Abstract
Brachial artery flow-mediated dilation (FMD) is impaired with aging and is associated with increased risk for cardiovascular disease (CVD). We determined if regular aerobic exercise improves brachial artery FMD in middle-aged/older (MA/O) men and postmenopausal women. In sedentary MA/O adults (age 55 – 79 years) without CVD, 8 weeks of brisk walking (6 days/week for ~50 min/day; randomized, controlled design) increased treadmill time ~20% in both MA/O men (n=11) and postmenopausal women (n=15) (P<0.01), without altering body composition or circulating CVD risk factors. Brachial artery FMD increased > 50% in the MA/O men (4.6 ± 0.6 to 7.1 ± 0.6%, P < 0.01), but did not change in the postmenopausal women (5.1 ± 0.8 vs. 5.4 ± 0.7%, P = 0.50). No changes occurred in the non-exercising controls. In a separate cross-sectional study (n =167), brachial artery FMD was ~50% greater in endurance exercise-trained (6.4 ± 0.4%, n = 45) vs. sedentary (4.3 ± 0.3%, n = 60) MA/O men (P < 0.001), whereas there were no differences between endurance-trained (5.3 ± 0.7%, n = 20) and sedentary (5.6 ± 0.5%, n = 42) postmenopausal women (P = 0.70). Brachial artery lumen diameter, peak hyperemic shear rate and endothelium independent dilation did not differ with exercise intervention or in the endurance-exercise vs. sedentary groups. Regular aerobic exercise is consistently associated with enhanced brachial artery FMD in MA/O men, but not in postmenopausal women. Some postmenopausal women without CVD may be less responsive to habitual aerobic exercise than MA/O men.
Keywords: aging, endothelium-dependent dilation, postmenopausal women
Introduction
Brachial artery flow-mediated dilation (FMD) is a measure of endothelium-dependent dilation and vascular endothelial function, and is an independent predictor of future cardiovascular disease (CVD)-related events in adults with and without existing CVD at baseline [1, 2, 3, 4, 5]. Brachial artery FMD is impaired in middle-aged and older adults [6, 7, 8, 9, 10] and this is associated with increased risk for CVD [3, 5]. As such, interventions that can improve brachial artery FMD in this group may have important clinical implications for the prevention of age-related CVD.
Regular aerobic exercise is associated with a lower risk of CVD compared with the sedentary state [11, 12, 13, 14]. Results of cross-sectional studies indicate that aerobic-endurance exercise-trained middle-aged/older men have greater brachial artery FMD compared with their non-exercising peers [6, 7, 8, 15, 16]. However, this has not been established in postmenopausal women, as little data are available [17]. Moreover, endurance exercise-trained adults generally have lower body fatness and CVD risk factors than sedentary adults [18, 19, 20, 21], and these influences can affect FMD independent of habitual exercise status [22, 23]. Prospective intervention studies have reported inconsistent effects of regular aerobic exercise on brachial FMD in previously sedentary middle-aged/older men and women [24, 25, 26]. Therefore, the efficacy of habitual aerobic exercise for improving brachial artery FMD has not been established in these groups.
In the present study, we hypothesized that regular aerobic exercise would improve brachial artery FMD in previously sedentary middle-aged/older men and postmenopausal women. To address this, we first conducted a randomized, controlled, parallel group design intervention trial to determine if regular, moderate-intensity aerobic exercise improves brachial artery FMD in previously sedentary middle-aged and older adults without CVD. We then determined if a higher-intensity/more prolonged habitual aerobic exercise “stimulus” is associated with enhanced brachial artery FMD, independent of sex, by comparing healthy sedentary and endurance exercise-trained middle-aged/older men and postmenopausal women.
Methods
Subjects
A total of 211 middle-aged and older (55–79 years of age) adults (intervention study, n=44; cross-sectional study, n=167) were recruited from the community using local newspaper and radio advertisements, university email advertisements and direct mailings. All subjects were non-diabetic, non-smokers and were free of other clinical diseases as assessed by medical history, physical examination, blood chemistry and resting and maximal exercise ECG and blood pressure. They were not taking prescription medications, herbal supplements, antioxidants or aspirin. Women were postmenopausal for at least 1 year and had not taken hormone replacement therapy for at least the previous 6 months. Sedentary status was defined as no regular exercise (i.e., <30 minutes/day, <2 days/week) for at least the prior 2 years. Endurance exercise-trained subjects had been performing vigorous habitual aerobic-endurance exercise (competitive distance running, cycling and/or triathlons) ≥5 days per week, >45 min/day for at least the previous 5 years. All study procedures complied with the Declaration of Helsinki and the informed consent and study documents were approved by the Human Research Committee of the University of Colorado at Boulder. The nature, benefits, and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation.
Measurements
All measurements were performed at the University of Colorado at Boulder Clinical and Translational Research Center (CTRC) after a 12-hour overnight fast and 24-hour abstention from alcohol and exercise.
Subject characteristics
Body mass index (BMI) was calculated from height and weight to the nearest 0.1 kg, and waist and hip circumferences were measured by anthropometry [27]. Total body fat was determined using dual energy x-ray absorptiometry (DPX-IQ, GE/Lunar, Inc.) [28]. Blood pressure was measured after 15 minutes of supine rest at least three times and averaged using a semi-automated device (Dynamap XL, Johnson and Johnson). Diet composition and caloric intake were estimated from 3-day food intake records (The Food Processor 8.2, ESHA Research) analyzed by a CTRC bionutritionist [27]. Maximal oxygen consumption was assessed during incremental treadmill exercise performed to exhaustion and habitual physical activity was assessed from estimates of daily energy expenditure during leisure-time and occupational activities as previously described by our laboratory [29].
Circulating factors
All assays were performed by the University of Colorado at Denver and Health Sciences Center Adult CTRC core laboratory. Fasting serum concentrations of total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, glucose, and white blood cell count were determined using standard assays. Plasma concentrations of c-reactive protein (CRP) were measured using a high-sensitivity Chemistry Immuno Analyzer (AU400e, Olympus America, Inc.). Plasma interleukin-6 (IL-6), tumor-necrosis factor-α (TNF-α; both R&D Systems, Inc), oxidized LDL (Alpco, Inc.), endothelin-1 (Peninsula Laboratories, Inc) were measured by ELISA. Total antioxidant status was measured by a colormetric assay where ABTS® (2,2’-Azino-di-[3-ethylbenzthiazoline sulphonate]) is incubated with a peroxidase (metmyoglobin) and hydrogen peroxide to produce the radical cation ABTS® forming a stable blue-green color (measured at 600 nm) (Randox Laboratories, Inc). Antioxidants in the sample cause suppression of this color production to a degree which is proportional to their concentration. Serum adiponectin and leptin were measured by radioimmunoassay (Linco Research, Inc.) and plasma norepinephrine by high performance liquid chromatography.
