Abstract
The activity of NK cells is controlled by inhibitory and activating receptors. The inhibitory receptors interact mostly with MHC class I proteins, however, inhibitory receptors such as CD300a, which bind to non-MHC class I ligands, also exist. Recently it was discovered that phosphatidylserine (PS) is a ligand for CD300a and that the interaction between PS expressed on apoptotic cells and CD300a inhibits the uptake of apoptotic cells by phagocytic cells. Whether PS can inhibit NK-cell activity through CD300a is unknown. Here we have generated specific antibodies directed against CD300a and we used these mAbs to demonstrate that various NK-cell clones express different levels of CD300a. We further demonstrated that both CD300a and its highly homologous molecule CD300c bind to the tumor cells equally well and that they recognize PS and additional unknown ligand/s expressed by tumor cells. Finally we showed that blocking the PS-CD300a interaction resulted in increased NK-cell killing of tumor cells. Collectively, we demonstrate a new tumor immune evasion mechanism that is mediated through the interaction between PS and CD300a and we suggest that CD300c, similarly to CD300a, also interacts with PS.
Keywords: CD300, ligand, phosphtidylserine (PS), tumor cell
Introduction
Natural killer (NK) cells represent the third (following B and T cells) largest lymphoid cell population in mammals [1]. The function of NK cells occurs naturally and unlike T or B cells, NK cells do not require sensitization for their activity, although recent reports demonstrates that NK cells possess a certain type of memory [2-5]. NK cells are characterized by the expression of activating and inhibitory receptors that mediate their function [6]. The inhibitory receptors recognizes mainly MHC class I proteins [7, 8], however, inhibitory receptors that interact with proteins other than MHC class I, such as CD300a, also exist [9]. The CD300a molecule contains four ITIM sequences in its cytoplasmic domain. It possesses a single V-like Ig domain that is 80% similar at the amino acid level to another family member, CD300c. However, unlike CD300a, CD300c contains a short cytoplasmic domain that lacks ITIM sequences and also includes a glutamic acid residue in its trans-membrane domain, suggesting an association with an as yet undefined signaling molecule [10-13]. Because of the high similarity between the extracellular portion of CD300a and CD300c none of the commercially available antibodies that are directed against these proteins can discriminated between them [14, 15].
Until recently the ligand/s recognized by CD300a were unknown however, Nakahashi-Oda et al. [16] and Simhadri et al. [17] recently reported that phosphatidylserine (PS) is a ligand for CD300a. PS is a membrane phospholipid that is ubiquitously present in membranes; it is normally asymmetrically distributed in the plasma membrane of mammalian cells so that essentially all of the PS is restricted to the cytosolic surface [18].
During several important biological processes this asymmetry collapses and PS becomes exposed on the cell surface. For example, PS becomes externalized on the cell surface during activation of platelets, during the blood coagulation cascade [19, 20] and during the early stages of apoptosis [18, 21, 22]. The externalization of PS appears to be the signal by which apoptotic cells are recognized and subsequently removed by phagocytes [23-25]. The recognition of PS by a phagocyte cell occurs through several different mechanisms: via direct recognition by members of the TIM family of receptors (TIM-1, TIM-3 and TIM-4) [26-29], BAI1[30] and Stabilin-2 [31] and via indirect recognition by soluble PS-binding molecules including MFG-E8 [32], Gas6 [33] and protein S [34]. Several studies have shown that in the tumor microenvironment there is significant stress imposed on the tumor endothelium by acidity, reactive oxygen species (ROS), and by transient hypoxia, which results in the redistribution of PS to the cell surface [35, 36]. Indeed, expression of PS was detected in gastric carcinoma [37], ovarian carcinoma [38] and melanoma [39].
Here we identified a new tumor immune evasion mechanism that is based on the inhibition of NK-cell activity through the CD300a-PS interaction.
Results
Specific recognition of CD300a by newly generated mAbs
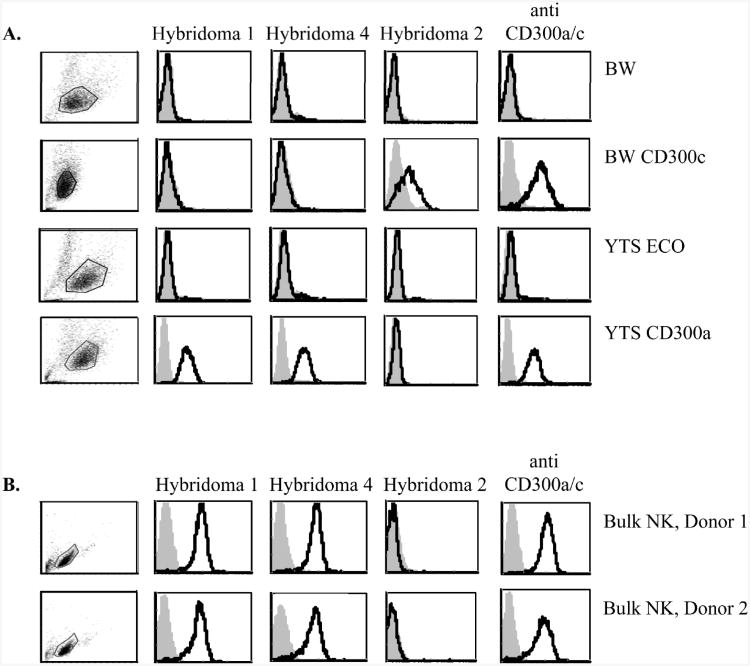
Currently there is no mAb able to discriminate between CD300a and CD300c (data not shown and [14, 15]). Therefore, to study the function of CD300a and CD300c we generated specific anti-CD300a and CD300c antibodies. Mice were immunized with fusion proteins that include the extracellular portions of CD300a and CD300c proteins fused to human IgG1 and hybridomas were generated according to standard techniques. To test the mAb specificity we stained YTS cells transfected to express CD300a, BW cells transfected to express CD300c and the corresponding parental cell lines (that are negative for CD300a and CD300c, Figure 1A) with three hybridomas (for an unknown reason we could not obtained transfectants of YTS cells expressing CD300c or tranfectants of BW cells expressing CD300a and therefore we screened for the expression of CD300a and CD300c on two different cell lines). As can be seen in Figure 1A, Hybridoma 1 and Hybridoma 4 specifically recognize CD300a and not CD300c, while Hybridoma 2 specifically recognizes CD300c.
