Abstract
Purpose
Klinefelter syndrome (KS) is related to testicular insufficiency, which causes low testosterone levels in serum. Generally, sex hormone levels and bone mineral density (BMD) are lower in patients with KS than normal. We investigated the effects of testosterone replacement on serum testosterone levels and BMD in KS patients.
Materials and Methods
From December 2005 to March 2008, 18 KS patients with a 47, XXY karyotype were treated with initial intramuscular injections of long-acting testosterone undecanoate (Nebido®, 1000 mg/4 mL) at baseline and second injections after six weeks. An additional four injections were administered at intervals of 12 weeks after the second injection. BMD was measured at the lumbar spine (L2-4), the left femoral neck and Ward's triangle, using dual energy X-ray absorptiometry. Medical histories, physical examinations and prostate specific antigen, hematology and serum chemistry were conducted for each patient. In addition, total testosterone and sex hormone-binding globulin levels were measured.
Results
Following testosterone replacement, mean serum total testosterone increased significantly from baseline (0.90 vs. 4.51 ng/mL, p<0.001), and total testosterone rose to normal levels after replacement in all patients. The mean BMD of the lumbar spine increased significantly (0.91 vs. 0.97 g/cm2, p<0.001). Similar increases of BMD were also observed at the femoral neck, but this increase was not significant.
Conclusion
These findings suggest that testosterone replacement therapy may be effective in treating BMD deficiency in men with testosterone deficiency, especially those with Klinefelter syndrome.
Keywords: Bone mineral density, Klinefelter syndrome, testosterone
INTRODUCTION
Osteoporosis and osteopenia in men are considered to be less frequent than in women. It might be due in part to a man's greater bone size, greater body mass, greater accrual of bone during growth, absence of a clear decrease in endogenous sex hormones as seen in menopause, and shorter average life span compared with women.1 However, it is frequent in men with secondary osteoporosis due to hypogonadism, glucocorticoid excess, alcoholism, hypercalciuria, malabsorption and hyperthyroidism.
Klinefelter syndrome (KS) is a common sex chromosome disorder in which males are born with an extra copy of the X chromosome. It occurs at a frequency of approximately 1 in 500 men and results in impaired spermatogenesis and androgen deficiency.2 KS is a type of primary hypogonadism, and 44-48% of KS patients have osteopenia and 6-14% osteoporosis that is a well known complication of male hypogonadism.2-4 Androgen deficiency in men is known to lead to a significant decrease in bone mineral density (BMD).5 Similarly, osteoporosis is related to lower level of serum testosterone in KS.6,7 Although increasing the testosterone levels is presumed to have a beneficial effect on BMD, only few data are available in these patients after testosterone replacement therapy, and data are still controversial.8-12
The aim of this study was to measure BMD, as well as biochemical and hormonal parameters in men with Klinefelter syndrome undergoing testosterone replacement therapy and determine whether testosterone replacement therapy reversed the detrimental effects of hypogonadism on bone density.
MATERIALS AND METHODS
Patients
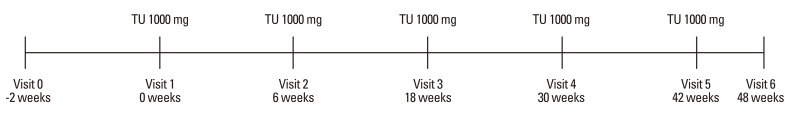
From December 2005 to March 2008, 18 Korean KS patients, ranging from 30 to 44 years of age, were studied. They had a careful medical history and physical examination taken, and the accuracy of data was verified by checking all available medical records. The diagnosis of KS was based on clinical features including androgen deficiency symptoms, low testosterone levels, and karyotype showing 47, XXY. Exclusion criteria were treatment with any medication known to affect the skeleton, known prostate cancer or clinically significant benign prostate hyperplasia, androgen-dependent carcinoma of the male mammary gland, other significant medical or psychological conditions, sleep apnea or snoring requiring treatment, or diabetes mellitus. Informed consent to the study was obtained from all the subjects. Intramuscular long-acting testosterone undecanoate (Nebido®, Bayer Pharma, Berlin, Germany, 1000 mg/4 mL) was administered to the deep gluteus muscle at week 0 (baseline), week 6 and every 12 weeks thereafter (Fig. 1). Blood samples were obtained at baseline and at injection time points for prostate specific antigen (PSA), hematology (including hemoglobin and hematocrit) and serum chemistry. Measurements of height and weight were taken, and body mass index was calculated [body mass index (BMI): kg/m2].
Fig. 1.
Testosterone injection schedule. TU, testosterone undecanoate.
