Abstract
Background/Aims
Microalbuminuria is a marker for early kidney disease and cardiovascular risk. The purposes of this study were to determine the prevalence of microalbuminuria in an HIV-infected clinic population, to test the predictive value of a single urine albumin-creatinine ratio (ACR) to identify persistent microalbuminuria and to examine covariates of microalbuminuria.
Methods
We conducted a prospective cohort study of HIV-infected subjects (n=182) without proteinuria (P/C ratio ≥0.5 g/g), elevated serum creatinine, diabetes, or chronic inflammatory conditions. Subjects completed three research visits within nine months. Microalbuminuria was defined as the geometric mean ACR of 25–355 mg/g for women and 17–250 mg/g for men.
Results
The prevalence of microalbuminuria was 14%. The negative predictive value of a single urine ACR determination was 98%, whereas the positive predictive value was only 74%. Microalbuminuria was similar among Black (15%) and non-Black (14%) subjects (p=0.8). Subjects with microalbuminuria were more likely to have hypertension (p=0.02) and metabolic syndrome (p=0.03). While duration of HIV infection and the level of HIV viremia were similar between groups, those with microalbuminuria were more likely to have a CD4 count <200 cells/μL (p=0.0003). In a multivariate logistic regression analysis, the only significant independent predictors of microalbuminuria were low CD4 count (p=0.018) and current ritonavir exposure (p=0.04).
Conclusion
The prevalence of microalbuminuria in an HIV-infected clinic population was similar to earlier reports, and was associated with hypertension and impaired immune function. A single normal ACR determination effectively excludes microalbuminuria, whereas an elevated ACR requires confirmation.
Keywords: HIV infection, microalbuminuria, urinary albumin-creatinine ratio
Introduction
Early in the HIV epidemic, the major forms of HIV-associated chronic kidney disease (CKD) were HIV-associated nephropathy and HIV-associated immune complex kidney disease (1). With the widespread adoption of antiretroviral therapy (ART), the emerging challenges of chronic HIV infection are conditions associated with chronic inflammation and aging, including cardiovascular disease, cancer, diabetes, and chronic liver, bone and chronic kidney disease of diverse etiologies. These kidney diseases include HIV-associated glomerular diseases, glomerular diseases associated with hypertension and diabetes, and tubular injury due to medication. In the HIV-infected population, measurements of urine total protein and albumin may be important tools to detect chronic kidney disease and be part of the evaluation for metabolic syndrome and cardiovascular risk, although their roles remain to be defined.
Six studies have addressed the prevalence of microalbuminuria, using quantitative methods to measure urinary albumin/creatinine ratio (ACR), in cohorts of 100 or more HIV infected subjects and using current thresholds to define microalbuminuria (2–7). These studies have been published in the past five years, and describe cohorts in the post-ART era. Four studies presented data from a single time-point and reported microalbuminuria prevalence ranging from 11–20%, while three studies reported persistent microalbuminuria prevalence on two-three samples as 4–16%. None of the studies reported the predictive value of a single elevated urine ACR to identify persistent microalbuminuria.
Our study had three objectives. First, we wished to determine the period prevalence of microalbuminuria in an HIV-infected clinic population, for the first time excluding patients with other inflammatory conditions (e.g. chronic infection, cancer) and transient causes of physiologic albuminuria (e.g. fever, exercise), in order to arrive at an estimate of the albuminuria rate associated with chronic HIV infection per se, including albuminuria associated with metabolic syndrome and kidney disease. Second, we wished to evaluate the variability of albuminuria over time, and specifically to determine the coefficient of variation (CV) and the positive and negative predictive value of a single urine specimen for persistent microalbuminuria. Third, we wished to examine the clinical and laboratory correlates of microalbuminuria in this population.