Brachial artery FMD and endothelium-independent dilation
Brachial artery FMD and endothelium-independent dilation in response to 0.4 mg sublingual glyceryl trinitrate (GTN) were determined using duplex ultrasonography (Powervision 6000, Toshiba, Inc.) with a multi-frequency linear-array transducer using as previously described by our laboratory [6, 7, 10, 22, 27, 30] and others [31, 32, 33]. Briefly, brachial artery images were obtained longitudinally ~3–6 cm above the antecubital crease once the anterior and posterior intima layers were visualized. The transducer was clamped in place and images were obtained for 30 seconds at baseline and then continuously for 2 minutes after 5 minutes of blood flow occlusion induced by inflation of a cuff positioned on the forearm (below the antecubital crease) at 250 mmHg. Baseline, occlusion (i.e., the average diameter for the 15 seconds immediately before rapid cuff deflation), and peak brachial artery diameters were analyzed using commercially available software (Vascular Research Tools 5.0, Medical Imaging Applications, LLC) by the same investigator who was blinded to subject group assignment. Pulsed doppler signals were recorded at an angle of insonation of 68 degrees with a sample volume the entire width of the artery as previously described [30]. Time-averaged peak velocity was obtained from recording the first 10 velocity envelopes. Brachial artery peak hyperemic shear rate was calculated as 8 times (due to wide sample volume) the peak velocity immediately following 5 minutes of forearm occlusion, divided by occlusion diameter and FMD responses were expressed as absolute (mmΔ) and relative (%Δ) maximal change from baseline diameter per recent recommendations [31, 32]. The coefficient of variation in our laboratory for trial-to-trial reliability for brachial artery baseline lumen diameter, peak lumen diameter and FMD are 0.3, 0.6, and 8.1%, respectively [6].
Intervention study
Eighty-six subjects signed informed consent for the intervention study. During screening, 26 subjects were excluded because of abnormal exercise ECG (n=12), abnormal blood chemistries (n=5), systolic blood pressure >140 mmHg (n=4), body mass index ≥30 kg/m2 (n=4) or moderate cognitive impairment/dementia (n=1). Another 16 subjects dropped out due to “lack of time” (n=10) or no reason given (n=6) resulting in enrollment of 44 subjects. Of these, 22 subjects were randomized to regular aerobic exercise; 15 to attention-control; and 7 other subjects with untreated hypercholesterolemia (n=5, plasma LDL-cholesterol 4.4 ± 0.2 mmol/L; 5F) or hypertriglyceridemia (n=2, 2.6 ± 0.1 mmol/L; 2M) also were assigned to the exercise group (after approval by their personal physician) because we did not feel it ethical to randomize these subjects to attention control.
Subjects in the exercise group were asked to walk for 8 weeks, 6–7 days/week for 40–50 minutes/day at 70–75% of maximal heart rate measured during the maximal treadmill exercise test. Adherence was documented with the use of heart rate monitors and diaries. The aerobic exercise intervention used is associated with excellent subject adherence and does not produce weight loss or other improvements in conventional risk factors for CVD that could complicate interpretation of results [29, 34].
During the 8-week intervention/control period, three subjects dropped out of the exercise group (minor knee pain related to previous injury, n=2; hip strain, n=1) and five dropped out of the control group (did not want to be in control group, n=3; noncompliance, n=2). Thus, 36 subjects (15M, 21F) completed the study: 26 in the exercise group (11M, 15F), including all 7 subjects with hyperlipidemia, and 10 attention control (4M, 6F).
Cross-sectional study
One hundred and sixty-seven middle-aged/older men and postmenopausal women were studied: 102 sedentary adults (60 men and 42 women) and 65 endurance exercise-trained adults (45 men and 20 women).
Statistical Analysis
All analyses were performed on SPSS 16.0, (SPSS, Inc.). All data are presented as mean ± standard error. Statistical significance was set a priori at P<0.05. For the intervention study, an independent t-test was performed to determine differences between the exercise and attention-control groups at baseline. A 2×2 repeated measures analysis of variance (ANOVA) was performed to identify a group (Exercise, Control) × time (Baseline, 8 weeks) interaction between FMD and all other variables. To determine if there were sex differences in the exercise group after 8 weeks of exercise, a 2×2 repeated measures ANOVA was used to identify any sex (Men, Women) × time (Baseline, 8 weeks) interactions. If significant interactions were present, a paired t-test with Bonferonni correction was performed to identify differences of within-group factors. For the cross-sectional group comparisons, an independent t-test was performed to identify differences in FMD and other variables between sedentary and exercise-trained subjects. For potential FMD-influencing variables that were significantly different between sedentary and exercise-trained men, but not women, an analysis of covariance was performed with the variable entered as the covariate and FMD as the dependent variable. Pearson correlations were performed between FMD and other variables to assess relations of interest.
Results
Exercise intervention in previously sedentary men and women
Characteristics of all subjects before and after the intervention and control periods are shown in Table 1 and Supplemental Tables I and II. There were no differences in the intervention and control groups at baseline. In the overall intervention group, subjects exercised ~6 days/week for 49 minutes/day at 71% of maximal heart rate for 8.7 weeks; there were no differences in the men and women (Table 2). The exercise intervention increased treadmill exercise time by 19% (P < 0.01) and tended to increase maximal oxygen consumption (P = 0.06), without affecting any other factor (Table 1 and Supplemental Tables I and II). The increases in these measures of maximal exercise capacity in response to the intervention were similar in the men and women (Table 3). As in the overall group, no other factors changed in response to the exercise intervention in the subgroups of men and women (Table 3 and Supplemental Table II).
Table 1.
