Abstract
Secoisolariciresinol diglucosides (SDGs) (S,S)-SDG-1 (major isomer in flaxseed) and (R,R)-SDG-2 (minor isomer in flaxseed) were synthesized from vanillin via secoisolariciresinol (6) and glucosyl donor 7 through a concise route that involved chromatographic separation of diastereomeric diglucoside derivatives (S,S)-8 and (R,R)-9. Synthetic (S,S)-SDG-1 and (R,R)-SDG-2 exhibited potent antioxidant properties (EC50 = 292.17 ± 27.71 μM and 331.94 ± 21.21 μM, respectively) which compared well with that of natural (S,S)-SDG-1 (EC50 = 275.24 ± 13.15 μM). These values are significantly lower than those of ascorbic acid (EC50 = 1129.32 ± 88.79 μM) and α-tocopherol (EC50 = 944.62 ± 148.00 μM). Compounds (S,S)-SDG-1 and (R,R)-SDG-2 also demonstrated powerful scavenging activities against hydroxyl [natural (S,S)-SDG-1: 3.68 ± 0.27; synthetic (S,S)-SDG-1: 2.09 ± 0.16; synthetic (R,R)-SDG-2: 1.96 ± 0.27], peroxyl [natural (S,S)-SDG-1: 2.55 ± 0.11; synthetic (S,S)-SDG-1: 2.20 ± 0.10; synthetic (R,R)-SDG-2: 3.03 ± 0.04] and DPPH [natural (S,S)-SDG-1: EC50 = 83.94 ± 2.80 μM; synthetic (S,S)-SDG-1: EC50 = 157.54 ± 21.30; synthetic (R,R)-SDG-2: EC50 = 123.63 ± 8.67] radicals. These results confirm previous studies with naturally occurring (S,S)-SDG-1 and establish both (S,S)-SDG-1 and (R,R)-SDG-2 as potent antioxidants and free radical scavengers for potential in vivo use.
Keywords: Antioxidant, SDG, Free radical scavenger, Flaxseed lignans, ROS
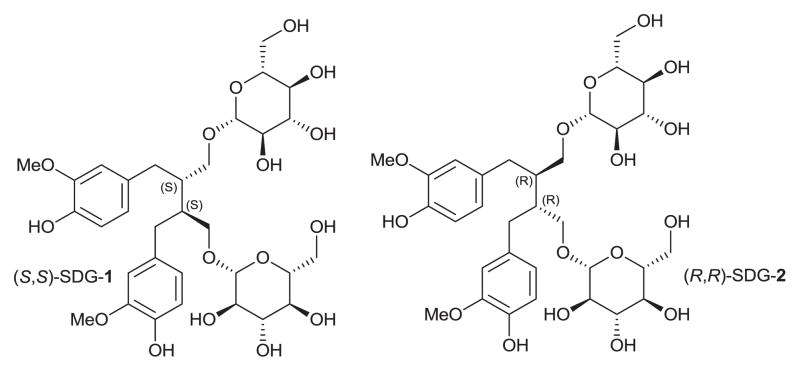
Secoisolariciresinol diglucoside [SDG, (S,S)-SDG-1] is a major component of the lignans in flaxseed (Figure 1).1 Previous studies have shown that flaxseed (S,S)-SDG-1 is a potent antioxidant in vitro and in vivo,2–6 and a powerful in vitro scavenging agent against free hydroxyl radicals.7 Increased generation of reactive oxygen species (ROS) such as superoxide anion (O2−), hydroxyl radical (·OH), and hydrogen peroxide leads to tissue damage under various pathological conditions.8–10 These species result in cellular damage through oxidative modification of cellular membrane lipids, proteins, and the genomic DNA.11 Therefore, SDG as an antioxidant may have therapeutic potential under various experimental and disease conditions that are associated with oxidative tissue damage such as in cancer patients undergoing radiotherapy.
Figure 1.
Molecular structures of secoisolariciresinol diglucosides (S,S)-SDG-1 and (R,R)-SDG-2.
Ionizing radiation inflicts damage to biological systems through generation of reactive oxygen and nitrogen species (e.g. peroxidation of membrane lipids, oxidation and nitration of proteins, DNA and RNA strand breaks).12 Antioxidant compounds that scavenge free radicals, enhance endogenous antioxidant enzyme levels, and boost DNA repair may be useful in countering radiation damage. Despite extensive research efforts toward the development of synthetic compounds as radioprotectors, there is still a need for safe and more effective agents. In view of their properties, natural products and their analogs are increasingly considered as promising leads for the discovery and development of radioprotectors.
