Summary
In recent years even with remarkable scientific advancements and significant increase of global R&D spending, drugs are frequently withdrawn from markets. This is primarily due to their side-effects or toxicities. Drug molecules often interact with multiple targets, coined as polypharmacology, and the unintended drug-target interactions could cause side-effects. Polypharmacology remains to be one of the major challenges in drug development, and it opens novel avenues to rationally design next generation of more effective but less toxic therapeutic agents. This review outlines the latest progress and challenges in polypharmacology studies.
Keywords: Polypharmacology, Off-target, Side-effects, Drug repurposing, Drug-target network, Multi-targeting design
Introduction
Drug discovery and development is a complex and expensive process [1,2]. Due to the exponential growth of molecular data and fast advancement in technologies, the efforts of drug discovery have been tremendously amplified [3-6]. The philosophy of drug design has been transformed from “one drug one target” to “one drug multiple targets”, coined as polypharmacology [7-12]. Polypharmacology is emerging as the next paradigm of drug discovery [7,13-18]. Polypharmacological phenomena includes: (a) single drug acting on multiple targets of a unique disease pathway, or (b) single drug acting on multiple targets pertaining to multiple disease pathways. In addition, polypharmacology for complex diseases is likely to employ multiple drugs acting on distinct targets, that are part of a networks regulating various physiological responses [7,19]. The Polypharmacological approaches aim to discover the unknown off targets for the existing drugs (also known as drug repurposing) [15,20,21]. The approach needs the systematic integration of the data derived from different disciplines including computational modeling, synthetic chemistry, in vitro/in vivo pharmacological testing, and clinical studies[22,23].
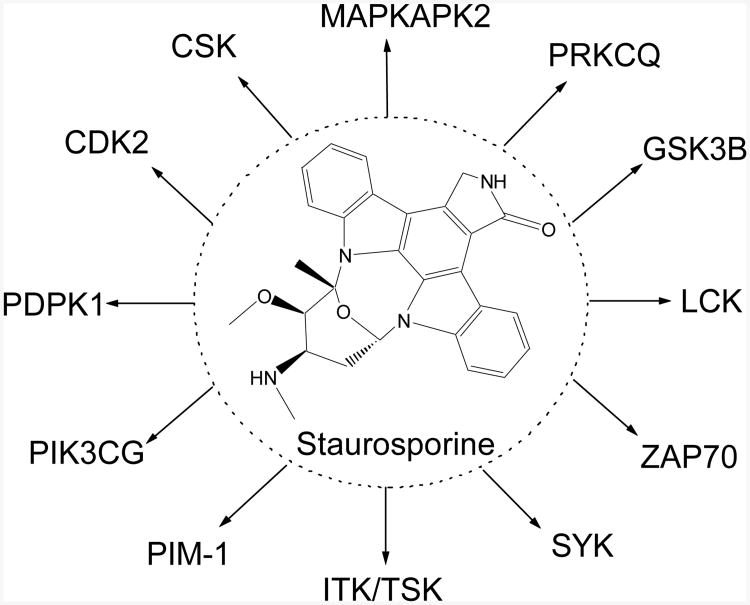
Although not designed on purpose, numerous drugs are known for their multi-targeting activities. One such example is Aspirin, often used as an analgesic to relieve minor pains or as an antipyretic to reduce fever [24]; also acts as an anti-inflammatory medication to treat rheumatoid arthritis [25], pericarditis[26], and Kawasaki diseases[27]. Additionally, it has been used in the prevention of transient ischemic attacks [28], strokes, heart attacks[29], pregnancy loss [30], and even cancer[31]. Another example is Sildenafil (Viagra), a phosphodiesterase (PDE) inhibitor. It was initially developed for hypertension and ischemic heart disease. However, it is now more frequently used to treat erectile dysfunction[32]. Certainly, kinase inhibitors are the elephant in the room regarding polypharmacology research. As a target class, kinases have received considerable attention over the last 20 years. There are currently 14 kinase inhibitors on the market for cancer treatment, with many more in clinical development [33,34]. Most of these cancer therapeutics inhibit more than one kinases, although they maintain reasonable selectivity over the serine/threonine and phosphoinositide (PI) kinase classes [34-38]. On the other hand, polypharmacology can be considered a double-edged sword and cause clinical problems when it is not fully understood. Australia's Therapeutic Goods Administration (TGA, the Australian equivalent of the FDA) cancelled the registration of Lumiracoxib (a COX-2 selective inhibitor/non-steroidal anti-inflammatory drug) in Australia due to concerns that it may cause liver failure. Later Merck voluntarily withdrew Rofecoxib from the market because of the increased risk of heart attack and stroke associated with the long-term, high-dosage use. Staurosporine, a potent protein kinase C inhibitor, is also known to interact with many other kinases (Figure 1) and this excluded its use in clinical practice.
Figure 1.
The kinase targets of staurosporine.