Subject characteristics for exercise intervention study: all subjects
| Exercise (n=26) | Control (n=10) | 2×2 ANOVA | |||
|---|---|---|---|---|---|
| Baseline | 8 Weeks | Baseline | 8 Weeks | P value | |
| Age (years) | 63 ± 1 | --- | 60 ± 1 | --- | --- |
| Weight (kg) | 73.5 ± 2.9 | 71.6 ± 3.1 | 72.7 ± 2.6 | 72.4 ± 2.6 | 0.44 |
| Body mass index (kg/m2) | 25.3 ± 0.7 | 24.9 ± 0.7 | 25.1 ± 0.8 | 25.1 ± 0.9 | 0.28 |
| Total body fat (%) | 33.5 ± 1.9 | 32.6 ± 2.0 | 36.0 ± 2.9 | 34.9 ± 3.0 | 0.76 |
| Waist circumference (cm) | 84.9 ± 2.7 | 84.3 ± 2.6 | 81.3 ± 3.3 | 82.8 ± 3.1 | 0.38 |
| Waist:hip ratio | 0.84 ± 0.02 | 0.83 ± 0.02 | 0.80 ± 0.04 | 0.81 ± 0.04 | 0.22 |
| Systolic blood pressure (mmHg) | 114 ± 3 | 112 ± 2 | 113 ± 3 | 109 ± 4 | 0.26 |
| Diastolic blood pressure (mmHg) | 69 ± 1 | 69 ± 1 | 69 ± 3 | 68 ± 3 | 0.88 |
| Resting heart rate (beats/min) | 61 ± 1 | 59 ± 1 | 59 ± 1 | 57 ± 2 | 0.90 |
| Total cholesterol (mmol/L) | 5.5 ± 0.2 | 5.0 ± 0.2 | 5.0 ± 0.2 | 4.7 ± 0.2 | 0.50 |
| LDL cholesterol (mmol/L) | 3.3 ± 0.1 | 3.0 ± 0.1 | 3.1 ± 0.2 | 3.0 ± 0.2 | 0.58 |
| HDL cholesterol (mmol/L) | 1.6 ± 3 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 | 0.66 |
| Triglycerides (mmol/L) | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.2 | 1.1 ± 0.1 | 0.50 |
| Glucose (mmol/L) | 4.9 ± 0.1 | 5.0 ± 0.1 | 4.8 ± 0.1 | 4.8 ± 0.1 | 0.64 |
| C-reactive protein (mg/L) | 1.3 ± 0.3 | 1.4 ± 0.4 | 1.0 ± 0.2 | 1.4 ± 0.4 | 0.62 |
| VO2 max (ml/kg/min) | 27.8 ± 1.1 | 29.5 ± 1.1 | 25.7 ± 1.5 | 23.9 ± 3.1 | 0.06 |
| Exercise test duration (min) | 9.6 ± 0.2 | 11.4 ± 0.3** | 10.0 ± 0.4 | 10.0 ± 0.6 | <0.01 |
Values are mean ± standard error.
P<0.01 vs. Baseline; LDL, low-density lipoprotein; HDL, high-density lipoprotein; VO2 max, rate of oxygen consumption at maximal exercise.
Table 2.
Exercise intervention study adherence
| All (n=26) | Men (n=11) | Women (n=15) | P value (Men vs. Women) |
|
|---|---|---|---|---|
| Frequency (days/week) | 5.9 ± 0.2 | 6.0 ± 0.3 | 5.8 ± 0.2 | 0.61 |
| Duration (min/session) | 48.6 ± 1.1 | 49.8 ± 2.0 | 47.7 ± 1.3 | 0.36 |
| Intensity (%HRmax/session) | 71.1 ± 0.8 | 72.0 ± 1.1 | 70.4 ± 1.0 | 0.31 |
| Total number of weeks (weeks/subject) | 8.7 ± 0.3 | 8.6 ± 0.5 | 8.7 ± 0.4 | 0.87 |
| Total minutes per week (min/week) | 286 ± 11 | 297 ± 20 | 277 ± 12 | 0.37 |
Values are mean ± standard error.
Table 3.
Subject characteristics for exercise intervention study: men and women
| Men (n=11) | Women (n=15) | 2×2 ANOVA | |||
|---|---|---|---|---|---|
| Baseline | 8 Weeks Exercise | Baseline | 8 Weeks Exercise | P value | |
| Age (years) | 63 ± 2 | --- | 63 ± 1 | --- | --- |
| Time since menopause (years) | --- | --- | 9.4 ± 1.7 | --- | --- |
| Positive history of HRT, no. (%) | --- | --- | 6 (40%) | --- | --- |
| Weight (kg) | 84.0 ± 4.6 | 82.5 ± 3.9 | 65.9 ± 2.2† | 63.6 ± 2.6 | 0.54 |
| Body mass index (kg/m2) | 26.3 ± 1.2 | 25.8 ± 1.3 | 24.6 ± 0.7 | 24.2 ± 0.8 | 0.70 |
| Total body fat (%) | 28.7 ± 3.0 | 27.3 ± 3.1 | 37.1 ± 2.0† | 36.5 ± 2.1 | 0.36 |
| Waist circumference (cm) | 94.2 ± 6.2 | 92.7 ± 4.1 | 77.6 ± 1.9† | 77.7 ± 2.5 | 0.25 |
| Waist:hip ratio | 0.94 ± 0.03 | 0.91 ± 0.02 | 0.77 ± 0.01† | 0.77 ± 0.01 | 0.18 |
| Systolic blood pressure (mmHg) | 113 ± 4 | 112 ± 4 | 114 ± 4 | 113 ± 3 | 0.88 |
| Diastolic blood pressure (mmHg) | 71 ± 2 | 70 ± 1 | 68 ± 2 | 67 ± 2 | 0.92 |
| Resting heart rate (beats/min) | 60 ± 2 | 57 ± 3 | 61 ± 1 | 60 ± 2 | 0.24 |
| Total cholesterol (mmol/L) | 5.2 ± 0.1 | 4.6 ± 0.2 | 5.7 ± 0.2 | 5.2 ± 0.2 | 0.73 |
| LDL-cholesterol (mmol/L) | 3.2 ± 0.1 | 2.8 ± 0.2 | 3.5 ± 0.9 | 3.1 ± 0.2 | 0.93 |
| HDL-cholesterol (mmol/L) | 1.3 ± 0.1 | 1.3 ± 3 | 1.8 ± 0.4† | 1.7 ± 0.1 | 0.63 |
| Triglycerides (mmol/L) | 1.5 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.1† | 1.0 ± 0.8 | 0.13 |
| Glucose (mmol/L) | 5.1 ± 0.1 | 5.1 ± 0.1 | 4.9 ± 0.1 | 4.9 ± 0.1 | 0.98 |
| C-reactive protein (mg/L) | 1.5 ± 0.5 | 1.0 ± 0.2 | 1.2 ± 0.3 | 1.7 ± 0.7 | 0.25 |
| VO2 max (ml/kg/min) | 30.0 ± 2.0 | 31.6 ± 1.8 | 26.2 ± 1.1 | 28.0 ± 1.3 | 0.88 |
| Exercise test duration (min) | 9.9 ± 0.3 | 11.6 ± 0.4 | 9.4 ± 0.3 | 11.3 ± 0.3 | 0.64 |
Values are mean ± standard error.