Plants contain a plethora of secondary metabolites with wide-ranging pharmacological properties. Phenolic acids, flavonoids, and phenylpropanoids are the most prominent metabolite classes known for antioxidant activity.13 Dietary and medicinal plants possessing antioxidant properties have been shown to play an important role in preventing many human diseases associated with oxidative stress.14,15
In previous studies, we have investigated the role of whole grain dietary flaxseed in radiation-induced damage.16–18 Flaxseed ameliorated the radiation-induced inflammation and oxidative stress in mice, and irradiated mice fed with flaxseed diets enriched with (S,S)-SDG-1 showed improved hemodynamic indices and survival over the control. Importantly, a flaxseed diet enriched in (S,S)-SDG-1 also improved polymorphonuclear cell infiltration and decreased lung inflammation, demonstrating its protective effects against radiation-induced lung damage in vivo.
Secoisolariciresinol (the aglycon of (S,S)-SDG-1 and (R,R)-SDG-2) has been of interest to several synthetic groups. A double Stobbe condensation19 has been used to prepare racemic secoisolariciresinol and enantioenriched secoisolariciresinols have been prepared from aldol reactions on chiral γ-butyrolactones20 as well as diasteroselective alkylation of Evans auxiliary appended hydroferulic acid derivatives.21 In a recent report the synthesis of (S,S)-SDG-1 was claimed.3
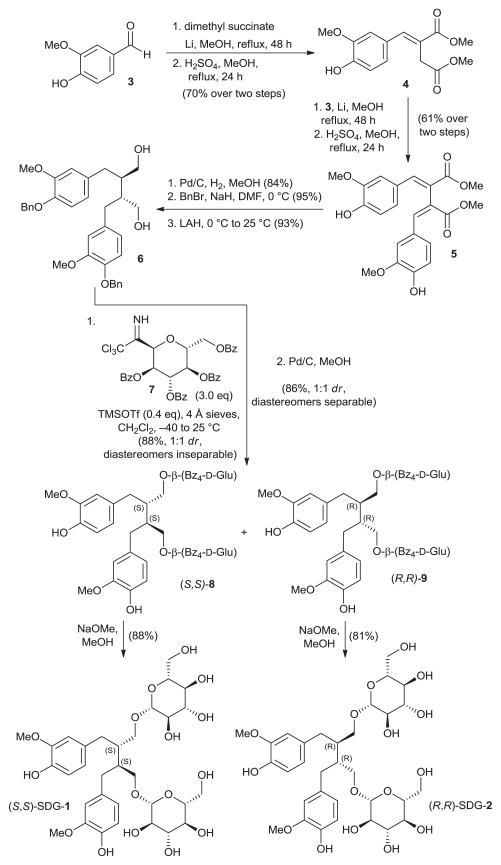
The synthesis of secoisolariciresinol diglucosides (S,S)-SDG-1 and (R,R)-SDG-2 proceeded from vanillin (3) as shown in Figure 2. Thus, following a modified literature procedure,19 3 was subjected to Stobbe condensation with dimethyl succinate in the presence of lithium wire in refluxing methanol, and the resulting mixture of carboxylic acids was esterified with methanol under acidic conditions to furnish dimethyl ester 4 in 70% overall yield (single isomer, unassigned olefin geometry). A second Stobbe condensation involving product 4 with vanillin under the same lithium-mediated conditions, followed by esterification with methanol under the same acidic conditions, led to diene 5 in 61% overall yield (single isomer, unassigned olefin geometry). The latter compound underwent diastereoselective hydrogenation (Pd/C, H2, 84% yield), phenolic benzylation (NaH, BnBr, 95% yield), and ester reduction (LAH, 93% yield) to provide bis-benzyl secoisolariciresinol 6. The relative stereochemistry of 6 was confirmed by X-ray crystallographic analysis (see Figure 3 for ORTEP representation). Attempts to attach the glucose moieties onto 6 employing peracetyl-protected glucosyl donors led predominately to acetylation of the primary hydroxyl groups of the substrate. The glycosidation of 6 was finally achieved through the use of the perbenzoyl-protected trichloroacetimidate 722 under the influence of TMSOTf, furnishing a diastereomeric mixture of inseparable bis-β-glucosides (88% combined yield, 1:1 dr). Cleavage of the benzyl ethers by hydrogenolysis provided a mixture of diastereomeric bis-phenols (86% yield, 1:1 dr) which proved separable by preparative thin layer chromatography (PTLC, multiple elutions) to afford pure (S,S)-8 and (R,R)-9. Each perbenzoylated bis-glucoside was treated with NaOMe in MeOH to give the corresponding secoisolariciresinol diglucoside (S,S)-SDG-1 (88% yield) and (R,R)-SDG-2 (81% yield), whose spectral data matched those previously reported for these compounds.23
Figure 2.