Current Technologies and Progress
Polypharmacology studies are critical in drug discovery and development. However, the exhaustive coverage of all targets by experimental methods is still an industry-wide challenge. Apparently the most competent approach would be to utilize the -omics (proteomics, cheminformatics, etc.) technologies [39,40]. The enormous molecular data generated in the post-genomic era has significantly accelerated the polypharmacology research. Systems biology approaches integrated with pharmacology are being frequently used to identify new off-targets [16,17,23,41]. There are a large number of public and private molecular databases available and are continuously growing in both size and number; some of them are listed in Table 1. They integrate diverse information of molecular pathways, crystal structures, binding experiments, side-effects, and drug targets. There are also number of other small molecule databases including: ZINC [42], PubChem [43], Ligand Expo [44], KEGG DRUG [45], etc., with enormous information about their disease relevance, chemical properties, and biological activities. These databases can be used not only to predict the protein targets of a small molecule, but also to obtain insights of designing polypharmacological agents in a prospective manner.
Table 1.
Some of the representative databases for polypharmacology studies.
| Name | Description | Website |
|---|---|---|
| DrugBank [24] | Combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. Database contains 6711 drug entries including 1447 FDA-approved small molecule drugs, 131 FDA-approved biotech (protein/peptide) drugs, 85 nutraceuticals and 5080 experimental drugs. | http://www.drugbank.ca/ |
| STITCH [46] | Contains interactions for between 300,000 small molecules and 2.6 million proteins from 1133 organisms. Chemicals are linked to other chemicals and proteins by evidence derived from experiments, databases and the literature. | http://stitch.embl.de/ |
| BindingDB [47] | Database of measured binding affinities, focusing chiefly on the interactions of protein considered to be drug-targets with small, drug-like molecules. BindingDB contains 832,773 binding data, for 5,765 protein targets and 362,123 small molecules. | http://www.bindingdb.org/ |
| Supertarget [48] | An extensive web resource for analyzing 332828 drug-target interactions | http://insilico.charite.de/supertarget/ |
| IUPHAR-DB [49] | Detailed pharmacological, functional and pathophysiological information on G Protein-Coupled Receptors, Voltage-Gated Ion Channels, Ligand-Gated Ion Channels and Nuclear Hormone Receptors. | http://www.iuphar-db.org/ |
| WOMBAT [50] | Contains 331,872 entries (268,246 unique SMILES), representing 1,966 unique targets captured from 15,320 papers published in medicinal chemistry journals between 1975 and 2009. | http://www.sunsetmolecular.com/ |
| KEGG[45] | Resource for understanding high-level functions and utilities of the biological system, such as the cell, the organism and the ecosystem, from molecular-level information, especially large-scale molecular datasets generated by genome sequencing and other high-throughput experimental technologies. | http://www.genome.jp/kegg/ |
| Comparative Toxicogenomics Database [51] | Includes curated data describing cross-species chemical–gene/protein interactions and chemical– and gene–disease associations. | http://ctdbase.org/ |
| PubChem's BioAssay Database [52] | The public repository of bioassay database contains 500,000 descriptions of assay protocols, covering 5000 protein targets, 30,000 gene targets and providing over 130 million bioactivity outcomes. | http://pubchem.ncbi.nlm.nih.gov/ |
| ChEMBL [53] | Contains 2D structures, calculated properties (e.g. logP, Molecular Weight, Lipinski Parameters, etc.) and abstracted bioactivities (e.g. binding constants, pharmacology and ADMET data). | https://www.ebi.ac.uk/chembl/ |
With the increasing availability of the above databases, various methods have been applied to predict molecular polypharmacology. Recently Keiser and colleagues used the similarity ensemble approach (SEA) [54,55] in a large scale to predict the activity of 656 marketed drugs on 73 unintended ‘side-effect’ targets, and confirmed half of the predictions with the IC50 activity values ranging from 1nM to 30μM [55]. Among these new associations was the finding that the abdominal pain side-effect of chlorotrianisene was due to its newly discovered inhibition of cyclooxygenase-1 (COX-1). Oprea et al. also reported results from their text mining of 7,684 approved drug and mapped the “adverse reactions” of 988 unique drugs onto 174 side-effects[15,20]. They were then clustered with principal component analysis into a self-organizing map and integrated it into a Cytoscape network. They expected that this type of data could streamline drug repurposing [20]. Barabasi et al. used a polypharmacology approach to build a bipartite graph composed of US Food and Drug Administration-approved drugs and proteins linked by drug-target binary associations [56]. Zhang et al. demonstrated that polypharmacology can not only speed the wide identification of drug targets but also find new applications for the existing drugs [57]. Additionally Apsel et al. reported systematic discovery of molecules that potently inhibit both tyrosine kinases and phosphatidylinositol-3-OH kinases [9]. Crystal structures revealed that the dual selectivity of these molecules is controlled by a hydrophobic pocket conserved in both enzyme classes and accessible through a rotatable bond in the drug skeleton [9]. In another polypharmacology prediction study, Cheng et al. proposed two different weighted network-based inference methods for chemical-protein interaction prediction [58]. Bork and colleagues used phenotypic side-effect similarities to infer whether two drugs share a target. They applied to 746 marketed drugs with a network of 1,018 side-effect followed up with experimental validations, and found that 11 out of 13 implied drug-target interactions reveal inhibition constant equal or less than 10um [59]. Mattingly and coworkers with the aid of text mining tools developed CTD [51] (comparative toxicogenomics database) which includes curated data describing cross-species chemical–gene/protein interactions and chemical– and gene–disease associations. The database is intended to provide insights into complex chemical–gene and protein interaction networks.