P<0.05 vs. Men Baseline; HRT, hormone replacement therapy; LDL, low-density lipoprotein; HDL, high-density lipoprotein; VO2 max, oxygen consumption during maximal exercise.
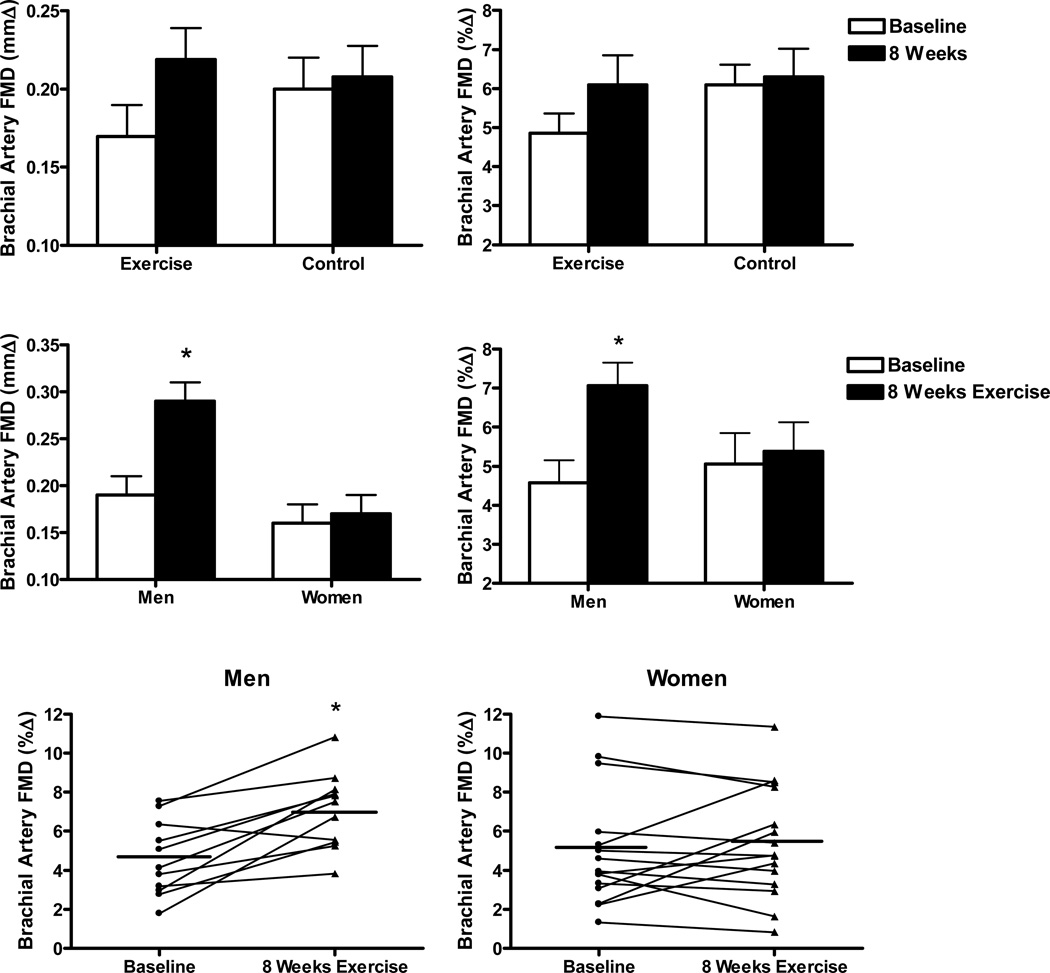
In the overall group, 8 weeks of aerobic exercise tended to increase brachial artery FMD (mean 26%) compared with attention-control (mmΔ: P=0.06, %Δ: P=0.07, top panels Figure 1), whereas there was no change in endothelium-independent dilation (Supplement Table I). When the exercise intervention subjects were stratified by sex, the change in brachial artery FMD was greater (P<0.01) in the men than in the women (middle panels Figure 1). Men demonstrated mean 52% (mmΔ) and 54% (%Δ) increases in brachial artery FMD after the exercise intervention, with 10 of the 11 subjects having higher values after compared with before the intervention (lower panels Figure 1). In contrast, there was no mean change in brachial artery FMD in the women (middle panels Figure 1). Of the 15 women, 5 had higher values and 10 had similar or lower values after compared with before the intervention (lower panels Figure 1). Within the overall group of women, the change in FMD with the exercise intervention was not significantly related to age, number of years post-menopause, history of hormone replacement therapy use or any other variable at baseline or in response to walking. The change in brachial artery FMD with exercise intervention was not significantly related to baseline FMD in either the men or the women (all P>0.05). The 7 subjects with hyperlipidemia responded similarly to the overall male and female subgroups: the 2 men had higher brachial artery FMD after the exercise intervention (mmΔ: 0.26 ± 0.03 vs. 0.16 ± 0.04; %Δ: 6.7 ± 1.2 vs. 3.9±1.2), whereas the 5 women demonstrated no change (mmΔ: 0.16 ± 0.03 vs. 0.16 ± 0.04; %Δ: 5.0 ± 0.9 vs. 4.9 ± 1.3). Brachial artery lumen diameter and peak hyperemic shear rates did not differ before and after the intervention in the either the men or the women (Supplemental Tables I and II).
Figure 1.
Top panels: Brachial artery FMD [absolute (mmΔ) and percent (%Δ) change] at baseline and after 8 weeks of daily vigorous walking (Exercise, n=26) or Control (n=10). Middle panels: Brachial artery FMD [absolute (mmΔ) and percent (%Δ) change] at baseline and after 8 weeks of brisk walking in men (n=11) and women (n=15). Bottom panels: Brachial artery FMD [percent change (%Δ)] individual responses after 8 weeks of brisk walking in men (n=11) and postmenopausal women (n=15). *P<0.01 compared with Baseline.