Methods
Subjects
Subjects were patients who attended HIV clinic at the National Institute of Allergy and Infectious Diseases (NIAID) outpatient clinic in Bethesda, MD, or the Washington Hospital Center Infectious Diseases clinic in Washington, DC between January 2007 and January 2011. All patients attending the two clinics were prescreened for eligibility (n=761). Potentially eligible participants were notified of the available research studies at routine clinic appointments and returned for subsequent research visits if interested. Subjects with known current pregnancy, opportunistic infection within the past three months, malignancy other than non-melanoma skin cancer or cutaneous Kaposi sarcoma, recent cytokine therapy (e.g. IL-2 or IFN-alpha therapy) pre-existing end stage renal disease or proteinuria, serum creatinine >1.4 mg/dL or urine protein/creatinine ratio ≥0.5 g/g, previously diagnosed diabetes, or fasting serum glucose > 125 mg/dL were excluded from participation. The institutional review boards of the National Institute of Diabetes and Digestive and Kidney Disease, and Medstar Research Institute approved the study protocols. Participants gave written informed consent. The study was registered with the NIH as NCT00524992.
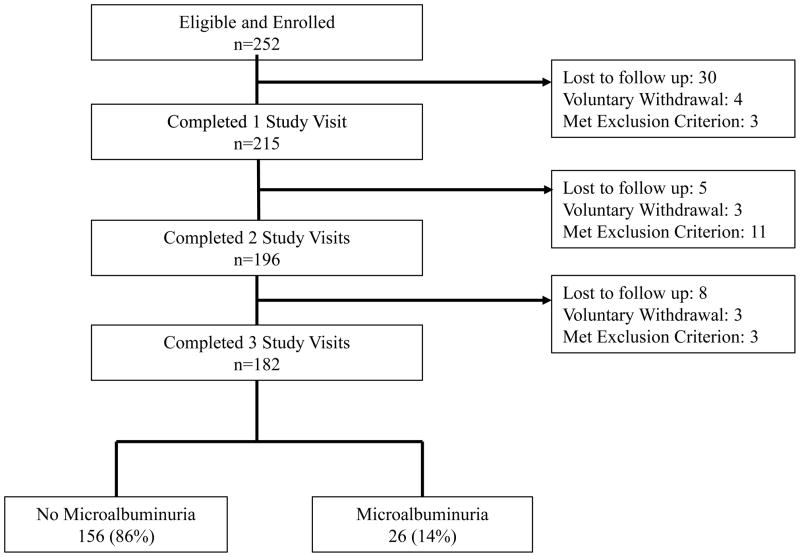
Of the 252 HIV-infected patients who were eligible at pre-screen and who agreed to participate, 182 participants completed three research visits within a nine-month period and are included in the analyses (See Figure 1 patient flow diagram). The median time between visits was 84 days (range 48–248 days). There was no significant difference in age, sex, race, BMI, duration of HIV infection, recent CD4 count, or frequency of hypertension between subjects who completed three visits and non-completers (data not shown).
Figure 1. Protocol Schema and Subject Participation.
A total of 252 subjects met eligibility criteria and were enrolled. Among 215 subjects who completed the first study visit, 182 (84.6%) completed all three study visits, 14 (6.7%) developed an exclusion criterion, and 19 (8.8%) were lost to follow-up or withdrew voluntarily.
Physical assessment at baseline included resting blood pressure, height, weight, waist and hip circumferences. Fasting blood and urine samples were collected at the first and third visits; only urine samples were collected at the second visit. Subjects were required to be afebrile and to refrain from strenuous physical exercise for 24 hours prior to each visit. A complete history of current and past antiretroviral therapy and current medications was recorded for all subjects. Hypertension was defined as 1.) current anti-hypertensive therapy, or 2.) systolic blood pressures greater than 140 mmHg or diastolic blood pressures greater than 90 mmHg on two consecutive readings. Laboratory measurements included serum creatinine, glucose, insulin C-reactive protein, as well as urine protein, albumin and creatinine. The geometric mean of three urine albumin-to-creatinine (ACR) ratios was determined for each participant. Microalbuminuria was defined as a geometric mean of urine ACR 25–355 mg/g for women and 17–250 mg/g for men. CD4 count and HIV viral load were obtained at the baseline visit if not obtained within the prior 90 days. Hepatitis C Virus (HCV) antibody status was evaluated in participants if HCV status was unknown or if HCV antibody status had not been checked within the past twelve months. Metabolic syndrome was defined using the NCEP ATP III (2005 revision) definition, which does not use albuminuria as a component of the definition.
