Abstract
Purpose
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is an accurate and minimally invasive technique used routinely for investigation of mediastinal and hilar lymphadenopathy. However, few studies have addressed its role in comparison to the traditional diagnostic approaches of transbronchial lung biopsy (TBLB), endobronchial biopsy (EBB), and bronchoalveolar lavage (BAL) in the diagnosis of sarcoidosis. We evaluated the usefulness of EBUS-TBNA in the diagnosis of sarcoidosis compared to TBLB, EBB, and BAL.
Materials and Methods
Consecutive patients with suspected sarcoidosis (stage I and II) on chest radiography and chest computed tomography were included. All 33 patients underwent EBUS-TBNA, TBLB, EBB, and BAL during the same session between July 2009 and June 2011. EBUS-TBNA was performed at 71 lymph node stations.
Results
Twenty-nine of 33 patients, were diagnosed with histologically proven sarcoidosis; two patients were compatible with a clinical diagnosis of sarcoidosis during follow-up; and two patients were diagnosed with metastatic carcinoma and reactive lymphadenopathy, respectively. Among 29 patients with histologically proven sarcoidosis in combination with EBUS-TBNA, TBLB, and EBB, only EBUS-TBNA and TBLB revealed noncaseating granuloma in 18 patients and one patient, respectively. The overall diagnostic sensitivities of EBUS-TBNA, TBLB, EBB, and BAL (CD4/CD8 ≥3.5) were 90%, 35%, 6%, and 71%, respectively (p<0.001). The combined diagnostic sensitivity of EBUS-TBNA, TBLB, and EBB was 94%.
Conclusion
EBUS-TBNA was the most sensitive method for diagnosing stage I and II sarcoidosis compared with conventional bronchoscopic procedures. EBUS-TBNA should be considered first for the histopathologic diagnosis of stage I and II sarcoidosis.
Keywords: Endobronchial ultrasound, transbronchial needle aspiration, sarcoidosis, mediastinal lymphadenopathy
INTRODUCTION
Sarcoidosis is a systemic inflammatory disease of unknown etiology that occurs throughout the world and affects people of all ages and races. The diagnosis of sarcoidosis is based on a compatible clinical and radiological pictures, supported by pathological evidence of noncaseating granulomas in the absence of organisms and other granulomatous diseases, such as tuberculosis and malignancy.1 These granulomas can occur with varying rates in any organ system, but are most commonly found in the lungs, with thoracic lymphadenopathy detected in up to 85% of cases.2 Pulmonary sarcoidosis is the most frequent form, and flexible bronchoscopy with tissue sampling is recommended as a first step to obtain a tissue diagnosis and exclude possible alternative diagnoses.
Flexible bronchoscopy permits transbronchial lung biopsy (TBLB) and endobronchial biopsy (EBB). Bronchoalveolar lavage (BAL) is also routinely recommended as an additional procedure. Despite the use of combined TBLB and EBB, approximately one-third of all bronchoscopies do not result in a diagnosis of sarcoidosis.3 BAL fluid lymphocytosis and a CD4/CD8 ratio ≥3.5 substantiate the diagnosis of sarcoidosis, but BAL is not used for a pathologic diagnosis.4
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a less invasive procedure that has an excellent diagnostic yield.5,6 Recent studies have reported that EBUS-TBNA has an excellent diagnostic yield in the diagnosis of sarcoidosis (85-93%).7-10
In this study, we evaluated the usefulness of EBUS-TBNA in the diagnosis of sarcoidosis compared with that of TBLB, EBB, and BAL.
MATERIALS AND METHODS
Patients
This retrospective study was conducted at the Samsung Medical Center (Seoul, South Korea) between July 2009 and June 2011. Thirty-three patients with enlarged intrathoracic lymph nodes (>1 cm on short axis) on chest computed tomography (CT) scan and suspected sarcoidosis were included. Lymph node stations were determined according to the new international lymph node map proposed by the International Association for the Study of Lung Cancer.11 All patients underwent EBUS-TBNA, TBLB, EBB, and BAL during the same session. This study was approved by the Institutional Review Board of Samsung Medical Center, which waived requirements for informed consent given the retrospective nature of the study.