Cross-sectional study comparisons of sedentary and endurance exercise-trained men and postmenopausal women
Characteristics of the sedentary and exercise-trained groups are shown in Table 4 and Supplemental Table III. Age, resting blood pressure, plasma total and LDL-cholesterol, fasting blood glucose, plasma oxidized LDL and brachial artery lumen diameter and peak shear rates did not differ in the sedentary and endurance exercise-trained groups. The exercise-trained groups had (P < 0.05) or tended to have lower body mass, measures of abdominal and total body fatness, plasma concentrations of triglycerides and C-reactive protein and resting heart rate, and higher plasma HDL-cholesterol, maximal oxygen consumption and leisure-time physical activity.
Table 4.
Subject characteristics for cross-sectional analysis: men and women
| Men (n=105) | Women (n=62) | |||||
|---|---|---|---|---|---|---|
| Sedentary (n=60) |
Exercise- trained (n=45) |
P value | Sedentary (n=42) |
Exercise- trained (n=20) |
P value | |
| Age (yrs) | 62 ± 1 | 64 ± 0.4 | 0.07 | 61 ± 1 | 59 ± 1 | 0.06 |
| Time since menopause (years) | --- | --- | --- | 8.1 ± 1.1 | 6.1 ± 1.2 | 0.25 |
| Positive history of HRT, no. (%) | --- | --- | --- | 17 (40) | 8 (40) | 0.95 |
| Weight (kg) | 84.6 ± 1.6 | 73.4 ± 1.1 | <0.001 | 70.4 ± 1.6 | 54.0 ± 1.2 | <0.001 |
| Body mass index (kg/m2) | 26.8 ± 0.5 | 23.5 ± 0.5 | <0.001 | 25.9 ± 0.5 | 20.2 ± 0.4 | <0.001 |
| Total body fat (%) | 28.6 ± 1.0 | 18.6 ± 0.8 | <0.001 | 39.9 ± 1.1 | 21.6 ± 0.8 | <0.001 |
| Waist circumference (cm) | 95.7 ± 1.6 | 84.2 ± 0.9 | <0.001 | 80.5 ± 1.6 | 68.0 ± 0.6 | <0.001 |
| Waist:Hip ratio | 0.95 ± 0.01 | 0.89 ± 0.01 | <0.001 | 0.77 ± 0.01 | 0.75 ± 0.01 | 0.06 |
| Systolic blood pressure (mmHg) | 125 ± 2 | 125 ± 2 | 0.90 | 116 ± 3 | 112 ± 2 | 0.28 |
| Diastolic blood pressure (mmHg) | 77 ± 1 | 75 ± 1 | 0.24 | 70 ± 1 | 75 ± 2 | 0.93 |
| Resting heart rate (beats/min) | 64 ± 3 | 54 ± 1 | <0.01 | 65 ± 2 | 54 ± 2 | <0.001 |
| Total cholesterol (mmol/L) | 5.2 ± 0.1 | 5.3 ± 0.1 | 0.65 | 5.4 ± 0.2 | 5.3 ± 0.1 | 0.68 |
| LDL-cholesterol (mmol/L) | 3.3 ± 0.1 | 3.2 ± 0.1 | 0.58 | 3.3 ± 0.1 | 3.1 ± 0.1 | 0.27 |
| HDL-cholesterol (mmol/L) | 1.2 ± 0.03 | 1.6 ± 0.1 | <0.001 | 1.7 ± 0.1 | 1.8 ± 0.1 | 0.21 |
| Triglycerides (mmol/L) | 1.5 ± 0.1 | 1.1 ± 0.1 | <0.001 | 1.1 ± 0.1 | 0.9 ± 0.1 | 0.24 |
| Glucose (mmol/L) | 5.3 ± 0.1 | 5.2 ± 0.1 | 0.58 | 4.8 ± 0.1 | 4.8 ± 0.1 | 0.77 |
| C-reactive protein (mg/L) | 1.3 ± 0.2 | 0.8 ± 0.2 | 0.16 | 1.5 ± 0.2 | 0.6 ± 0.2 | 0.02 |
| VO2 max (ml/kg/min) | 30.0 ± 0.6 | 41.9 ± 0.8 | <0.001 | 24.7 ± 0.7 | 41.1 ± 1.1 | <0.001 |
| Leisure-time PA (MET hrs/wk) | 26 ± 3 | 75 ± 6 | <0.001 | 17 ± 3 | 99 ± 12 | <0.001 |
Values are mean ± standard error. VO2 max, oxygen consumption during maximal exercise; LDL, low-density lipoprotein; HDL, high-density lipoprotein; PA, physical activity.
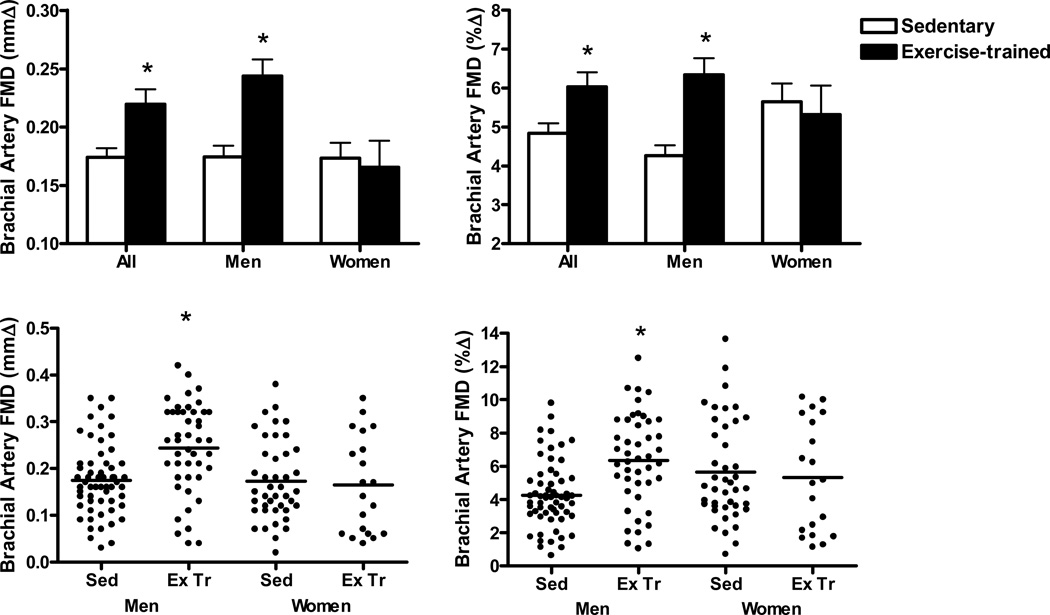
In the overall group, brachial artery FMD was 25% greater in the exercise-trained compared with the sedentary subjects (P < 0.01, Figure 2), whereas there was no difference in brachial artery endothelium-independent dilation (Supplemental Table III). Brachial artery FMD was 40 (mmΔ) to 49 (%Δ) % greater in the exercise-trained compared with the sedentary men (P < 0.001, Figure 2). In contrast, there were no differences in brachial artery FMD in sedentary and exercise-trained women (mmΔ, P=0.75; %Δ, P=0.70; Figure 2). The differences in brachial artery FMD between the sedentary and exercise-trained men remained significant (P < 0.01) after correcting for factors that differed significantly between those groups, but only tended to differ in the women (i.e., waist:hip ratio, HDL-cholesterol, triglycerides, norepinephrine and baseline brachial artery diameter)(Table 4 and Supplemental Table III). There was no difference in FMD among exercise-trained postmenopausal women with a history (n=8) vs. no history (n=12) of hormone replacement therapy use (5.6 ± 1.2 vs. 5.1 ± 1.0%, P=0.73). Brachial artery endothelium-independent dilation was not different in the sedentary vs. exercise-trained groups of men and women (Supplemental Table III).