Measurements
All samples for serum creatinine and urine albumin and creatinine determinations were processed and analyzed the same day as collection at a single lab at the NIH Department of Laboratory Medicine (Bethesda, MD). Urine albumin was determined via the Microalbumin (MALB) method, based on a particle-enhanced turbidimetric inhibition immunoassay adapted to the Dimension clinical chemistry system (Dade Behring, Newark, DE). Urine and serum creatinine measurements were determined using the CREA method on the Dimension clinical chemistry system (Dade Behring, Newark, DE); for serum creatinine, standards used were traceable to Cleveland Clinic standards. For all subjects with three specimens, the ACR CV was calculated.
Statistical Analysis
Data are expressed either as the mean ± standard deviation (SD) or as a number and percentage. Common logarithmic transformation was applied to non-normally distributed variables (e.g. HIV RNA levels) to normalize the skewed distribution. Demographic characteristics and laboratory values of patients with and without microalbuminuria were compared using the Pearson’s χ2 test and Student’s t-test for categorical and continuous variable, respectively. The positive and negative predictive value of a single ACR to identify those with persistent microalbuminuria was calculated using patient classification as having or not having microalbuminuria at baseline relative to the classification based on the geometric mean of the ACR using all three visits. Odds ratio (OR) was determined by logistic regression analyses and associations between continuous variables were assessed using linear regression coefficients. A multivariate logistic regression model including age, race, sex, and variables found to be associated with microalbuminuria in univariate analyses (hypertension, low CD4 count defined as CD4 count <200 cell/μL, and current use of ritonavir) was performed. All statistical analyses were conducted using SAS JMP version 8.0 (SAS Institute, Cary, NC). P-values were two-sided and p values of <0.05 were considered to be statistically significant.
Results
Subjects (n=182) were predominantly male (64.3%) with a mean age of 45 years, and predominantly Black (58.8%) or White (30.8%) by self-identification (Table 1). The geometric mean ACR was 12 ± 17 mg/g. Considering the samples for each individual, the median CV was 37% and the mean CV was 45%. The prevalence of microalbuminuria, defined as a geometric mean of urine ACR > 25 mg/g for women and > 17 mg/g for men, was 14%. There was no difference between subjects with and without microalbuminuria with regard to age, sex, and ethnicity. In particular, there was no increased propensity for microalbuminuria among Black subjects (15%) compared to non-Black subjects (14%) (χ2=0.06, p=0.8).
Table 1.
Demographic and Clinical Characteristics by Microalbuminuria Status
| Characteristic or laboratory value | All (n = 182) | No Microalbuminuria (n = 156) | Microalbuminuria (n = 26) | p-value |
|---|---|---|---|---|
| Age, years | 45 ± 10 | 44 ± 10 | 46 ± 8 | 0.52 |
| Sex, n (%) | 0.15 | |||
| Male | 117 (64.3) | 97 (62.2) | 20 (76.9) | |
| Female | 65 (35.7) | 59 (37.8) | 6 (23.1) | |
| Ethnicity, n (%) | 0.84 | |||
| Caucasian | 56 (30.8) | 49 (31.4) | 7 (26.9) | |
| Black | 107 (58.8) | 91 (58.3) | 16 (61.5) | |
| Asian | 5 (2.7) | 4 (2.6) | 1 (3.9) | |
| Hispanic | 11 (6.0) | 10 (6.4) | 1 (3.9) | |
| Mixed Race | 3 (1.6) | 2 (1.3) | 1 (3.9) | |
| Duration HIV (years) | 14 ± 7 | 13 ± 7.0 | 14 ± 6 | 0.51 |
| HCV-infection, n (%) | 14 (7.7) | 12 (7.7) | 2 (7.7) | 1.00 |
| Smoker, n (%) | 52 (28.6) | 42 (26.9) | 10 (38.5) | 0.23 |
| Tenofovir use, n (%) | 118 (64.8) | 99 (63.5) | 19 (73.1) | 0.34 |
| Ritonavir use, n (%) | 101 (55.5) | 81 (51.9) | 20 (76.9) | 0.02 |
| Lipid Medications, n (%) | 27 (14.8) | 22 (14.1) | 5 (19.2) | 0.50 |
| BMI (kg/m2) | 26.9 ± 6.0 | 27.0 ± 5.7 | 26.3 ± 8.0 | 0.70 |
| Waist-to-Hip Ratio | 0.92 ± 0.07 | 0.92 ± 0.07 | 0.93 ± 0.09 | 0.48 |
| Hypertension, n (%) | 45 (24.7) | 34 (21.8) | 11 (42.3) | 0.02 |