Hormonal measurements
Baseline sex hormone-binding globulin (SHBG) and serum or total testosterone (TT) levels were measured at 2 weeks before injection, and 30 weeks and 48 weeks after the first injection. Venous blood samples were collected between 08:00 and 10:00 hours. The serum was separated by centrifugation and subsequently stored at -20℃ until assayed. Serum TT level was measured by enzyme sandwich immunoassay using Vitros® ECi Immunodiagnostic System (Ortho Clinical Diagnostics, Rochester, NY, USA). Free and bioavailable testosterone was calculated by using the "Free & Bioavailable Testosterone calculator" (http://www.issam.ch/freetesto.htm). SHBG in serum was measured by using IRMA-Count® (a solid-phase immunoradiometric assay designed for the quantitative measurement of SHBG in serum) (Diagnostic Products Corporation, Los Angeles, CA, USA).
Bone density determination
BMD (the area density in g/cm2) was assessed using dual-energy X-ray absorptiometry operating in the fan-beam mode (QDR-2000 densitometer, Hologic Inc., Waltham, MA, USA). Quality control scans were taken daily, using an anthropometric spine phantom supplied by the manufacturer; the long-term (>1 year) coefficient of variation was 0.40%. BMD was measured on visit 0 and 48 weeks. Lumbar BMD was assessed at L2-4 on the basis of the postero-anterior view. Fractured vertebrae were excluded from analysis. The femur neck and Ward's triangle BMD was measured at the upper left femur. The mean precision error of absorptiometry measurements was <1.5% for the lumbar spine, and <2% for the femur neck and Ward's triangle BMD. T scores were calculated using the manufacturer's references. Individual values of vertebral and femoral BMD in KS patients were also expressed as Z-score, calculated on the basis of this reference range. T-score compares with young normal mean BMD, and the Z-score compares with age-matched controls and race-matched controls.
Statistical analysis
The characteristics of the patients were summarized by descriptive statistics. Student's t-test (paired) and Wilcoxon rank sum test were used to compare continuous variables. Data storage and statistical analysis were performed using the SPSS 12.0K statistical program (SPSS Inc., Chicago, IL, USA). A p-value <0.05 was used to define statistical significance. Results are presented as mean±SD unless otherwise indicated.
RESULTS
Eighteen patients were followed up (mean age 35.9±3.3 years). During follow-up period, there was a slight decrease in weight and a slight increase in height, which resulted in a slight decrease in BMI without significance.
The effects of testosterone replacement on Klinefelter syndrome are presented in Table 1. A small and significant increase was observed in PSA, but PSA levels were lower than normal limit. SHBG decreased from 44.8 nmol/L to 36.6 nmol/L, but it was not statistically significant (p=0.072). Total testosterone rose to normal levels after replacement (0.9 ng/mL to 4.511 ng/mL, p<0.001). Likewise, free and bioavailable testosterones increased from 0.015 ng/mL to 0.974 ng/mL, and 0.347 ng/mL to 2.284 ng/mL, respectively, and these were all statistically significant.
Table 1.
Effects of Testosterone Replacement on Bone Mineral Density in Patients with Klinefelter's Syndrome
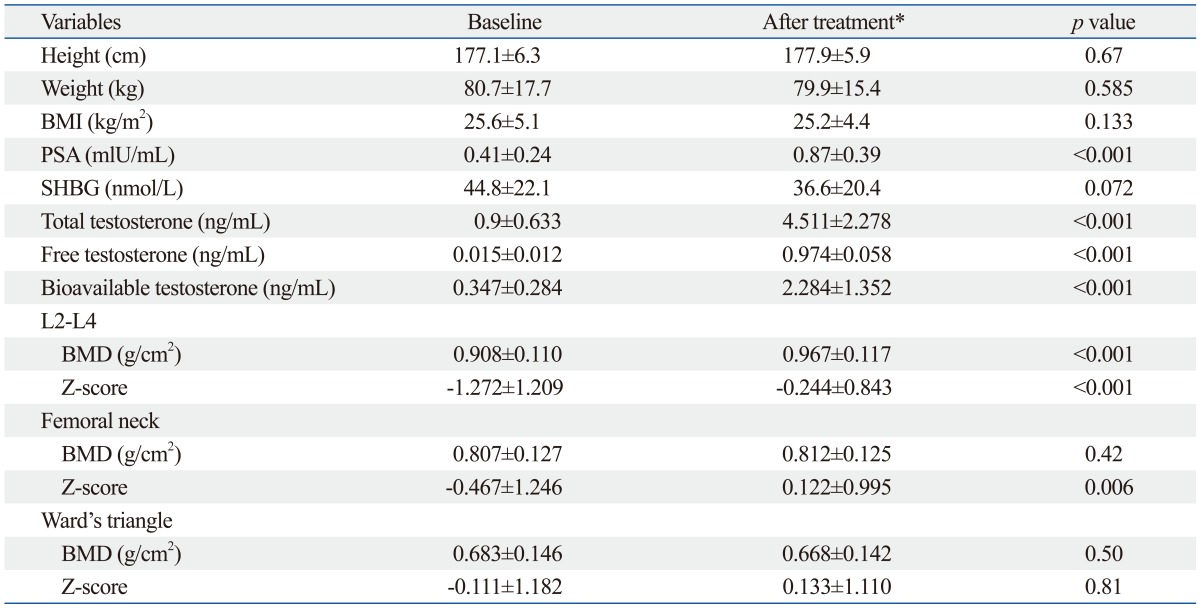
BMI, body mass index; PSA, postate-specific antigen; SHBG, sex hormone-binding globulin; BMD, bone mineral density.
