musacchio A, Saraste M, Courtneidge SA, Wierenga RK (1993) Crystal structure of the SH3 domain in human Fyn; comparison of the three-dimensional structures of SH3 domains in tyrosine kinases and spectrin. EMBO J 12: 2617–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nore BF, Vargas L, Mohamed AJ, Branden LJ, Backesjo CM, Islam TC, Mattsson PT, Hultenby K, Christensson B, Smith CI (2000) Redistribution of Bruton's tyrosine kinase by activation of phosphatidylinositol 3-kinase and Rho-family GTPases. Eur J Immunol 30: 145–154 [DOI] [PubMed] [Google Scholar]
- Pleiman CM, Hertz WM, Cambier JC (1994) Activation of phosphatidylinositol-3′ kinase by Src-family kinase SH3 binding to the p85 subunit. Science 263: 1609–1612 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Kung HJ (2000) Signaling network of the Btk family kinases. Oncogene 19: 5651–5661 [DOI] [PubMed] [Google Scholar]
- Rameh LE, Arvidsson A, Carraway KL III, Couvillon AD, Rathbun G, Crompton A, VanRenterghem B, Czech MP, Ravichandran KS, Burakoff SJ, Wang DS, Chen CS, Cantley LC (1997) A comparative analysis of the phosphoinositide binding specificity of pleckstrin homology domains. J Biol Chem 272: 22059–22066 [DOI] [PubMed] [Google Scholar]
- Rawlings DJ, Scharenberg AM, Park H, Wahl MI, Lin S, Kato RM, Fluckiger AC, Witte ON, Kinet JP (1996) Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science 271: 822–825 [DOI] [PubMed] [Google Scholar]
- Robinson DN, Cooley L (1997) Genetic analysis of the actin cytoskeleton in the Drosophila ovary. Annu Rev Cell Dev Biol 13: 147–170 [DOI] [PubMed] [Google Scholar]
- Roulier EM, Panzer S, Beckendorf SK (1998) The Tec29 tyrosine kinase is required during Drosophila embryogenesis and interacts with Src64 in ring canal development. Mol Cell 1: 819–829 [DOI] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC (1982) Genetic transformation of Drosophila with transposable element vectors. Science 218: 348–353 [DOI] [PubMed] [Google Scholar]
- Saito K, Scharenberg AM, Kinet JP (2001) Interaction between the Btk PH domain and phosphatidylinositol-3,4,5-trisphosphate directly regulates Btk. J Biol Chem 276: 16201–16206 [DOI] [PubMed] [Google Scholar]
- Salim K, Bottomley MJ, Querfurth E, Zvelebil MJ, Gout I, Scaife R, Margolis RL, Gigg R, Smith CI, Driscoll PC, Waterfield MD, Panayotou G (1996) Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton's tyrosine kinase. EMBO J 15: 6241–6250 [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite AB, Witte ON (2000) The role of Bruton's tyrosine kinase in B-cell development and function: a genetic perspective. Immunol Rev 175: 120–127 [PubMed] [Google Scholar]
- Simon MA, Drees B, Kornberg T, Bishop JM (1985) The nucleotide sequence and the tissue-specific expression of Drosophila c-src. Cell 42: 831–840 [DOI] [PubMed] [Google Scholar]
- Smith CI, Islam TC, Mattsson PT, Mohamed AJ, Nore BF, Vihinen M (2001) The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. BioEssays 23: 436–446 [DOI] [PubMed] [Google Scholar]
- Spradling AC, Rubin GM (1982) Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218: 341–347 [DOI] [PubMed] [Google Scholar]
- Takahashi F, Endo S, Kojima T, Saigo K (1996) Regulation of cell-cell contacts in developing Drosophila eyes by Dsrc41, a new, close relative of vertebrate c-src. Genes Dev 10: 1645–1656 [DOI] [PubMed] [Google Scholar]
- Tateno M, Nishida Y, Adachi-Yamada T (2000) Regulation of JNK by Src during Drosophila development. Science 287: 324–327 [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513–609 [DOI] [PubMed] [Google Scholar]
- Toker A, Cantley LC (1997) Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 387: 673–676 [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD (2001) Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem 70: 535–602 [DOI] [PubMed] [Google Scholar]
- Varnai P, Rother KI, Balla T (1999) Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton's tyrosine kinase pleckstrin homology domain visualized in single living cells. J Biol Chem 274: 10983–10989 [DOI] [PubMed] [Google Scholar]
- Vincent WSd, Gregory RJ, Wadsworth SC (1989) Embryonic expression of a Drosophila src gene: alternate forms of the protein are expressed in segmental stripes and in the nervous system. Genes Dev 3: 334–347 [DOI] [PubMed] [Google Scholar]
- Woods ML, Kivens WJ, Adelsman MA, Qiu Y, August A, Shimizu Y (2001) A novel function for the Tec family tyrosine kinase Itk in activation of beta 1 integrins by the T-cell receptor. EMBO J 20: 1232–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Spradling AC (1992) hu-li tai shao, a gene required for ring canal formation during Drosophila oogenesis, encodes a homolog of adducin. Genes Dev 6: 2443–2454 [DOI] [PubMed] [Google Scholar]
- Zallen JA, Cohen Y, Hudson AM, Cooley L, Wieschaus E, Schejter ED (2002) SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J Cell Biol 156: 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]