Structure-based methods including inverse docking [60-62] are also widely used to predict protein targets of small molecules. A panel of tractable targets involved in a disease network are screened against the approved drug molecules with docking. The targets with available 3D structure are normally used. The top ranked targets (excluding the original known targets) can be treated as the lead off-targets for further testing. For instance, Zou and coworkers [60] used inverse docking approach to identify the potential direct target oxidosqualene cyclase (part of the cholesterol synthetic pathway) of PRIMA-1 (known for its ability to restore mutant p53 tumor suppressor functions). Experimentally they have shown that both PRIMA-1 and Ro 48-8071 (a known potent OSC inhibitor) could significantly reduce the viability of breast cancer cells relative to normal mammary cells. Similarly Tawa et al [63] tested 3D-shape/chemical similarity analysis program ROCS (Rapid Overlay of Chemical Structures) to generate off-target profiles of drugs from DrugBank[24] and KEGG[45]. A systems pharmacology approach was used by Dar and co-workers to indentify polypharmacological agents with an optimal balance of activity and less toxicity against the kinases Ret, Raf, Src, Tor and S6K [23]. These examples demonstrate the importance of this highly promising field, and it is clear that developing multi-target therapeutics agents represents an important avenue to advance drug discovery. Some of the methodologies are summarized in Table 2.
Table 2.
Broad classification of polypharmacological methods.
| Methodology | Description | Reference |
|---|---|---|
| Systems biology/pharmacology approaches | Uses experimental and computational approaches to have the systems-level understanding of diseases and both the therapeutic and adverse mechanisms of drug actions. | [16,17,23,41] |
| Side-effect similarity | Drugs/Targets are mapped based on phenotypic side-effect similarities. | [59][55] |
| Similarity ensemble approach (SEA) | Relates proteins based on the set-wise chemical similarity among their ligands. It can be used to rapidly search large compound databases and to build cross-target similarity maps. | [54,55] |
| Knowledge based approach | Form the associations and depict as a network between various biomolecules stored in various databases. | [7,13,19,22,64] |
| Text mining tools | Text mining tools were used to dig the mapping information from literature and public databases | [65-68] |
| Docking/Inverse docking | Docking a ligand against several targets | [10,60-62,69] |
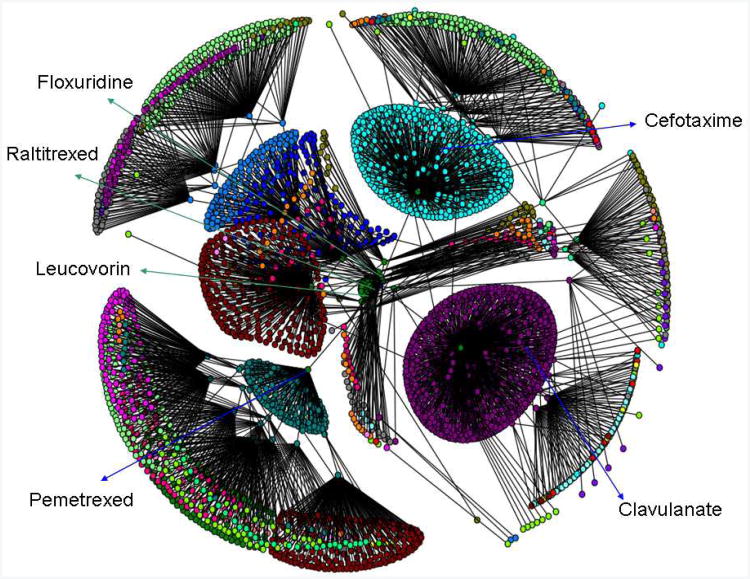
Recently, our group, among others [10,13,70,71], have embarked on the development of novel methods to explore the ligand-target interactions using molecular networks [70,72]. These approaches are to deduce polypharmacological relations among drugs and the protein crystal structures reported in PDB [73]. The drug molecules were obtained from the available public databases such as DrugBank[24], and the corresponding target information was obtained from PDB [73]. After data curation with our in-house programs, databases were constructed for both drugs and targets, and networks were created to evaluate their relationships. The structural similarity among the drug molecules that are likely to bind to the same targets has also been evaluated using JChem software [74]. Using our method, the polypharmacology network created for a query compound Raltitrexed (brand name Tomudex; an anti-metabolite and inhibitor of thymidylate synthase and folylpolyglutamate synthase) is depicted in Figure 2. The complexity of the network is reduced by hiding the targets and only showing the ligand molecules connected to Raltitrexed in three levels. The network shows multiple clusters of compounds that have close relationships with each other based on their shared targets. Ralititrexed is connected to multiple molecules such as Floxuridine and Leucovorin which share common target (thymidylate synthase) in the first level of network, meaning they might have similar biological activities or they bind to similar targets. The network is further extended to more levels by ligand-target pairing.