Figure 1. Specific recognition of CD300a by newly generated hybridomas.
(A, B) FACS analysis of (A) BW cells, BW expressing CD300c, YTS ECO cells and YTS ECO expressing CD300a cells and of (B) bulk NK-cell cultures derived from two different donors. The various hybridomas are indicated above the histograms. The anti-CD300a/c antibody recognizes both CD300a and CD300c. Staining is indicated by black-line histogram and background (filled gray histogram) is the staining with allophycocyanin-conjugated F(ab')2 goat anti-mouse IgG only. The gating strategy is shown in the left part of the figure. Data shown are representative of one out of three independent experiments performed.
We next stained primary bulk NK-cell cultures derived from various donors with the different antibodies and as can be seen in Figure 1B while all bulk NK cells were stained with the CD300a-specific mAbs; Hybridoma 1 and Hybridoma 4, no staining was detected with Hybridoma 2 (Figure 1B and data not shown). We have therefore continued our research with the CD300a specific Hybridomas 1 and 4.
Anti-CD300a antibodies inhibit NK-cell killing in re-directed cytotoxicity assays
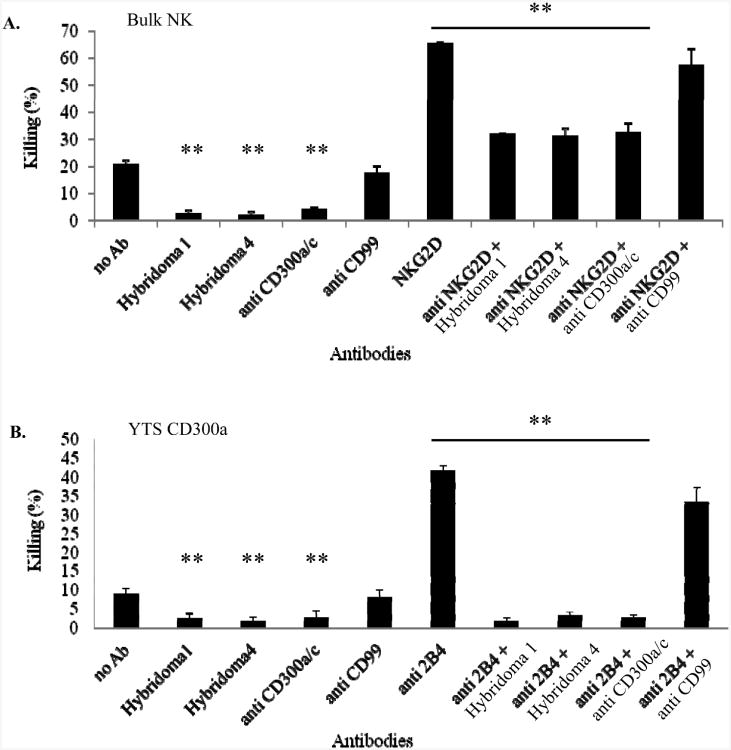
To test whether the triggering of CD300a by Hybridomas 1 and 4 will inhibit NK-cell cytotoxicity we performed redirected killing assays (Figure 2). Primary bulk NK cells (Figure 2A) or YTS CD300a cells (Figure 2B) were incubated with P815 cells that were pre-coated with and without anti-NKG2D mAb (Figure 2A) and anti 2B4 (Figure 2B), in the presence or absence of the Hybridoma 1, Hybridoma 4, Hybridoma that recognizes both CD300a and CD300c (anti CD300a/c, CMRF02.6 [15]) and with anti-CD99 mAb (which was used as a negative control). Basal killing of P815 cells was observed with both the primary bulk NK cultures and the YTS CD300a cells (the identity of the killer receptor/s involved in the killing of P815 cells by NK cells or by YTS is unknown) and this basal killing was inhibited by all anti-CD300a antibodies (Figure 2); the two specific ones Hybridomas 1 and 4 and the anti CD300a/c mAb. Furthermore, the NKG2Dmediated redirected killing of the bulk NK-cell cultures was inhibited by all anti-CD300a and anti-CD300a/c antibodies (Figure 2A). Similar results were obtained when the redirected killing of the bulk NK-cell cultures was induced by other antibodies such as anti-CD16 and anti-2B4 (data not shown).
Figure 2. The new CD300a hybridomas inhibit Bulk NK re-directed killing.
(A) Bulk NK cells and (B) YTS CD300a cells were incubated with P815 cells that were pre-coated with the various antibodies (indicated in the x axis). The E:T ratio was 3:1 for the bulk NK and 20: 1 for the YTS CD300a cell line. Data are shown as means percentage and +SD of three replicates and are representative of one out of three independent experiments performed. **p≤0.02, t-test.
The killing of the YTS cell line is executed mainly by the 2B4 receptor [40] and as can be seen in Figure 2B the 2B4-mediated redirected killing of YTS CD300s cells was also inhibited by all antibodies apart from the control anti-CD99 mAb.
Human NK-cell clones express various levels of CD300a
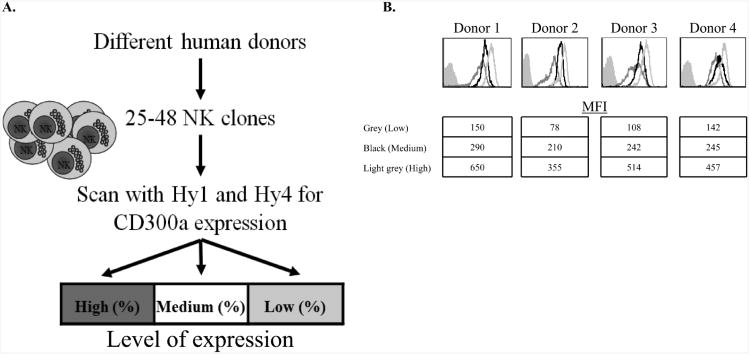
To study the clonal distribution of CD300a we grew NK clones derived from 4 different donors at various time points over a period of one year (between 25-48 NK-cell clones were obtained from each donor, Figure 3A). The different NK clones expressed CD300a at various levels and we thus divided them into 3 groups based on the levels of CD300a expression (Figure 3A). NK-cell clones expressing CD300a at levels below Median Fluorescence Intensity (MFI) of 200 were considered low expressing, clones expressing CD300a in an MFI of 200-300 were considered medium and clones expressing CD300a in an MFI higher than 300 MFI were considered high. The percentages of NK-cell clones in each group are indicated in Table 1 and examples of the expression of CD300a on the various clones are shown in Figure 3B.