Synthesis of secoisolariciresinol diglucosides (S,S)-SDG-1 and (R,R)-SDG-2. For details see supplementary data.
Figure 3.
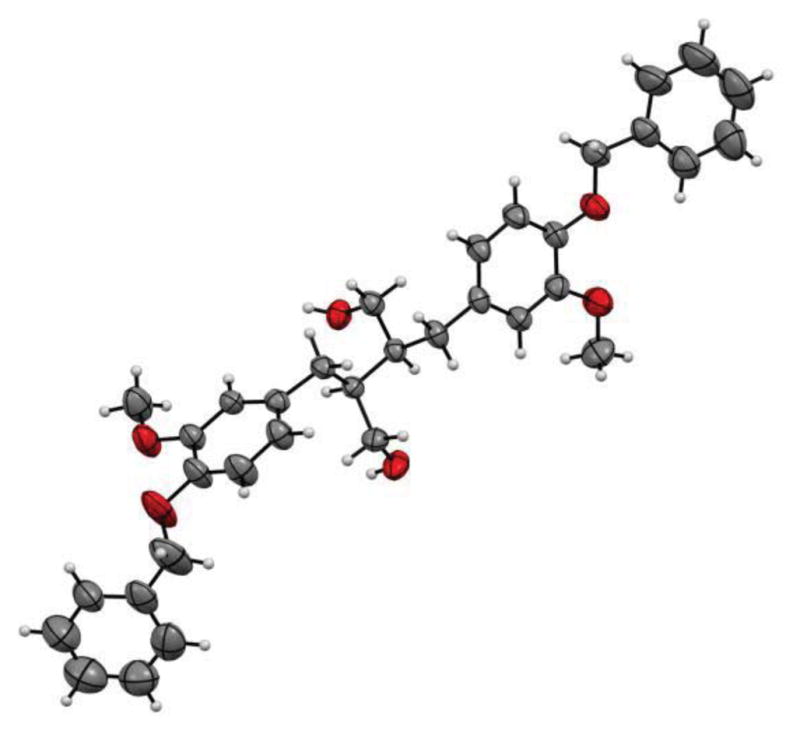
ORTEP representation of dihydroxy compound 6 (CCDC-940963).
As antioxidants, polyphenols and other lignan components have been reported to modulate the activity and expression of enzymes involved in reactive oxygen species detoxification, quench free radicals, and chelate transition metals, resulting in complexes which are redox inactive in the Fenton reaction.24 Since such a compound may have diverse antioxidant properties, we chose to evaluate our synthetic agents using various assays.25 In light of these observations, we proceeded to evaluate the reducing power of synthesized (S,S)-SDG-1 and (R,R)-SDG-2 as well as their free radical scavenging activity against hydroxyl, peroxyl, and DPPH free radicals.
The reducing power of synthetic (S,S)-SDG-1, synthetic (R,R)-SDG-2, natural (S,S)-SDG-1, ascorbic acid, and α-tocopherol was determined by the reduction of K3FeCN6 in the presence of FeCl3, as measured by the absorbance of the resulting ferric-ferrous complex (Figure 4).26 The reducing power of synthetic (S,S)-SDG-1, synthetic (R,R)-SDG-2, and natural (S,S)-SDG-1 were significantly concentration-dependent at higher concentrations; however, at all concentrations tested the SDGs had comparable or higher reducing power than known antioxidants ascorbic acid and α-tocopherol, with a notable increase in potency in the 200–500 μM range. A linear relationship between reducing power and substrate concentration was observed at lower concentrations (1–100 μM), allowing regression line equations to be established for the five compounds. This allowed the half maximal effective concentration (EC50) for reducing power to be calculated (Figure 5). The EC50 (mean ± std. dev.) values for (S,S)-SDG-1 and (R,R)-SDG-2 were 292.17 ± 27.71 μM and 331.94 ± 21.21 μM, respectively. These values were comparable to that of natural (S,S)-SDG-1 (EC50 = 275.24 ± 13.15 μM) but approximately three-fold higher than that exhibited by ascorbic acid (EC50 = 1129.32 ± 88.79 μM) and α-tocopherol (EC50 = 944.62 ± 148.00 μM).
