Abstract
Microparticles (MPs) are membrane-bound vesicles shed normally or as a result of various (pathological) stimuli. MPs contain a wealth of bio-active macromolecules. The aminophospholipid phosphatidylserine (PS) is present on the surface of many MPs. As PS and phosphatidylethanolamine (PE) are related, yet distinct aminophospholipids, the purpose of this study was to systematically and directly assess PE exposure on MPs. We examined MPs from various human cellular sources (human breast cancer, endothelial, red and white blood cells) by flow cytometry using a PE-specific probe, Duramycin, and two PS-specific probes, annexin V and lactadherin. PS and PE exposure percentage was comparable on vascular and blood cell-derived MPs (80-90% of MP-gated events). However, the percentage of malignant breast cancer MPs exposing PE (~90%) was significantly higher than PS (~50%). Thus, while PS and PE exposure can result from a general loss of membrane asymmetry, there may also be distinct mechanisms of PE and PS exposure on MPs that vary by cellular source.
Keywords: Microparticles, Microvesicles, Phosphatidylethanolamine, Duramycin, Membrane asymmetry
1. Introduction
Circulating microparticles (MPs) are membrane-bound vesicles shed from normal, stressed, diseased, and/or dying cells. MPs contain a wealth of informative molecules, including surface markers from parent cells, nucleic acids, signaling peptides and lipids, and other pathological macromolecules [1]. MPs have been implicated in the pathophysiology of a range of diseases, including cardiovascular [2,3], malignant [4,5] and infectious diseases [6,7]. MPs may also serve as prognostic markers for these diseases [1-7].
MPs have a bilayered phospholipid membrane [8]. Characterization of the inter-leaflet distribution and dynamics of phospholipids in MP membranes is an important step toward understanding the formation and effects of different types of MPs. In particular, since the outer leaflet of the phospholipid bilayer of circulating MPs is exposed to the vascular space, it is an interface where physicochemical interactions with blood components and the endothelium take place. Additionally, the molecules present in the outer surface, once defined, provide accessible markers for detecting and characterizing MPs using molecular probes [9].
Under normal, resting cellular conditions, phospholipids in the bilayer of eukaryotic plasma membrane are asymmetrically distributed. Phosphatidylcholine (PC) and sphingomyelin (SM) are mostly present in the outer leaflet; other species such as phosphatidylserine (PS), phosphatidylethanolamine (PE) and phosphatidylinositides (PIs) are predominantly distributed in the inner leaflet [10]. The dynamic distribution of phospholipids in the cellular plasma membrane is mediated by energy-dependent and -independent enzymes, putatively including the type IV P- type ATPases (P4 ATPases), scramblases, and floppases [10]. Transient or irreversible redistribution of phospholipid(s) across the membrane bilayer has been shown to play important roles in cellular events, such as coagulation [11], cytokinesis [12], sperm capacitation [13], and apoptosis [14].
While the MP membrane is derived from the plasma membrane of the host cell, it remains unclear whether the asymmetry of phospholipid distribution across the bilayer is preserved in MPs [15,16]. The presence of externalized PS on MP surfaces indicates an altered phospholipid distribution profile compared to the plasma membrane of a resting mammalian cell. However, it is unknown if the altered phospholipid distribution profile in MPs is phospholipid species-selective; i.e. is PS externalized with or without the concurrent externalization of other aminophospholipids, such as PE? As a major phospholipid species, PE typically accounts for 20 — 50% of total phospholipid content in the mammalian plasma membrane [10]. As supported by a growing body of evidence, PE and PS serve distinctly different functions and are regulated by different mechanisms, even though both are members of aminophospholipids. For instance, the current consensus is that PE alone is anticoagulant, but it accelerates coagulation in the presence of PS [17]. Additionally, distinct trans-bilayer flip-flop mechanisms have been documented with specificity for PE or PS [18]. The presence of PE at the outer membrane surface, if specifically proven, may provide a marker for MP detection and characterization.
Although the externalized PS is well documented, that of PE remains largely unknown. The goal of this study was to systematically investigate the presence of PE at the surface of MPs derived from different types of human cells: erythrocytes, endothelial cells, macrophages, and malignant breast cancer cells. A PE-specific molecular probe used in the current study, duramycin, is a 19 amino acid lantibiotic produced by Streptoverticillium cinnamoneus. Duramycin binds PE with high specificity and affinity [19]. The significance is that PE is a major phospholipid species in the plasma membrane, and the presence (or absence) of PE will have important implications in coagulation and other functions.