Figure 3. CD300a exhibit clonal pattern of expression on different NK-cell clones.
(A) Schematic representation of the experimental procedure. (B) Representative staining of the low (dark gray line histogram) medium (black line histogram) and high (light gray line histogram) clones from each donor. Background (filled gray histogram) is the staining with allophycocyanin-conjugated F(ab')2 goat anti-mouse IgG only.
Table 1. Three levels of expression of CD300a on NK cellsa.
| Level of expression | High (%) | Medium (%) | Low (%) |
|---|---|---|---|
| Donor1 | 36.67 | 10 | 53.33 |
| Donor1 | 10.42 | 52.08 | 37.5 |
| Donor1 | 44.44 | 48.15 | 7.41 |
| Donor1 | 85.71 | 10.71 | 3.57 |
| Donor2 | 6.98 | 34.88 | 58.14 |
| Donor2 | 22.22 | 66.67 | 11.11 |
| Donor3 | 54.17 | 31.25 | 14.58 |
| Donor3 | 19.05 | 57.14 | 23.81 |
| Donor4 | 13.04 | 58.7 | 28.26 |
| Donor4 | 18.18 | 72.73 | 9.09 |
Summary of the percentages of clones expressing CD300a derived from four different donors at various time points during a 1-year period. NK-cell clones were divided into three groups based on the levels of CD300a expression.
As can be seen in Table 1 the expression of CD300a varies significantly among different donors. For example, 13.04% and 18.18% of the NK-cell clones of donor 4 (NK cells from this donor were isolated at two time points during the one-year period of this study) expressed CD300a at high levels, while at same time point 54.17% of NK cells derived from donor 3 and 85% of NK cells derived from donor 1 expressed CD300a at high levels.
Furthermore, even within the same donor the expression levels of CD300a vary significantly, depending on the time in which they were isolated. For example, at some point 85.71% of donor 1 NK cells expressed CD300a at high levels while in other time point only 10.42% of the NK-cell clones of donor 1 expressed CD300a at high levels (Table 1).
Tumor cells express a trypsin sensitive ligand for CD300
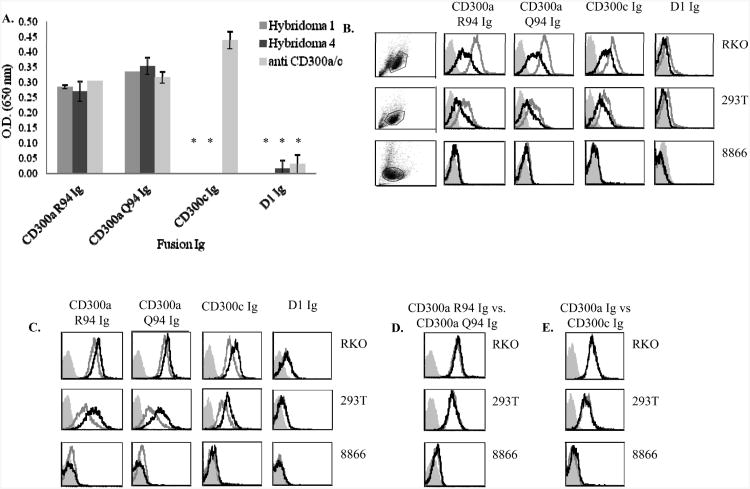
A single nucleotide polymorphism (SNP) in CD300a which encodes for a nonsynonymous mutation in the IgV-like domain (the corresponding amino acids are either Argenin (R) or Glutamine (Q)) has been linked to susceptibility to psoriasis [17, 41]. To test whether CD300a variants having either R94 or Q94 in CD300a will be differentially recognized by Hybridomas 1 and Hybridoma 4 we constructed fusion proteins in which the extracellular portion of CD300a containing either R (CD300a R94 Ig) or Q (CD300a Q94 Ig) was fused to human IgG1. These fusion proteins were used in ELISA assays for recognition by the different anti-CD300a Hybridomas. As can be seen in Figure 4A, all mAbs tested; hybridomas 1 and 4 and the anti-CD300a/c antibody recognized the CD300a R94 Ig and CD300a Q94 Ig proteins equally well. CD300c Ig was recognized by the anti-CD300a/c mAb only, whereas the control D1 Ig fusion protein was not recognized by any of the mAb tested (Figure 4A).
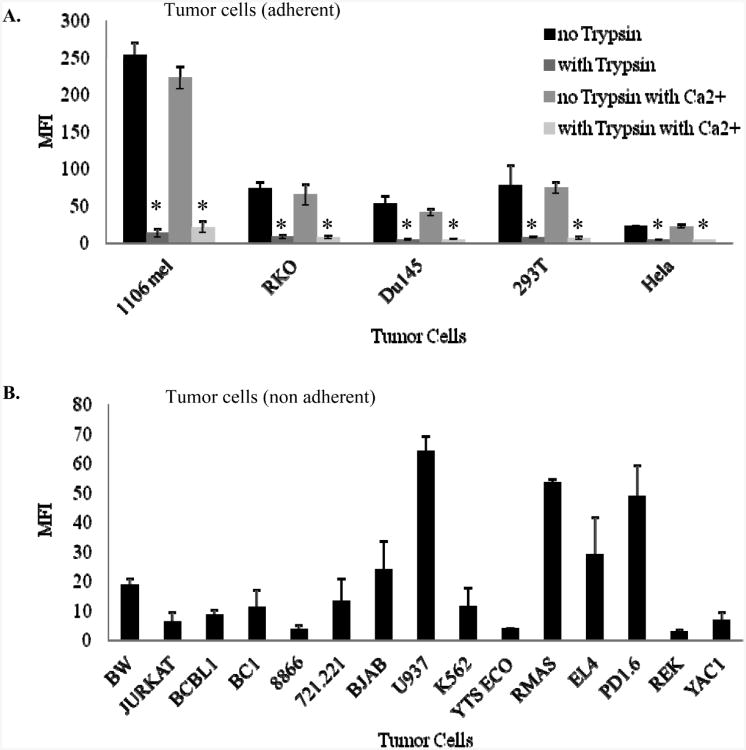
Figure 4. Tumor cells express a trypsin sensitive ligand for CD300.