Figure 2.
Graphical depiction of the drug network for Raltitrexed, derived based on their shared targets. The complexity of the network is reduced by hiding the targets and only showing the drug molecules that are directly or in directly related to Raltitrexed in three levels. Each level is shown in different colors.
Challenges for Polypharmacology
Despite their evident growth, polypharmacological approaches are attributed with several challenges. The major limitation is that we only partially understand the pathways/mechanism of many diseases at the molecular levels. It is exceedingly difficult to derive the full polypharmacological networks without the complete data. Additionally more accurate mining techniques and mapping methodologies are needed to analyze the complex data. On the other hand, understanding the convoluted associations is also a challenging task after the complex networks are constructed [75]. Other methods also face various issues. For instance, inverse docking approaches suffer from several drawbacks including difficulty of addressing target flexibility and meager performance of scoring algorithms [61,76]; In text mining it is still difficult to constantly access/update the enormous information provided by different and non-synchronized databases [68]. Addressing these issues in respective areas will certainly help to improve polypharmacology studies.
Expert commentary
Currently it is acknowledged that polypharmacology goes well beyond target families. The fact is that the toxicities or targets of many phenotypes drugs are either largely unknown or insufficiently understood in most cases. If we can use known drug properties to understand the underlying mechanisms, it will shift the paradigm of chemogenomic studies and drug development. However, it is too costly to conduct these studies experimentally. Therefore we need more predictive algorithms as well as increased integration of available data. The synergy among different disciplines could help the polypharmacology studies for the drug discovery community. There have been several promising attempts [9,20,55,59], and various methods [10,14,58,71,72,77] and databases [24,46,47,50,78] have been developed. Despite the existing challenges in the area, polypharmacology approaches are showing their promise and will certainly have the potential to transform our next-generation drug discovery and development.
Five-year view
Polypharmacology can be employed to identify new drug off-targets. This is particularly important for prediction of possible adverse effects of for new drugs in development [55,59]. On the other hand, it can also be used for drug repurposing where new indications of existing drugs/agents can be identified [20,79-83]. This will significantly improve the current stalling drug discovery engine. As a matter of fact, NIH and FDA have launched such programs to identify new uses for existing agents developed by pharmas[84]. Therefore, during the next five or more years, we will see wide collaborations between academia and pharmaceutical sector for repositioning of existing therapeutic agents. Additionally continuous growth in the computational methods for polypharmacology prediction will be witnessed along with their widespread applications in drug discovery. We expect that the unexpected, potentially disastrous adverse properties of pipeline products will be modeled during their early development. Down the road in the next few years, more sophisticated and comprehensive polypharmacology approaches will boom and the rational design of more potent but less toxic multi-targeting agents may occur, although it is still extremely difficult at the current stage.
Key issues.
The concept of polypharmacology involves the interaction of drug molecules with multiple targets, which may interfere with a single or multiple disease pathways.
The polypharmacological studies could uncover new off-targets for the existing drugs.
The approach could provide us with the explanation for the drug side-effects and disastrous toxicities.
Polypharmacology can be used for drug repurposing by finding new indications (or new therapeutic targets) for existing drugs.
Computational approaches for polypharmacology modeling will witness rapid growth and wide application in drug discovery.
There exists various (a) similarity based, (b) network based, and (c) structure based approaches to uncover polypharmacological relationships.
High level data curation/integration and methodology development from various drug discovery disciplines would be needed for accurate prediction of polypharmacology prediction and rational design of multi-targeting agents.
Various challenges still exist for polypharmacology modeling, and rational design of multi-targeting agents is extremely complex.
Acknowledgments
We specially thank John Morrow for proof-reading the manuscripts. This work was partially supported by the University Cancer Foundation via the Institutional Research Grant program, NCI SPORE P50CA140388 and 5P50CA127001, and NIH 5R01CA138702-03. The authors declare no competing financial interests.