| Metabolic Syndrome, n (%) | 25 (13.7) | 18 (11.5) | 7 (26.9) | 0.03 |
| Systolic blood pressure (mmHg) | 123 ± 14 | 123 ± 14 | 120 ± 14 | 0.25 |
| Diastolic blood pressure (mmHg) | 76 ± 10 | 76 ± 10 | 78 ± 11 | 0.27 |
| Hypertension Medication, n (%) | 41 (22.5) | 31 (19.9) | 10 (38.5) | 0.04 |
| ACE Inhibitors and/or ARB use, n (%) | 19 (10.4) | 13 (8.3) | 6 (23.1) | 0.02 |
HCV, Hepatitis C Virus; BMI, body mass index; ACE, Angiotensin Converting Enzyme; ARB, Angiotensin II Receptor Blocker
In order to assess the prevalence of microalbuminuria in the setting of HIV infection relative to the general population, we calculated the age, sex and race specific rates of microalbuminuria that would be expected using the rates generated by the NHANES 2001–2010 data among non-diabetic adults which defined microalbuminuria as an ACR 30–300 mg/g (8). Based on NHANES prevalence estimates, we would have expected 12.6 cases of microalbuminuria from 182 non-diabetic adults of the similar age, sex and race for a prevalence of 7%, in contrast to our observed prevalence of 14%.
As various methods have been used to assess ACR values and define microalbuminuria, we performed several sensitivity analyses; these analyses also serve to allow our data to be compared with published data from other cohorts. First, considering only a single urine ACR, microalbuminuria was present on 17.6% of first ACR values, on 12.6% of second ACR values, and on 15.9% of third ACR values. Fifteen subjects (8.2%) had ACR values in the microalbuminuric range at all three visits. Second, using the definition of geometric mean ACR 30–299 mg/g (not sex specific), the prevalence of microalbuminuria was 9.4% (11/117) in males and 4.6% (3/65) in females with an overall estimate of 7.7% (14/182). Third, ACR values are distributed according to a logarithmic function, with right skew. For this reason, the geometric mean is the most appropriate method to summarize data from one individual and serves to reduce effect of a single high outlier value on the mean value. Nevertheless, simple means have been used in the past to summarize urine ACR values. Using the simple mean ACR of 30–299 mg/g, the prevalence of microalbuminuria was 13.7% in males and 7.7% in females, and 11.5% for the group overall. Fourth, so-called “high normal” or elevated ACR values of 10–29 mg/g have been associated with increased cardiovascular risk and are defined as low-level albuminuria in new albuminuria taxonomy (9). In our cohort, 35 subjects (19.2%) had geometric mean ACR values of 10–29 mg/g, and thus, using these cutoffs, 26.9% of subjects had high-normal or elevated ACR values.
In order to determine the accuracy of a single urine determination of ACR to identify individuals with microalbuminuria, we calculated the positive and negative predicative value of the first specimen relative to the geometric mean ACR from three consecutive determinations. The positive predictive value of a single urine ACR to identify subjects with microalbuminuria was 74%, whereas the negative predictive value of a single determination was 98%.
Participants with microalbuminuria were more likely to meet criteria for metabolic syndrome, using a definition of metabolic syndrome that lacked albuminuria as a component (26.9% vs. 11.5%, p=0.03). Subjects with microalbuminuria were more likely to have hypertension (p=0.02) and to be on antihypertensive therapy (p=0.04) compared to those without microalbuminuria. However, there was no difference between those with and without microalbuminuria in measured blood pressure. There was a weak positive correlation identified between diastolic blood pressure and geometric mean ACR (r=0.19, p=0.01). Angiotensin converting enzyme inhibitor and/or angiotensin receptor blocker use was also increased among those with microalbuminuria (32% vs. 12%, p=0.02). Participants with microalbuminuria did not have significantly greater fasting lipid values or increased BMI or waist-to-hip ratio compared to those without microalbuminuria (Table 1 and Table 2).
Table 2.