Diagnosis of sarcoidosis
Diagnosis of sarcoidosis was established if the clinical and radiological findings were supported by pathological tissue demonstrating noncaseating granulomas.12 Other granulomatous diseases were ruled out by the patient's history and microbiological results. Patients were categorized as "indefinite" if the definite diagnosis could not be made by biological and pathological evaluations. In these cases, patients were followed up for ≥6 months. A clinical diagnosis of sarcoidosis was confirmed when patients met two or more of the following criteria: 1) stability or regression during follow up, 2) response to corticosteroid therapy, 3) BAL lymphocytosis with a CD4/CD8 ratio ≥3.5, and 4) demonstration of extrapulmonary manifestations of sarcoidosis (erythema nodosum, uveitis, neurologic or cardiac dysfunction).1,4,13,14
Bronchoscopic procedure
All patients received four diagnostic modalities during the same session: EBUS-TBNA, TBLB, EBB, and BAL. All bronchoscopic and EBUS-TBNA procedures were carried out on an inpatient basis under local anesthesia with mild conscious sedation. Two milliliters of 4% lidocaine was nebulized and sprayed into the pharynx. Bolus doses of 1.3% lidocaine were administered through the channel during the procedures as needed. Patients were monitored by electrocardiogram, pulse oximetry, and for blood pressure. Conventional flexible bronchoscopy (BF-1T260 bronchovideoscope; Olympus, Tokyo, Japan) was used for BAL, EBB and TBLB. First, BAL was performed in the right middle lobe. Then, at least three biopsy specimens were obtained by EBB from the bronchial wall of the right upper lobe and at least five biopsy specimens were obtained by TBLB from the right lower lobe if no parenchymal lesion was visible on a chest CT scan. If we did not obtain enough tissue from three biopsies from EBB and five biopsies from TBLB, we performed more biopsies until we obtained enough specimens for histopathological analysis. If the chest CT scan showed evidence of pulmonary parenchymal involvement (stage II), a TBLB specimen was obtained from the targeted region.
EBUS-TBNA procedure
Following conventional bronchoscopy, EBUS-TBNA was performed using a flexible convex-probe ultrasonic-puncture bronchoscope with a linear scanning transducer at a frequency of 7.5 MHz (CP-EBUS, BFUC206F-OL8; Olympus, Tokyo, Japan). A dedicated ultrasound scanner (EU-C2000; Olympus, Tokyo, Japan) was used for image processing. Color Doppler mode was used to avoid puncture of vessels as the occasion demanded. Each target nodal station was punctured at least twice, until one or more tissue core specimens were obtained with a dedicated 22-gauge needle (NA-201SX-4022; Olympus, Tokyo, Japan). The aspirated material was smeared onto glass slides. Smears were air dried and fixed in 95% alcohol. Histological specimens were fixed with 10% neutral buffered formalin and stained with hematoxylin and eosin. Aspirated material and histological specimens were sent for histopathological and microbiological examination, including cytological analysis, special staining for acid-fast bacilli, and culture for Mycobacteria and Mycobacteria tuberculosis polymerase chain reaction. Immunohistochemical staining was also performed when needed. Rapid onsite cytopathological examination was not available. Patients diagnosed with benign lymphadenopathy by EBUS-TBNA subsequently underwent clinical and radiological follow-up for at least 6 months. All patients underwent chest radiography after the procedure to ensure that pneumothorax, pneumomediastinum, or pulmonary hemorrhage did not occur.
Statistical analysis
Data are presented as medians for continuous variables and as numbers and percentages for categorical variables. The primary endpoint was the difference in diagnostic sensitivity among the four modalities. Diagnostic sensitivity was calculated using standard definitions. Yields from the diagnostic modalities were compared using the generalized estimating equation or McNemar's test. All p-values ≤0.05 were considered significant. Data were analyzed using PASW statistics software version 18 (SPSS Inc., Chicago, IL, USA).
