Abstract
The TGFβ/BMP signaling pathways are involved in glaucomatous damage to the trabecular meshwork (TM) leading to elevated intraocular pressure (IOP), which is a major risk factor for the development and progression of glaucoma. The BMP antagonist gremlin is elevated in glaucomatous TM cells and tissues and can directly elevate IOP. Gremlin utilizes the TGFβ2/SMAD pathway to induce TM extracellular matrix (ECM) proteins. The purpose of this study is to determine whether expression of the ECM cross-linking lysyl oxidase (LOX) genes is regulated by gremlin in cultured human TM cells. Human TM cells were treated with recombinant gremlin, and expression of the LOX genes was examined by quantitative RT-PCR and western immunoblotting. TM cells were pretreated with TGFBR inhibitors (LY364947 or SB431542), an inhibitor of the SMAD signaling pathway (SIS3), or with JNK (SP600125) and p38 MAPK (SB203580) inhibitors to identify the signaling pathway(s) involved in gremlin induction of LOX protein expression. All five LOX genes (LOX and LOXL1–4) were induced by gremlin. Gremlin induction of LOX genes and protein expression was blocked by TGFBR inhibitors as well as by inhibitors of the SMAD3, JNK and p38 MAPK signaling pathways. We conclude that gremlin employs both canonical TGFβ/SMAD and the non-canonical JNK and p38 MAPK signaling pathways to induce LOX genes and proteins in cultured human TM cells. Increased LOX levels may be at least partially responsible for gremlin-mediated IOP elevation and increased aqueous humor outflow resistance leading to glaucoma.
Keywords: primary open angle glaucoma, trabecular meshwork, extracellular matrix, gremlin, lysyl oxidase, lysyl oxidase like, transforming growth factor beta
1. Introduction
Glaucoma is a leading cause of irreversible visual impairment and blindness in the world, with primary open-angle glaucoma (POAG) being the major form of glaucoma (Quigley and Broman, 2006). Elevated intraocular pressure (IOP) is a major risk factor for the development and progression of glaucoma (AGIS, 2000; Weinreb and Khaw, 2004). Ocular hypertension is attributed to increased aqueous humor (AH) outflow resistance in the trabecular meshwork (TM), a tissue in the anterior segment of the eye. Elevated IOP has been associated with increased deposition of extracellular matrix (ECM) material within the TM. Therefore, glaucoma can be viewed as a fibrotic disorder of the TM.
Numerous studies have shown that TGFβ2 levels are elevated in the AH (Inatani et al., 2001; Tripathi et al., 1994) and TM of POAG patients (Tovar-Vidales et al., 2011). TGFβ2 is a profibrotic growth factor linked to fibrotic diseases of the lung, kidney, skin, and liver. TGFβ2 has a number of effects on the TM, most notably increasing AH outflow resistance and elevating IOP in perfusion cultured human and porcine eyes (Bachmann et al., 2006; Fleenor et al., 2006; Gottanka et al., 2004) as well as in rodent eyes (Shepard et al., 2010). TGFβ2 also modulates TM ECM metabolism. This growth factor increases the expression of a variety of ECM proteins, including fibronectin (FN), collagen (COL), elastin (ELN), and proteoglycans as well as plasminogen activator inhibitor-1 (PAI1) and tissue inhibitor of metalloproteinase-1 (TIMP1) (Fuchshofer and Tamm, 2012). PAI-1 and TIMP-1 suppress proteolytic degradation of the ECM (Fuchshofer and Tamm, 2012). In addition, TGFβ2 increases expression of the ECM cross-linking enzymes transglutaminase-2 (TGM2) (Tovar-Vidales et al., 2008; Welge-Lussen et al., 2000), lysyl oxidase (LOX), and LOX like 1–4 (LOXL1–4) (Sethi et al., 2011b). Thus, parallels can be easily drawn between the profibrotic effects of TGFβ in the TM and the TGFβ-mediated fibrosis that occurs in other cells and tissues.
We have previously reported that TM cells express several members of the bone morphogenetic (BMP) family, including BMP ligands (BMP2, BMP4, BMP5 and BMP7), BMP receptors (BMPR1a, BMPR1b, BMPR2), and BMP antagonists such as gremlin (Wordinger et al., 2002, 2007). BMPs are members of the TGFβ superfamily of proteins that control multiple functions in a variety of cell types. Interestingly, BMP4 and BMP7 block the TGFβ2-induction of a variety of ECM proteins in TM cells, including fibronectin, collagens IV & VI, TSP-1, and PAI1 (Fuchshofer et al., 2007; Wordinger et al., 2007).
BMP antagonists, such as gremlin, tightly regulate BMP cellular activity. Gremlin directly binds to specific BMP ligands and blocks BMP binding to their receptors (Wordinger et al., 2008). We have reported greater levels of gremlin in glaucomatous TM cells and tissues (Wordinger et al., 2007). Elevated gremlin levels also occur in fibrotic disease of the kidney (Wordinger et al., 2008). We previously reported that gremlin antagonized BMP4 inhibition of TGFβ2-induced ECM proteins like FN and PAI1 in TM cells and also elevated IOP in perfusion cultured human anterior segments (Wordinger et al., 2007). Recently, we also demonstrated that gremlin alone can induce fibrosis-like activities in TM cells. Gremlin induced expression of FN, COL1, ELN and PAI1 genes and proteins in cultured TM cells utilizing the TGFβ2/SMAD signaling pathway (Sethi et al., 2011a).