Figure 2.
Top panels: Brachial artery FMD [absolute (mmΔ) and percent (%Δ) change] in sedentary (n=105) and endurance exercise-trained (n=62) adults (All), sedentary (n=60) and exercise-trained (n=45) men, and sedentary (n=42) and exercise-trained (n=20) postmenopausal women. Bottom Panels: Individual brachial artery FMD values of sedentary (Sed, n=60) and exercise-trained (Ex Tr, n=45) men, and sedentary (n=42) and exercise-trained (n=20) postmenopausal women. *P<0.01 compared with Sedentary.
Discussion
Our main finding was that 8 weeks of essentially daily brisk walking improved mean brachial artery FMD in previously sedentary middle-aged/older men, but not postmenopausal women. Endothelium-independent dilation was unaffected by the exercise intervention in both men and women, indicating that the improvements in FMD after the exercise intervention in the men were specific for the vascular endothelium. The differences in responsiveness to aerobic exercise in the overall groups of men and women were observed in the absence of any sex-specific differences in the exercise intervention “stimulus”, improvements in maximal exercise capacity or changes in other factors known to influence brachial artery FMD.
To our knowledge, the present results are the first to show that regular moderate-intensity aerobic exercise can improve brachial artery FMD in previously sedentary middle-aged/older men without clinical CVD. Our finding of a >50% mean increase in brachial artery FMD with our exercise intervention in previously sedentary middle-aged/older men is consistent with results of cross-sectional comparisons of small groups of sedentary and endurance-trained men in previous studies by our laboratory [6, 7, 22] and others [8, 15, 16], as well as with the ~50% difference in brachial artery FMD observed in the present cross-sectional study between larger groups of sedentary and exercise-trained men (Figure 2). We found that the responses to walking were consistent in that 10 out of the 11 men showed higher values after compared with before the intervention. Importantly, 8 weeks of walking improved brachial artery FMD to 7.1%, which is close to the average values observed in young healthy men in our laboratory (7.3 ± 0.3%, n=58, age 23 ± 0.5 years; see Supplemental Figure I) [6, 7, 22]. Thus, our aerobic exercise intervention essentially restored brachial artery FMD in previously sedentary middle-aged/older men to levels seen on average in young men. These improvements were independent of changes in other factors, suggesting direct physiological effects of aerobic exercise [35] as observed previously with this intervention [29, 34]. Together with our laboratory’s previous finding that regular brisk walking increases forearm blood flow responses to acetylcholine [29], the present results provide evidence that moderate-intensity habitual aerobic exercise improves endothelium-dependent dilation in both conduit arteries and resistance vessels of previously sedentary middle-aged/older men.
In contrast to the effects observed in men, we found no association between habitual aerobic exercise and brachial artery FMD in postmenopausal women. Specifically, the exercise intervention did not improve brachial artery FMD on average in previously sedentary estrogen-deficient postmenopausal women and there were no differences in FMD between sedentary and exercise-trained postmenopausal women in our cross-sectional analyses. The latter results argue against the idea that the habitual aerobic exercise stimulus may need to be greater and/or more sustained in postmenopausal women than in middle-aged/older men to observe an effect on brachial artery FMD. No variable was related to changes in brachial FMD in response to the exercise intervention, and no factor explained the sedentary-exercise group differences in FMD between the men and women in the cross-sectional analyses. The age-associated impairment in brachial artery FMD at baseline was similar in the men and women in the intervention study and the lack of differences in FMD between sedentary and exercise-trained postmenopausal women was not associated with differences in baseline brachial artery diameter nor influenced by age (no between-group differences in 50–59 vs. 60–69 years of age). VO2max values for the sedentary men and women were consistent with previous studies published from our laboratory [29, 34]. The differences in VO2max between sedentary and exercise-trained subjects was greater for the postmenopausal women than the men, suggesting that the absence of differences in FMD between sedentary and exercising women could not be explained by lesser differences in maximal aerobic exercise capacity.
Our intervention study data are consistent with recent observations of Casey and colleagues [24] reporting no change in brachial artery FMD with 2 days/week of aerobic exercise in postmenopausal women. Our findings extend this work by showing that the absence of an effect was not the result of insufficient frequency of exercise. The present results differ from previous reports of greater brachial FMD in the exercise-trained versus sedentary states in postmenopausal women [17, 26] and an absence of an exercise effect in middle-aged and older men [26]. Small numbers of subjects in each group [17, 26], high variability in mean FMD values in men [26], the absence of an age-associated impairment in baseline (pre-exercise training) FMD in men [26], and differences in age and/or time since menopause in the older women [17, 26] or the measurement of FMD [17] may explain the disparate results.