RESULTS
Data from 33 consecutive patients with suspected sarcoidosis who underwent combined EBUS-TBNA with TBLB, EBB, and BAL were analyzed. Twelve patients were male and the median age of all patients was 46 years (range, 21-72 years). Based on radiological findings, 11 patients had stage I sarcoidosis, and 22 patients had stage II sarcoidosis.
In total 71 lymph nodes were examined in 33 patients. Sixty (85%) enlarged lymph nodes were located in the mediastinum and the remaining 11 were hilar and interlobar. The subcarinal node (station 7) was the most frequently sampled (33/71, 46%). A median of two lymph nodes per patient (range, 1-3 lymph nodes) was aspirated with a median of three passes per node (range, 1-5 passes). The median short axis diameter of the enlarged lymph nodes, as measured by CT, was 16 mm (range, 10-31 mm). Fifty-one of 71 (71.5%) lymph nodes had noncaseating granulomas. The characteristics of the lymph nodes sampled by EBUS-TBNA are summarized in Table 1.
Table 1.
Characteristics of Lymph Nodes Sampled by EBUS-TBNA
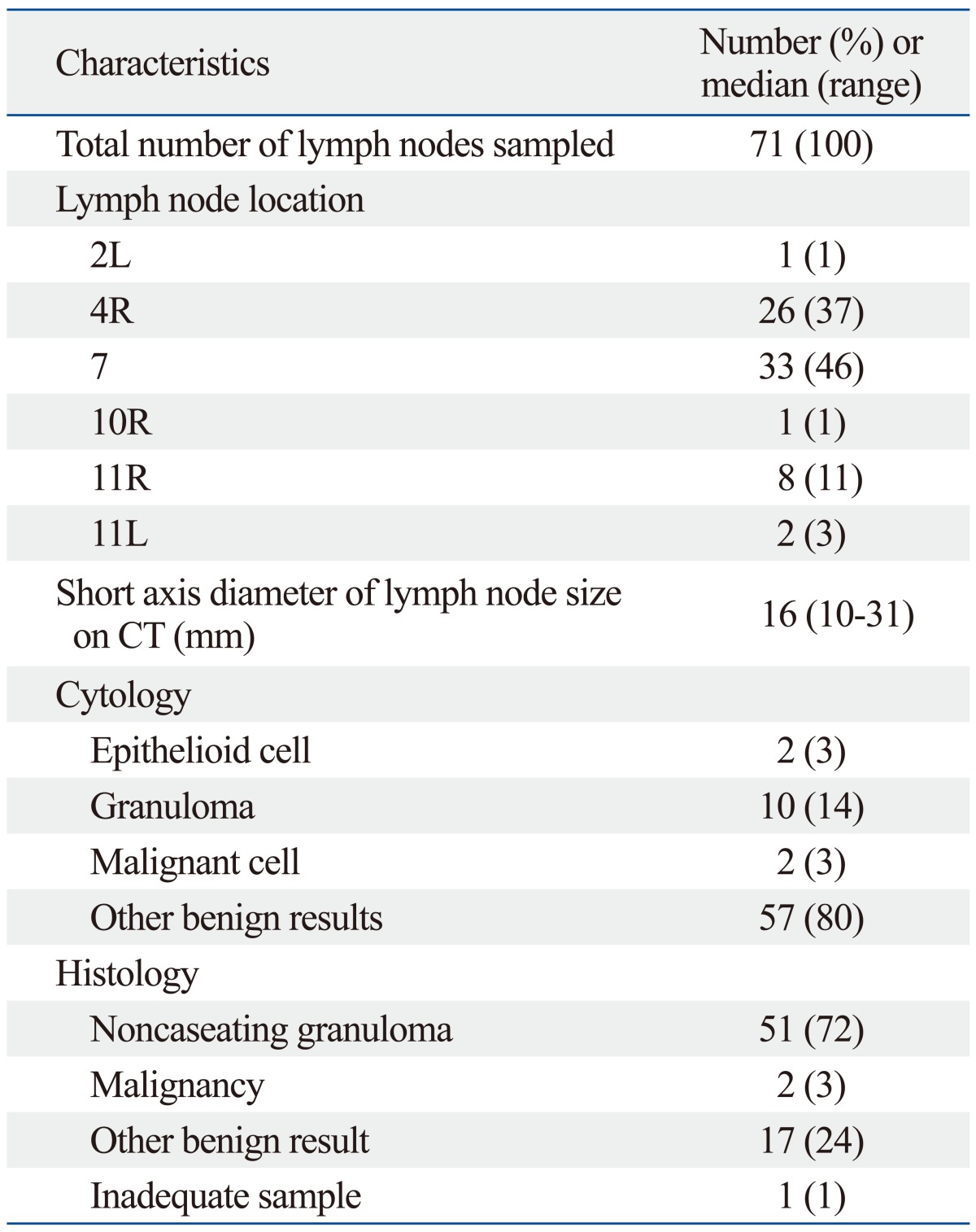
EBUS-TBNA, endobronchial ultrasound transbronchial needle aspiration.