The LOX family contains 5 genes, LOX and LOXL1–4, encoding enzymes that covalently cross-link elastin and collagens via generation of aldehydes on lysine residues. This cross-linking reaction provides additional mechanical strength to the ECM and also makes the ECM more resistant to degradation. LOX and LOXL are associated with several abnormalities related to an imbalance in ECM synthesis and/or degradation such as fibrotic disorders of connective tissues of the heart (atrial fibrosis and myocardial fibrosis), vasculature (atherosclerosis, vascular aneurysms and arterial fibrosis), lungs (pulmonary fibrosis), skin (fibrosis, hypertrophic scarring, keloids, and scleroderma), kidney (diabetic nephropathy, nephritis), liver (liver stiffness preceding liver fibrosis), mouth (inflamed oral tissue, gingival atrophy), and colon (intestinal fibrotic disease) (Sethi et al., 2012). The TM expresses enzymatically active LOX and LOXL proteins, and TGFβ2 utilizes both canonical SMAD as well as c-Jun N-terminal Kinase (JNK) signaling pathways to induce LOX genes and proteins in cultured human TM cells (Sethi et al., 2011b). Both SMAD and mitogen activated protein kinase (MAPK) signaling pathways, including JNK signaling, have been associated with fibrosis (Ma et al., 2009). However, very little is known about the role of gremlin and the signaling mechanism(s) used to induce LOX and LOXL.
The purpose of the present study was to determine: (1) whether gremlin induces LOX gene expression in the TM cells, and (2) which signaling pathway(s) regulate gremlin-induced LOX expression in the TM.
2. Materials and methods
2.1. TM cell culture
Human TM cells were isolated from carefully dissected human TM tissue explants derived from donor eyes and characterized as previously described (Fleenor et al., 2006; Wordinger et al., 2002, 2007). All donor tissues were obtained from regional eye banks and managed according to the guidelines in the Declaration of Helsinki for research involving human tissue. Isolated TM cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen-Gibco, Grand Island, NY) containing L-glutamine (0.292 mg/ml; Gibco BRL Life Technologies), penicillin (100 units/ml)/streptomycin (0.1 mg/ml); (Gibco BRL Life Technologies), and 10% fetal bovine serum (Gibco BRL Life Technologies).
2.2. TM cell treatments
TM cells were grown to 100% confluency and then kept in serum-free medium for 24 h prior to gremlin or inhibitor treatment to avoid the confounding effects of serum proteins. TM cells were incubated with fresh medium containing specific signaling inhibitors for 1–6 h, prior to the addition of varying concentrations of recombinant gremlin protein (R&D System, Minneapolis, MN). Small molecule inhibitors LY364947 (5 μM, Tocris biosciences, Ellisville, MO) and SB431542 (5 μM, Sigma–Aldrich, St. Louis, MO) were used to examine the effects of inhibition of TGFβ Receptor-1/2. SMAD-3 phosphorylation inhibitor SIS3 (10 μM, Sigma–Aldrich, St. Louis, MO), JNK inhibitor SP600125 (10 μM, Sigma–Aldrich, St. Louis, MO), and p38 MAPK inhibitor SB203580 (5 μM, Tocris Biosciences, Ellisville, MO) were used to examine effects of inhibition on canonical SMAD, JNK, and p38 signaling pathways, respectively (Sethi et al., 2011b).
2.3. RNA isolation
Total cellular RNA was extracted from cultured TM cells using TRI Reagent RT extraction (MRC Inc., Cincinnati, OH), and the SuperScript VILO cDNA Synthesis kit (Invitrogen) was used for first strand cDNA synthesis. PCR primers for the various LOX genes were designed using Primer3 software (http://frodo.wi.mit.edu/primer3/). The primer pairs are listed in Table 1.
Table 1.
List of the primers used for PCR studies.
| Gene | Primer (5′ → 3′) | |
|---|---|---|
| LOX | Left | CGACCCTTACAACCCCTACA |
| Right | AAGTAGCCAGTGCCGTATCC | |
| LOXL1 | Left | AGAGCCTCTCTGTCCACCAG |
| Right | GTACACCTGCCCGTTGTTCT | |
| LOXL2 | Left | CCTGGGGAGAGGACATACAA |
| Right | CTCGCAGGTGACATTCTTCA | |
| LOXL3 | Left | CAACGCGGCCTTCTACAG |
| Right | GGTGTCATTGGCACGATAGA | |
| LOXL4 | Left | CGACAGCCACTACTACAGGAAA |
| Right | CTGGTGGATCCAGAAGGAGTT | |
| GAPDH | Left | CCACCCATGGCAAATTCCATGGCA |
| Right | TCTAGACGGCAGGTCAGGTCCACC |
2.4. Quantitative real time PCR
Real-time PCR was performed as described previously (Sethi et al., 2011b). Briefly, 2.5 μl of cDNA was used in a reaction consisting of 1.5 units per reaction of antibody-bound Taq enzyme (Jump Start; Sigma–Aldrich, St. Louis, MO), 10× PCR buffer, 1.5 mM MgCl2, 200 nM dNTP mix, 100 nM PCR primers (Table 1), 2.5 μl green nucleic acid dye (EvaGreen; Biotium, Hayward, CA), as well as 30 nM passive reference dye (Rox; USB, Cleveland, OH) per 50-μl reaction. PCR was performed on a real-time thermal cycler (model Mx3000p; Stratagene, La Jolla, CA), with cycling parameters of initial denaturation at 95 °C; 40 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 60 s, and a denaturation cycle for the creation of a dissociation curves. Reactions for each sample were run in duplicate, cycle thresholds (Ct) were normalized to GAPDH expression as a housekeeping gene, and comparative quantitation was performed using MxPro ver. 4.0 software (Stratagene). The delta delta Ct method was used for quantification of the data. Only individual PCR samples with single-peak dissociation curves were selected for data analysis.
2.5. Protein extraction and western blot analysis
Secreted proteins
LOX proteins secreted by TM cells were determined by western immunoblot analysis. Conditioned medium was collected from human TM cells after 24-h treatment with gremlin in serum-free medium containing 0.5 mg/ml BSA. Proteins were separated on a 10% denaturing polyacrylamide gel and transferred by electrophoresis to a PVDF membrane. Membranes were blocked with 5% Fat-free Dry Milk in tris-buffered saline tween buffer (TBST) for 1 h and then incubated overnight with primary antibodies (Table 2). The membranes were washed with TBST and processed with corresponding horseradish peroxidase-conjugated secondary antibodies (Table 2). The proteins were then visualized in a Fluor ChemTM 8900 imager (Alpha Innotech, San Leandro, CA) using ECL detection reagent SuperSignal West Femto Maximum Sensitivity Substrate (Pierce Biotechnology Rockford, IL).
