Abstract
Bipolar Disorder is a major cause of disability and a high risk for suicide. The pathophysiology of the disorder remains largely unknown. Medication choice for bipolar depression patients involves trial and error. Our group reported previously that brain serotonin 1A (5HT1A) receptor binding measured by positron emission tomography (PET) is higher in bipolar depression. We now investigated whether pretreatment 5HT1A levels correlates with antidepressant medication outcome. 41 medication-free DSM-IV diagnosed, bipolar patients in a major depressive episode (MDE) had brain PET scans performed using [11C]WAY-100635 and a metabolite corrected arterial input function. The patients then received naturalistic psychopharmacologic treatment as outpatients and a follow up Hamilton Depression Rating Scale (HDRS) after 3 months of treatment. Patients with 24 item HDRS scores less than 10 were considered to have remitted. A linear mixed effects model was used to compare BPF (binding potential, proportional to the total number of available receptors) in 13 brain regions of interest between remitters and non-remitters. 34 patients completed 3 months of treatment and ratings; 9 had remitted. Remitters and non-remitters did not differ in age, sex or recent medication history with serotonergic medications. Remitters had higher [11C]WAY-100635 BPF across all brain regions compared with non-remitters (p=0.02). Higher pre-treatment brain 5HT1A receptor binding was associated with remission after 3 months of pharmacological treatment in bipolar depression. Prospective treatment studies are warranted to determine whether this test predicts outcome of specific types of treatment.
Keywords: Bipolar Disorder, PET, [11C]WAY-100635, Antidepressant, Mood stabilizer
Introduction
Bipolar disorder is the sixth leading cause of disability-adjusted-life-years of all diseases according to the World Health Organization (2002). About 1% of the population meets lifetime criteria for bipolar I disorder and 1.2% for bipolar II disorder (Bauer & Pfennig, 2005; Weissman et al, 1996). It carries an increased risk for suicide, which accounts for approximately 10% of deaths in those with this diagnosis (Harris & Barraclough, 1997). The risk of suicide is elevated during episodes of major depression and mixed mood states, making depression a key risk factor. There is debate about the medication options for treatment of depression, and no laboratory or clinical tools exist for treatment selection. Progress in developing such a laboratory tool is handicapped by the fact that the disease pathophysiology remains largely unknown, and the mechanisms of action of the medications effective as either mood stabilizers or antidepressants in bipolar depression have not been fully elucidated (Belmaker, 2004).
Only two medications are currently approved by the Food and Drug Administration (FDA) for the treatment of bipolar depression, quetiapine and the olanzapine-fluoxetine combination pill. Other medications are used off label. Antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), used alone may increase the rate of switching to hypomania or mania, although this is debated. The main cause of disability in bipolar disorder is arguably due to the depressed state, and this state is most associated with suicide. Bipolar patients also spend the most symptomatic periods in the depressed state. A better understanding of bipolar depression's pathophysiology and treatment mechanism is therefore particularly needed (Baldessarini et al, 2010; Salvi et al, 2008).
Our group previously reported higher brain binding potential (BPF) of [11C]WAY-100635 in unmedicated male bipolar depressed subjects compared with healthy volunteers (Sullivan et al, 2009). This result likely reflects higher levels of serotonin 1A (5-HT1A) receptors in the brain, including autoreceptors in the midbrain serotonin raphe nuclei. In this analysis, patients' history of suicide attempts and depression severity did not correlate with BPF. Our group also previously found that subjects with major depressive disorder who remitted after naturalistic treatment had altered pre-treatment BPF of [11C]WAY-100635 compared with nonremitters (Parsey et al, 2006a). Bipolar depression cannot be readily distinguished from depressive episodes of major depressive disorder, raising the question whether pre-treatment [11C]WAY-100635 binding can predict antidepressant response of bipolar depression. Therefore, in the current study, we sought to determine whether pre-treatment [11C]WAY-100635 BPF is associated with clinical response after three months of antidepressant medication treatment of bipolar depression. This is a secondary analysis of PET data that has been previously published combined with never previously reported clinical outcome data.