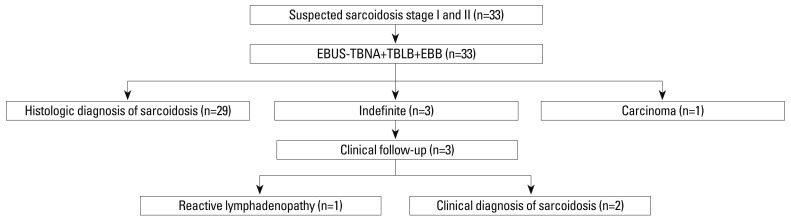
Of 33 patients, 29 (88%) had noncaseating granulomas detected by TBLB or EBUS-TBNA, and one patient had a metastatic adenocarcinoma. Three patients had no evidence of granuloma or malignancy with EBUS-TBNA, TBLB or EBB, so these patients were followed up for at least 6 months (a median follow-up period of 19 months) and were reviewed. Among the patients with an indefinite diagnosis, two were clinically diagnosed with sarcoidosis and one patient was diagnosed with reactive lymphadenopathy (Fig. 1). Therefore, 31 patients were diagnosed with sarcoidosis. Among 29 patients with histologically proven sarcoidosis in combination with EBUS-TBNA, TBLB, and EBB, only EBUS-TBNA and TBLB revealed noncaseating granuloma in 18 and one patient, respectively. Of 33 patients who underwent EBUS-TBNA, noncaseating granulomas were detected by histology in 28 patients and by cytology in 8 patients. Twenty-two of 31 patients (71%) had BAL lympho-cytosis with CD4/CD8 ≥3.5, but two of 31 patients (6%) had a decreased CD4/CD8 ratio (CD4/CD8 <1.0).
Fig. 1.
Diagnostic flowchart for 33 patients with suspected stage I and stage II sarcoidosis. EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; TBLB, transbronchial lung biopsy; EBB, endobronchial biopsy.
The diagnostic performance of EBUS-TBNA and the bronchoscopic procedures are summarized in Table 2. The overall diagnostic sensitivities of EBUS-TBNA, TBLB, EBB, and BAL (CD4/CD8 ≥3.5) were 90%, 35%, 6%, and 71%, respectively (p<0.001). The combined diagnostic sensitivity of EBUS-TBNA, TBLB, and EBB was 94%. No significant difference in diagnostic sensitivity was observed between EBUS-TBNA alone or in combination with EBUS-TBNA, TBLB, and EBB (p=0.990). Further analysis was performed in relation to radiologic staging of sarcoidosis on chest CT scan. Seven of nine (78%) stage I patients were diagnosed with sarcoidosis by EBUS-TBNA, whereas TBLB was only useful for diagnosing sarcoidosis in three (33%) patients. One stage II patient had a negative EBUS-TBNA result but a positive TBLB result. In our study, EBUS-TBNA was the most sensitive diagnostic method in patients with stage I and stage II sarcoidosis.
Table 2.