Table 2.
List of antibodies used for western immunoblotting studies.
| Antibody | Ab. Dilution | Source |
|---|---|---|
| Rabbit anti-LOX | 1:10,000 | Novus Biologicals (Cat. # NB100-2530) |
| Rabbit anti-LOXL1 | 1:500 | Abnova (Cat. # H00004016-D01P) |
| Mouse anti-LOXL2 | 1:2000 | R&D Systems (Cat. # MAB2639) |
| Rabbit anti-LOXL3 | 1:1000 | SigmaeAldrich (Cat # SAB2107508) |
| Mouse anti-LOXL4 | 1:250 | Abcam (Cat. # ab25904) |
| Rabbit anti-GAPDH | 1:1000 | Cell Signaling (Cat. # 2118) |
| Donkey anti-mouse IgG | 1:10,000 | Santa Cruz Biotechnology (Cat. # sc-2314) |
| Goat anti-rabbit IgG | 1:10,000 | Santa Cruz Biotechnology (Cat. # sc-2004) |
Cell-associated proteins
Total cellular protein was extracted from TM cells using mammalian protein extraction buffer (MPER, Pierce Biotechnology), containing protease inhibitor (Pierce Biotechnology) and phosphatase inhibitor (Pierce Biotechnology) cocktails. Protein concentration was determined using the Bio-Rad Dc protein assay system (Bio-Rad Laboratories, Richmond, CA). The cellular proteins were separated on denaturing polyacrylamide gels and then transferred to PVDF membranes by electrophoresis. Blots were blocked with 5% Fat-free Dry Milk in TBST for 1 h and then incubated overnight with primary antibodies (Table 2). The membranes were washed with TBST and processed with corresponding horseradish peroxidase-conjugated secondary antibodies (Table 2). The proteins were then visualized in a Fluor ChemTM 8900 imager (Alpha Innotech) using ECL detection reagent SuperSignal West Femto Maximum Sensitivity Substrate (Pierce Biotechnology). To ensure equal protein loading, the same blot was subsequently developed for GAPDH expression.
2.6. Statistical analysis
For comparing results between two groups, the two-tailed student’s t test was performed. One-way ANOVA was employed for comparison of results between more than two groups.
3. Results
3.1. Gremlin induces LOX mRNA and protein expression in TM cells
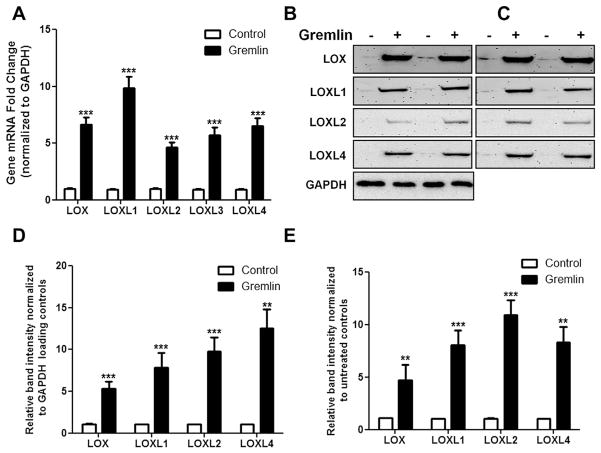
We previously reported that gremlin induces the ECM proteins FN, COL1, PAI1 and ELN in TM cells (Sethi et al., 2011a). Therefore, we first determined the effect of gremlin on LOX and LOXL expression. Treatment with gremlin (1 μg/ml) for 24 h significantly induced LOX and LOXL1–4 mRNA expression (n = 3, p < 0.05) (Fig. 1A). Gremlin also induced protein expression of cell associated and secreted LOX, LOXL1, LOXL2, and LOXL4 in cell lysates (Fig. 1B and D) and conditioned medium (Fig. 1C and E). LOXL3 was not assayed due to lack of a commercially consistently reliable antibody.
Fig. 1.
Gremlin induces LOX mRNA and proteins in TM cells. (A) Induction of LOX, LOXL1, LOXL2, LOXL3 and LOXL4 mRNA in six TM cell strains treated with gremlin (1 μg/ml) for 24 h vs. untreated control samples. Values represent the fold induction of LOX genes normalized to GAPDH. Three replicates of each sample were employed. Gremlin significantly induced all 5 LOX genes in all the six cell lines. (B–C) Western immunoblots of cell-associated (B) and secreted (C) LOX, LOXL1, LOXL2 and LOXL4 proteins in two TM cell strains treated with gremlin (1 μg/ml) for 24 h. Cell-associated LOX proteins were normalized to GAPDH (B), while equal volumes of conditioned medium were loaded for secreted LOXs (C). Gremlin increased the expression of both cell associated and secreted LOXs. Similar results were observed in four additional TM cell strains. The western blot images are representative of three independent experiments. (D–E) Densitometry analysis of western immunoblots for cell associated (D) and secreted (E) LOXs. Two-tailed student’s t test was used to compare control and gremlin-treated samples **0.0001 < p < 0.01 and ***p < 0.0001.