2. Methods and Materials
2.1 Cells and reagents
Duramycin, dimethylformamide (DMF), triethylamine, lipopolysaccharide (LPS), DETANONOate, and DPBS (with and without calcium) were obtained from Sigma (St. Louis, MO, USA). Sulfo-N-hydroxysulfosuccinimide ester biotin (NHS-Biotin) was obtained from Solulink, streptavidin Alexafluor-633 (SA633), streptavidin Alexafluor-647 (SA647), annexin V-FITC, Human Aortic Endothelial Cells (HAEC), MDA-MB-231 human breast cancer cells (MDA), and DMEM were purchased from Invitrogen (Carlsbad, CA). EBM2 was from Lonza (Basel, Switzerland). FBS was from Thermo-Fischer (Waltham, MA, USA). Nanolink ® or Magnabind ® beads were from Solulink (San Diego, CA, USA) or Thermo, respectively. Outdated (42- to 43-day old) units of Leukocyte-Reduced Blood Cell (LRBC) in citrate-phosphate-dextrose with additive solution 1 were obtained from the Blood Center of Wisconsin (Milwaukee, WI, USA). The peripheral blood of 3 consented volunteers was collected into sodium citrate in accordance with IRB-approved protocols. Monocytes were then isolated by Ficoll-Histopaque centrifugation for 30-40 minutes at 400x g (per manufacturer instructions). Other materials included Accucheck® counting beads (Roche Diagnostics, Indianapolis, IN, USA), 3 μm sizing beads (Bangs Laboratories, Fisher, IN, USA), mouse anti-human Tissue Factor antibody (American Diagnostica, Stamford, CT, USA), Tissue Factor (Innovin, Dade Behring, Germany), lactadherin-FITC (Haematologic Technologies, Essex Junction, VA, USA) and phycoerythrin-labeled mouse anti-human CD31 (PECAM-1) or anti-human CD235a (glycophorin A) and rat isotype control antibodies (BD Pharmingen, Franklin Lakes, NJ, USA).
2.2 Duramycin-biotin preparation
Duramycin-biotin was prepared as described previously [20]. Briefly, duramycin was incubated with NHS-biotin overnight at room temperature in DMF at 1:1.2 molar ratio in the presence of triethylamine. The duramycin-conjugate was purified with high-performance liquid chromatography (HPLC) and confirmed with mass spectrometry. The lyophilized product was stored at −80 °C until use, when it was resuspended in calcium-free PBS.
2.3 MP isolation
MP-rich supernatant from all sources were clarified by centrifugation at 500x g for 10-15 minutes followed by 1500x g for 15 minutes to remove cells, and large debris. Aged blood MPs were examined in the supernatant from 42-44 day-old donor leukocyte-reduced units. MDA MPs were obtained from media culturing MDA-MB-231 cells at 37 °C for 24-48 hours. To obtain LPS-stimulated HAEC MPs, HAECs (not older than 9 passages) were stimulated with 1-10 ng/ml LPS for 6-24 hours after which culture supernatant was removed and processed as described above. Monocyte-derived MPs were generated by incubating peripheral monocytes with 1-10ng/ml LPS for 6 hours.
Because of potential interference of anticoagulant in donor blood units with calcium for Annexin V binding, a portion of MP aliquots were further ultracentrifuged at 20000-22000x g at 10°C for 0.5-1 hour, after which the pellet was washed and resuspended in calcium-free PBS. Additionally, as calcium added to phosphate-buffered solutions can form precipitates [21], some MP pellets were resuspended in normal saline. Although effort was made to perform flow cytometry on the day of isolation, some aliquots were flash frozen and stored at −80 to −20°C until further use. See Online Supplement for additional details.
2.4 Microscopy
2.4.1 Microscopy of microparticles
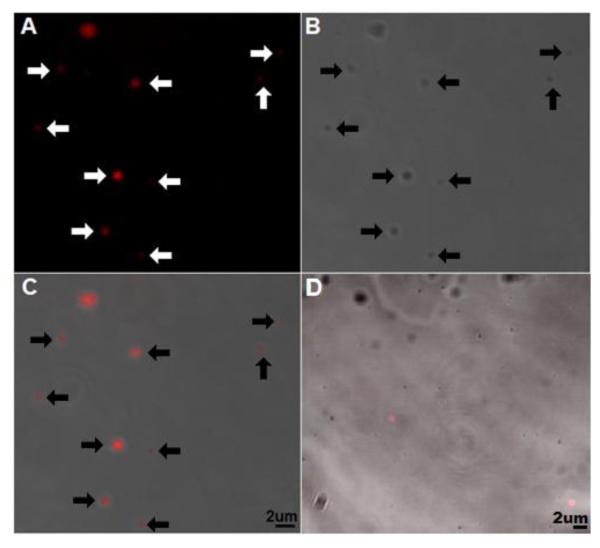
MDA MPs were incubated with 500nM duramycin-biotin followed by 250nM streptavidin Alexafulor-647 (SA647) (or SA647 alone as control) for 7-10 minutes each. Following incubation, the MPs were centrifuged at 20000x g for 10 minutes and resuspended in 1:1 PBS:Tissue-Tek O.C.T. (Sakura Finetek, Torrence, CA) and visualized with confocal microscopy (Fig. 1). Images were captured using a Zeiss LSM510 laser scanning confocal inverted microscope.
Figure 1.
Confocal imaging of duramcyin-labeled cancer MPs
To label exposed phosphatidylethanolamine (PE) MDA MB-231 breast cancer cell (MDA) microparticles (MPs) were stained with 500nM duramycin-biotin and 250nM streptavidin Alexafluor-647, pelleted, and resuspended in 1:1 PBS:O.C.T. A) 647 signal, B) phase contrast, C) and overlay show fluorescence localizes to MPs (arrows added for emphasis). D) Fluorescent control (duramycin-biotin omitted) overlay. C-D) Lower right corner of overlays, bar indicates 2 μm. O.C.T., optimal cutting temperature embedding medium. Images representative of 2 separate experiments.