Diagnostic Yields of EBUS-TBNA, TBLB, EBB, and BAL for Sarcoidosis
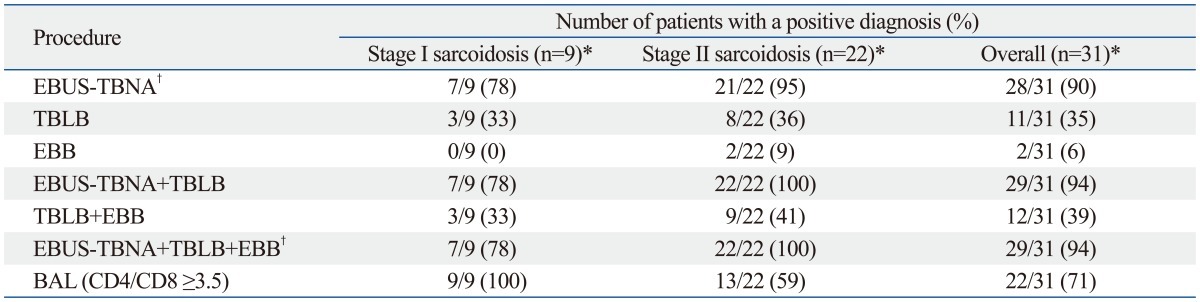
EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; TBLB, transbronchial lung biopsy; EBB, endobronchial biopsy; BAL, bronchoalveolar lavage.
*p<0.001 comparing yields from EBUS-TBNA with those from TBLB, EBB, and BAL.
†p=0.990 comparing yields from EBUS-TBNA alone with those from combination EBUS-TBNA, TBLB, and EBB.
Three patients experienced pneumothorax, requiring overnight admission, but no complication due to pneumomediastinum or pulmonary hemorrhage was observed.
DISCUSSION
Our results confirm that EBUS-TBNA of mediastinal or hilar lymph nodes had a high diagnostic yield for detecting noncaseating granulomas in cases of suspected sarcoidosis. In our study, EBUS-TBNA had diagnostic sensitivities of 90% for all stages, 78% for stage I, and 95% for stage II, which were significantly higher than those of TBLB and EBB. The combination of EBUS-TBNA with conventional bronchoscopic procedures added little to its diagnostic sensitivity.
Tissue specimens should be obtained from the most readily accessible organ using the least invasive method.1 Because the lungs are involved in most patients, TBLB has remained the conventional procedure for confirming a histological diagnosis and its diagnostic yield was reported as 40-90% in previous studies.15-17 However, the diagnostic yield of TBLB depends upon the skill of the person performing the procedure, the number of biopsy samples taken, and the degree of interstitial involvement at the time of biopsy.15 Optimal results are accomplished if 4-5 biopsies are taken for stage II disease and up to 10 biopsies for stage I disease.15,18 TBLB is associated with complications, as 5% of patients showed pneumothorax and pulmonary hemorrhage in a previous study.19 The risk for complications increases proportionally with the number of biopsy specimens. Hence, we routinely obtained at least five biopsy specimens by TBLB from each patient. No significant difference was observed in the diagnostic sensitivity of TBLB for stage I and stage II sarcoidosis in our study. The diagnostic sensitivities of TBLB for stage I, stage II, and overall were 33%, 36%, and 35%, respectively. Three of 33 (9%) patients experienced pneumothorax, but no pulmonary hemorrhage or pneumomediastinum occurred.
EBB could have additional value in patients with normal-appearing bronchial mucosa and those with sarcoidosis.20 However, EBB had a low diagnostic yield and did not increase the diagnostic yield when used in combination with TBLB and EBUS-TBNA in our study. BAL fluid analysis is also performed to diagnose sarcoidosis. An increased in the number of lymphocytes can be detected in 90% of subjects with sarcoidosis at the time of diagnosis, regardless of the stage of sarcoidosis. Although its sensitivity was low (53-59%), CD4/CD8 ratio increased to ≥3.5 in about 55% of patients with sarcoidosis and showed a high specificity of 93-96% for sarcoidosis.4,14 However, BAL fluid may be normal in 10-15% of patients, and the ratio decreases to <1.0 in 12% of patients with sarcoidosis.21 The most important limitation of BAL fluid analysis is that it is not useful for a pathological diagnosis. Two of 31 (6%) patients demonstrated a decrease in CD4/CD8 ratio (CD4/CD8 <1.0) in our study.