References
(Papers of special note have been highlighted with *)
- 1.Dimasi Ja. Risks in new drug development: approval success rates for investigational drugs. Clin Pharmacol Ther. 2001;69(5):297–307. doi: 10.1067/mcp.2001.115446. [DOI] [PubMed] [Google Scholar]
- 2.Dickson M, Gagnon Jp. Key factors in the rising cost of new drug discovery and development. Nat Rev Drug Discov. 2004;3(5):417–429. doi: 10.1038/nrd1382. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins Al, Groom Cr. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 4.Zambrowicz Bp, Sands At. Knockouts model the 100 best-selling drugs--will they model the next 100? Nat Rev Drug Discov. 2003;2(1):38–51. doi: 10.1038/nrd987. [DOI] [PubMed] [Google Scholar]
- 5.Yook Sh, Oltvai Zn, Barabasi Al. Functional and topological characterization of protein interaction networks. Proteomics. 2004;4(4):928–942. doi: 10.1002/pmic.200300636. [DOI] [PubMed] [Google Scholar]
- 6.Lipinski C, Hopkins A. Navigating chemical space for biology and medicine. Nature. 2004;432(7019):855–861. doi: 10.1038/nature03193. [DOI] [PubMed] [Google Scholar]
- 7*.Hopkins Al. Network pharmacology: the next paradigm in drug discovery. Nature Chemical Biology. 2008;4(11):682–690. doi: 10.1038/nchembio.118. An early review discussed polypharmacology and suggested that exquisitely selective compounds, compared with multi-targeting drugs, may have lower clinical efficacy Polypharmacology is possible to tackle the two major sources of attrition in drug development - efficacy and toxicity. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins Al. Drug discovery: Predicting promiscuity. Nature. 2009;462(7270):167–168. doi: 10.1038/462167a. [DOI] [PubMed] [Google Scholar]
- 9*.Apsel B, Blair Ja, Gonzalez B, et al. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nature Chemical Biology. 2008;4(11):691–699. doi: 10.1038/nchembio.117. A systematic attempt to design of multi-targeted agents that potently inhibit both tyrosine kinases and phosphatidylinositol-3-OH kinases Through iterative chemical synthesis, X-ray crystallography and kinome-level biochemical profiling, the authors identified compounds that inhibit a spectrum of new target combinations in the two kinase families. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon Z, Peragovics A, Vigh-Smeller M, et al. Drug effect prediction by polypharmacology-based interaction profiling. J Chem Inf Model. 2012;52(1):134–145. doi: 10.1021/ci2002022. [DOI] [PubMed] [Google Scholar]
- 11.Brianso F, Carrascosa Mc, Oprea Ti, Mestres J. Cross-Pharmacology Analysis of G Protein-Coupled Receptors. Curr Top Med Chem. 2011 doi: 10.2174/156802611796391285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Paolini Gv, Shapland Rh, Van Hoorn Wp, Mason Js, Hopkins Al. Global mapping of pharmacological space. Nat Biotechnol. 2006;24(7):805–815. doi: 10.1038/nbt1228. This paper presents the global mapping of pharmacological space by the integration of several vast sources of medicinal chemistry structure-activity relationships (SAR) data. [DOI] [PubMed] [Google Scholar]
- 13.Yildirim Ma, Goh Ki, Cusick Me, Barabasi Al, Vidal M. Drug-target network. Nat Biotechnol. 2007;25(10):1119–1126. doi: 10.1038/nbt1338. [DOI] [PubMed] [Google Scholar]
- 14.Durrant Jd, Amaro Re, Xie L, et al. A multidimensional strategy to detect polypharmacological targets in the absence of structural and sequence homology. PLoS Comput Biol. 2010;6(1):e1000648. doi: 10.1371/journal.pcbi.1000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oprea Ti, Mestres J. Drug Repurposing: Far Beyond New Targets for Old Drugs. Aaps J. 2012 doi: 10.1208/s12248-012-9390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boran Adw, Iyengar R. Systems approaches to polypharmacology and drug discovery. Current Opinion in Drug Discovery & Development. 2010;13(3):297–309. [PMC free article] [PubMed] [Google Scholar]
- 17.Boran Adw, Iyengar R. Systems Pharmacology. Mount Sinai Journal of Medicine. 2010;77(4):333–344. doi: 10.1002/msj.20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie L, Xie L, Kinnings Sl, Bourne Pe. Novel Computational Approaches to Polypharmacology as a Means to Define Responses to Individual Drugs. Annual Review of Pharmacology and Toxicology. 2012;52(52):361–+. doi: 10.1146/annurev-pharmtox-010611-134630. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins Al. Network pharmacology. Nature Biotechnology. 2007;25(10):1110–1111. doi: 10.1038/nbt1007-1110. [DOI] [PubMed] [Google Scholar]
- 20.Oprea Ti, Nielsen Sk, Ursu O, et al. Associating Drugs, Targets and Clinical Outcomes into an Integrated Network Affords a New Platform for Computer-Aided Drug Repurposing. Mol Inform. 2011;30(2-3):100–111. doi: 10.1002/minf.201100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achenbach J, Tiikkainen P, Franke L, Proschak E. Computational tools for polypharmacology and repurposing. Future Medicinal Chemistry. 2011;3(8):961–968. doi: 10.4155/fmc.11.62. [DOI] [PubMed] [Google Scholar]