(A) Fusion proteins (indicated in the X axis) were incubated on ELISA plates and ELISA assays were performed with the indicated mAbs. Data are shown as means and ± SD of three replicates and are representative out of three independent experiments performed. **p≤0.004, ***p≤0.001, t- test. (B) 8866, RKO and 293T cells were harvested with or without trypsin (black and grey line histogram respectively) and FACS staining was performed. Background (filled gray histogram) is the staining with allophycocyanin-conjugated F(ab')2 goat anti-human IgG only. (C) The various tumor cells indicated in the figure were harvested without trypsin and treated with medium with or without calcium (black and grey open histograms respectively). Background (filled gray histogram) is the staining with allophycocyanin-conjugated F(ab')2 goat anti-human IgG only. (D) Binding of CD300a R94 Ig and CD300a Q94 Ig (black and grey open histogram respectively) to various tumor cell lines. (E) Binding of CD300a Ig and CD300c Ig (black and grey open histogram respectively) to various tumor cell lines. (B-D) Data shown are representative of one out of three independent experiments performed.
It was shown recently that PS is a ligand for CD300a [16 ]. To test whether tumor cells such as RKO, 293T and 8866 cells which serves as targets for NK cells express a ligand for CD300a and CD300c we stained them with the two CD300a fusion proteins, (containing either R94 or Q94), with CD300c Ig and with the control D1 Ig. The adherent cells RKO and 293T were harvested with and without trypsin. As can be seen in Figure 4B both adherent cells expressed a ligand for CD300a (as they were recognized by CD300a Ig containing R94 or Q94) and for CD300c (since they were recognized by CD300c Ig), whereas the non-adherent cells 8866 did not express ligand/s for these receptors. Interestingly, following trypsin treatment the expression of the CD300a/c ligand/s was significantly reduced (Figure 4B).
It was demonstrated that the binding of CD300a to PS is calcium dependent [17]. Therefore, we incubated the various tumors indicated in Figure 4C in medium with and without calcium and observed an enhancement in the binding of all fusion proteins except from the control Ig fusion protein, D1 Ig. The binding of CD300a R94 Ig and CD300a Q94 Ig to tumor cells was very similar, almost identical (Figure 4D), regardless of whether the cells were treated with or without trypsin and with or without calcium. Interestingly, the binding of CD300c was also very similar to that of CD300a (Figure 4E) and it remained similar in the presence or in the absence of the various treatments.
Tumor cells express PS
As previous reports have shown that PS is a ligand for CD300a [16, 17] we next tested whether PS is expressed by tumor cells and as can be seen in Figure 5, it is indeed expressed by both adherent (Figure 5A) and non-adherent (Figure 5B) cells. The expression of PS is variable and some adherent cells express PS at low levels, while others PS at high levels (Figure 5A). A similar picture is observed with regard to the non-adherent cells (Figure 5B). Furthermore, some mouse cells (such as RMAS, EL4, PD1.6) also express PS, while others (such as YAC1) are PS negative (Figure 5B). All cells that express PS at high levels were recognized by CD300a Ig and CD300c Ig (data not shown).
Figure 5. Tumor cell lines express Phosphatidylserine (PS).
Various tumor cell lines, (A) adherent and (B) non-adherent were stained with anti-PS antibody. (A) The adherent cells were harvested with or without trypsin and incubated with medium with or without calcium. The MFI average of three independent experiments is shown as means and ± SD. * p≤0.01, t-test.
Since we have demonstrated above that the expression of the tumor ligand/s for CD300a is trypsin-sensitive and calcium-dependent we have next tested whether the expression of PS will be influenced by these treatments. Adherent cells were harvest with or without trypsin and as can be seen in Figure 5A the levels of PS were significantly reduced following trypsin treatment. The calcium treatment did not affect the expression of PS either on the adherent cells (Figure 5A), or on the non-adherent cells (data not shown), suggesting that calcium is probably important for the CD300a and CD300c binding to PS and not for the expression of PS itself.
PI-negative tumor cells are recognized by CD300a and CD300c
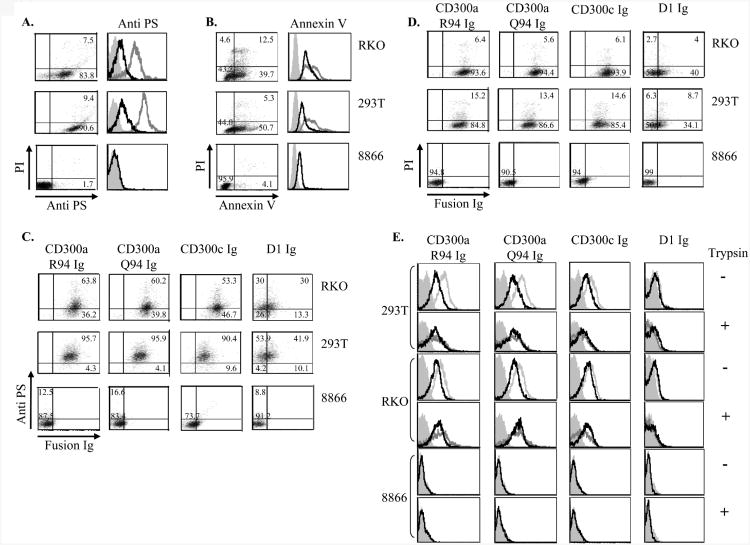
It was reported that PS is expressed on dying cells and that it inhibits the engulfment of the apoptotic cells by macrophages [17]. To test whether the tumor cells that are recognized by CD300a are apoptotic we double stained the tumor cells with anti-PS and with propidium iodide (PI). As can be seen in Figure 6A both 293T and RKO cells lines express PS and are recognized by CD300a Ig, however, most of these cells were PI-negative, indicating that these cells are not found in a late apoptotic stage. The 8866 cell line which was not recognized by CD300a Ig did not express PS (Figure 6A). Thus, we concluded that either these cells are always a bit apoptotic or that alternatively PS is expressed by these tumor cells naturally, regardless of their apoptotic status.
Figure 6. CD300a and CD300c interact with PS expressed by tumor linese.