3.2. Gremlin induces LOX genes and proteins in a concentration-and time-dependent fashion
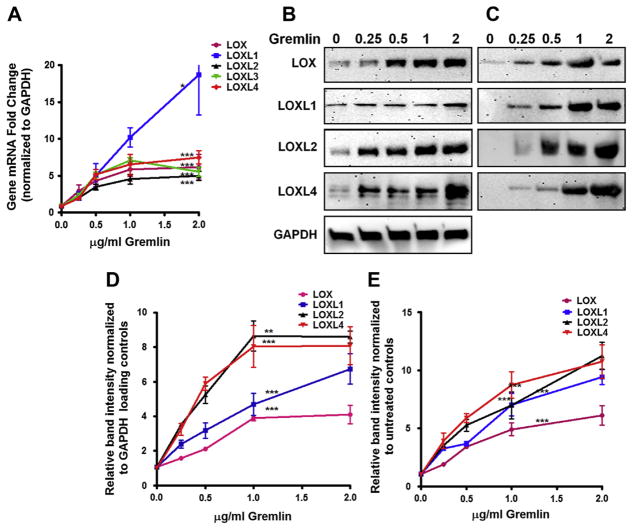
TM cell strains (n = 3) were treated with increasing concentrations of gremlin (0–2 μg/ml) for 24 h. The mRNA and protein expression of LOX and LOXL1–4 were determined using qRT-PCR and western immunoblotting, respectively. Gremlin induced the expression of all 5 LOX genes (Fig. 2A), as well as cell-associated (Fig. 2B and D) and secreted LOX proteins (Fig. 2C and E) in a concentration-dependent manner. TM cells were also treated with gremlin for 6,12 and 24 h to determine the time dependence of LOX and LOXL mRNA induction. Gremlin significantly (p < 0.01) induced LOX and LOXL mRNA expression, although the time course of induction varied for each gene (Fig. 3A). By six hours, gremlin significantly induced all LOX genes except LOXL3. Similarly, TM cell strains (n = 3) were treated with gremlin (1 μg/ml) for 3, 6, 12, 24 and 48 to evaluate the effects of exogenous gremlin on LOX protein expression. Gremlin induced both cell-associated (Fig. 3B and D) and secreted (Fig. 3C and E) LOX proteins as early as 6 h and maintained this induction for up to 48 h. We were unable to get consistent western immunoblot results for LOXL3. The gremlin induction of LOXL2 and LOXL4 proteins peaked as soon as 3 h and was maintained at 48 h. Therefore, gremlin induction of LOX and LOXL mRNA and proteins was both time and concentration dependent.
Fig. 2.
Concentration-dependent gremlin-induction of LOX mRNA and proteins. Concentration dependent induction of LOX mRNA (A), cell-associated protein (B), and secreted proteins (C) by 0–2 μg/ml gremlin in cultured TM cell strains (n = 3). qRT-PCR values (A) represent fold LOX induction normalized to GAPDH and compared to controls. Three replicates of each sample were employed. Each line represents the individual LOX gene. Concentration dependent induction of cell associated (B) and secreted LOX proteins (C) by 0–2 μg/ml gremlin in cultured TM cell strains. Similar results were observed in two additional TM cell strains. The western blot images are representative of two independent experiments in each TM cell strain. (D–E) Densitometry analysis of western immunoblots for cell associated (D) and secreted (E) LOXs are plotted as lines. One-Way ANOVA was used for statistical analyses for comparing the samples. *0.01 < p < 0.05, **0.0001 < p < 0.01 and ***p < 0.0001.
Fig. 3.
Time-dependent gremlin-induction of LOX proteins. Time course induction (0–48 h) of LOX mRNA (A), cell-associated proteins (B), and secreted LOX proteins (C) after treatment of cultured TM cell strains (n = 3) with gremlin (1 μg/ml). qRT-PCR values (A) represent fold LOX induction normalized to GAPDH compared to controls. Three replicates of each sample were employed for qRT-PCR. Each line represents the individual LOX gene. Time dependent induction of cell associated (B) and secreted LOX proteins (C) by gremlin in cultured TM cell strains. Similar results were observed in two additional TM cell strains. The western blot images are representative of two independent experiments in each TM cell strain. GAPDH served as loading control (B), while equal volumes of conditioned medium were used for secreted proteins (C). (D–E) Densitometry analysis of western immunoblots for cell associated (D) and secreted (E) LOXs are plotted as continuous lines. One-Way ANOVA was used for statistical analyses for comparing the samples. *0.01 < p < 0.05, **0.0001 < p < 0.01 and ***p < 0.0001.
3.3. TGFβ signaling in gremlin induction of LOX proteins
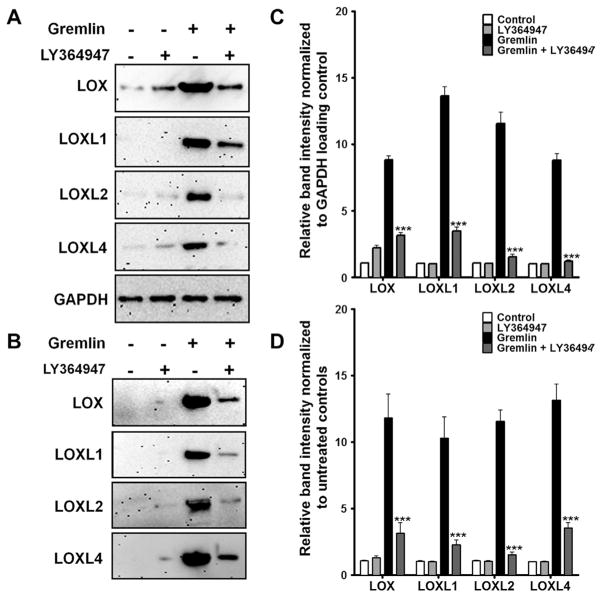
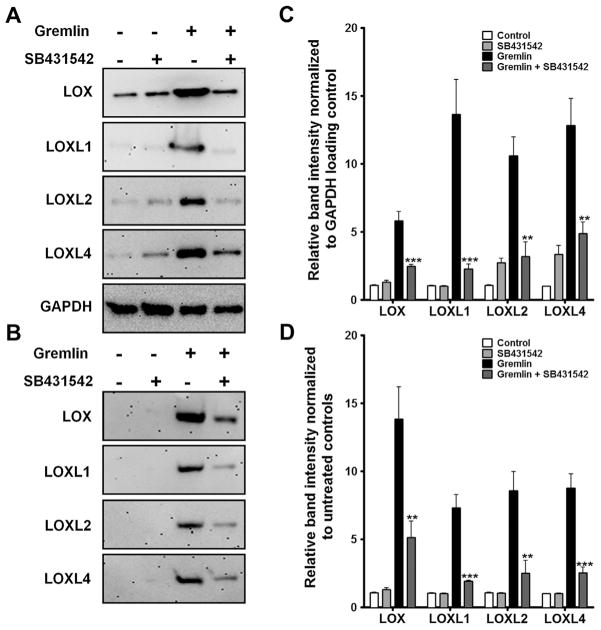
We previously used various small molecule inhibitors to explore the involvement of TGFβ signaling pathway(s) in gremlin-mediated ECM induction. We discovered that gremlin utilizes TGFβ receptors to induce ECM proteins (Sethi et al., 2011a). We employed a similar approach to study the role of TGFβ receptors/signaling pathways in regulating gremlin induction of LOX and LOXL proteins. SB431542 is a selective TGFBR1 and TGFBR2 receptor inhibitor, while LY364947 is relatively more selective for the TGFBR2 receptor than the TGFBR1 receptor. We pretreated TM cell strains (n = 3) for 1 h with or without 5 μM SB431542 or LY364947 followed by treatment with recombinant gremlin (1 μg/ml) for 24 h. Gremlin elevated cell-associated and secreted LOX and LOXL protein expression compared to untreated or inhibitor only-treated samples. Pre-treatment with either of the two inhibitors, LY364947 (Fig. 4) or SB431542 (Fig. 5) blocked gremlin-mediated LOX induction in all TM cell strains tested (p < 0.001). Treatment with the inhibitors alone did not have any effect on the expression of LOX or LOXL proteins. The inhibitors alone or in combination with gremlin also did not affect the health of TM cells as measured by LDH cytotoxicity assay (Data not shown).