- 22.Yamanishi Y, Araki M, Gutteridge A, Honda W, Kanehisa M. Prediction of drug-target interaction networks from the integration of chemical and genomic spaces. Bioinformatics. 2008;24(13):i232–240. doi: 10.1093/bioinformatics/btn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dar Ac, Das Tk, Shokat Km, Cagan Rl. Chemical genetic discovery of targets and anti-targets for cancer polypharmacology. Nature. 2012;486(7401):80–84. doi: 10.1038/nature11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knox C, Law V, Jewison T, et al. DrugBank 3.0: a comprehensive resource for 'omics' research on drugs. Nucleic Acids Res. 2011;39(Database issue):D1035–1041. doi: 10.1093/nar/gkq1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson Mr, Simpson Nr, Masheter Hc. New drugs. 8. Flufenamic acid in rheumatoid arthritis. Comparison with aspirin and the results of extended treatment. Ann Phys Med. 1966;8(6):208–213. [PubMed] [Google Scholar]
- 26.Berman J, Haffajee Ci, Alpert Js. Therapy of symptomatic pericarditis after myocardial infarction: retrospective and prospective studies of aspirin, indomethacin, prednisone, and spontaneous resolution. Am Heart J. 1981;101(6):750–753. doi: 10.1016/0002-8703(81)90610-4. [DOI] [PubMed] [Google Scholar]
- 27.Durongpisitkul K, Gururaj Vj, Park Jm, Martin Cf. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995;96(6):1057–1061. [PubMed] [Google Scholar]
- 28.Grundmann K, Jaschonek K, Kleine B, Dichgans J, Topka H. Aspirin non-responder status in patients with recurrent cerebral ischemic attacks. J Neurol. 2003;250(1):63–66. doi: 10.1007/s00415-003-0954-y. [DOI] [PubMed] [Google Scholar]
- 29.Amory Jk, Amory Dw. Dosing frequency of aspirin and prevention of heart attacks and strokes. Am J Med. 2007;120(4):e5. doi: 10.1016/j.amjmed.2006.04.023. author reply e7. [DOI] [PubMed] [Google Scholar]
- 30.Daya S. Recurrent spontaneous early pregnancy loss and low dose aspirin. Minerva Ginecol. 2003;55(5):441–449. [PubMed] [Google Scholar]
- 31.Baron Ja. Aspirin and Cancer: Trials and Observational Studies. J Natl Cancer Inst. 2012 doi: 10.1093/jnci/djs338. [DOI] [PubMed] [Google Scholar]
- 32.Debusk Rf, Pepine Cj, Glasser Db, Shpilsky A, Deriesthal H, Sweeney M. Efficacy and safety of sildenafil citrate in men with erectile dysfunction and stable coronary artery disease. Am J Cardiol. 2004;93(2):147–153. doi: 10.1016/j.amjcard.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Barf T, Kaptein A. Irreversible protein kinase inhibitors: balancing the benefits and risks. J Med Chem. 2012;55(14):6243–6262. doi: 10.1021/jm3003203. [DOI] [PubMed] [Google Scholar]
- 34.Baumann Kh, Du Bois A, Meier W, et al. A phase II trial (AGO 2.11) in platinum-resistant ovarian cancer: a randomized multicenter trial with sunitinib (SU11248) to evaluate dosage, schedule, tolerability, toxicity and effectiveness of a multitargeted receptor tyrosine kinase inhibitor monotherapy. Annals of Oncology. 2012;23(9):2265–2271. doi: 10.1093/annonc/mds003. [DOI] [PubMed] [Google Scholar]
- 35.Winum Jy, Maresca A, Carta F, Scozzafava A, Supuran Ct. Polypharmacology of sulfonamides: pazopanib, a multitargeted receptor tyrosine kinase inhibitor in clinical use, potently inhibits several mammalian carbonic anhydrases. Chemical Communications. 2012;48(66):8177–8179. doi: 10.1039/c2cc33415a. [DOI] [PubMed] [Google Scholar]
- 36.Ulahannan Sv, Brahmer Jr. Antiangiogenic Agents in Combination with Chemotherapy in Patients with Advanced Non-Small Cell Lung Cancer. Cancer Investigation. 2011;29(4):325–337. doi: 10.3109/07357907.2011.554476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konopa K, Jassem J. The Role of Pemetrexed Combined with Targeted Agents for Non-Small Cell Lung Cancer. Current Drug Targets. 2010;11(1):2–11. doi: 10.2174/138945010790030965. [DOI] [PubMed] [Google Scholar]
- 38.Keedy Vl, Sandler Ab. Inhibition of angiogenesis in the treatment of non-small cell lung cancer. Cancer Science. 2007;98(12):1825–1830. doi: 10.1111/j.1349-7006.2007.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmid A, Blank Lm. Hypothesis-driven omics integration. Nature Chemical Biology. 2010;6(7):485–487. doi: 10.1038/nchembio.398. [DOI] [PubMed] [Google Scholar]
- 40.Joyce Ar, Palsson Bo. The model organism as a system: integrating 'omics' data sets. Nature Reviews Molecular Cell Biology. 2006;7(3):198–210. doi: 10.1038/nrm1857. [DOI] [PubMed] [Google Scholar]
- 41.Zhao S, Iyengar R. Systems Pharmacology: Network Analysis to Identify Multiscale Mechanisms of Drug Action. Annual Review of Pharmacology and Toxicology. 2012;52(52):505–521. doi: 10.1146/annurev-pharmtox-010611-134520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irwin Jj, Shoichet Bk. ZINC--a free database of commercially available compounds for virtual screening. J Chem Inf Model. 2005;45(1):177–182. doi: 10.1021/ci049714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wheeler Dl, Barrett T, Benson Da, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2008;36(Database issue):D13–21. doi: 10.1093/nar/gkm1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng Z, Chen L, Maddula H, et al. Ligand Depot: a data warehouse for ligands bound to macromolecules. Bioinformatics. 2004;20(13):2153–2155. doi: 10.1093/bioinformatics/bth214. [DOI] [PubMed] [Google Scholar]