(A) The indicated tumor cells were double stained with PI and PS (left). Values in quadrants indicate percentages. FACS analysis of the indicated different tumor cell lines harvested with or without trypsin (black and gray open histogram respectively) and stained with anti-PS mAb (right histograms). Background (filled gray histogram) is the staining with allophycocyanin-conjugated F(ab')2 goat anti-mouse IgG only. (B) The indicated tumor cells were double stained with PI and with AnnexinV (left). Values in quadrants indicate percentages. FACS analysis of the indicated tumor cell lines harvested with or without trypsin (black and gray open histogram respectively) and stained with Annexin V (right). (C, D) The various tumor cells indicated in the figure were double stained with anti-PS (C) or with PI (D) together with the indicated fusion proteins. The numbers in the figure indicate percentages. (E) The various tumor cells (each tumor cell line is represented by two histogram rows) were treated without trypsin (-, upper histogram row in each tumor cell line) or with Trypsin (+ lower histogram row in each tumor cell line) incubated with or without 0.5 μg MGF-E8 (black and gray open histogram respectively) and then incubated with the indicated fusion protein. Data shown are representative of one out of three independent experiments performed.
Another reason accounting for the expression of PS on RKO and on 293T cells might be that the anti-PS antibody we used is not specific to PS only. Therefore, we confirmed the PS expression on the tumor cells by using Annexin V. As can be seen in Figure 6B, PS is indeed express by RKO and by 293T cells as they were stained by Annexin V, while 8866 cells were negative.
We showed above (Figures 4 and 5) that the binding of the anti-PS mAb to the tumor cells was reduced by trypsin treatment. Therefore, we treated the tumor cells with trypsin, stained them with Annexin V and observed that the Annexin V binding was reduced upon trypsin treatment (Figure 6B, right). The 8866 cells were sometimes recognized by Annexin V (Figure 6B, right). However, because the 8866 cells were never recognized by the anti-PS antibody and because the Annexin V staining was not reduced following trypsin treatment (Figure 6B, right) we considered this binding to 8866 cells non-specific.
To further study the CD300a Ig and CD300c Ig binding to tumor cells we next performed double staining of the tumor cells with PS together with CD300a Ig and CD300c Ig. As can be seen in Figure 6C most of the PS-positive cells were recognized by CD300a and CD300c Ig. However, staining of CD300a Ig and CD300c Ig was detected also in a subset of PS-negative cells (especially in RKO, Figure 6C), suggesting that CD300a and CD300c recognize additional ligand/s other than PS that are expressed on RKO and 293T cells. Finally, we showed that the RKO and 293T cells that were recognized by CD300a and CD300c Ig are not found in the late apoptotic stage as they were almost entirely PI-negative (Figure 6D).
To demonstrate directly that CD300a Ig and CD300c Ig interact with PS we blocked the PS recognition by using MGF-E8 that was previously shown to block the CD300a-PS interaction on macrophages [16, 32]. Importantly, following incubation of RKO and 293T cells with MGF-E8, the binding of CD300a Ig and CD300c Ig was reduced (Figure 6E), indicating that both proteins interact with PS. When RKO and 293T cells were treated with trypsin, the binding of CD300a Ig and CD300c Ig was reduced but still detected and this binding could not be blocked with MGF-E8 (Figure 6E). No binding of CD300a Ig or CD300c Ig was detected to 8866 cells in the presence or in the absence of MGF-E8, with or without trypsin (Figure 6E). Thus, we concluded that both CD300a and CD300c recognize two different ligands on RKO and 293T cells: 1) a trypsin sensitive ligand PS and 2) a trypsin-insensitive unknown ligand.
Blocking of PS enhanced the killing of tumor cells by NK cells
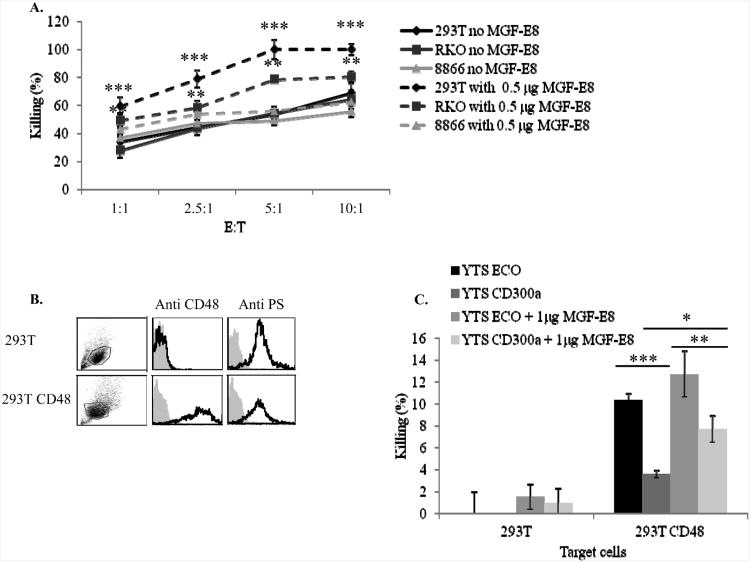
We next performed NK-cell cytotoxicity assays. Because both anti-CD300a mAbs that we have generated (Hybridoma 1 and Hybridoma 4) are not blocking antibodies (data not shown) we blocked the interaction of CD300a with PS by using MGF-E8. As can be seen in Figure 7A, when PS was blocked the killing of the cells expressing PS (RKO and 293T) was significantly enhanced, while the killing of the PS-negative cell line, 8866 remained unchanged, suggesting that the interactions between CD300a and PS indeed inhibits NK-cell cytotoxicity .
Figure 7. Blocking of PS enhanced the killing of tumor cells by NK cells.
(A) The indicated cells were incubated with or without 0.5 μg/well of MGF-E8 and then incubated with bulk NK-cell cultures at different E:T ratios. **p≤ 0.01, ***p≤ 0.0006, t-test. (B) 293T and 293T CD48 were stained with anti CD48 and anti-PS antibody (indicated above the histograms). The black line histogram represents the staining with the appropriate mAb, while the gray filled histogram is the background staining with allophycocyanin-conjugated F(ab')2 goat anti-mouse IgG only. The gating strategy is shown in the left part of the figure. (C) The parental YTS ECO cells and YTS CD300a cells were incubated with 293T or 293T CD48 in the presence or absence of 1 μg MGF-E8. The E:T ratio was 20:1. *p≤ 0.05, **p≤ 0.04, ***p≤ 0.008, t-test. Data are shown as means percentage and ± SD of three replicate and are representative of one out of three independent experiments performed.