2.4.2 Microscopy of PE on erythrocytes
Duramycin is thought to be cytotoxic by inducing pore formation at a higher concentration [22]. Thus, to avoid any potential of hemolysis, erythrocytes were incubated with only 250nM duramycin-biotin for 2-3 minutes, gently centrifuged at 200x g, washed, stained with 62.5nM SA633 for an additional 2-3 minutes, gently centrifuged at 200x g washed and resuspended in PBS. Confocal images were obtained as described above.
2.5 Flow analysis of MPs
2.5.1 Flow cytometer acquisition and gating
Flow cytometry was performed on LSRII®, FACS DiVa®, or Accuri C6® (BDIS, San Jose, CA, USA), for either a minimum of 10,000 events for isolated MPs or 100,000 events for uncentrifuged donor blood samples. The use of different flow cytometers was done to ensure this novel MP-staining approach was robust, and not instrument-specific. For consistency, we used the same 1 μm beads (Thermo) to establish the MP gate upper limits on the 3 different machines. There was no significant difference in percentages of PE+ cancer MPs among the cytometers. The lower limit of the gate was the minimum forward-scatter threshold, which was adjusted so that sterile-filtered PBS resulted in a maximum of 100 events per minute (an arbitrary acceptable ‘noise’ level) and kept constant for subsequent experiments. To ensure that apoptotic bodies and other large membrane vesicles were not included in the MP gate, events larger than roughly1 μm were excluded based on forward-scatter data of 1 μm beads (Supplemental Fig. 1A-C). However, as shown by Furie et al [23], flow cytometry cannot confidently be used to differentiate bead size below 3 μm based on light-scatter, thus making it difficult to size MPs (which are smaller than 1 μm) from light-scatter characteristics. Thus, to confirm that our MP gate included sub-micron membrane vesicles, we filtered MP-rich supernatant with two different sub-micron pore filters (0.20 μm and 0.45 μm-pore size) (Supplemental Fig. 1D-F).
After establishing the MP gate based on light scatter, the phospholipid-positive gates were determined based on fluorescent streptavidin alone to account for non-specific labeling.
2.5.2 MP surface marker and phospholipid staining
To empirically confirm the MP gate contained MPs, co-staining of surface-marker and aminophospholipid were performed. For the staining of externalized PE, duramycin-biotin and fluorescently-labeled SA were added at a molar ratio of 4 to 1 in the labeling mixture, since a single SA tetramer binds up to 4 biotin molecules. Duramycin-biotin was added to MP-rich supernatant and incubated on ice for 10-15 minutes. Following the incubation, fluorescently-labeled streptavidin was added and incubated on ice in the dark for an additional 10-15 minutes before flow cytometry. We performed pH and calcium titration experiments to ensure optimal phospholipid staining conditions. Duramycin and lactadherin staining was unaffected by the presence or absence of calcium (data not shown), unlike annexin V binding (Supplemental Fig. 2A-B). Experiments utilizing various phospholipid probes were done in parallel or under similar dilutions, as the probe/MP phospholipid ratio was critical (Supplemental Fig. 2C). Duramycin staining was highest near physiologic pH (Supplemental Fig. 2D). Annexin V-FITC or lactadherin-FITC was incubated with MPs for 20-30 minutes on ice in the dark to stain for the presence of surface PS. Phycoerythrin-labeled anti-PECAM-1 (for platelets or endothelial cell-derived MPs) or glycophorin-A (for RBC-derived MPs) antibodies were added to appropriate MP types and incubated for 20-30 minutes covered on ice prior to flow cytometry.
2.5.3 Flow cytometry analysis
Analysis of flow cytometry was performed using FlowJo (TreeStar, Ashland, OR, USA) for experiments done on the FACSDiva or LSRII, or C-Flow Plus (BDIS) for experiments performed on the Accuri C6® due to minor compatibility issues examining Accuri C6-generated data using FlowJo.
2.6 Plasma coagulation assays
Plasma coagulation assays were done using ultracentrifuged and 0.22μm filtered, sodium citrate-anticoagulated plasma donated from 2 different healthy, consented donors on a STart 4 coagulometer (Diagnostica Stago, Parsippany, NJ, USA). MDA MP-containing media or controls were diluted 1:10 in a calcium-free HEPES + 5% BSA buffer with various concentrations of duramycin-biotin from 0 (control) to 100 μM. The media were then added 5:4 to plasma, followed by the addition of CaCl2 at 10mM to initiate coagulation. Control coagulation assays were done in triplicate at minimum from the 2 different plasma sources; no difference was found and thus duplicates of each sample were combined for each concentration of duramycin-biotin tested. A positive control using Tissue Factor (Innovin) diluted 1:100 in the plasma resulted in near immediate coagulation (data not shown). Coagulation time was measured up to ten minutes; samples that did not clot after 10 minutes were considered as “no clot.”
2.7 Statistical analyses
We assumed a normal distribution of mean phospholipid probe labeling across experiments. Therefore, parametric statistical analysis was done using the 2-tailed Student’s t-test. Significance was considered at a p-value <0.05. Results are presented as mean ± SEM unless explicitly stated otherwise. For experiments with considerable variability (i.e. greater than 100% difference between groups), data were log transformed prior to statistical analysis.