- 45.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40(Database issue):D109–114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhn M, Von Mering C, Campillos M, Jensen Lj, Bork P. STITCH: interaction networks of chemicals and proteins. Nucleic Acids Res. 2008;36(Database issue):D684–688. doi: 10.1093/nar/gkm795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu T, Lin Y, Wen X, Jorissen Rn, Gilson Mk. BindingDB: a web-accessible database of experimentally determined protein-ligand binding affinities. Nucleic Acids Res. 2007;35(Database issue):D198–201. doi: 10.1093/nar/gkl999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hecker N, Ahmed J, Von Eichborn J, et al. SuperTarget goes quantitative: update on drug-target interactions. Nucleic Acids Res. 2012;40(Database issue):D1113–1117. doi: 10.1093/nar/gkr912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharman Jl, Mpamhanga Cp, Spedding M, et al. IUPHAR-DB: new receptors and tools for easy searching and visualization of pharmacological data. Nucleic Acids Res. 2011;39(Database issue):D534–538. doi: 10.1093/nar/gkq1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olah M, Mracec M, Ostopovici L, et al. WOMBAT: World of molecular bioactivity. In: Oprea Ti., editor. Chemoinformatics in Drug Discovery. Wiley-VCH; New York: 2004. pp. 223–239. [Google Scholar]
- 51.Davis Ap, King Bl, Mockus S, et al. The Comparative Toxicogenomics Database: update 2011. Nucleic Acids Res. 2011;39(Database issue):D1067–1072. doi: 10.1093/nar/gkq813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Yl, Xiao Jw, Suzek To, et al. PubChem's BioAssay Database. Nucleic Acids Research. 2012;40(D1):D400–D412. doi: 10.1093/nar/gkr1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaulton A, Bellis Lj, Bento Ap, et al. ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012;40(Database issue):D1100–1107. doi: 10.1093/nar/gkr777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keiser Mj, Roth Bl, Armbruster Bn, Ernsberger P, Irwin Jj, Shoichet Bk. Relating protein pharmacology by ligand chemistry. Nat Biotechnol. 2007;25(2):197–206. doi: 10.1038/nbt1284. [DOI] [PubMed] [Google Scholar]
- 55*.Lounkine E, Keiser Mj, Whitebread S, et al. Large-scale prediction and testing of drug activity on side-effect targets. Nature. 2012;486(7403):361–367. doi: 10.1038/nature11159. An exemplified large scale study where the similarity ensemble approach was used to predict the activity of 656 marketed drugs on 73 unintended ‘side-effect’ targets. Experimental evaluations confirmed that half of the predictions were correct with the activity values ranging from 1nM to 30μM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barabasi Al, Oltvai Zn. Network biology: understanding the cell's functional organization. Nat Rev Genet. 2004;5(2):101–113. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Ah, Sun H, Yang B, Wang Xj. Predicting new molecular targets for rhein using network pharmacology. Bmc Systems Biology. 2012;6 doi: 10.1186/1752-0509-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Cheng F, Zhou Y, Li W, Liu G, Tang Y. Prediction of chemical-protein interactions network with weighted network-based inference method. PLoS One. 2012;7(7):e41064. doi: 10.1371/journal.pone.0041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59*.Campillos M, Kuhn M, Gavin Ac, Jensen Lj, Bork P. Drug target identification using side-effect similarity. Science. 2008;321(5886):263–266. doi: 10.1126/science.1158140. A study of using phenotypic side-effect similarities to infer whether two drugs share a target With 746 marketed drugs on a network of 1,018 side effect, 261 drug-target relations are formed by chemically dissimilar drugs from different therapeutic indications The authors experimentally validated 13 implied drug-target relations, of which 11 reveal inhibition constants equal to less than 10μM. [DOI] [PubMed] [Google Scholar]
- 60.Grinter Sz, Liang Y, Huang Sy, Hyder Sm, Zou X. An inverse docking approach for identifying new potential anti-cancer targets. J Mol Graph Model. 2011;29(6):795–799. doi: 10.1016/j.jmgm.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hui-Fang L, Qing S, Jian Z, Wei F. Evaluation of various inverse docking schemes in multiple targets identification. J Mol Graph Model. 2010;29(3):326–330. doi: 10.1016/j.jmgm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Chen Yz, Zhi Dg. Ligand-protein inverse docking and its potential use in the computer search of protein targets of a small molecule. Proteins. 2001;43(2):217–226. doi: 10.1002/1097-0134(20010501)43:2<217::aid-prot1032>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 63.Abdulhameed Mdm, Chaudhury S, Singh N, Sun H, Wallqvist A, Tawa Gj. Exploring Polypharmacology Using a ROCS-Based Target Fishing Approach. Journal of Chemical Information and Modeling. 2012;52(2):492–505. doi: 10.1021/ci2003544. [DOI] [PubMed] [Google Scholar]