Figure 4.
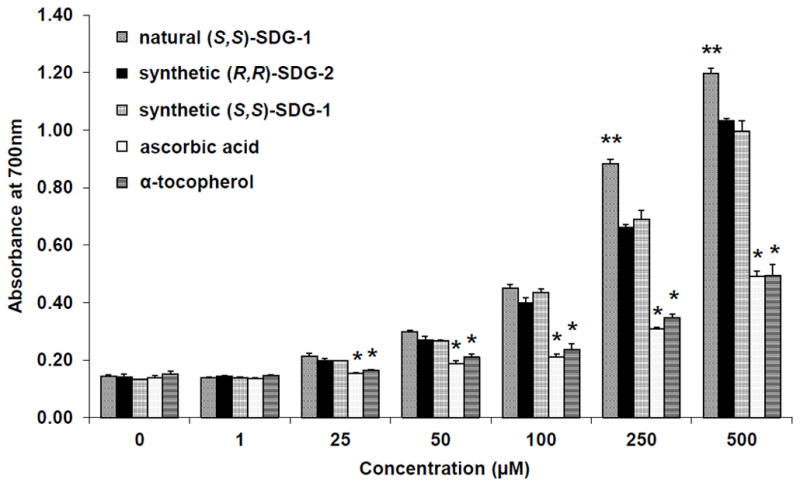
Reducing power of synthetic (S,S)-SDG-1 and (R,R)-SDG-2. The increase in absorbance at 700nm indicates increase in reducing power. The results are presented as mean ± standard deviation (n = 3). *p < 0.05 significantly lower than natural (S,S)-SDG-1, synthetic (R,R)-SDG-2, and synthetic (S,S)-SDG-1; **p < 0.05 significantly higher than synthetic (R,R)-SDG-2, synthetic (S,S)-SDG-1, ascorbic acid and α-tocopherol. The somewhat higher potency of natural (S,S)-SDG-1 may be due to an unknown impurity in our samples, however the NMR spectra of both natural and synthetic (S,S)-SDG-1 did not reveal major impurities.
Figure 5.
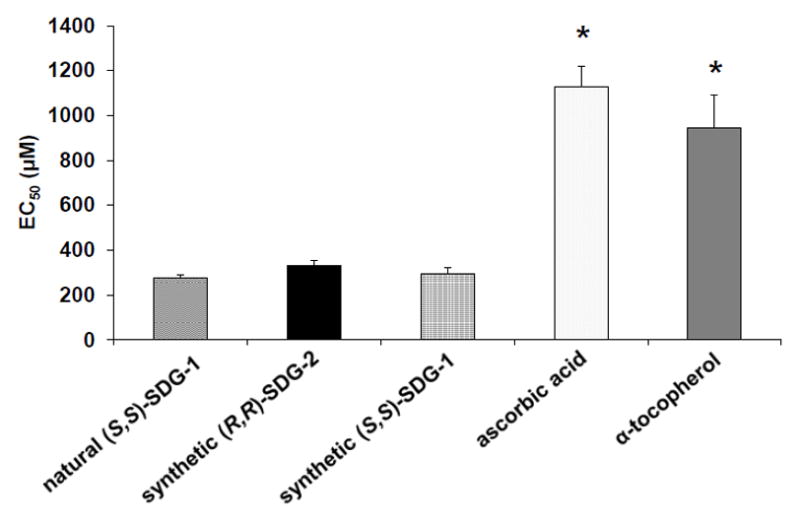
Reducing power of synthetic (S,S)-SDG- 1 and (R,R)-SDG-2. The rate of reaction is linear in the concentration range of 1–100 μM. Equation of the linear regression was used to determine EC50. The results are presented as mean ± standard deviation (n = 3). Natural (S,S)-SDG-1, synthetic (R,R)-SDG-2, and synthetic (S,S)-SDG-1 were not significantly different from each other. *p < 0.05 significantly higher than natural (S,S)-SDG-1, synthetic (R,R)-SDG-2, and synthetic (S,S)-SDG-1.