Data are given as means±SD.
*After treatment: 48 weeks after the first injection.
The results of BMD (g/cm2) and Z-scores at the lumbar spine, femoral neck, and Ward's triangle in the treated 18 KS patients are shown in Table 1. At the lumbar spine, BMD and Z-score significantly increased from 0.908 g/cm2 to 0.967 g/cm2 and from -1.272 to -0.244, respectively. Similar increases were also observed at femoral neck, but significant difference only in Z-score (BMD: 0.807 g/cm2 to 0.812 g/cm2, p=0.42, Z-score: -0.467 to 0.122, p=0.006). At the BMDs and Z-score of the Ward's triangle, there were no significant changes (BMD: 0.683 g/cm2 to 0.668 g/cm2, p=0.5, Z-score: -0.111 to 0.133, p=0.81).
DISCUSSION
We previously suggested that testosterone replacement may be an effective treatment for increasing BMD in men with KS and a low serum testosterone level.7 In this study, we evaluated whether testosterone replacement therapy reversed the detrimental effects of KS on bone density. Several previous reports indicate that testosterone replacement increased BMD in middle-aged men or androgen deficiency.13,14 Isidori, et al.14 reported that testosterone improved BMD among 1083 subjects at lumbar spine by +3.7% (CI: 1.0-6.4%) compared to placebo, but not at the femoral neck after a minimum of 12-36 months of treatment. However, the effects of androgen replacement on bone mineral density in patients with KS have been studied very little. When studying 29 men with KS, there was no correlation between serum testosterone levels and bone density.15 Wong, et al.12 suggested that sufficient testosterone replacement does not reverse the decrease of bone mass, associated with hypogonadism, in patients with KS. They pointed out several possible explanations for these findings as follows: first, short-acting testosterone replacement by parenteral and oral replacement could not dispense physiological testosterone concentrations and normal circadian patterns; and many patients with KS were diagnosed after pubertal development. After pubertal development, epiphyseal bone closure and skeletal maturation had taken place. Therefore, androgen replacement was instituted late and after puberty. The third possibility is that it may be take a long time for restore the bone density and mass to normal. Contrary to these findings, only few available data indicate that increasing the testosterone levels has a beneficial effect on BMD.8-12
Our present findings suggest that testosterone replacement is an effective treatment for increasing BMD in men with KS and a low serum testosterone level. In this study, long-acting testosterone undecanoate was administered. The use of a long-acting form of the hormone, which provides a constant level of testosterone, may be effective compared with the use of a shorter acting formulation. The undecanoate ester of natural testosterone is a long acting, intramuscular, injectable testosterone that extends the current maximum treatment dosing interval approximately 4-fold compared with that of other injectable products, thus eliminateing the need of daily topical application of gels and patches. Testosterone undecanoate (TU: 1000 mg) given at a variable dosing interval is used for treating men with hypogonadism. Studies in Europe have demonstrated that 1000 mg TU administered as an intramuscular injection at 10 to 14-week intervals is adequate to sustain normal testosterone levels in men with hypogonadism.16,17 This long acting formulation has been shown to be safe and well tolerated. In this study, follow-up over 48 weeks, when all subjects received TU, showed a good level of safety.
We observed in the present study that the testosterone replacement definitely increased BMD and Z-score of the lumbar spine, whereas it was not effective or minimal in femoral neck and Ward's triangle. This finding may imply that the loss of bone occurs mainly in trabecular bone rather than cortical bone since trabecular bone is metabolically more active than cortical bone.
The potential weakness of this study is that diet such as calcium, vitamin D and the like were not assessed among the subjects, which could influence BMD. In addition, testosterone replacement was supplemented after puberty. Therefore, for better results, it would be necessary to give testosterone replacement before patients' puberty.