Materials and Methods
Subjects
Forty-one patients who met DSM-IV criteria for a major depressive episode and criteria for either bipolar I or bipolar II disorder were included in the study as assessed with Structured Clinical Interview for Axis I disorders (SCID-I/P) (First, 1994), psychiatric interview and chart review. Thirty-two of these subjects had been included in the previous study from our group on bipolar disorder (Sullivan et al, 2009). Fifty-one healthy volunteers were enrolled after assessment with SCID-I-NP detected no axis I diagnosis; they also had no history of mood disorders, psychotic disorders or suicide in first-degree relatives. SCID interviews and diagnostic assessments were performed by MA or PhD level psychologists, and the diagnosis was confirmed by a research psychiatrist. Forty-seven of the control subjects had been included in a previous study (Sullivan et al, 2009). Bipolar subjects were aged between 18-65 years old and had scores of ≥16 on the 17 item Hamilton Depression Rating Scale. Patients were off antidepressant and mood stabilizer treatment for at least 14 days (42 days if on fluoxetine, and 21 days without oral antipsychotics) before PET scanning. No patients received injectable antipsychotics. Small doses of benzodiazepines were permitted until 72 hours before PET scans. Exclusion criteria included significant medical conditions, positive urine toxicology, positive pregnancy test, planned pregnancy, lifetime exposure to 3,4-methylenedioxymethamphetamine (MDMA), or history of alcohol or substance use disorder in lifetime for controls or within the previous 6 months for bipolar disorder patients. Written informed consent was obtained for all participants after a description of the study. The study was approved by the Institutional Review Board of the New York State Psychiatric Institute and Columbia University Medical Center, and in accordance with the Helsinki Declaration of 1975. Control subjects were scanned between 1999 and 2007 and bipolar patients were scanned between 1999 and 2008.
Clinical care
Clinical assessments were performed at baseline and after 3 months using both the HDRS-24 (Hamilton, 1960) and the Beck Depression Inventory (BDI) (Beck et al, 1961) to assess depression, the Young Mania Rating Scale (Young et al, 1978) to assess mania and the Global Assessment Scale (Endicott et al, 1976) to assess functional impairment. After PET scanning, the patients received outpatient psychiatric care and received a range of different medications. Remission was defined by a 24 item HDRS score of < 10 after three months of treatment.
Genotyping
The 5-HT1A promotor allele C(-1019)G was genotyped for each subject as previously described (Huang et al, 2004; Wu & Comings, 1999)
Chemistry and Input Function Measurement
[Carbonyl-C-11]WAY100635 [N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl) cyclohexane carboxamide] was prepared as previously described (Parsey et al, 2000). Arterial input function, metabolites, and free plasma fraction (fP) were measured as previously described (Parsey et al, 2005; Parsey et al, 2000).
Acquisition and analysis
MRI and PET data were acquired as previously described (Parsey et al, 2005). Subject motion correction and de-noising were applied as previously described (Sullivan et al, 2009). Regions of interest (ROI's), including the ventral prefrontal cortex (VPFC), anterior cingulate cortex (ACN), posterior cingulate cortex (CIN), amygdala (AMY), hippocampus (HIP), parahippocampal gyrus (PHG), insular cortex (INS), temporal cortex (TEM), parietal cortex (PAR) and occipital cortex (OCC), were drawn on the MRI of each subject as previously described (Parsey et al, 2000). Cylindrical areas were drawn in the cerebellar white matter as a reference region and in the approximate area of the raphe nucleus as an additional region of interest (RN) as previously described (Parsey et al, 2005). Regional volumes of distribution of [C-11]WAY100635 were calculated and a two-tissue compartment (2T) model using arterial input function as previously described to estimate the outcome measure, BPF = Bavail/KD where Bavail is the total number of available receptors and 1/KD is the affinity of the tracer for the receptor (Parsey et al, 2000).
Statistics
Group differences of BPF were determined using a linear mixed models analysis with subject as the random effect and fixed effects including region, sex (Parsey et al, 2005; Parsey et al, 2006b; Parsey et al, 2002; Sullivan et al, 2009) and genotype of the 5-HT1A receptor C-1019G polymorphism (Parsey et al, 2006b). When multiple regions were considered in a single analysis, the analysis was performed on the natural log of BPF to remedy some slight skewness in the outcome measure, to stabilize the variance across regions, and to allow for proportional differences in binding levels. Standard errors (SE) were computed for each BPF value using a bootstrap algorithm that takes into account errors in metabolite, plasma and brain data; observations were weighted accordingly (Ogden & Tarpey, 2006). Statistical differences of continuous demographic or clinical measures were calculated using Student's t-test, and categorical data were analyzed using Fisher's exact test. Significance was defined as p less than 0.05 and all tests were two sided. SPSS 11 for Mac OSX (www.spss.com) and R (www.R-project.org) were used for calculations.