Fig. 4.
TGFβ receptor II inhibitor LY364947 blocks gremlin-induction of LOX proteins. Western immunoblots determining the effect of TGFBR inhibitor LY364947 on gremlin induction of cell-associated (A) and secreted (B) LOX proteins. Western immunoblots of TM cells pretreated with 5 μM of LY364947 followed by treatment with 1 μg/ml of gremlin for 24 h. Untreated and inhibitor only treated cells served as negative controls. GAPDH was used as a loading control for cell associated proteins. Blots shown are representative data obtained in one TM strain. The experiment was performed independently two times in three different TM cell strains. (C–D) Densitometry analysis of western immunoblots for cell associated (C) and secreted (D) LOXs are plotted as bar graphs. Two-tailed student’s t test was used to compare gremlin- and gremlin + LY364549-treated samples. ***p < 0.0001.
Fig. 5.
TGFβ receptor I/II inhibitor SB431542 blocks gremlin-induction of LOX proteins. Western immunoblots determining the effect of TGFBR inhibitor SB431542 on gremlin induction of cell-associated (A) and secreted (B) LOX proteins. Western immunoblots of TM cells pretreated with 5 μM of SB431542 followed by treatment with 1 μg/ml of gremlin for 24 h. Untreated and inhibitor only treated cells served as negative controls. GAPDH was used as loading control for cell associated proteins. Blots shown are representative data obtained in one TM strain. The experiment was performed independently two times in three different TM cell strains. (C–D) Densitometry analysis of western immunoblots for cell associated (C) and secreted (D) LOXs are plotted as bar graphs. Two-tailed student’s t test was used to compare gremlin- and gremlin + SB431542-treated samples. **0.0001 < p < 0.01, ***p < 0.0001.
We also previously reported that gremlin induced phosphorylation of SMAD2 and SMAD3 proteins within 15 min in TM cells and maintained this phosphorylation up to 4 h. SMAD2 and 3 together or individually form a complex with co-SMAD4 to regulate transcription of target genes (Shi and Massague, 2003). To determine whether SMAD3 transcriptionally regulates LOX proteins, we employed SIS3, a selective small molecule inhibitor of SMAD3. Three TM cell strains were pretreated with SIS3 (10 μM) six hours prior to treating with recombinant gremlin for an additional 24 h to study the expression of LOX and LOXL proteins. Untreated cells and SIS3 alone treated cells served as negative controls. Gremlin-induction of cell-associated (Fig. 6A and C) and secreted (Fig. 6B and D) LOX protein expression was inhibited by SIS3 pretreatment. Therefore, we concluded that gremlin induction of LOXs is mediated by SMAD3 signaling.
Fig. 6.
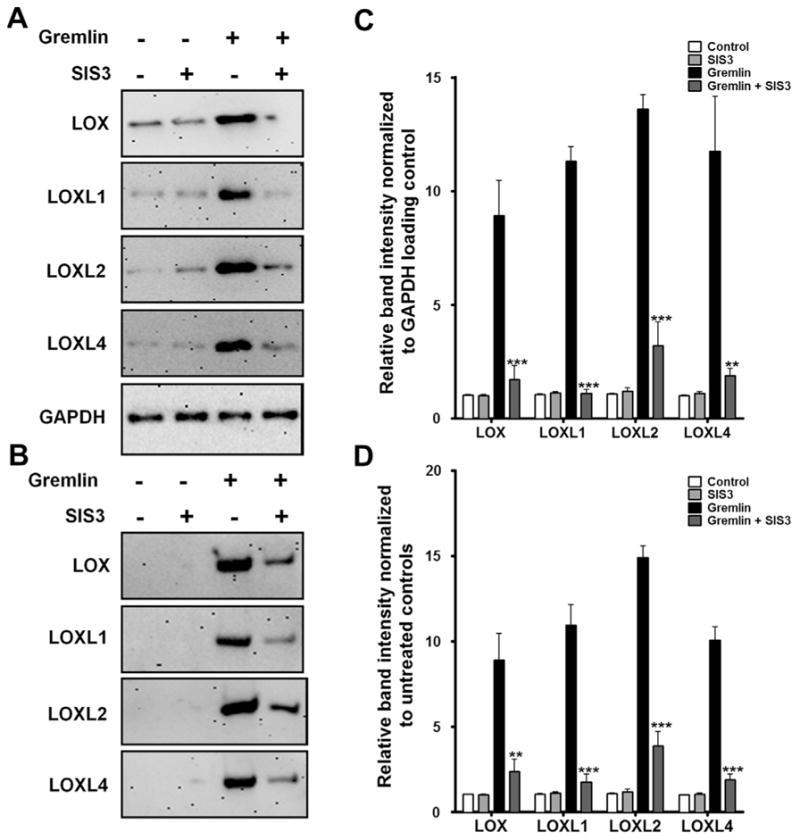
SMAD3 inhibitor SIS3 blocks gremlin-induction of LOX proteins. Representative western immunoblots of SMAD3 inhibitor SIS3 effects on gremlin induction of cell-associated (A) and secreted (B) LOX proteins. Western immunoblots of TM cells pretreated with 10 μM of SIS3 followed by treatment with 1 μg/ml of gremlin for 24 h. Untreated and inhibitor only treated cells served as negative controls for cell-associated proteins. GAPDH was used as loading control for cell associated proteins. Blots shown are representative data obtained in one TM strain. The experiment was performed independently two times in three different TM cell strains. (C–D) Densitometry analysis of western immunoblots for cell associated (C) and secreted (D) LOXs are plotted as bar graphs. Two-tailed student’s t test was used to compare gremlin− and gremlin + SIS3-treated samples. **0.0001 < p < 0.01, ***p < 0.0001.