3. Results
3.1 Microscopy of PE on membrane and membrane vesicles of breast cancer cells
As cancer cells are known to shed MPs [22], MDA MP-rich supernatant was stained with Duramycin-biotin and fluorescently-labeled streptavidin (SA) and visualized using confocal microscopy (Fig. 1). PE was found on small (<2 μm) membrane vesicles in these experiments. Based on these qualitative results, quantitative analyses were carried out using flow cytometry-based techniques.
3.2 Flow cytometry of duramycin binding to PE on MPs
In order to exclude cells, apoptotic bodies, and other large membrane fragments, we gated out flow cytometry events larger than roughly 1 μm in diameter. Size estimates were based upon the forward-scatter width and height of purchased beads and RBCs of known size (Supplemental Fig. 1A-C). Relative to uncentrifuged samples (Supplemental Fig. 1C), clarification of the original sample/media removed all cells and larger debris (>3 μm) while leaving small (less than ~1 μm) events (Supplemental Fig. 1D). Indeed, after appropriate multiplet exclusion gating, the vast majority (>90%) of flow cytometry events from all cell types were smaller than the 1 μm estimate.
However, flow cytometry size estimates smaller than 3 μm can be inaccurate [24]. To ensure that the gate included sub-micron sized membrane vesicles and not larger particles or debris, we filtered MP-rich supernatant with different pore-sized membranes and examined the filtrate with flow cytometry (Supplemental Fig. 1D-F). Differential filtration using sub-micron pores assured the MP gate, indeed, included sub-micron vesicles.
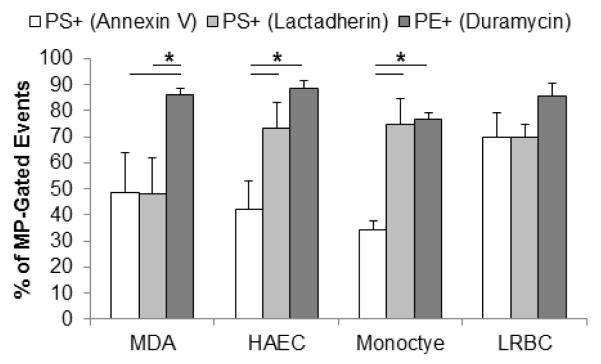
PE+ MP-gated events co-stained with surface markers (Fig. 2). MPs from LRBCs were mostly (90%) positive for the RBC marker, glycophorin-A, and/or PE (Fig. 2A). The vast majority (>85%) of the MP-gated events were positive for PECAM-1 and/or PE. This empirically confirmed the events in the light-scatter-based gate were MPs, and highlights the compatibility of phospholipid probe and other protein surface markers. Some endothelial-derived MPs invariably did not stain positive for PECAM-1, which has been reported before [25]. However, the majority of such PECAM-1-negative events were positive for PE (Fig. 2B), highlighting the need for phospholipid-based probes in addition to immunolabeling when examining MPs with flow cytometry.
Figure 2.
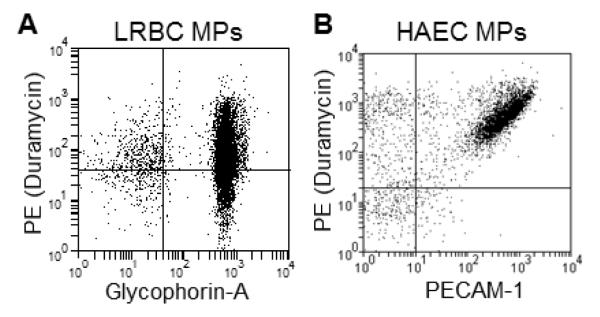
Duramycin binds MPs from various cellular sources
MP-rich supernatants from aged donor units (LRBC) and human aortic endothelial cells stimulated with LPS (HAECs) were examined with flow cytometry after staining with 500 nM duramycin-biotin, 125 nM fluorescent streptavidin and 1:200 fluorescently-labeled antibodies. A) PE and glycophorin A staining. B) PE with PECAM-1 staining. Representative of N=3. Gating was based on isotype and fluorescent (streptavidin-only) controls.
3.3 Most MP-gated events expose PE comparably or significantly greater than PS
Since the staining of surface PS using annexin V is a widely practiced method for MP identification, we sought to compare PS with PE staining on MPs. PS staining using lactadherin has been shown to be more sensitive than Annexin V [26]. Thus, parallel titration experiments were carried out using annexin V, lactadherin, and duramycin to identify optimal/saturating concentrations (Supplemental Fig. 3). Figure 3 is representative of flow cytometry titration experiments using duramycin to label MP-PE. Shown are the controls (fluorescently-labeled SA at the highest concentration used with duramycin-biotin omitted) and corresponding duramycinbiotin titrations.
Figure 3.
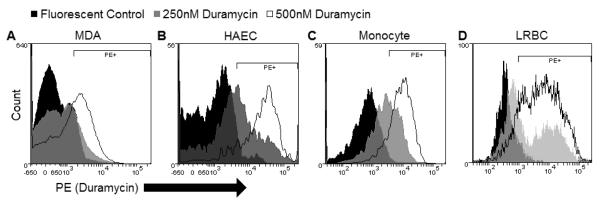
Duramycin titration on MPs
MP-rich supernatants from A) MDA, B) HAEC, C) monocyte, and D) LRBC were labeled with duramycin-biotin (or 125 nM fluorescent SA only) at various concentrations. Representative of 3-6 experiments.