Results
After three months of naturalistic treatment, 34 out of 41 patients followed up with a clinical assessment. Of the 34, nine had remitted. Remitters had higher pre-treatment [11C]WAY-100635 BPF compared with non-remitters across all brain regions, (Figure 1; F=5.96; df=1,32; p=0.020). There was no brain region by remitter status interaction (F=0.81; df=12,382; p=0.641). Post-hoc analyses without adjustment for multiple comparisons found significant remitter differences from non-remitters in all brain regions except amygdala and raphe nucleus. A linear mixed model of change in HAM-D score from baseline to the three month time points showed a trend correlation with BPF values (F=4.03; df=1,32; p=0.053) such that higher binding was associated with better antidepressant response.
Figure 1.
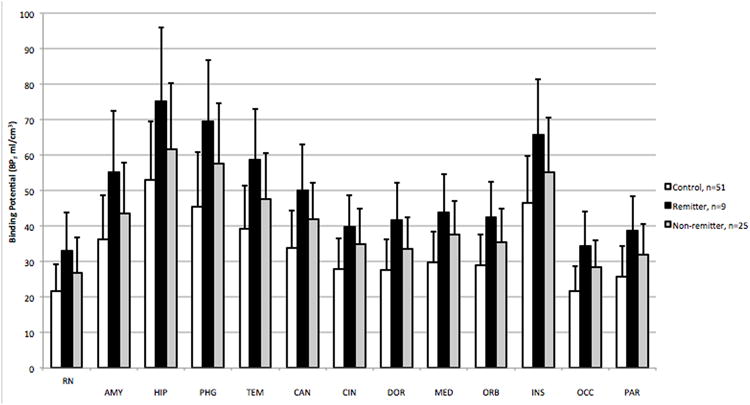
Bipolar Patients who remitted after 3 months of treatment for depression have higher binding potential of [11C]WAY-100635 in the brain than patients who did not remit (p = 0.02), likely reflecting higher 5HT1A receptor levels. Healthy control subjects are included here as a reference. VPFC = ventral prefrontal cortex, ACN = anterior cingulate cortex, CIN = posterior cingulate cortex, AMY = amygdala, HIP = hippocampus, PHG = parahippocampal gyrus, INS = insular cortex, TEM = temporal cortex, PAR = parietal cortex and OCC = occipital cortex. Standard errors (SE) were computed for each BPF value using a bootstrap algorithm that takes into account errors in metabolite, plasma and brain data; observations were weighted accordingly.
Several analyses were undertaken to assess possible confounds in the binding difference between groups. When the sex of the subjects and the genotype of 5-HT1A receptor C-1019G polymorphism were included in the model, the effect of remission status on BPF remained significant and the effect of neither sex nor genotype was significant in this model. Pre-treatment clinical symptomatology as assessed by scores on the YMRS and HAM-D did not differ between remitters and nonremitters. The remitted patients did not differ from non-remitted patients in age, sex, percentage of bipolar I patients, 5-HT1A receptor C-1019G genotype, percentage of patients with a history of past substance use disorder, or recent antidepressant exposure (Table1). There were also no significant differences in injected mass (p=0.43), injected dose (p=0.95) or specific activity (p=0.36) of the radiotracer between remitter and non-remitter groups. The patients reported taking a variety of medications during the three-month interval after their PET scans. Remitters and non-remitters had received similar proportions of medication classes: antidepressants (4/9 remitters, 14/25 non-remitters, p=0.73), serotonin based antidepressants (2/9 remitters and 13/25, non-remitters, p=0.24), mood stabilizers (7/9 remitters, 22/25 non-remitters, p=0.59) and atypical antipsychotics (5/9 remitters, 7/25 non-remitters, p=0.22). No significant differences between remitters and non-remitter groups of lifetime exposure to medications were found for any of these medication classes. Remitter and non-remitter groups also had similar percentages of patients who received psychotherapy (4/9 remitters, 14/25 non-remitters, p=0.73)
Table 1. Clinical and demographic variables for remitter and non-remitter bipolar depressed groups.