We do not believe that methodological issues explain the lack of an effect of habitual aerobic exercise in postmenopausal women. We have extensive experience with the brachial artery FMD technique [6, 7, 10, 27, 30, 36, 37] and used standardized procedures [31, 32, 33] in both women and men. We considered the possibility that the hyperemic stimulus to brachial artery FMD was smaller after the exercise intervention in postmenopausal women, and in the trained vs. sedentary postmenopausal women, and this could explain the lack of exercise effect on FMD. Peak shear rate positively related to FMD in postmenopausal women (%Δ: r=0.51, P<0.01; mmΔ: r=0.42, P<0.05), but values did not differ in the sedentary and exercise-trained women. We did not assess area under the curve shear rate (AUC SR) up to brachial artery peak dilation, which may [31, 38], or may not [39], be a more meaningful expression of the hyperemic stimulus for normalization of FMD than peak shear rate, in the subjects. However, in a pilot study recently conducted in our laboratory, AUC SR up to peak dilation was not related to FMD in 25 healthy older men (n=10, % Δ: r=0.25, P=0.48; mm Δ: r=0.17, P=0.65) and postmenopausal women (n=15, % Δ: r=0.23, P=0.42; mm Δ: r=0.20, P=0.48) (unpublished data). Moreover, there was no difference in AUC SR up to peak dilation in the sedentary (n=5) and exercise-trained (n=10) postmenopausal women (sed: 29.9 ± 16.4×103 vs. trained: 24.1 ± 11.0×103 sec−1). These observations suggest that differences in peak or AUC SR likely did not explain the lack of difference in FMD before and after the exercise intervention or between sedentary and exercise-trained postmenopausal women.
We wish to emphasize that the results of our study may apply only to estrogen-deficient postmenopausal women without a high CVD risk factor burden or overt clinical disease. It is interesting that habitual aerobic exercise does not produce adaptations in left ventricular structure and function in estrogen-deficient postmenopausal women such as those observed in older men [40] and young women [41], but improves carotid artery compliance in estrogen-replaced postmenopausal women similar to that observed in middle-aged and older men [34]. In the present study, we found no relation between history of hormone replacement therapy and changes in FMD with walking among our postmenopausal women, but none of our subjects were estrogen-replaced while participating. Alternatively, an aerobic exercise intervention may be more consistently effective for postmenopausal women with more severely impaired brachial artery FMD associated with multiple risk factors and/or clinical CVD [42, 43, 44]. That the 5 women in the present study with higher values for brachial artery FMD after compared with before completion of the walking program had baseline brachial FMD at or below the initial group mean is somewhat consistent with this possibility, although the lack of responsiveness to exercise in the women with hyperlipidemia is not. However, it is important to recognize that habitual aerobic exercise has beneficial effects on other cardiovascular functions [34], general CVD risk factor management [19, 21] and maintenance of overall physiological function in postmenopausal women without clinical disease [45, 46]. As noted recently, it also is possible that habitual exercise/physical activity plays a reduced role in this group in modulating some risk factors for CVD such as vascular endothelial dysfunction [47].
In conclusion, here we provide evidence that the efficacy of habitual aerobic exercise for improving or preserving brachial artery FMD with aging may differ in middle-aged/older men and estrogen-deficient postmenopausal women without clinical disease. Additional research clearly is needed to determine the effectiveness of aerobic exercise intervention for improving vascular endothelial dysfunction in postmenopausal women. This should include investigation of the cellular and molecular mechanisms that contribute to the sex-specific effects of aerobic exercise on vascular endothelial function in middle-aged/older adults and the potential modulatory influence of circulating estrogen. Determining the benefits of habitual exercise on vascular endothelial dysfunction in postmenopausal women is particularly important given that hormone replacement therapy remains clinically contraindicated for the primary prevention of CVD. Our findings here also indicate that more information is needed on the efficacy of other lifestyle (e.g., body weight control, dietary sodium restriction), pharmacological (e.g., statins) and novel neutraceutical (e.g, resveratrol) approaches as potential strategies for combating arterial aging in this group.
Supplementary Material
Acknowledgments
The authors thank Adam Bergquist, Livia Tsien and the University of Colorado at Boulder Clinical and Translational Research Center staff for technical assistance.
Sources of funding
This work was supported by National Institutes of Health awards AG013038, AG000279, RR00051 and AG031617; and American Heart Association award 0715735Z.
Footnotes
Author Contributions
Gary Pierce was the director of the study, performed FMD measurements, performed data analysis and interpretation, created all tables and figures, and wrote and edited first and final drafts of manuscript. Iratxe Eskurza was involved in the initial study design, provided helpful discussions of data analyses and reviewed/edited first and final drafts of manuscript. Ashley Walker analyzed FMD measurements and reviewed/edited first and final drafts of the manuscript. Tara Fay performed data entry, analyzed brachial artery doppler velocities and contributed to revisions of first and final drafts of manuscript. Douglas Seals was involved in the initial study design, data interpretation, editing first and final drafts of manuscript, and was the primary investigator on the grant funding for the study.