Laboratory Evaluation by Microalbuminuria Status
| Characteristic or laboratory value | All (n = 182) | No Microalbuminuria (n = 156) | Microalbuminuria (n = 26) | p-value |
|---|---|---|---|---|
| Serum creatinine (mg/dL) | 0.93 ± 0.20 | 0.9 ± 0.2 | 1.0 ± 0.2 | 0.37 |
| Total Cholesterol (mg/dL) | 178 ± 40 | 176 ± 40 | 186 ± 42 | 0.29 |
| LDL (mg/dL) | 102 ± 32 | 102 ± 31 | 102 ± 36 | 0.95 |
| HDL (mg/dL) | 49 ± 15 | 48 ± 13 | 51 ± 23 | 0.48 |
| Triglycerides (mg/dL) | 159 ± 122 | 151 ± 105 | 209 ± 187 | 0.13 |
| Fibrinogen | 330.1 ± 79.1 | 330.5 ± 80.5 | 327.6 ± 71.4 | 0.85 |
| C-reactive Protein | 2.1 ± 4.0 | 2.1 ± 3.8 | 2.1 ± 5 | 0.97 |
| D-dimer (μg/mL) | 0.5 ± 1.5 | 0.5 ± 1.6 | 0.4 ± 0.4 | 0.63 |
| HIV RNA (log copies/mL) | 2.1 ± 0.9 | 2.0 ± 0.8 | 2.3 ± 1.2 | 0.33 |
| HIV RNA < 50 copies/mL, n (%) | 127 (69.8) | 111 (71.2) | 16 (61.5) | 0.32 |
| CD4 count (cells/μL) | 513 ± 277 | 534 ± 273 | 392 ± 276 | 0.02 |
| CD4 count < 200 cells/μL, n (%) | 19 (10.4) | 11 (7.1) | 8 (30.8) | 0.0003 |
| Nadir CD4 count (cells/μL) | 168 ± 154 | 172 ± 152 | 148 ± 167 | 0.49 |
ACR, Albumin-to-creatinine ratio;
LDL, low-density lipoprotein;
HDL, high-density lipoprotein
While duration of HIV and HIV viremia were similar between groups, those with microalbuminuria had significantly lower mean CD4 counts (p=0.02) and were more likely to have a CD4 count < 200 cell/μL (p=0.0003) (Table 2). In order to assess potential associations between microalbuminuria and antiretroviral exposures, we compared current and cumulative exposures to each class of agents and each individual medication between those with and without microalbuminuria. Participant currently receiving ritonavir were more likely to have microalbuminuria compared to those not on ritonavir (19.8% vs. 7.4%, p=0.02). There were no significant differences in current or cumulative exposures to any class or other individual agent, including tenofovir, between the two groups (Table 1).
In a multivariate logistic regression analysis which evaluated low CD4 count (CD4 count <200 cells/μL), hypertension, current ritonavir use, age, sex and race, the only significant independent predictors of microalbuminuria were low CD4 count (p=0.018) and current ritonavir exposure (p=0.04).
Discussion
To date six large cohort studies of HIV-infected adults have evaluated microalbuminuria prevalence, using quantitative assessment of urine ACR and current definitions of normal values; these studies have reported microalbuminuria values ranging from 4% to 20% (Table 3). Two studies have included two or three baseline urine samples, and these found microalbuminuria prevalence of 4% and 9%. The present study was designed to exclude subjects with microalbuminuria associated with diabetes, physiological stress (e.g. fever, vigorous exercise) or chronic inflammatory conditions which might be associated with HIV infection but were not unique to HIV infection (cancer or chronic infections such as mycobacteria). Further, we used the most rigorous sampling method used to date in studies of HIV-associated microalbuminuria, with three urine samples collected over six to nine months in all completing subjects. We defined microalbuminuria based on the geometric mean of three samples, to account for the logarithmic relationship between ACR values and clinical outcomes, and we used sex-specific ACR thresholds to account for differences in muscle mass and hence urine creatinine concentrations between men and women. For these reasons, we consider the microalbuminuria period prevalence of 14% reported here to be a useful and reliable estimate of microalbuminuria in contemporary HIV clinic populations, excluding subjects with chronic inflammatory conditions and physiologic albuminuria. Using NHANES data we estimate this to be twice as high as would be expected in a non-diabetic population of similar age, sex and racial background. Although we were not able to match directly on blood pressure, the self-reported prevalence of hypertension in the non-diabetic NHANES population was 21%, whereas our HIV clinic population prevalence was similar at 24.7%. Therefore, we postulate that the increment in this prevalence over that of the general US population plausibly reflects the effects of HIV, immunosuppression, or anti-retroviral therapy.