| Remitters | Non-remitters | P value | |
|---|---|---|---|
| Age in years | 41.0 (10.1) | 36.9 (8.0) | 0.14a |
| Sex (% female) | 66.7 | 56.0 | 0.70b |
| HAMD baseline | 28.9 (7.4) | 27.2 (6.9) | 0.53a |
| HAMD 3 month | 5.8 (2.8) | 20.6 (7.3) | 1.71 e-6 |
| YMRS baseline | 5.9 (7.5) | 8.0 (8.5) | 0.52a |
| YMRS 3 month | 1.9 (3.4) | 3.1 (3.4) | 0.40a |
| Medication Status (%AE) | 77.8 | 64.0 | 0.68b |
| Bipolar subtype (% Bipolar I) | 44.4 | 76.0 | 0.40 |
| 5HT1AR CC polymorphism (%) | 33.3 | 24.0 | |
| 5HT1AR CG polymorphism (%) | 44.4 | 48.0 | |
| 5HT1AR GG polymorphism (%) | 22.2 | 28.0 | 0.31c |
Values for age, HAMD and YMRS are listed as group means with standard deviations in parentheses. HAMD: Hamilton Depression Rating Scale value. YMRS: Young Mania Rating Scale value. 5HT1AR polymorphism: Serotonin 1A receptor promoter C(-1019)G biallelic genotype. Bipolar Subtype: Percentage of bipolar patients with a bipolar I subtype diagnosis. AE: Percentage of patients who have been exposed to antidepressants recently (see methods).
Calculated with two tailed t test,
Calculated with Fisher's exact test.
Calculated using Fisher's exact test comparing count data for the three polymorphisms between the two groups
The bipolar patient cohort discussed here (remitter and non-remitter) only had an additional 8 subjects and the healthy control group included an additional 4 subjects that were not published in our previous published bipolar disorder study (Sullivan et al, 2009). The addition of these subjects supports our initial publication in that the expanded cohort of all bipolar depressed subjects has higher [11C]WAY-100635 BPF than healthy control subjects. There was a significant effect of diagnosis confirming higher binding in the bipolar group (F=10.33; df=1,83; p=0.002) and a significant interaction between diagnosis and region (F=1011.24; df=12,1008; p<0.001). There was a significant effect of sex of the patients on the model (F=4.17; df=1,79; p=0.045), but no effect of age, injected dose or injected mass. Male patients had higher binding compared with healthy control subjects in BPF, adjusting for region (F=9.37; df=1,34; p=0.004), but females only showed a trend adjusting for region (F=2.74; df=1,47; p=0.104). These sex specific differences were consistent with those previously reported (Sullivan et al, 2009).
Discussion
Unmedicated bipolar depressed patients that remitted after three months of treatment had higher pre-treatment BPF of [11C]WAY-100635 compared to bipolar depressed non-remitters. To our knowledge, this is the first molecular neuroimaging finding that is associated with clinical response of bipolar depression. These results are from a pilot, naturalistic treatment study and require a prospective study for confirmation. A prospective study would also control for variance in treatment the patients received after their PET scan.
Serotonin neurotransmission is abnormal in both depression and mania. Medications that increase serotonin intra-synaptically, such as SSRIs, have been effective in treating bipolar depression when used in conjunction with mood stabilizers (Baldessarini et al, 2010). Serotonin based antidepressants may precipitate mania in bipolar disorder, especially in the absence of a mood stabilizer. The 5-HT1A receptors are central to regulating the serotonin system in the brain. They are inhibitory somatodendritic autoreceptors in the raphe nucleus. They are also postsynaptic receptors on non-serotonergic neurons throughout serotonin neuron projection areas in other brain regions. Administration of SSRIs desensitizes and downregulates the autoreceptors in rodent studies, increasing serotoninergic firing in a time course of weeks that is comparable to that required for clinical medication response (Blier et al, 1998). We recently confirmed this effect in major depressive disorder treated with SSRI's (Gray et al, 2013).
The fact that bipolar patients who remitted from treatment had higher 5HT1A receptors in the brain tissue than patients who did not remit indicates that the serotonin neurotransmission system is important to the antidepressant response. Patients who remitted had 5HT1A receptor levels that were more different (higher) than healthy volunteer subjects than patients who did not remit. This result can be explained by two potential models.. The first model proposes that higher 5HT1A receptor density allows for a greater response to treatment within the serotoninergic system. More 5HT1A autoreceptors may mean greater capacity for downregulation and therefore greater increase in serotonin neuron firing after several weeks of medication treatment. The second mechanism posits that upregulation of post-synaptic 5HT1A receptors on postsynaptic target neurons is a compensatory mechanism in depressed bipolar patients in response to insufficient firing and serotonin release by serotonergic neurons. Therefore, those with greater upregulation of 5-HT1A receptors, receive greater clinical benefit from a medication-induced increase in serotonin release because of greater post-synaptic signaling capacity.