The ability of synthetic (S,S)-SDG-1 and (R,R)-SDG-2 to scavenge hydroxyl and peroxyl radicals as manifested by their inhibition of the oxidation of fluorescein was assessed by the hydroxyl radical averting capacity (HORAC, gallic acid standard) and peroxyl radical absorbance capacity assays (ORAC, Trolox standard), respectively (Table 1). Fluorescein oxidation by hydroxyl radicals was decreased by synthetic (S,S)-SDG-1 and synthetic (R,R)-SDG-2 in a concentration-dependent manner and was found to be two-fold higher than gallic acid. However, synthetic (S,S)-SDG-1 activity differed from natural (S,S)-SDG-1, likely due to trace impurities. Fluorescein oxidation by peroxyl radicals generated using 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) was greatly reduced in the presence of synthetic (S,S)-SDG-1, (R,R)-SDG-2 and natural (S,S)-SDG-1, with a two-fold increase in potency over the Trolox standard (Table 1).
Table 1.
Antioxidant capacity of synthetic (S,S)-SDG-1 and (R,R)-SDG-2. Hydroxyl radicals were generated from hydrogen peroxide by Fenton reaction. Peroxyl radicals were generated by AAPH (2,2′-azobis(2-amidinopropane) dihydrochloride). Oxidation of fluorescein was measured. Calculations used SDG concentrations that fitted the linear part of the calibration curve. The results are presented as mean ± standard deviation (n = 3). The somewhat higher potency of natural (S,S)-SDG-1 may be due to an unknown impurity in our samples, however the NMR spectra of both natural and synthetic (S,S)-SDG-1 did not reveal major impurities.
| Entry | Antioxidant | Against Hydroxyla Radicals (GAE)c | Against Peroxylb Radicals (TE)d |
|---|---|---|---|
| 1. | natural (S,S)-SDG-1 | 3.68±0.27 | 2.55±0.11 |
| 2. | synthetic (R,R)-SDG-2 | 1.96±0.27 | 2.20±0.10 |
| 3. | synthetic (S,S)-SDG-1 | 2.09±0.16 | 3.03±0.04 |
Determined by HORAC Assay;
Determined by ORAC Assay;
Gallic acid equivalents;
Trolox equivalents.
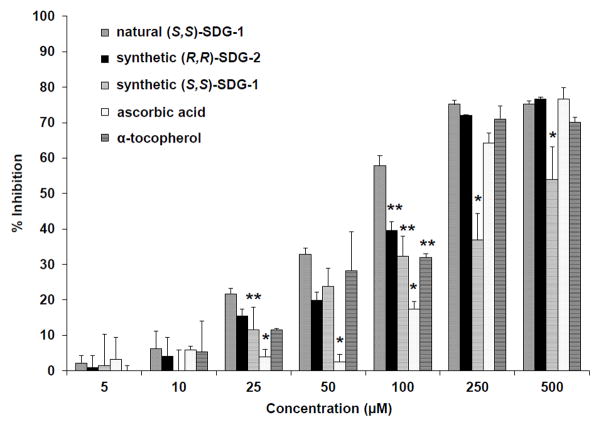
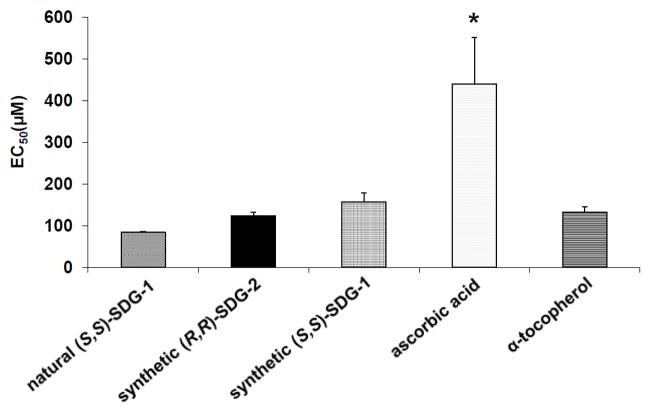
The free radical scavenging activities of synthetic (S,S)-SDG-1 and (R,R)-SDG-2 were determined using a 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay and was compared to those of natural (S,S)-SDG-1, ascorbic acid, and α-tocopherol (Figure 6). At low (5–25 μM) and mid-concentration (50–100 μM) ranges, the SDGs exhibited similar scavenging potentials; however at higher concentrations (250–500 μM), the inhibition by synthetic (S,S)-SDG-1 was significantly lower than those exerted by (R,R)-SDG-2 and natural (S,S)-SDG-1. Establishing regression lines for the potentials at low- and mid-concentration ranges (5–100 μM) allowed the free radical EC50 scavenging activity of these compounds to be determined. As shown in Figure 7, synthetic (R,R)-SDG-2 (123.63 ± 8.67 μM) and synthetic (S,S)-SDG-1 (157.54 ± 21.30 μM) were not significantly different. These values were similar to those exhibited by natural (S,S)-SDG-1 (83.94 ± 2.80 μM) and α-tocopherol (132.81 ± 12.57 μM) but considerably lower than that shown by ascorbic acid (439.56 ± 11.81 μM). These results are comparable to those recently reported for SDG.3
Figure 6.