The enhancement of NK-cell killing following MGF-E8 blocking can result from the interaction of PS with receptors other than CD300a that are present on human NK cells. Therefore, to demonstrate directly that the interaction between CD300a and PS inhibits tumor cell killing we used the YTS cell line. As mentioned above, the killing of target cells by YTS cells is mediated via the 2B4-CD48 interactions. Because both RKO and 293T cells are CD48 negative (Figure 7B) we expressed the CD48 protein in 293T cells and verified that the expression of PS was similar to the parental 293T cells (Figure 7B). Next we performed killing assays using YTS cells and the various 293T cells in the presence and in the absence of MGF-E8. As can be seen in Figure 7C in the absence of CD48 the parental 293T cells were not killed by YTS cells. In contrast, a moderate killing was executed by the parental YTS ECO cells against 293T CD48 cells and this moderate killing was significantly reduced when YTS CD300a were used. Importantly, the reduction in YTS CD300a killing was partially dependent on PS as incubation of 293T CD48 cells with MGF-E8 partially restored the killing by YTS CD300a cells (Figure 7C).
Discussion
The CD300 family consists of seven protein members that coordinate leukocyte responses [42]. Members of the human CD300 family have broad expression patterns and the function of the various members is largely unknown [43].
We started this research by generating two specific anti-CD300a antibodies and one specific mAb against CD300c. The anti-CD300c mAb specifically recognized the CD300c-Ig fusion and transfectants expressing CD300c, however it did not recognize CD300c on NK cells. One possible explanation for discrepancy is that on the surface of the NK cells the epitope that is recognized by our anti-CD300c mAb is masked by another protein, maybe the one that is associated with it charged amino acid that is present in the trans-membrane domain of the receptor.
We showed that NK cells can be divided into three groups based on the levels of CD300a expression, and that the percentages of such clones vary significantly among various individuals and even when the NK cells are taken from the same donor at various time points (the NK-cell clones were grown during the study period using the exact same conditions and staining were performed at exactly the same time following NK-cell isolation). Alternatively, it is possible that the levels of CD300a expression on the different NK clones might result from different cloning efficiencies. It should be noated however that no significance change at the expression of CD300a over time was observed when bulk NK cells were stained at various time points following their isolation (data not shown). It will be interesting to characterize in the future the expression pattern of CD300a on NK cells derived from various tissues such as lymph nodes, lungs, decidua and liver and to test which factors affect the levels of expression of CD300a.
Only recently it was found that PS interacts with CD300a and that this leads to inhibition of phagocytosis of apoptotic cells [17 ]. Our data support the above observations as we showed that the binding of CD300a-Ig to tumor cells is reduced when PS is blocked and that the blocking of PS enhances NK-cell-mediated cytotoxicity. Blocking of PS only partially blocked the binding of CD300a to tumor cells and only partially restored NK-cell cytotoxicity, indicating that tumor cells express an additional ligand for CD300a. Furthermore, we showed that the other ligand for CD300a expressed on tumor cells have different biochemical properties than that of PS as it was trypsin insensitive.
This additional ligand might be phosphatidylethanolamine (PE) which was also shown to be a ligand for CD300a [17]. We have tried to investigate this option and to study the function PE with regard to NK-cell activity. Because antibodies against PE are unavailable, we used Durmycin in order to block the PE interactions with CD300a; however, unfortunately, the drug was toxic to the cells.
The realization that CD300a present on NK cells interacts with PS might help in the discovery of new functions and interactions between NK cells and other cells. Bone marrow cells for example express high levels of PS as a part of the bone assembling and disassembling processes [44]. PS also plays a major role in coagulation and platelets express high level of PS following activation [18, 45].
We demonstrated here that tumor cell lines are protected from NK-cell-mediated cytotoxicity via the CD300a-PS interactions and that PI-negative tumor cells express PS. Our results are supported by previous findings showing that tumor cells exhibit elevated expression of PS in the outer leaflet of the cell membrane [37, 46, 47].
The CD300a protein has two isoforms (R94 and Q94) and we showed here that the tumor ligand/s for CD300a and CD300c is recognized equally well by both CD300a isoforms and by CD300c. In contrast, Simhadri et al. [17] demonstrated that CD300 R94 isoform binds PS at a higher affinity than that of the Q94 isoform. We think that the reasons for these differences derived from the different systems used to evaluate the binding of the various isoforms. Simhadri et al. used primarily SPR analysis and ELISA in which PS and PE containing liposomes were used and we have used cell culture systems. Indeed, when the CD300a isoforms were used by Simhadri et al. to stain dead PBLs the differences were minor [17].
We further showed that the tumor ligand/s for CD300a and also CD300c are sensitive to trypsin and since PS is a lipid we wondered why it is influenced by the trypsin proteases. Membrane lipid sidedness originates from vectorial biosynthesis of lipids in combination with transporter proteins that move lipids from one side of the membrane to the other [48, 49]. Thus, if PS is found on the cell membrane attached to these chaperons it will be trypsin sensitive. Indeed, several studies reported on a connection between membrane proteins and PS. For example, a link between Glycophorin-C (GPC) binding (ligation) and PS expression on erythrocytes has been suggested by its appearance on P. falciparum-infected erythrocytes [50]. In addition it was shown that human gastric carcinoma cells which over expressing BCRP (an ABC transporter) also overexpress PS and that treating these cells with Tryprostatin A decreased the expression of PS [37]. We demonstrated here that CD300c has binding properties similar to CD300a: the CD300c binding to tumor cells was trypsin-sensitive and calcium-dependent, the binding was correlated with expression of PS and in all cases the intensity of CD300c binding was almost identical to that CD300a. Finally, we demonstrated that treating the tumor cells with MGF-E8 diminished the binding of CD300c Ig. Thus, we suggest that by similarly to CD300a, CD300c also interacts with PS.
Materials and methods
Ethics Statement
The NK cells that were used in this study were obtained from the blood of healthy voluntaries. The intuitional Helsinki committee of Hadassah approved the study (Helsinki number 0030-12-HMO). All subjects provided a written informed consent.
Reagents, mAbs and cells
The anti CD300a: Hybridoma 1 and Hybridoma 4, anti CD300a/c (CMRF2.06) and anti CD300c Hybridoma 2. Hybridomas were generated using standard protocols through the injection of CD300a-Ig and CD300c-Ig fusion proteins into mice. The following reagents were used AnnexinV- FITC (IQ Products) and Milk fat globule EGF factor 8 (MGF-E8, R&D systems). The cell lines used in the present study were the mouse BW cells, BW transfected with CD300c, YTS cells, transfected with either the ecotropic murine retrovirus receptor alone (YTS ECO) or with the ecotropic receptor and CD300a (YTS CD300a) [15, 51]. Additional tumor cell lines used in the present study were RKO, 293T and 8866.