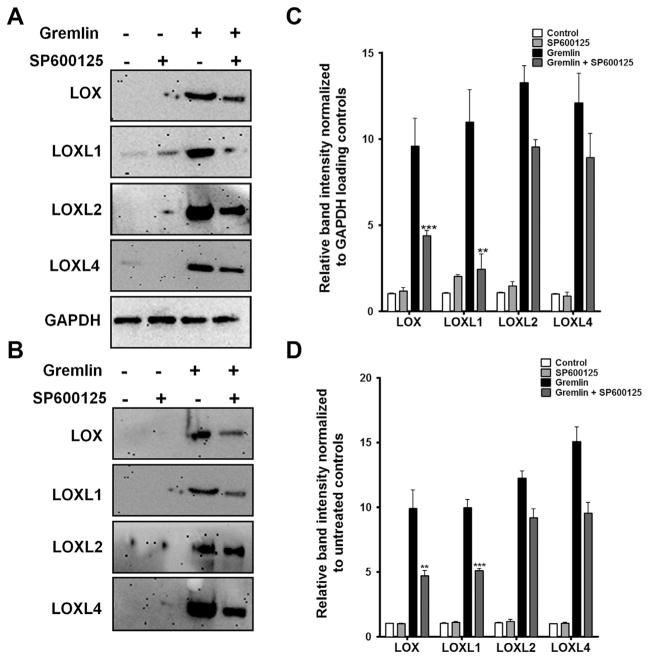
We previously demonstrated that TGFβ2 utilizes the JNK signaling pathway to regulate LOX and LOXL induction in TM cells (Sethi et al., 2011b). Therefore, we wanted to test whether gremlin also utilizes MAPK signaling pathways to regulate LOX protein expression. We used SP600125, a selective small molecule inhibitor of JNK, to determine the potential role of the JNK signaling pathway in gremlin-induction of LOX proteins. Three TM cell strains were pretreated with SP600125 (10 μM) for one hour prior to treating with recombinant gremlin for an additional 24 h. Untreated cells and SP600125-alone treated cells served as negative controls. Gremlin-induction of cell-associated (Fig. 7A and C) and secreted (Fig. 7B and D) LOX and LOXL1 protein expression was inhibited by SP600125 pretreatment. However, SP600125 only mildly blocked gremlin induction of cell-associated LOXL2 and LOXL4 proteins, but did not affect their secreted forms.
Fig. 7.
JNK inhibitor SP600125 blocks gremlin-induction of several LOX proteins. Representative western immunoblots on the effect of JNK inhibitor SP600125 on gremlin induction of cell-associated (A) and secreted (B) LOX proteins. Western immunoblots of TM cells pretreated with 10 μM of SP600125 followed by treatment with 1 μg/ml of gremlin for 24 h. Untreated and inhibitor only treated cells served as negative controls. GAPDH was used as loading control for cell associated LOXs. Blots shown are representative data obtained in one TM strain. The experiment was performed independently two times in three different TM cell strains. (C–D) Densitometry analysis of western immunoblots for cell associated (C) and secreted (D) LOXs are plotted as bar graphs. Two-tailed student’s t test was used to compare gremlin− and gremlin + SP600125-treated samples. **0.0001 < p < 0.01, ***p < 0.0001.
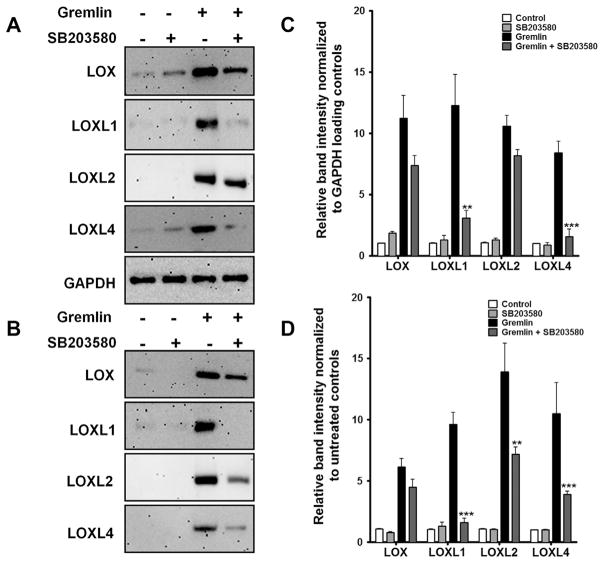
TGFβ2 also phosphorylates and activates p38 MAPK in cultured TM cells as early as 15 min and maintains this phosphorylation state for 4 h (Sethi et al., 2011b). To determine whether the p38 pathway regulates LOX protein expression, we employed SB203580, a selective small molecule inhibitor of p38. Three TM cell strains were pretreated with SB203580 (10 μM) for one hour prior to treating with recombinant gremlin for an additional 24 h. Untreated cells and SB203580-alone treated cells served as negative controls. Gremlin-induction of cell-associated LOXL1 and LOXL4 proteins was completely inhibited by SB203580 pretreatment (Fig. 8A and C). SB203580 mildly decreased gremlin induction of LOX and only marginally blocked gremlin-induced LOXL2. SB203580 also blocked gremlin induction of secreted LOXL1, LOXL2 and LOXL4 proteins and had little effect on blocking LOX secretion (Fig. 8B and D).