DPPH free radical scavenging activity of natural (S,S)-SDG-1, synthetic (S,S)-SDG-1 and (R,R)-SDG-2, ascorbic acid, and α-tocopherol. The radical scavenging activity was measured as a decrease in the absorbance of DPPH at 517 nm. The results are presented as mean ± standard deviation (n = 3). *p < 0.05 significantly lower than all other compounds, **p < 0.05 significantly lower than natural (S,S)-SDG-1.
Figure 7.
DPPH free radical scavenging activity of natural (S,S)-SDG-1, synthetic (S,S)-SDG-1 and (R,R)-SDG-2, ascorbic acid, and α-tocopherol. Equation of the linear regression was used to determine EC50. The rate of reaction is linear in the concentration range of 1–100 μM. The results are presented as mean ± standard deviation (n = 3). Natural (S,S)-SDG-1, synthetic (R,R)-SDG-2, synthetic (S,S)-SDG-1, and α-tocopherol were not significantly different. *p < 0.05 significantly higher than natural (S,S)-SDG-1, synthetic (R,R)-SDG-2, synthetic (S,S)-SDG-1, and α-tocopherol.
In summary, we have synthesized (S,S)-SDG-1 and (R,R)-SDG-2 and characterized their antioxidant properties. Both possess strong reducing power and high free radical scavenging activity for hydroxyl, peroxyl and DPPH free radicals. Synthetic (S,S)-SDG-1 and (R,R)-SDG-2 have been shown to be promising agents for use in modulating cellular redox states and for scavenging oxygen free radicals in vivo. Efforts are currently underway to develop scalable synthetic routes to (S,S)-SDG-1, (R,R)-SDG-2 and related analogs. Further in vitro and in vivo studies to determine the mechanism of action and usefulness of the SDGs as antioxidants and protectors against radiation-induced tissue damage are in progress.
Supplementary Material
Acknowledgments
We thank Drs. D.H. Huang, L. Pasternack, and R. Chadha for NMR spectroscopic and X-ray crystallographic assistance. This work was funded in part by The Skaggs Institute for Research, the National Science Foundation (graduate fellowship to N.S.), Merck (postdoctoral fellowship to R.A.V.), the Deutsche Akademie der Naturforscher Leopoldina (postdoctoral fellowship to P.H.), NIH-R01 CA133470 (M.C.S.), NIH-RC1AI081251 (M.C.S.), the University of Pennsylvania Research Foundation (M.C.S.) and by pilot project support from 1P30 ES013508-02 awarded to M.C.S. (its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH).