Ig-fusion proteins
The following Ig fusion proteins; CD300a R94-Ig, CD300a Q94-Ig CD300c-Ig, and D1-Ig, were generated as described previously [15]. The CD300a R94 isoform was cloned from the CIR cell line cDNA and CD300a Q94 isoform was cloned from bulk NK cell cDNA.
Cytotoxicity assays
For the redirected killing assay bulk NK or YTS cells were added to 35S-labeled P815 cells that were pre-coated with 0.2 μg/well of the various mAbs. The level of cytotoxicity was determined as previously described [52]. The cytotoxic activity of primary NK cells against the various targets were assessed in 5-h 35S release assays, as previously described. To block the PS-CD300a interaction tumor cells were incubated with or without 0.5-1 μg/well of MFG-E8(R&D Systems) for 30min at RT and then incubated with the bulk NK or with YTS cells.
Flow cytometry of tumor cells
Flow cytometry assays were performed as previously described [15]. Cells were harvest with or without trypsin and treated with or without calcium. Cells were incubated with calcium containing solution (10 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) pH 7.4, 140 mM NaCl, 2.5mM CaCl2, and 2 mM MgCl2) in RT for 15 minutes and then washed. Blocking with MGF-E8 was performed as followed: cells were incubated with 0.5 μg/well MGF-E8 for 30 min at RT. Next cells were incubated with 0.3 μg of fusion proteins for 30 min at RT, washed and stained with a secondary allophycocyanin-conjugated F(ab')2 goat anti-human antibodies (Jackson Immunoresearch Laboratories). Analysis of Annexin V (IQ products) binding was performed according to manufacture instructions.
Statistical analysis
T-test was used for the statistical analysis.
Acknowledgments
This study was supported by grants from the Israeli Science Foundation, The Croatia Israel Research Grant, by the Rosetrees trust, by the ICRF professorship grant by the Israeli ICORE, by the Association for International Cancer Research (AICR) and by the ERC advanced grant all to OM and by NIH grant 1R01AI083201-01 to S. Jonjic. O.M is a Crown professor of Molecular Immunology.
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703–714. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 2.Pyzik M, Vidal SM. Natural killer cells: NK cells stroll down the memory lane. Immunol Cell Biol. 2009;87:261–263. doi: 10.1038/icb.2009.10. [DOI] [PubMed] [Google Scholar]
- 3.Biron CA. Expansion, maintenance, and memory in NK and T cells during viral infections: responding to pressures for defense and regulation. PLoS Pathog. 2010;6:e1000816. doi: 10.1371/journal.ppat.1000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun JC, Lanier LL. Versatility in NK cell memory. Immunol Cell Biol. 2011;89:327–329. doi: 10.1038/icb.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune “memory”. J Immunol. 2011;186:1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmer J. Natural Killer Cells: At the Forefront of Modern Immunology Springer. 2009 [Google Scholar]
- 7.Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M, Bottino C, et al. Human natural killer cell receptors and co-receptors. Immunol Rev. 2001;181:203–214. doi: 10.1034/j.1600-065x.2001.1810117.x. [DOI] [PubMed] [Google Scholar]
- 8.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebbink RJ, Meyaard L. Non-MHC ligands for inhibitory immune receptors: novel insights and implications for immune regulation. Mol Immunol. 2007;44:2153–2164. doi: 10.1016/j.molimm.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Clark GJ, Fitzpatrick S, Kuo B, Modra C, Jamriska L, Hart DN. CMRF-35A, CMRF-35H: potential new CD. J Biol Regul Homeost Agents. 2002;16:233–235. [PubMed] [Google Scholar]
- 11.Clark GJ, Cooper B, Fitzpatrick S, Green BJ, Hart DN. The gene encoding the immunoregulatory signaling molecule CMRF-35A localized to human chromosome 17 in close proximity to other members of the CMRF-35 family. Tissue Antigens. 2001;57:415–423. doi: 10.1034/j.1399-0039.2001.057005415.x. [DOI] [PubMed] [Google Scholar]
- 12.Clark GJ, Ju X, Azlan M, Tate C, Ding Y, Hart DN. The CD300 molecules regulate monocyte and dendritic cell functions. Immunobiology. 2009;214:730–736. doi: 10.1016/j.imbio.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Clark GJ, Green BJ, Hart DN. The CMRF-35H gene structure predicts for an independently expressed member of an ITIM/ITAM pair of molecules localized to human chromosome 17. Tissue Antigens. 2000;55:101–109. doi: 10.1034/j.1399-0039.2000.550201.x. [DOI] [PubMed] [Google Scholar]
- 14.Green BJ, Clark GJ, Hart DN. The CMRF-35 mAb recognizes a second leukocyte membrane molecule with a domain similar to the poly Ig receptor. Int Immunol. 1998;10:891–899. doi: 10.1093/intimm/10.7.891. [DOI] [PubMed] [Google Scholar]
- 15.Lankry D, Simic H, Klieger Y, Levi-Schaffer F, Jonjic S, Mandelboim O. Expression and function of CD300 in NK cells. J Immunol. 2010;185:2877–2886. doi: 10.4049/jimmunol.0903347. [DOI] [PubMed] [Google Scholar]
- 16.Nakahashi-Oda C, Tahara-Hanaoka S, Honda S, Shibuya K, Shibuya A. Identification of phosphatidylserine as a ligand for the CD300a immunoreceptor. Biochem Biophys Res Commun. 2012;417:646–650. doi: 10.1016/j.bbrc.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Simhadri VR, Andersen JF, Calvo E, Choi SC, Coligan JE, Borrego F. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood. 2012;119:2799–2809. doi: 10.1182/blood-2011-08-372425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vance JE, Steenbergen R. Metabolism and functions of phosphatidylserine. Prog Lipid Res. 2005;44:207–234. doi: 10.1016/j.plipres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Bevers EM, Comfurius P, Zwaal RF. Changes in membrane phospholipid distribution during platelet activation. Biochim Biophys Acta. 1983;736:57–66. doi: 10.1016/0005-2736(83)90169-4. [DOI] [PubMed] [Google Scholar]