As shown in Figure 4, duramycin and lactadherin bound to greater percentages of MP-gated events than annexin V on HAEC- and monocyte-derived MPs (p<0.01) at the highest concentrations tested. There was a significantly higher frequency of PE+ than PS+ MDA-derived MPs. There was no significant difference in staining percentages between duramycin and lactadherin on non-cancer MPs. The percentage of PE+ MP-gated events on all cell types was consistently near 80-90%.
Figure 4.
Duramycin binds MPs from a variety of cells.
MP-rich supernatants from A) MDA, B) HAEC, C) monocyte, and D) LRBC were labeled with duramycin-biotin (or 125 nM fluorescent SA only) at or near saturating conditions (1:50 annexin V, 32nM lactadherin, and 500 nM duramycin). Annexin V stained 48, 42, 34, and 70%; lactadherin stained 48, 73, 75, and 70%; duramycin stained 86, 88, 76, and 85% of MP-gated events of MP-rich supernatant from MDA, HAEC, monocyte, and LRBC, respectively. SA, streptavidin Alexafluor.
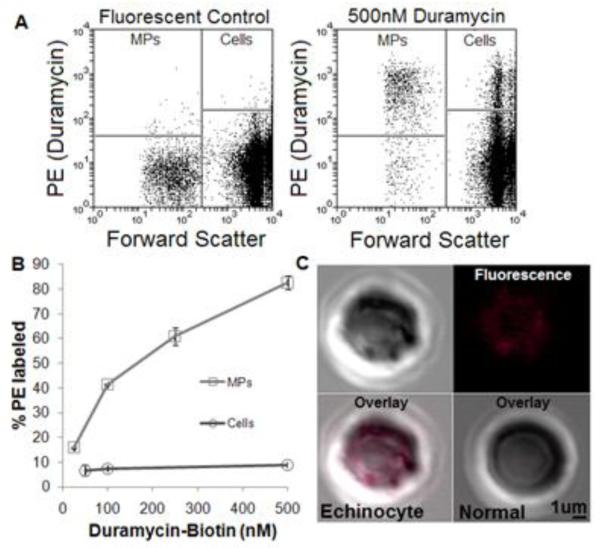
3.4 Duramycin binds MPs from RBCs more than RBCs
Because the addition of micro-molar amounts of PE-binding lantibiotic probes can induce cellular morphological changes [27], special consideration was taken to observe light scatter changes after the addition of duramycin to both MP-rich supernatant and cells. In donor blood-derived MPs and cells, morphological changes were minimal (data not shown).
Of note, duramycin bound to a higher percentage of MPs than cells (Figure 5). Most (>90%) of the cells remained PE-negative (Fig. 5A-B), which is indicative of a lack of appreciable PE externalization in viable cells.
Figure 5.
Duramycin-PE binding on RBCs and RBC MPs
A) Fluorescent (SA only) control (left) and duramycin-stained donor blood (right). B) Duramycin bound a larger portion of LRBC-derived MPs compared with cells over a wide range of concentrations. C) Confocal imaging of duramycin-stained RBCs show PE exposure on echinocytes (upper half and lower left) but not normal cells (lower right).
To further assess the staining pattern of the small percentage of PE+ cells, microscopy was employed. Brightfield coupled with confocal imaging showed that damaged or crenated erythrocytes rather than normal-appearing RBCs stained brightly for PE (Fig. 5C). Since PE is more abundant than PS in cell membranes and given the aged/ATP-depleted nature of RBCs in outdated donor blood, the small percentage of PE+ cells we observed are consistent with that of PS+ cells reported elsewhere [28,29].
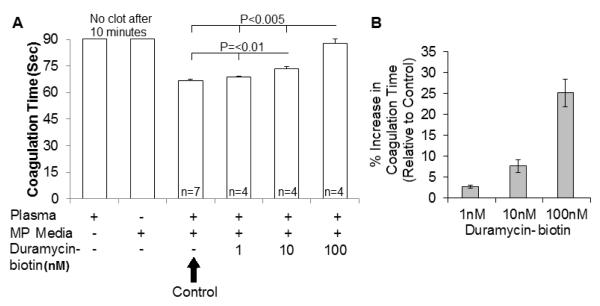
3.5 Duramycin inhibits coagulation induced by breast cancer microparticles
Breast cancer-derived MPs have been linked to the hypercoagulable state seen in cancer due to their expression of Tissue Factor (TF) and pro-coagulant phospholipids such as phosphatidylserine (PS) [4]. Duramycin has been shown to inhibit MP-caused coagulation using pancreatic cells and MPs [30]. To further explore the effects of duramycin on coagulation on a different cancer MP type, and at benign concentrations, we examined the effects of up to 100nM duramycin-biotin on the coagulation time of MDA MP-containing media.
Recalcification of MP-free (ultracentrifuged, filtered) plasma did not result in a clot within 10 minutes; nor did the breast cancer MP media clot within 10 minutes in the absence of plasma. The addition of breast cancer MP-rich media to plasma resulted in coagulation after the addition of calcium (Fig. 6A). The addition of sub-toxic duramycin concentration increased the plasma coagulation time of MPs from breast cancer cells in a dose-dependent manner (Fig. 6B).
Figure 6.