Table 3.
Microalbuminuria in studies of HIV-infected Adults
| Reference | Study group or location | Enrollment Period | N | Inclusion Criteria | Major Exclusion Criteria | Microalbuminuria definition | Microalbuminuria prevalence | Urine samples |
|---|---|---|---|---|---|---|---|---|
| Szczech et al., 2007 (2) | Fat redistribution and metabolic change in HIV infection (FRAM) | 1999 | 760 | Proteinuria ≥1 by dipstick | ACR ≥30 mg/g | 11% | 1 | |
| Baekken, et al., 2008 (3) | Microalbuminuria in HIV+ population in Oslo (MAHO) | 2004–2005 | 495 | Hypertension Diabetes | ACR 2.5–30 mg/mmol (22–265 mg/g) present on ≥2 samples | 9% | 93% had 3 samples over 12 months | |
| Szczech, et al., 2010 (4) | North Carolina | Not reported | 948 | Urine PCR ≥0.35 g/g | ACR >30 mg/g | 20% at baseline 11% persistent albuminuria on repeat ACR over 2 years |
75% had 2 samples over ≤2 years | |
| Wyatt, et al., 2011 (5) | Women’s Interagency Health (WIHS) | 1994–1995 | 1,073 | Naïve to antiretroviral therapy | None | ACR >30 mg/g | 4% | 2 samples over 6 months |
| Yanagisawa, et al., 2011 (6) | Japan | 2009 | 732 | None | ACR 30–299 mg/g | 13% | 1 | |
| Overton, et al., 2012 (7) | Study to understand the natural history of HIV/AIDS (SUN) | 2004–2006 | 670 | CD4 count <100 | ACR ≥30 mg/g | 11% | 1 | |
| Present study 2012 | Prospective evaluation of albuminuria in HIV (PEA-H) | 2007–2011 | 182 | Serum creatinine ≤1.4 mg/dL | Urine PCR ≥0.5 g/g Diabetes Chronic infection Malignancy Fever or vigorous exercise prior to urine |
ACR geometric mean; Men 17–250 mg/g Women 25–355 mg/g |
14% | 3 samples over 6–9 months |
Studies were selected which enrolled >100 HIV-infected subjects and assessed urine albumin using a quantitative method. Seven studies met these criteria; all enrolled only adult subjects, and 5 were performed in the USA and one study each in Norway and Japan. Studies are listed by year of publication.
Microalbuminuria is defined as 30–300 mg/g, except two studies as shown that included macroalbuminuria, and the present study, which used sex-specific thresholds for the geometric mean value of 3 urines samples.
ACR, urine albumin/creatinine ratio; PCR, urine protein/creatinine ratio
The variability of albumin/creatinine measurements in first morning or random urine samples assessed over periods of a few months has been evaluated rigorously in relatively few clinical settings. Single void ACR CV values have been reported as follows: type 1 diabetes, mean CV 49%; type 2 diabetes, median CV 32% (10); and general population, median 19% for first void and 36% for mid-morning collection (11). In the present study using untimed samples, we report a median CV of 37% and a mean CV of 45%, which is similar to these non-HIV populations. Concordance of one sample with the mean to predict microalbuminuria have been reported as follows: type 1 diabetes, 97% sensitivity and 82% specificity for concordance with the arithmetic mean (12) and for type 2 diabetes, 95% concordance with normal albumin excretion for the geometric mean and 83% concordance with microalbuminuria for the geometric mean (10). For HIV disease, a single study has reported that when an increased ACR is noted, it persists in only 41% of subjects on a second measurement (13). In the present manuscript, we found that a single sample with normal albumin had negative predictive value of 98% to exclude albuminuria, defined as the geometric mean of three samples, and a single sample with albuminuria had positive predictive value of 74% for albuminuria, also defined as the geometric mean of three samples.