- 64.Davis Ap, Murphy Cg, Rosenstein Mc, Wiegers Tc, Mattingly Cj. The Comparative Toxicogenomics Database facilitates identification and understanding of chemical-gene-disease associations: arsenic as a case study. BMC Med Genomics. 2008;1:48. doi: 10.1186/1755-8794-1-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirschman L, Burns Ga, Krallinger M, et al. Text mining for the biocuration workflow. Database (Oxford) 2012;2012:bas020. doi: 10.1093/database/bas020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krallinger M, Leitner F, Vazquez M, et al. How to link ontologies and protein-protein interactions to literature: text-mining approaches and the BioCreative experience. Database (Oxford) 2012;2012:bas017. doi: 10.1093/database/bas017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dowell Kg, Mcandrews-Hill Ms, Hill Dp, Drabkin Hj, Blake Ja. Integrating text mining into the MGI biocuration workflow. Database (Oxford) 2009;2009:bap019. doi: 10.1093/database/bap019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiegers Tc, Davis Ap, Cohen Kb, Hirschman L, Mattingly Cj. Text mining and manual curation of chemical-gene-disease networks for the comparative toxicogenomics database (CTD) BMC Bioinformatics. 2009;10:326. doi: 10.1186/1471-2105-10-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kolb P, Ferreira Rs, Irwin Jj, Shoichet Bk. Docking and chemoinformatic screens for new ligands and targets. Curr Opin Biotechnol. 2009;20(4):429–436. doi: 10.1016/j.copbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tian L, Zhang S. Mapping drug-target interaction networks. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2336–2339. doi: 10.1109/IEMBS.2009.5335053. [DOI] [PubMed] [Google Scholar]
- 71.Adams Jc, Keiser Mj, Basuino L, et al. A mapping of drug space from the viewpoint of small molecule metabolism. PLoS Comput Biol. 2009;5(8):e1000474. doi: 10.1371/journal.pcbi.1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morrow Jk, Tian L, Zhang S. Molecular networks in drug discovery. Crit Rev Biomed Eng. 2010;38(2):143–156. doi: 10.1615/critrevbiomedeng.v38.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berman Hm, Westbrook J, Feng Z, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28(1):235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Csizmadia F. JChem: Java applets and modules supporting chemical database handling from web browsers. J Chem Inf Comput Sci. 2000;40(2):323–324. doi: 10.1021/ci9902696. [DOI] [PubMed] [Google Scholar]
- 75.Wren Jd, Bekeredjian R, Stewart Ja, Shohet Rv, Garner Hr. Knowledge discovery by automated identification and ranking of implicit relationships. Bioinformatics. 2004;20(3):389–398. doi: 10.1093/bioinformatics/btg421. [DOI] [PubMed] [Google Scholar]
- 76.Cheng T, Li Q, Zhou Z, Wang Y, Bryant Sh. Structure-based virtual screening for drug discovery: a problem-centric review. Aaps J. 2012;14(1):133–141. doi: 10.1208/s12248-012-9322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nidhi, Glick M, Davies Jw, Jenkins Jl. Prediction of biological targets for compounds using multiple-category Bayesian models trained on chemogenomics databases. J Chem Inf Model. 2006;46(3):1124–1133. doi: 10.1021/ci060003g. [DOI] [PubMed] [Google Scholar]
- 78.Benson Ml, Smith Rd, Khazanov Na, et al. Binding MOAD, a high-quality protein-ligand database. Nucleic Acids Res. 2008;36(Database issue):D674–678. doi: 10.1093/nar/gkm911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ravot E, Lisziewicz J, Lori F. New uses for old drugs in HIV infection: the role of hydroxyurea, cyclosporin and thalidomide. Drugs. 1999;58(6):953–963. doi: 10.2165/00003495-199958060-00001. [DOI] [PubMed] [Google Scholar]
- 80.Chong Cr, Sullivan Dj., Jr New uses for old drugs. Nature. 2007;448(7154):645–646. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- 81.Chen D, Dou Qp. New uses for old copper-binding drugs: converting the pro-angiogenic copper to a specific cancer cell death inducer. Expert Opin Ther Targets. 2008;12(6):739–748. doi: 10.1517/14728222.12.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sannella Ar, Casini A, Gabbiani C, et al. New uses for old drugs. Auranofin, a clinically established antiarthritic metallodrug, exhibits potent antimalarial effects in vitro: Mechanistic and pharmacological implications. FEBS Lett. 2008;582(6):844–847. doi: 10.1016/j.febslet.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 83.Vazquez-Martin A, Lopez-Bonetc E, Cufi S, et al. Repositioning chloroquine and metformin to eliminate cancer stem cell traits in pre-malignant lesions. Drug Resist Updat. 2010;14(4-5):212–223. doi: 10.1016/j.drup.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Allison M. NCATS launches drug repurposing program. Nat Biotechnol. 2012;30(7):571–572. doi: 10.1038/nbt0712-571a. [DOI] [PubMed] [Google Scholar]