Duramycin inhibits breast cancer microparticle-mediated coagulation
A) Control plasma or MDA MP-containing media did not clot even after 10 minutes of adding calcium. Relative to MDA MPs in plasma, the addition of duramycin lengthened the time prior to clot formation. B) This resulted in a dose-dependent increase in the coagulation time (or decrease in the coagulability) of the breast cancer MPs. N>=4.
4. Discussion
In the current study, we directly demonstrated that PE is present on the surface of MPs from a variety of human cells, and that PE provides a sensitive molecular marker for detecting and characterizing MPs. PE is a highly abundant phospholipid species in mammalian cellular membranes. In a viable, resting cell, the aminophospholipids PS and PE are predominantly sequestered in the inner leaflet of the plasma membrane by the actions of energy-dependent translocases [10]. The presence of such aminophospholipids on the outer surface of MPs is indicative of compromised asymmetric phospholipid distribution across the two leaflets by one or more of possible scenarios including the suppression of flippases, activation of floppases and/or scramblases [10]. The content of PE in membranes is often several folds higher than that of PS depending on the cell type [10]. The large pool of PE potentially makes it a sensitive marker for characterizing MPs [31-33] as well as cells with compromised membrane integrity [34].
Numerous reports have highlighted important biological functions of MPs in a variety of human diseases and blood products, including coagulation via bearing tissue factor or PS, inflammatory cell activation, and even horizontal transfer of macromolecular cargo [3-6,35,36]. However, the variability in MP levels, as detected using currently available methods, has limited the development of clinical and scientific applications of MP-based utilities [37,38]. At this time, the externalization of PS is commonly used as a molecular marker for the detection of apoptosis and MPs using annexin V or its derivatives. A potential drawback of using annexin V is that calcium is an essential cofactor for annexin V-PS interactions. The addition of exogenous calcium may alter the biological system under investigation [17,39]. In contrast, the interactions between lactadherin and PS or duramycin and PE require no divalent cations, eliminating potential errors and allowing for direct addition of the phospholipid probe to citrate- or EDTA-anticoagulated blood, thereby enhancing the potential diagnostic and experimental utility of MP-based assays. Lactadherin and duramycin preferentially bind their respective phospholipid head groups on membranes with high affinity and specificity.
An interesting finding by using duramycin to target PE as a surface marker is demonstrated from a consistently greater percentage of MPs detected in a given sample compared to the standard PS-based method (annexin V), or even lactadherin for MDA MPs. This observation is likely a reflection of a greater abundance of PE than PS on MP surface, which is consistent with PE versus PS content in mammalian cellular membranes.
Both lactadherin and duramycin stained a similar percentage of total MP-gated events from non-cancerous cells; however, on MDA-derived MPs, a significantly higher percentage of MPs stained for PE+ than for PS+ (Fig. 3). This difference in PE/PS ratios on MP surface is implicative of distinct molecular mechanisms involved in the formation of extracellular vesicles, and different functions for MPs from different cellular origins.
Roughly 10-20% of MP-gate events were not labeled in parallel PS and PE staining experiments. These negative events likely represent non-MP debris or may possibly represent MPs that are truly negative for externalized PS and PE. Indeed, increasing the forward-scatter threshold resulted in less PE or PS-negative events (e.g. noise), but at the cost of discarding valid (surface marker positive) flow cytometry events. This highlights the utility using lactadherin or duramycin for distinguishing MPs from artifact via flow cytometry.
There have been a few reports suggesting PE is in MPs, but no systematic measurement has been done using MPs from various cellular sources. Of particular notable is a recent study by Wehman et al. examining a PE-specific translocases in the plasma membrane and its inactivation in vesicle formation using C. elegans as a model system [40]. Our data directly demonstrate that PE is abundantly present on the outer surface of mammalian MPs. Externalized PE can be used as a sensitive surface marker for MPs, which enables efficient differentiation of MPs from artifact using flow cytometry. MPs from one type of malignant breast cancer cells more frequently expressed PE than PS. More studies are needed to elucidate the possibly distinct mechanisms and functions of different phospholipids in MPs.
Supplementary Material
Highlights.
Phosphatidylethanolamine (PE) is detectable using Duramycin at surface of microparticles (MPs).
Surface PE was systematically characterized in MPs from different sources.
Different types of MPs consistently express PE but not PS.
Externalized PE is a reliable molecular marker for detecting MPs.
Coagulation time is prolonged by blocking MP surface PE.
Acknowledgements
The authors would like to thank Hope Campell for help with flow cytometry and Steven Johnson, Zhixin Li, and Paul Ellery for help with cell culture and MP isolation, and Alan Mast for critical guidance as well as reagents. This work was funded by NIH grant 5R01HL102085 (MZ), U54 HL090503 (CAH) and P01-HL44612 (CAH) and MSTP training grant T32GM080202 (MCL, TJK) of the Medical College of Wisconsin.
Abbreviations
- MPs
microparticles
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- SA
Streptavidin Alexafluor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures MCL — Has submitted an invention disclosure to the institutional technology transfer office regarding targeting of MPs using duramycin.