References
- 1.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J. Am. Coll. Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 2.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shiplman A, Menzoian JO, Watkins MT, Raffetto JD, et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler. Thromb. Vasc. Biol. 2007;27:2113–2119. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 4.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J. Am. Coll. Cardiol. 2008;51:997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 5.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eskurza I, Monahan K, Robinson J, Seals DR. Effect of acute and chronic ascorbic acid augmentation on flow-mediated dilation with physically active and sedentary aging. J. Physiol. 2004;556:215–224. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eskurza I, Myerburgh L, Khan Z, Seals DR. Tetrahydrobiopterin augments endothelial-dependent dilation in sedentary but not habitually exercising older adults. J. Physiol. 2005;568.3:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franzoni F, Ghiadoni L, Galetta F, Plantinga Y, Lubrano V, Huang Y, Salvetti G, Regoli F, Taddei S, Santoro G, et al. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am. J. Hypertens. 2005;18:510–516. doi: 10.1016/j.amjhyper.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J. Am. Coll. Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 10.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ. Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 11.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. J.A.M.A. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 12.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N. Engl. J. Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 13.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity an reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. J.A.M.A. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 15.Rinder MR, Spina RJ, Ehsani AA. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J. Appl. Physiol. 2000;88:761–766. doi: 10.1152/jappl.2000.88.2.761. [DOI] [PubMed] [Google Scholar]
- 16.Rywik TM, Blackman MR, Yataco AR, Vaitkevicius PV, Zink RC, Cottrell EH, Wright JG, Katzel LI, Fleg JL. Enhanced endothelial vasoreactivity in endurance-trained older men. J. Appl. Physiol. 1999;87:2136–2142. doi: 10.1152/jappl.1999.87.6.2136. [DOI] [PubMed] [Google Scholar]
- 17.Hagmar M, Eriksson MJ, Lindholm C, Schenck-Gustafsson K, Hirschberg AL. Endothelial function in post-menopausal former elite athletes. Clin. J. Sport. Med. 2006;16:247–252. doi: 10.1097/00042752-200605000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson ET, Davy KP, Jones PP, Desouza CA, Seals DR. Blood pressure risks factors in healthy postmenopausal women: physical activity and hormone replacement. J. Appl.Physiol. 1997;82:652–660. doi: 10.1152/jappl.1997.82.2.652. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson ET, Davy KP, Seals DR. Hemostatic, metabolic, and androgenic risk factors for coronary heart disease in physically active and less active postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 1995;15:669–677. doi: 10.1161/01.atv.15.5.669. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Clevenger CM, Jones PP, Seals DR, DeSouza CA. Influence of body fatness on the coronary risk profile of physically active postmenopausal women. Metabolism. 1998;47:1112–1120. doi: 10.1016/s0026-0495(98)90286-4. [DOI] [PubMed] [Google Scholar]
- 21.Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Effects of endurance training on glucose tolerance and plasma lipid levels in older men and women. J.A.M.A. 1984;252:645–649. [PubMed] [Google Scholar]
- 22.Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: influence of habitual exercise. Am. J. Hypertens. 2009;22:250–256. doi: 10.1038/ajh.2008.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arcaro G, Zamboni M, Rossi L, Turcato E, Covi G, Armellini F, Bosello O, Lechi A. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int. J. Obes. Relat. Metab. Disord. 1999;23:936–942. doi: 10.1038/sj.ijo.0801022. [DOI] [PubMed] [Google Scholar]
- 24.Casey DP, Pierce GL, Howe KS, Mering MC, Braith RW. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur. J. Appl. Physiol. 2007;100:403–408. doi: 10.1007/s00421-007-0447-2. [DOI] [PubMed] [Google Scholar]
- 25.Thijssen DH, de Groot PCE, Smits P, Hopman MT. Vascular adaptations to 8-week cycling training in older men. Acta. Physiol. 2007;190:221–228. doi: 10.1111/j.1748-1716.2007.01685.x. [DOI] [PubMed] [Google Scholar]
- 26.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. J. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1109–H1116. doi: 10.1152/ajpheart.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohrt WM. Preliminary evidence that DEXA provides an accurate assessment of body composition. J. Appl. Physiol. 1998;84:372–377. doi: 10.1152/jappl.1998.84.1.372. [DOI] [PubMed] [Google Scholar]
- 29.DeSouza CA, Shapiro L, Clevenger C, Dinenno FA, Monahan K, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 30.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, Friberg P, Deanfield JE. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J. Am. Coll. Cardiol. 2008;51:1959–1964. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 33.Corretti M, Anderson T, Benjamin E, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J. Amer. Coll. Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 34.Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy, and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc. Res. 2003;57:861–868. doi: 10.1016/s0008-6363(02)00777-0. [DOI] [PubMed] [Google Scholar]
- 35.Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exercise and Sport Sciences Reviews. 2009;37:196–202. doi: 10.1097/JES.0b013e3181b7b6e3. [DOI] [PubMed] [Google Scholar]
- 36.Eskurza I, Kahn Z, Seals DR. Xanthine oxidase does not contribute to impaired peripheral artery conduit artery endothelium-dependent dilatation with aging. J. Physiol. 2006;571:661–668. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by L-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J. Appl. Physiol. 2007;102:63–71. doi: 10.1152/japplphysiol.00660.2006. [DOI] [PubMed] [Google Scholar]
- 38.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J. Appl. Physiol. 2007;102:1510–1519. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- 39.Thijssen DH, Bullens LM, van Bemmel MM, Dawson EA, Hopkins N, Tinken TM, Black MA, Hopman MT, Cable NT, Green DJ. Does arterial shear explain the magnitude of flow-mediated dilation?: a comparison between young and older humans. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H57–H64. doi: 10.1152/ajpheart.00980.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spina RJ, Ogawa T, Miller TR, Kohrt WM, Ehsani AA. Effect of exercise training on left ventricular performance in older women free of cardiopulmonary disease. J. Am. J. Cardiol. 1993;71:99–104. doi: 10.1016/0002-9149(93)90718-r. [DOI] [PubMed] [Google Scholar]
- 41.Spina RJ, Ogawa T, Martin WH, 3rd, Coggan AR, Holloszy JO, Ehsani AA. Exercise training prevents decline in stroke volume during exercise in young healthy subjects. J. Appl. Physiol. 1992;72:2458–2462. doi: 10.1152/jappl.1992.72.6.2458. [DOI] [PubMed] [Google Scholar]
- 42.Lavrencic A, Salobir B, Keber I. Physical training improves flow-mediated dilation in patients with the polymetabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2000;20:551–555. doi: 10.1161/01.atv.20.2.551. [DOI] [PubMed] [Google Scholar]
- 43.Fuchsjager-Mayrl G, Pleiner J, Wiesinger G, Sieder A, Quittan M, Nuhr M, Francesconi C, Seit H, Francesconi M, Schmetterer L, et al. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care. 2002;25:1795–1801. doi: 10.2337/diacare.25.10.1795. [DOI] [PubMed] [Google Scholar]
- 44.Vona M, Codeluppi GM, Iannino T, Ferrari E, Bogousslavsky J, von Segesser LK. Effects of different types of exercise training followed by detraining on endothelium-dependent dilation in patients with recent myocardial infarction. Circulation. 2009;119:1601–1608. doi: 10.1161/CIRCULATIONAHA.108.821736. [DOI] [PubMed] [Google Scholar]
- 45.Van Pelt RE, Jones PP, Davy KP, Desouza CA, Tanaka H, Davy BM, Seals DR. Regular exercise and the age-related decline in resting metabolic rate in women. J. Clin. Endocrinol. Metab. 1997;82:3208–3212. doi: 10.1210/jcem.82.10.4268. [DOI] [PubMed] [Google Scholar]
- 46.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. J.A.M.A. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 47.Parker BA, Kalasky MJ, Proctor DN. Evidence for sex differences in cardiovascular aging and adaptive responses to physical activity. Eur. J. Appl. Physiol. 2010 May 18; doi: 10.1007/s00421-010-1506-7. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.