The most common feature of sarcoidosis is hilar and mediastinal lymphadenopathy.22 Therefore, a tissue diagnosis using these areas is reasonable, as they are a likely target for confirming the diagnosis. EBUS-TBNA has emerged as an accurate, minimally invasive, and safe procedure for evaluating mediastinal lymphadenopathy.5,6 EBUS-TBNA can visualize the paratracheal, subcarinal, and hilar lymph nodes, which can be sampled, resulting in a high diagnostic yield.23 Oki, et al.9 reported that EBUS-TBNA was diagnostic in 13 of 14 (93%) patients with sarcoidosis by showing histological evidence of noncaseating granulomas in 18 of 23 (78%) lymph nodes examined. Wong, et al.7 assessed 65 patients with mediastinal lymphadenopathy with suspected sarcoidosis and reported that the sensitivity of EBUS-TBNA was 92%. Nakajima, et al.24 reported a higher diagnostic yield for EBUS-TBNA (91.4%) than for TBLB (40.0%) in a recent retrospective study comparing EBUS-TBNA, TBLB, and BAL in 38 patients. This retrospective study did not assess the role of EBB. Navani, et al.10 reported that the overall diagnostic sensitivities of EBUS-TBNA, TBLB, and EBB were 85%, 31%, and 11%, respectively, in 27 patients diagnosed with sarcoidosis. These data were similar to those of the present study, as our overall sensitivities of EBUS-TBNA, TBLB, and EBB were 90%, 35%, and 6%, respectively.
Our study had several limitations. First, the retrospective study design and inclusion of a small number of patients from a single center introduced the potential for selection bias. Second, three patients showed no evidence of granuloma or malignancy following EBUS-TBNA, TBLB and EBB. Among the patients with an indefinite diagnosis, two patients were clinically diagnosed with sarcoidosis, which was not supported by a pathological tissue confirmation. Third, the complication rate of pneumothorax (9%) was relatively high after TBLB in this study. In this study, we did not perform TBLB under fluoroscopic guidance and this may be related to relatively high rate of pneumothorax in this study.
In conclusion, EBUS-TBNA was the most sensitive method for diagnosing stage I and II sarcoidosis compared with conventional bronchoscopic procedures. EBUS-TBNA should be considered first for the histopathologic diagnosis of stage I and II sarcoidosis.
ACKNOWLEDGEMENTS
This work was supported by the Samsung Biomedical Research Institute (C-B0-312-1).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 2.Lynch JP, 3rd, Kazerooni EA, Gay SE. Pulmonary sarcoidosis. Clin Chest Med. 1997;18:755–785. doi: 10.1016/s0272-5231(05)70417-2. [DOI] [PubMed] [Google Scholar]
- 3.Shorr AF, Torrington KG, Hnatiuk OW. Endobronchial involvement and airway hyperreactivity in patients with sarcoidosis. Chest. 2001;120:881–886. doi: 10.1378/chest.120.3.881. [DOI] [PubMed] [Google Scholar]
- 4.Winterbauer RH, Lammert J, Selland M, Wu R, Corley D, Springmeyer SC. Bronchoalveolar lavage cell populations in the diagnosis of sarcoidosis. Chest. 1993;104:352–361. doi: 10.1378/chest.104.2.352. [DOI] [PubMed] [Google Scholar]
- 5.Yasufuku K, Chiyo M, Sekine Y, Chhajed PN, Shibuya K, Iizasa T, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004;126:122–128. doi: 10.1378/chest.126.1.122. [DOI] [PubMed] [Google Scholar]
- 6.Herth FJ, Eberhardt R, Vilmann P, Krasnik M, Ernst A. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006;61:795–798. doi: 10.1136/thx.2005.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong M, Yasufuku K, Nakajima T, Herth FJ, Sekine Y, Shibuya K, et al. Endobronchial ultrasound: new insight for the diagnosis of sarcoidosis. Eur Respir J. 2007;29:1182–1186. doi: 10.1183/09031936.00028706. [DOI] [PubMed] [Google Scholar]