MZ — No conflicts of interest
TJK — No conflicts of interest
JEW — No conflict of interest
CAH — Consultant for Bayer Pharmaceuticals
References
- [1].Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010;107:1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- [2].Amabile N, Rautou P-E, Tedgui A, Boulanger CM. Microparticles: key protagonists in cardiovascular disorders. Semin. Thromb. Hemost. 2010;36:907–916. doi: 10.1055/s-0030-1267044. [DOI] [PubMed] [Google Scholar]
- [3].Tushuizen ME, Diamant M, Sturk A, Nieuwland R. Cell-derived microparticles in the pathogenesis of cardiovascular disease: friend or foe? Arterioscler. Thromb. Vasc. Biol. 2011;31:4–9. doi: 10.1161/ATVBAHA.109.200998. [DOI] [PubMed] [Google Scholar]
- [4].Rak J. Microparticles in cancer. Semin. Thromb. Hemost. 2010;36:888–906. doi: 10.1055/s-0030-1267043. [DOI] [PubMed] [Google Scholar]
- [5].Zwicker JI. Predictive value of tissue factor bearing microparticles in cancer associated thrombosis. Thromb. Res. 2010;125(Suppl 2):S89–91. doi: 10.1016/S0049-3848(10)70022-0. [DOI] [PubMed] [Google Scholar]
- [6].Meziani F, Delabranche X, Asfar P, Toti F. Bench-to-bedside review: Circulating microparticles - a new player in sepsis? Crit Care. 2010;14:236. doi: 10.1186/cc9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pankoui Mfonkeu JB, Gouado I, Fotso Kuaté H, Zambou O, Amvam Zollo PH, Grau GER, Combes V. Elevated cell-specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS ONE. 2010;5:e13415. doi: 10.1371/journal.pone.0013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morel O, Jesel L, Freyssinet J-M, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler. Thromb. Vasc. Biol. 2011;31:15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- [9].György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, Nagy G, Falus A, Buzás EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life. Sci. 2011;68:2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fadeel B, Xue D. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 2009;44:264–77. doi: 10.1080/10409230903193307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lhermusier T, Chap H, Payrastre B. Platelet membrane phospholipid asymmetry: from the characterization of a scramblase activity to the identification of an essential protein mutated in Scott syndrome. J. Thromb. Haemost. 2011;9:1883–1891. doi: 10.1111/j.1538-7836.2011.04478.x. [DOI] [PubMed] [Google Scholar]
- [12].Emoto K, Inadome H, Kanaho Y, Narumiya S, Umeda M. Local change in phospholipid composition at the cleavage furrow is essential for completion of cytokinesis. J. Biol. Chem. 2005;280:37901–37907. doi: 10.1074/jbc.M504282200. [DOI] [PubMed] [Google Scholar]
- [13].Harrison RAP, Gadella BM. Bicarbonate-induced membrane processing in sperm capacitation. Theriogenology. 2005;63:342–351. doi: 10.1016/j.theriogenology.2004.09.016. [DOI] [PubMed] [Google Scholar]
- [14].Zhao M. Lantibiotics as probes for phosphatidylethanolamine. Amino Acids. 2009;41:1071–1079. doi: 10.1007/s00726-009-0386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Key NS. Analysis of Tissue Factor Positive Microparticles. Thromb Res. 2010;125:S42–S45. doi: 10.1016/j.thromres.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Connor DE, Exner T, Ma DDF, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb. Haemost. 2010;103:1044–1052. doi: 10.1160/TH09-09-0644. [DOI] [PubMed] [Google Scholar]
- [17].Smirnov MD, Ford DA, Esmon CT, Esmon NL. The effect fo membrane composition on the hemostatic balance. Biochemistry. 1999;38:359–8. doi: 10.1021/bi982538b. [DOI] [PubMed] [Google Scholar]
- [18].Bosch I, Dunussi-Joannopoulos K, Wu RL, Furlong ST, Croop J. Phosphatidylcholine and phsophatidylethanolmaine behave as substrates of the human MDR1 P-glycoprotein. Biochemistry. 1997;36:5685–94. doi: 10.1021/bi962728r. [DOI] [PubMed] [Google Scholar]
- [19].Iwamoto K, Hayakawa T, Murate M, Makino A, Ito K, Fujisawa T, Kobayashi T. Curvature-dependent recognition of ethanolamine phospholipids by duramycin and cinnamycin. Biophys. J. 2007;93:1608–1619. doi: 10.1529/biophysj.106.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhixin Li, Wells CW, North PE, Kumar S, Duris CB, McIntyre JA, Zhao Ming. Phosphatidylethanolamine at the luminal endothelial surface--implications for hemostasis and thrombotic autoimmunity. Clin. Appl. Thromb. Hemost. 2011;17:158–163. doi: 10.1177/1076029609350620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mckinnon BT. FDA Safety Alert: Hazards of Precipitation Associated With Parenteral Nutrition. Nutrition in Clinical Practice. 1996;11:59–65. doi: 10.1177/011542659601100259. [DOI] [PubMed] [Google Scholar]
- [22].Zebedin E, Koenig X, Radenkovic M, Pankevych H, Todt H, Freissmuth M, Hilber K. Effects of duramycin on cardiac voltage-gated ion channels. Naunyn Schmiedebergs Arch. Pharmacol. 2008;377:87–100. doi: 10.1007/s00210-007-0248-5. [DOI] [PubMed] [Google Scholar]