Fig. 8.
p38 MAPK inhibitor SB203580 blocks gremlin-induction of several LOX proteins. Representative western immunoblots of p38 MAPK inhibitor SB203580 effects on gremlin induction of cell-associated (A) and secreted (B) LOX proteins. Western immunoblots of TM cells pretreated with 10 μM of SB203580 followed by treatment with 1 μg/ml of gremlin for 24 h. Untreated and inhibitor only treated cells served as negative controls. GAPDH was used as loading control for cell associated LOXs. Blots shown are representative data obtained in one TM strain. The experiment was performed independently two times in three different TM cell strains. (C–D) Densitometry analysis of western immunoblots for cell associated (C) and secreted (D) LOXs are plotted as bar graphs. Two-tailed student’s t test was used to compare gremlin− and gremlin + SB203580-treated samples. **0.0001 < p < 0.01, ***p < 0.0001.
Collectively, these data illustrate a complex regulation of LOX proteins in TM cells, which may have significant implications in fibrosis and glaucoma pathogenesis.
4. Discussion
LOX and LOXL oxidize peptidyl lysine and hydroxylysine residues in collagen and lysine residues in elastin to produce peptidyl alpha-aminoadipic-delta-semialdehydes. These aldehyde residues can spontaneously combine with vicinal peptidyl aldehydes or with epsilon-amino groups of peptidyl lysine to form the covalent cross-links that stabilize and decrease the solubility of fibrillar collagen and elastin fibers in the ECM (Kagan and Li, 2003). Also, LOX and LOXL are involved in several connective tissue fibrotic diseases (Sethi et al., 2012). It is likely that these enzymes also play a crucial role in other fibrotic diseases such as glaucoma.
Elastin and collagen proteins are elevated in the glaucomatous TM (Acott and Kelley, 2008), and a recent report used atomic force microscopy to demonstrate that glaucomatous TM tissues were significantly stiffer than the TM from non-glaucoma eyes (Last et al., 2011). Greater cross-linking of ECM proteins within the TM could lead to greater tissue stiffness. Overexpression of profibrotic molecules like TGFβ2 has been shown to increase ECM crosslinking (Sethi et al., 2011b; Tovar-Vidales et al., 2011; Welge-Lussen et al., 2000).
Gremlin’s role in development and in several fibrotic diseases is quite well known. It is not uncommon to find developmental genes re-expressed in pathological conditions, including cancer and inflammation. Prior to this work, our understanding of gremlin’s involvement in TM fibrosis is currently limited to TGFβ signaling-mediated gremlin induction of ECM proteins (Sethi et al., 2011a). However, the role of gremlin in regulating ECM crosslinking has not been studied previously.
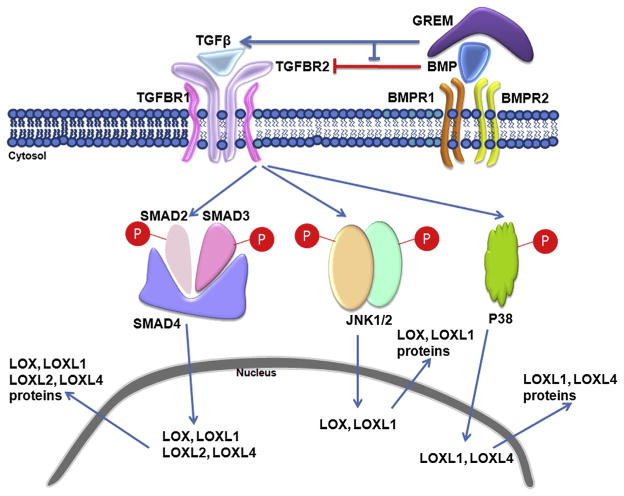
Our current results demonstrate that exogenous gremlin induces mRNA and protein expression of LOX and LOXL genes in multiple TM cell strains. We also demonstrated that gremlin utilizes the TGFβ signaling pathway to regulate LOX and LOXL proteins and that both the canonical SMAD as well as non-SMAD JNK1/2 and p38 MAPK signaling pathways are involved in this gremlin induction. The results have been illustrated in Fig. 9, which suggests that gremlin functions by inhibiting the endogenous BMP antagonism of TGFβ signaling in cultured TM cells. This leads to LOX and LOXL induction. These data highlight the significance of ECM cross-linking by BMP antagonists like gremlin in the TM cells and highlight that perturbations of the TGFβ–BMP signaling pathways regulation of the ECM environment may lead to TM fibrosis and glaucoma. Since TGFβ and BMP pathways are endogenously active in TM cells (Wordinger et al., 2002, 2007), the data raise important questions about the existence and regulation of a plethora of downstream players including other intracellular and extracellular proteins that are involved in maintaining TM homeostasis, disturbance of which may lead to diseases like glaucoma.
Fig. 9.
Proposed mechanism of gremlin regulation of LOXs in TM. Gremlin blocks BMP signaling pathway, which is unable to inhibit the endogenous TGFβ signaling in TM cells. The endogenously active TGFβ pathway can activate Smad, P38 and JNK pathways to induce different LOX genes and proteins in TM cells.
We have previously reported that both TGFβ2 and gremlin phosphorylate and activate SMAD2/3 signaling in TM cells. Knocking down the SMAD signaling pathway blocked TGFβ2 induction of LOX and LOXL (Sethi et al., 2011b). By blocking SMAD signaling with SIS3, we also observed that gremlin induction of LOX proteins is inhibited. These data not only imply that gremlin employs the canonical SMAD pathway to regulate LOXs, but also emphasizes the profibrotic effects of SMAD signaling in the TM. We have previously observed that TM cells maintain basal phosphorylation levels of both JNK and p38 MAPK (Sethi et al., 2011a, 2011b).