- 20.Zwaal RF, Comfurius P, Bevers EM. Lipid-protein interactions in blood coagulation. Biochim Biophys Acta. 1998;1376:433–453. doi: 10.1016/s0304-4157(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 21.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. 2010 doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bratton DL, Henson PM. Apoptotic cell recognition: will the real phosphatidylserine receptor(s) please stand up? 2008 doi: 10.1016/j.cub.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 24.Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem. 1997;272:26159–26165. doi: 10.1074/jbc.272.42.26159. [DOI] [PubMed] [Google Scholar]
- 25.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 26.DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim YL, Lee HH, Karisola P, et al. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J Immunol. 2010;184:1918–1930. doi: 10.4049/jimmunol.0903059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santiago C, Ballesteros A, Martinez-Munoz L, Mellado M, Kaplan GG, Freeman GJ, Casasnovas JM. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 30.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 31.Park SY, Jung MY, Kim HJ, Lee SJ, Kim SY, Lee BH, Kwon TH, et al. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15:192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 32.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 33.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, Shacter E. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat Immunol. 2003;4:87–91. doi: 10.1038/ni871. [DOI] [PubMed] [Google Scholar]
- 35.Zulueta JJ, Yu FS, Hertig IA, Thannickal VJ, Hassoun PM. Release of hydrogen peroxide in response to hypoxia-reoxygenation: role of an NAD(P)H oxidase-like enzyme in endothelial cell plasma membrane. Am J Respir Cell Mol Biol. 1995;12:41–49. doi: 10.1165/ajrcmb.12.1.7529030. [DOI] [PubMed] [Google Scholar]
- 36.Stafford JH, Thorpe PE. Increased exposure of phosphatidylethanolamine on the surface of tumor vascular endothelium. Neoplasia. 2011;13:299–308. doi: 10.1593/neo.101366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woehlecke H, Pohl A, Alder-Baerens N, Lage H, Herrmann A. Enhanced exposure of phosphatidylserine in human gastric carcinoma cells overexpressing the half-size ABC transporter BCRP (ABCG2) Biochem J. 2003;376:489–495. doi: 10.1042/BJ20030886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong HP, Holth A, Kleinberg L, Ruud MG, Elstrand MB, Trope CG, Davidson B, et al. Evaluation of cell surface expression of phosphatidylserine in ovarian carcinoma effusions using the annexin-V/7-AAD assay: clinical relevance and comparison with other apoptosis parameters. Am J Clin Pathol. 2009;132:756–762. doi: 10.1309/AJCPAVFA8J3KHPRS. [DOI] [PubMed] [Google Scholar]
- 39.Kirszberg C, Lima LG, Da Silva de Oliveira A, Pickering W, Gray E, Barrowcliffe TW, Rumjanek VM, et al. Simultaneous tissue factor expression and phosphatidylserine exposure account for the highly procoagulant pattern of melanoma cell lines. Melanoma Res. 2009;19:301–308. doi: 10.1097/CMR.0b013e32832e40fe. [DOI] [PubMed] [Google Scholar]
- 40.Chuang SS, Kim MH, Johnson LA, Albertsson P, Kitson RP, Nannmark U, Goldfarb RH, et al. 2B4 stimulation of YT cells induces natural killer cell cytolytic function and invasiveness. Immunology. 2000;100:378–383. doi: 10.1046/j.1365-2567.2000.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speckman RA, Wright Daw JA, Helms C, Duan S, Cao L, Taillon-Miller P, Kwok PY, et al. Novel immunoglobulin superfamily gene cluster, mapping to a region of human chromosome 17q25, linked to psoriasis susceptibility. Hum Genet. 2003;112:34–41. doi: 10.1007/s00439-002-0851-y. [DOI] [PubMed] [Google Scholar]
- 42.Clark GJ, Ju X, Tate C, Hart DN. The CD300 family of molecules are evolutionarily significant regulators of leukocyte functions. Trends Immunol. 2009;30:209–217. doi: 10.1016/j.it.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Takatsu H, Hase K, Ohmae M, Ohshima S, Hashimoto K, Taniura N, Yamamoto A, et al. CD300 antigen like family member G: A novel Ig receptor like protein exclusively expressed on capillary endothelium. Biochem Biophys Res Commun. 2006;348:183–191. doi: 10.1016/j.bbrc.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 44.Wu LN, Genge BR, Wuthier RE. Analysis and molecular modeling of the formation, structure, and activity of the phosphatidylserine-calcium-phosphate complex associated with biomineralization. J Biol Chem. 2008;283:3827–3838. doi: 10.1074/jbc.M707653200. [DOI] [PubMed] [Google Scholar]
- 45.Lentz BR. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog Lipid Res. 2003;42:423–438. doi: 10.1016/s0163-7827(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 46.Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002;62:6132–6140. [PubMed] [Google Scholar]
- 47.Riedl S, Rinner B, Asslaber M, Schaider H, Walzer S, Novak A, Lohner K, et al. In search of a novel target - phosphatidylserine exposed by non-apoptotic tumor cells and metastases of malignancies with poor treatment efficacy. Biochim Biophys Acta. 2011;1808:2638–2645. doi: 10.1016/j.bbamem.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprong H, van der Sluijs P, van Meer G. How proteins move lipids and lipids move proteins. Nat Rev Mol Cell Biol. 2001;2:504–513. doi: 10.1038/35080071. [DOI] [PubMed] [Google Scholar]
- 49.Zwaal RF, Comfurius P, Bevers EM. Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci. 2005;62:971–988. doi: 10.1007/s00018-005-4527-3. [DOI] [PubMed] [Google Scholar]
- 50.Head DJ, Lee ZE, Poole J, Avent ND. Expression of phosphatidylserine (PS) on wild-type and Gerbich variant erythrocytes following glycophorin-C (GPC) ligation. Br J Haematol. 2005;129:130–137. doi: 10.1111/j.1365-2141.2005.05407.x. [DOI] [PubMed] [Google Scholar]
- 51.Yoneda N, Tatsumi E, Kawano S, Teshigawara K, Oka T, Fukuda M, Yamaguchi N. Detection of Epstein-Barr virus genome in natural-killer-like cell line, YT. Leukemia. 1992;6:136–141. [PubMed] [Google Scholar]
- 52.Mandelboim O, Reyburn HT, Vales-Gomez M, Pazmany L, Colonna M, Borsellino G, Strominger JL. Protection from lysis by natural killer cells of group 1 and 2 specificity is mediated by residue 80 in human histocompatibility leukocyte antigen C alleles and also occurs with empty major histocompatibility complex molecules. J Exp Med. 1996;184:913–922. doi: 10.1084/jem.184.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]