Our approach to quantifying the binding potential of the PET radiotracer uses a metabolite and free fraction corrected arterial input function to calculate BPF values using cerebellar white matter as a reference region. In contrast other groups use a different outcome measure, BPND, and a different reference region, the cerebellar gray matter. Our approach offers advantages as it avoids the complication of nonspecific binding of the radiotracer in the cerebellum and the susceptibility of the cerebellum to signal artifacts. We previously reported higher 5HT1A binding in depressed bipolar patients than healthy control subjects, as confirmed here (Sullivan et al, 2009). We have also reported higher binding potential in not recently medicated patients with major depressive disorder when compared to healthy control subjects (Parsey et al, 2010; Parsey et al, 2006b). Thus, we find a comparable biochemical change in both bipolar and unipolar depressions. Other groups have reported both lower (Drevets et al, 1999; Drevets et al, 2007; Sargent et al, 2000) and no difference in binding of the radiotracer in major depressive disorder (Mickey et al, 2008). We have proposed that a major reason for disagreement in the results is likely is due to the outcome measure and choice of reference region as discussed elsewhere (Parsey et al, 2010; Shrestha et al, 2011).
Only two published studies have found clinical outcomes in bipolar disorder that correlate with neuroimaging findings in bipolar disorder. One study reported that bipolar disorder with a history of poor clinical course in the five years before the scan had larger volume of white matter hyperintensities than patients who had had a better clinical course in that time (Regenold et al, 2008). A second reported that worse clinical course for two years before the scan was associated with higher rate of subcortical punctate white matter hyperintensities, but not periventricular white matter hyperintensities (Moore et al, 2001). Our results are the first molecular neuroimaging finding that correlates with clinical remission of bipolar depression after three months of treatment.
This study has limitations inherent in being a pilot study of naturalistic treatment. Sample size is modest, treatment medication and dosage pattern are variable and not fully characterized, and the relationship of imaging outcome to clinical response was a post hoc analysis. The small sample size makes it difficult to know if the effect is due to a small subsample of remitters with high binding or if binding across the full range contributes to treatment outcome. If higher [11C]WAY-100635 BPF in the brain does prove to be predictive of clinical outcome for bipolar depression, it may have clinical utility if future studies can show that it predicts preferentially better outcome with specific types of treatment and thereby aid in treatment selection. Such data may prove to be the first step in identifying a neuroimaging signature that can predict which patients will respond to a particular treatment type. The next step would be to determine if these findings hold for both serotonergic and nonserotonergic treatments. Thus, these data could prove to be the first step in designing a brain imaging test to determine a personalized treatment plan with greatest likelihood of inducing remission.
Figure 2.
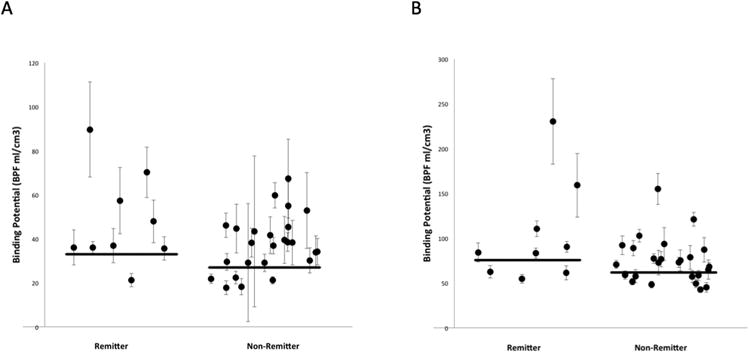
Scatter plots of BPF values within two representative regions of interest, the raphe nucleus (A) and the hippocampus (B). Data are divided into remitter and non-remitter groups. Error bars indicate weights that reflect the level of uncertainty for each value. Horizontal lines mark the weighted mean values for each group. X values are computer generated random numbers for each subject.
Acknowledgments
We are grateful to the staff of the Brain Imaging Division and Clinical Evaluation Core of the Conte Translational Center for the Neurobiology of Suicidal Behavior, the Columbia Kreitchman PET center and the Radioligand Laboratory for their contributions to the project. We would also like to thank all of the subjects who volunteered for the project. The work was funded by United States Public Health Service grants MH62185 (Conte Center), K08-MH67015 (GMS) and the American Foundation for Suicide Prevention. Part of this work was presented at the annual meeting of the Society for Biological Psychiatry, Philadelphia, Pennsylvania, May 3-5, 2012. Dr Mann has past unrelated grants from Novartis and GSK.