- 8.Garwood S, Judson MA, Silvestri G, Hoda R, Fraig M, Doelken P. Endobronchial ultrasound for the diagnosis of pulmonary sarcoidosis. Chest. 2007;132:1298–1304. doi: 10.1378/chest.07-0998. [DOI] [PubMed] [Google Scholar]
- 9.Oki M, Saka H, Kitagawa C, Tanaka S, Shimokata T, Kawata Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration is useful for diagnosing sarcoidosis. Respirology. 2007;12:863–868. doi: 10.1111/j.1440-1843.2007.01145.x. [DOI] [PubMed] [Google Scholar]
- 10.Navani N, Booth HL, Kocjan G, Falzon M, Capitanio A, Brown JM, et al. Combination of endobronchial ultrasound-guided transbronchial needle aspiration with standard bronchoscopic techniques for the diagnosis of stage I and stage II pulmonary sarcoidosis. Respirology. 2011;16:467–472. doi: 10.1111/j.1440-1843.2011.01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–577. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 12.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 13.Thomas KW, Hunninghake GW. Sarcoidosis. JAMA. 2003;289:3300–3303. doi: 10.1001/jama.289.24.3300. [DOI] [PubMed] [Google Scholar]
- 14.Costabel U. CD4/CD8 ratios in bronchoalveolar lavage fluid: of value for diagnosing sarcoidosis? Eur Respir J. 1997;10:2699–2700. doi: 10.1183/09031936.97.10122699. [DOI] [PubMed] [Google Scholar]
- 15.Gilman MJ, Wang KP. Transbronchial lung biopsy in sarcoidosis. An approach to determine the optimal number of biopsies. Am Rev Respir Dis. 1980;122:721–724. doi: 10.1164/arrd.1980.122.5.721. [DOI] [PubMed] [Google Scholar]
- 16.Koonitz CH, Joyner LR, Nelson RA. Transbronchial lung biopsy via the fiberoptic bronchoscope in sarcoidosis. Ann Intern Med. 1976;85:64–66. doi: 10.7326/0003-4819-85-1-64. [DOI] [PubMed] [Google Scholar]
- 17.de Boer S, Milne DG, Zeng I, Wilsher ML. Does CT scanning predict the likelihood of a positive transbronchial biopsy in sarcoidosis? Thorax. 2009;64:436–439. doi: 10.1136/thx.2008.105031. [DOI] [PubMed] [Google Scholar]
- 18.Roethe RA, Fuller PB, Byrd RB, Hafermann DR. Transbronchoscopic lung biopsy in sarcoidosis. Optimal number and sites for diagnosis. Chest. 1980;77:400–402. doi: 10.1378/chest.77.3.400. [DOI] [PubMed] [Google Scholar]
- 19.Bilaçeroğlu S, Perim K, Günel O, Cağirici U, Büyükşirin M. Combining transbronchial aspiration with endobronchial and transbronchial biopsy in sarcoidosis. Monaldi Arch Chest Dis. 1999;54:217–223. [PubMed] [Google Scholar]
- 20.Shorr AF, Torrington KG, Hnatiuk OW. Endobronchial biopsy for sarcoidosis: a prospective study. Chest. 2001;120:109–114. doi: 10.1378/chest.120.1.109. [DOI] [PubMed] [Google Scholar]
- 21.Kantrow SP, Meyer KC, Kidd P, Raghu G. The CD4/CD8 ratio in BAL fluid is highly variable in sarcoidosis. Eur Respir J. 1997;10:2716–2721. doi: 10.1183/09031936.97.10122716. [DOI] [PubMed] [Google Scholar]
- 22.Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–1234. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]
- 23.Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest. 2004;125:322–325. doi: 10.1378/chest.125.1.322. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Yasufuku K, Kurosu K, Takiguchi Y, Fujiwara T, Chiyo M, et al. The role of EBUS-TBNA for the diagnosis of sarcoidosis--comparisons with other bronchoscopic diagnostic modalities. Respir Med. 2009;103:1796–1800. doi: 10.1016/j.rmed.2009.07.013. [DOI] [PubMed] [Google Scholar]