Footnotes
Full synthetic details, compound characterization and descriptions of the biological assays may be found in the supplementary material.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Axelson M, Sjovall J, Gustafsson BE, Setchell KDR. Nature. 1982;298:659. doi: 10.1038/298659a0. [DOI] [PubMed] [Google Scholar]
- 2.Kitts DD, Yuan YV, Wijewickreme AN, Thompson LU. Mol Cell Biochem. 1999;202:91. doi: 10.1023/a:1007022329660. [DOI] [PubMed] [Google Scholar]
- 3.Moree S, Khanum SA, Rajesha J. Free Rad Antiox. 2011;1:31. [Google Scholar]
- 4.Hu C, Yuan YV, Kitts DD. Food Chem Toxicol. 2007;45:2219. doi: 10.1016/j.fct.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Moree SS, Rajesha J. Mol Cell Biochem. 2013;373:179. doi: 10.1007/s11010-012-1487-4. [DOI] [PubMed] [Google Scholar]
- 6.Rajesha J, Murthy KN, Kumar MK, Madhusudhan B, Ravishankar GA. J Agric Food Chem. 2006;54:3794. doi: 10.1021/jf053048a. [DOI] [PubMed] [Google Scholar]
- 7.Prasad K. Mol Cell Biochem. 1997;168:117. doi: 10.1023/a:1006847310741. [DOI] [PubMed] [Google Scholar]
- 8.Adolphe JL, Whiting SJ, Juurlink BH, Thorpe LU, Alcorn J. Br J Nutr. 2010;103:929. doi: 10.1017/S0007114509992753. [DOI] [PubMed] [Google Scholar]
- 9.Seifried HE. J Nutr Biochem. 2007;18:168. doi: 10.1016/j.jnutbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Seifried HE, Anderson DE, Fisher EI, Milner JA. J Nutr Biochem. 2007;18:567. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B. Free Radical Biol Med. 1989;7:645. doi: 10.1016/0891-5849(89)90145-7. [DOI] [PubMed] [Google Scholar]
- 12.Riley PA. Int J Radiat Biol. 1994;65:27. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 13.Rice-Evans CA, Miller NJ. Biochem Soc Trans. 1996;24:790. doi: 10.1042/bst0240790. [DOI] [PubMed] [Google Scholar]
- 14.Scalbert A, Johnson IT, Saltmarsh M. Am J Clin Nutr. 2005;81:215S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 15.Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Crit Rev Food Sci Nutr. 2005;45:287. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 16.Pietrofesa R, Turowski J, Tyagi S, Dukes F, Arguiri E, Busch TM, Gallagher-Colombo SM, Solomides CC, Cengel KA, Christofidou-Solomidou M. BMC Cancer. 2013;13:179. doi: 10.1186/1471-2407-13-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christofidou-Solomidou M, Tyagi S, Pietrofesa R, Dukes F, Arguiri E, Turowski J, Grieshaber PA, Solomides CC, Cengel KA. Radiat Res. 2012;178:568. doi: 10.1667/RR2980.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JC, Krochak R, Blouin A, Kanterakis S, Chatterjee S, Arguiri E, Vachani A, Solomides CC, Cengel KA, Christofidou-Solomidou M. Cancer Biol Ther. 2009;8:47. doi: 10.4161/cbt.8.1.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee E, Ahamed VSJ, Kumar MS, Rhee SW, Moon SS, Hong IS. Bioorg Med Chem Lett. 2011;21:6245. doi: 10.1016/j.bmcl.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi S, Sugahara T, Matsugi J, Someya T, Masuda T, Kishida T, Akiyama K, Maruyama M. Biosci Biotechnol Biochem. 2007;71:2283. doi: 10.1271/bbb.70275. [DOI] [PubMed] [Google Scholar]
- 21.Allais F, Pla TJL, Ducrot P. Synthesis. 2011;9:1456. [Google Scholar]
- 22.Mühlhausen U, Schirrmacher R, Piel M, Lecher B, Briegert M, Piee-Staffa A, Kaina B, Rösch F. J Med Chem. 2006;49:263. doi: 10.1021/jm050588q. [DOI] [PubMed] [Google Scholar]
- 23.For (S,S)-SDG-1: Chimichi S, Bambagiotti-Alberti M, Coran SA, Giannellini V, Biddau B. Magn Reson Chem. 1999;37:860.Qiu S, Lu Z, Luyengi L, Lee SK, Pezzuto JM, Farnsworth NR, Thompson LU, Fong HHS. Pharm Biol. 1999;37:1.For (R,R)-SDG-2: Ford JD, Huang K, Wang H, Davin LB, Lewis NG. J Nat Prod. 2001;64:1388. doi: 10.1021/np010367x.Ford JD, Huang K, Wang H, Davin LB, Lewis NG. J Nat Prod. 2002;65:800. doi: 10.1021/np010367x.
- 24.Heim KE, Tagliaferro AR, Bobilya DJ. J Nutr Biochem. 2002;13:572. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 25.Prior RL, Cao G. Free Radical Biol Med. 1999;27:1173. doi: 10.1016/s0891-5849(99)00203-8. [DOI] [PubMed] [Google Scholar]
- 26.Yen GC, Pin-Der D. J Am Oil Chem Soc. 1993;70:383. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.