Footnotes
The authors declare no conflict of interest.
References
- The World Health Report 2002:Reducing Risks, Promoting Healthy Life. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- Baldessarini RJ, Vieta E, Calabrese JR, Tohen M, Bowden CL. Bipolar depression: overview and commentary. Harv Rev Psychiatry. 2010;18:143–157. doi: 10.3109/10673221003747955. [DOI] [PubMed] [Google Scholar]
- Bauer M, Pfennig A. Epidemiology of bipolar disorders. Epilepsia. 2005;46(4):8–13. doi: 10.1111/j.1528-1167.2005.463003.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–486. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- Blier P, Pineyro G, el Mansari M, Bergeron R, de Montigny C. Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann N Y Acad Sci. 1998;861:204–216. doi: 10.1111/j.1749-6632.1998.tb10192.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- First MS, RL, Gibbon M, Williams JBW. SCID-I/P, Version 2.0. In Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition. New York: 1994. [Google Scholar]
- Gray NA, Milak MS, Delorenzo C, Ogden RT, Huang YY, Mann JJ, Parsey RV. Antidepressant Treatment Reduces Serotonin-1A Autoreceptor Binding in Major Depressive Disorder. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205–228. doi: 10.1192/bjp.170.3.205. [DOI] [PubMed] [Google Scholar]
- Huang YY, Battistuzzi C, Oquendo MA, Harkavy-Friedman J, Greenhill L, Zalsman G, Brodsky B, Arango V, Brent DA, Mann JJ. Human 5-HT1A receptor C(-1019)G polymorphism and psychopathology. Int J Neuropsychopharmacol. 2004;7:441–451. doi: 10.1017/S1461145704004663. [DOI] [PubMed] [Google Scholar]
- Mickey BJ, Ducci F, Hodgkinson CA, Langenecker SA, Goldman D, Zubieta JK. Monoamine oxidase A genotype predicts human serotonin 1A receptor availability in vivo. J Neurosci. 2008;28:11354–11359. doi: 10.1523/JNEUROSCI.2391-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PB, Shepherd DJ, Eccleston D, Macmillan IC, Goswami U, McAllister VL, Ferrier IN. Cerebral white matter lesions in bipolar affective disorder: relationship to outcome. Br J Psychiatry. 2001;178:172–176. doi: 10.1192/bjp.178.2.172. [DOI] [PubMed] [Google Scholar]
- Ogden RT, Tarpey T. Estimation in regression models with externally estimated parameters. Biostatistics. 2006;7:115–129. doi: 10.1093/biostatistics/kxi044. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, Mikhno A, Milak M, Zanderigo F, Sullivan GM, Oquendo MA, Mann JJ. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry. 2010;68:170–178. doi: 10.1016/j.biopsych.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006a;31:1745–1749. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, Van Heertum RL, Arango V, Mann JJ. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006b;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;954:173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Slifstein M, Hwang DR, Abi-Dargham A, Simpson N, Mawlawi O, Guo NN, Van Heertum R, Mann JJ, Laruelle M. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tisssue input functions. J Cereb Blood Flow Metab. 2000;20:1111–1133. doi: 10.1097/00004647-200007000-00011. [DOI] [PubMed] [Google Scholar]
- Regenold WT, Hisley KC, Phatak P, Marano CM, Obuchowski A, Lefkowitz DM, Sassan A, Ohri S, Phillips TL, Dosanjh N, Conley RR, Gullapalli R. Relationship of cerebrospinal fluid glucose metabolites to MRI deep white matter hyperintensities and treatment resistance in bipolar disorder patients. Bipolar Disord. 2008;10:753–764. doi: 10.1111/j.1399-5618.2008.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi V, Fagiolini A, Swartz HA, Maina G, Frank E. The use of antidepressants in bipolar disorder. J Clin Psychiatry. 2008;69:1307–1318. doi: 10.4088/jcp.v69n0816. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Shrestha S, Hirvonen J, Hines CS, Henter I, Svenningsson P, Pike VW, Innis RB. Serotonin-1A receptors in major depression quantified using PET: Controversies, confounds, and recommendations. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Ogden RT, Oquendo MA, Kumar JS, Simpson N, Huang YY, Mann JJ, Parsey RV. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol Psychiatry. 2009;66:223–230. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- Wu S, Comings DE. A common C-1018G polymorphism in the human 5-HT1A receptor gene. Psychiatr Genet. 1999;9:105–106. doi: 10.1097/00041444-199906000-00010. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]