- [23].Furie B, Zwicker J, LaRocca T, Kos C, Bauer B, Furie B. Tissue factor-bearing microparticles and cancer-associated thrombosis. Haemotologica Reports 2005. 2005;1:5–8. [Google Scholar]
- [24].Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. Journal of Thrombosis and Haemostasis. 2008;6:1517–1524. doi: 10.1111/j.1538-7836.2008.02987.x. [DOI] [PubMed] [Google Scholar]
- [25].Jimenez JJ, Jy W, Mauro LM, Soderland C, Horstman LL, Ahn YS. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb. Res. 2003;109:175–180. doi: 10.1016/s0049-3848(03)00064-1. [DOI] [PubMed] [Google Scholar]
- [26].Hou J, Fu Y, Zhou J, Li W, Xiw R, Cao F, Gilbert GE, Shi J. Lactadherin functions as a probe for phosphatidylserine exposure and as an anticoagulant in the study of stored platelets. Vox. Sang. 2011;100:187–95. doi: 10.1111/j.1423-0410.2010.01375.x. [DOI] [PubMed] [Google Scholar]
- [27].Makino A, Baba T, Fujimoto K, Iwamoto K, Yano Y, Terada N, Ohno S, Sato SB, Ohta A, Umeda M, Matsuzaki K, Kobayashi T. Cinnamycin (Ro 09-0198) promotes cell binding and toxicity by inducing transbilayer lipid movement. J. Biol. Chem. 2003;278:3204–3209. doi: 10.1074/jbc.M210347200. [DOI] [PubMed] [Google Scholar]
- [28].Gao C, Xie R, Yu C, Wang Q, Shi F, Yao C, Xie R, Zhou J, Gilbert GE, Shi J. Procoagulant activity of erythrocytes and platelets through phosphatidylserine exposure and microparticles release in patients with nephrotic syndrome. Thrombosis and Haemostasis. 2012;107 doi: 10.1160/TH11-09-0673. [DOI] [PubMed] [Google Scholar]
- [29].Bratosin D, Mitrofan L, Palii C, Estaquier J, Montreuil J. Novel fluorescence assay using calcein-AM for the determination of human erythrocyte viability and aging. Cytometry A. 2005;66:78–84. doi: 10.1002/cyto.a.20152. [DOI] [PubMed] [Google Scholar]
- [30].Yates KR, Welsh J, Echrish HH, Greenman J, Maraveyas A, Madden LA. Pancreatic cancer cell and microparticle procoagulant surface characterization: involvement of membrane-expressed tissue factor, phosphatidylserine and phosphatidylethanolamine. Blood Coagul. Fibrinolysis. 2011;22:680–687. doi: 10.1097/MBC.0b013e32834ad7bc. [DOI] [PubMed] [Google Scholar]
- [31].Weerheim AM, Kolb AM, Sturk A, Nieuwland R. Phospholipid composition of cell-derived microparticles determined by one-dimensional high-performance thin-layer chromatography. Anal. Biochem. 2002;302:191–198. doi: 10.1006/abio.2001.5552. [DOI] [PubMed] [Google Scholar]
- [32].Biró E, Akkerman JWN, Hoek FJ, Gorter G, Pronk LM, Sturk A, Nieuwland R. The phospholipid composition and cholesterol content of platelet-derived microparticles: a comparison with platelet membrane fractions. J. Thromb. Haemost. 2005;3:2754–2763. doi: 10.1111/j.1538-7836.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- [33].Abid Hussein MN, Böing AN, Biró E, Hoek FJ, Vogel GMT, Meuleman DG, Sturk A, Nieuwland R. Phospholipid composition of in vitro endothelial microparticles and their in vivo thrombogenic properties. Thromb. Res. 2008;121:865–871. doi: 10.1016/j.thromres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- [34].Zhao M, Li Z, Bugenhagen S. 99mTc-labeled duramycin as a novel phosphatidylethanolamine-binding molecular probe. J. Nucl. Med. 2008;49:1345–1352. doi: 10.2967/jnumed.107.048603. [DOI] [PubMed] [Google Scholar]
- [35].Simak J, Gelderman MP. Cell membrane microparticles in blood and blood products: potentially pathogenic agents and diagnostic markers. Transfus Med Rev. 2006;20:1–26. doi: 10.1016/j.tmrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- [36].Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48:180–186. doi: 10.1161/01.HYP.0000231507.00962.b5. [DOI] [PubMed] [Google Scholar]
- [37].Enjeti AK, Lincz LF, Seldon M. Detection and measurement of microparticles: an evolving research tool for vascular biology. Semin. Thromb. Hemost. 2007;33:771–779. doi: 10.1055/s-2007-1000369. [DOI] [PubMed] [Google Scholar]
- [38].Ayers L, Kohler M, Harrison P, Sargent I, Dragovic R, Schaap M, Nieuwland R, Brooks SA, Ferry B. Measurement of circulating cell-derived microparticles by flow cytometry: sources of variability within the assay. Thromb. Res. 2011;127:370–377. doi: 10.1016/j.thromres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- [39].Shi J, Pipe SW, Rasmussen JT, Heegaard CW, Gilbert GE. Lactadherin blocks thrombosis and hemostasis in vivo: correlation with platelet phosphatidylserine exposure. J. Thromb. Haemost. 2008;6:1167–1174. doi: 10.1111/j.1538-7836.2008.03010.x. [DOI] [PubMed] [Google Scholar]
- [40].Wehman AM, Poggioli C, Schweinsberg P, Grant BD, Nance J. The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C elegans embryos. Curr. Biol. 2011;21:1951–1959. doi: 10.1016/j.cub.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.