In conclusion, male hypogonadism including KS has been recognized as one of the major causes of secondary osteoporosis in men, nevertheless, most cases seem to be left undiagnosed. Bone loss is a distinctive feature of KS. We suggest that testosterone replacement may be effective for preventing BMD deficiency. Therefore, early diagnosis and testosterone replacement therapy coupled with specific treatment for osteoporosis could be useful in preventing future severe bone loss and associated skeletal morbidity.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084318).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Seeman E. During aging, men lose less bone than women because they gain more periosteal bone, not because they resorb less endosteal bone. Calcif Tissue Int. 2001;69:205–208. doi: 10.1007/s00223-001-1040-z. [DOI] [PubMed] [Google Scholar]
- 2.Klinefelter HF. Background of the recognition of Klinefelter's syndrome as a distinct pathologic entity. Am J Obstet Gynecol. 1973;116:436–437. doi: 10.1016/s0002-9378(15)31307-7. [DOI] [PubMed] [Google Scholar]
- 3.Seeman E, Melton LJ, 3rd, O'Fallon WM, Riggs BL. Risk factors for spinal osteoporosis in men. Am J Med. 1983;75:977–983. doi: 10.1016/0002-9343(83)90878-1. [DOI] [PubMed] [Google Scholar]
- 4.van den Bergh JP, Hermus AR, Spruyt AI, Sweep CG, Corstens FH, Smals AG. Bone mineral density and quantitative ultrasound parameters in patients with Klinefelter's syndrome after long-term testosterone substitution. Osteoporos Int. 2001;12:55–62. doi: 10.1007/s001980170158. [DOI] [PubMed] [Google Scholar]
- 5.Orwoll ES, Klein RF. Osteoporosis in men. Endocr Rev. 1995;16:87–116. doi: 10.1210/edrv-16-1-87. [DOI] [PubMed] [Google Scholar]
- 6.Horowitz M, Wishart JM, O'Loughlin PD, Morris HA, Need AG, Nordin BE. Osteoporosis and Klinefelter's syndrome. Clin Endocrinol (Oxf) 1992;36:113–118. doi: 10.1111/j.1365-2265.1992.tb02910.x. [DOI] [PubMed] [Google Scholar]
- 7.Seo JT, Lee JS, Oh TH, Joo KJ. The clinical significance of bone mineral density and testosterone levels in Korean men with non-mosaic Klinefelter's syndrome. BJU Int. 2007;99:141–146. doi: 10.1111/j.1464-410X.2006.06584.x. [DOI] [PubMed] [Google Scholar]
- 8.Choi HR, Lim SK, Lee MS. Site-specific effect of testosterone on bone mineral density in male hypogonadism. J Korean Med Sci. 1995;10:431–435. doi: 10.3346/jkms.1995.10.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Rosa M, Paesano L, Nuzzo V, Zarrilli S, Del Puente A, Oriente P, et al. Bone mineral density and bone markers in hypogonadotropic and hypergonadotropic hypogonadal men after prolonged testosterone treatment. J Endocrinol Invest. 2001;24:246–252. doi: 10.1007/BF03343854. [DOI] [PubMed] [Google Scholar]
- 10.Stepan JJ, Burckhardt P, Hána V. The effects of three-month intravenous ibandronate on bone mineral density and bone remodeling in Klinefelter's syndrome: the influence of vitamin D deficiency and hormonal status. Bone. 2003;33:589–596. doi: 10.1016/s8756-3282(03)00205-9. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Swerdloff RS. Androgen replacement therapy. Curr Ther Endocrinol Metab. 1997;6:331–337. [PubMed] [Google Scholar]
- 12.Wong FH, Pun KK, Wang C. Loss of bone mass in patients with Klinefelter's syndrome despite sufficient testosterone replacement. Osteoporos Int. 1993;3:3–7. doi: 10.1007/BF01623169. [DOI] [PubMed] [Google Scholar]
- 13.Aminorroaya A, Kelleher S, Conway AJ, Ly LP, Handelsman DJ. Adequacy of androgen replacement influences bone density response to testosterone in androgen-deficient men. Eur J Endocrinol. 2005;152:881–886. doi: 10.1530/eje.1.01920. [DOI] [PubMed] [Google Scholar]
- 14.Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 2005;63:280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith DA, Walker MS. Changes in plasma steroids and bone density in Klinefelter's syndrome. Calcif Tissue Res. 1977;22(Suppl):225–228. doi: 10.1007/BF02064069. [DOI] [PubMed] [Google Scholar]
- 16.Schubert M, Minnemann T, Hübler D, Rouskova D, Christoph A, Oettel M, et al. Intramuscular testosterone undecanoate: pharmacokinetic aspects of a novel testosterone formulation during long-term treatment of men with hypogonadism. J Clin Endocrinol Metab. 2004;89:5429–5434. doi: 10.1210/jc.2004-0897. [DOI] [PubMed] [Google Scholar]
- 17.von Eckardstein S, Nieschlag E. Treatment of male hypogonadism with testosterone undecanoate injected at extended intervals of 12 weeks: a phase II study. J Androl. 2002;23:419–425. [PubMed] [Google Scholar]