Crosstalk and interaction between SMAD and MAPK pathways has been observed in several cell types and in a variety of normal and pathological conditions (de la Cruz-Merino et al., 2009; Javelaud and Mauviel, 2005). Our data indicate that the basal level of MAPK kinase activity may be important in regulating LOX and LOXL in TM cells. Whether the basal MAPK kinase activity regulates LOX enzymatic activity is a question that needs to be addressed.
Several additional questions are raised by our current results. First, it was surprising to find that all 5 LOX family genes are induced by gremlin. The LOX and LOXL enzymes may have different specific roles in the TM including differences in substrate specificity and/or specific localization patterns. The potential relationship between the LOX proteins in regulating AH outflow in gremlin-induced ocular hypertension and POAG is not known. It is also not clear which LOX protein is important for normal TM homeostasis and if any of the LOX proteins are directly involved in pathogenesis of glaucoma. Future in vivo studies are needed to address this question. The role of MAPK signaling in TM fibrosis and in regulating TM LOX enzymatic activity also needs further study. Our current results provide a foundation to address these issues in future studies.
Acknowledgments
The authors would like to acknowledge grant support from the National Institute of Health-National Eye Institute (EY-017374). The authors would also acknowledge Ankur Jain and Tara Tovar-Vidales at the North Texas Eye Research Institute, UNT Health Science Center for his help in this project. We would also like to thank Lions Eye Institute for Transplant and Research (Tampa, FL) for providing donor eyes used for preparing primary TM cell cultures.
Abbreviations
- POAG
Primary open-angle glaucoma
- IOP
Intraocular pressure
- AH
Aqueous Humor
- TM
Trabecular Meshwork
- ECM
Extracellular matrix
- TGFβ
Transforming growth factor beta
- FN
Fibronectin
- COL
Collagen
- ELN
Elastin
- PAI1
Plasminogen activator inhibitor-1
- TIMP1
Tissue inhibitor of metalloproteinase-1
- TGM2
Transglutaminase 2
- LOX
Lysyl Oxidase
- LOXL
Lysyl Oxidase like
- BMP
Bone morphogenetic proteins
- BMPR
Bone morphogenetic proteins receptor
- MAPK
Mitogen activated protein kinase
- JNK
c-Jun N-terminal Kinase
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008;86:543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AGIS. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration The AGIS Investigators. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- Bachmann B, Birke M, Kook D, Eichhorn M, Lutjen-Drecoll E. Ultra-structural and biochemical evaluation of the porcine anterior chamber perfusion model. Invest Ophthalmol Vis Sci. 2006;47:2011–2020. doi: 10.1167/iovs.05-1393. [DOI] [PubMed] [Google Scholar]
- de la Cruz-Merino L, Henao-Carrasco F, Garcia-Manrique T, Fernandez-Salguero PM, Codes-Manuel de Villena M. Role of transforming growth factor beta in cancer microenvironment. Clin Transl Oncol. 2009;11:715–720. doi: 10.1007/s12094-009-0433-8. [DOI] [PubMed] [Google Scholar]
- Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47:226–234. doi: 10.1167/iovs.05-1060. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Tamm ER. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012;347:279–290. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Yu AH, Welge-Lussen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:715–726. doi: 10.1167/iovs.06-0226. [DOI] [PubMed] [Google Scholar]
- Gottanka J, Chan D, Eichhorn M, Lutjen-Drecoll E, Ethier CR. Effects of TGF-beta2 in perfused human eyes. Invest Ophthalmol Vis Sci. 2004;45:153–158. doi: 10.1167/iovs.03-0796. [DOI] [PubMed] [Google Scholar]
- Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001;239:109–113. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- Javelaud D, Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene. 2005;24:5742–5750. doi: 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Last JA, Pan T, Ding Y, Reilly CM, Keller K, Acott TS, Fautsch MP, Murphy CJ, Russell P. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:2147–2152. doi: 10.1167/iovs.10-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma FY, Sachchithananthan M, Flanc RS, Nikolic-Paterson DJ. Mitogen activated protein kinases in renal fibrosis. Front Biosci (Schol Ed) 2009;1:171–187. doi: 10.2741/s17. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A, Jain A, Zode GS, Wordinger RJ, Clark AF. Role of TGFbeta/Smad signaling in gremlin induction of human trabecular meshwork extracellular matrix proteins. Invest Ophthalmol Vis Sci. 2011a;52:5251–5259. doi: 10.1167/iovs.11-7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A, Mao W, Wordinger RJ, Clark AF. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011b;52:5240–5250. doi: 10.1167/iovs.11-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A, Wordinger RJ, Clark AF. Focus on molecules: lysyl oxidase. Exp Eye Res. 2012;104:97–98. doi: 10.1016/j.exer.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010;51:2067–2076. doi: 10.1167/iovs.09-4567. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Tovar-Vidales T, Clark AF, Wordinger RJ. Transforming growth factor-beta2 utilizes the canonical Smad-signaling pathway to regulate tissue transglutaminase expression in human trabecular meshwork cells. Exp Eye Res. 2011;93:442–451. doi: 10.1016/j.exer.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Vidales T, Roque R, Clark AF, Wordinger RJ. Tissue transglutaminase expression and activity in normal and glaucomatous human trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2008;49:622–628. doi: 10.1167/iovs.07-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994;59:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- Welge-Lussen U, May CA, Lutjen-Drecoll E. Induction of tissue transglutaminase in the trabecular meshwork by TGF-beta1 and TGF-beta2. Invest Ophthalmol Vis Sci. 2000;41:2229–2238. [PubMed] [Google Scholar]
- Wordinger RJ, Agarwal R, Talati M, Fuller J, Lambert W, Clark AF. Expression of bone morphogenetic proteins (BMP), BMP receptors, and BMP associated proteins in human trabecular meshwork and optic nerve head cells and tissues. Mol Vis. 2002;8:241–250. [PubMed] [Google Scholar]
- Wordinger RJ, Fleenor DL, Hellberg PE, Pang IH, Tovar TO, Zode GS, Fuller JA, Clark AF. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Zode G, Clark AF. Focus on molecules: gremlin. Exp Eye Res. 2008;87:78–79. doi: 10.1016/j.exer.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]