Our data suggest that when ACR is measured in HIV subjects and care is taken to avoid active infections or cancer, or physiologic causes of albuminuria, that a single ACR value is generally predictive of the mean ACR over a period of six to nine months, with CV and predictive values similar to other disease settings. Why are these data important? The current recommendation by the Infectious Disease Society of America is to measure urine protein annually by urinalysis dipstick in all patients with HIV disease (14), who currently number 1.1 million individuals. The use of ACR as a screening tool is recommended for early detection of kidney disease in high risk individuals, such as those with diabetes, hypertension, or family history of kidney disease, but not in the general population (15). While cost-benefit studies for urine ACR in the HIV population have not been published, we feel that it may be prudent to carry out annual measurement of urine protein/creatinine ratio or urine albumin/creatinine ratio, or preferably both, to provide guidance as to the presence of CKD and the risk for cardiovascular events (16). The stability of urine ACR in the HIV population shown here suggests that annual measurement is sufficient when the ACR is normal, and should be repeated to confirm an elevated value. Prior studies in the HIV population have demonstrated that, when classified accurately, microalbuminuria is predictive of the development as overt proteinuria and renal dysfunction (4, 17).
The risk factors for microalbuminuria we identified included traditional HIV specific markers such as CD4 lymphocyte count as well as cardiovascular risk factors such as hypertension and metabolic syndrome. It is likely that hypertension and metabolic syndrome act by altering endothelial integrity, resulting in increases glomerular filtration of albumin (18). Of interest, we did not identify an increased prevalence of microalbuminuria related to African American race despite a higher risk for glomerulopathy among HIV-infected Black subjects and a higher rate of microalbuminuria among Black subjects in the US population (19, 20).
The association between microalbuminuria and cardiovascular disease risk has been noted in prior studies of both HIV-infected and non-HIV-infected individuals, including a 2007 study by Szczech and colleagues (2) in which higher albumin excretion rates were strongly associated with higher systolic blood pressure and insulin resistance. In addition, two small studies demonstrated improvements in microalbuminuria with the use of telmisartan to treat hypertension in HIV (21, 22). In the general population, metabolic syndrome has been linked to high rates of albuminuria (23), and among large HIV-infected cohorts, metabolic syndrome is increasingly prevalent (24). We identified an increased frequency of metabolic syndrome (26.9%) among those with microalbuminuria compared to those without, suggesting a contribution of this pathophysiologic constellation to kidney dysfunction in HIV.
An association between microalbuminuria and low CD4 count has been reported in earlier studies (2, 4). For example, in a cross-sectional cohort study of 760 HIV-infected men and women (2), Szczech and colleagues identified that both low current CD4 count and lower nadir CD4 count were significantly associated with elevated ACR based on a spot urine evaluation. Data from the present study confirm that impaired immune function is a risk factor for early renal impairment. HIV-associated nephropathy is believed to result from direct viral effects of HIV on renal epithelial cells and podocytes (25), however in this cohort of largely virally suppressed patients, there was no relationship between microalbuminuria and HIV viremia.
Although the relationship between tenofovir use and renal dysfunction has been noted in a number of studies (26–29), few reports have implicated tenofovir in the development of proteinuria (30). As observed by Hall and colleagues (30), our analysis showed no increased propensity for microalbuminuria in patients currently receiving tenofovir. Of interest, we found that HIV-infected subjects with microalbuminuria were more likely to be currently receiving ritonavir compared to those without microalbuminuria. This effect remained statistically significant in multivariate models that also took into account effects of age, sex, race, hypertension and CD4 count. In contrast, Szczech and colleagues identified that current NNRTI use was associated with increased ACR, and not PI use (2). To our knowledge, a relationship between ritonavir and microalbuminuria has not been noted in earlier studies. A recently published study looking at 670 HIV-infected subjects reported that current exposure to ritonavir was associated with renal dysfunction defined as eGFR <90mL/min/1.73 m2 (7). Further, Ando and colleagues observed that HIV-infected patients with low grade ACR elevations were more likely to progress to renal dysfunction (eGFR<60mL/min/1.73 m2) and that use of a PI in combination with tenofovir was also a significant risk factor for progression (17). Albuminuria may be an important early indicator of renal toxicity from certain antiviral medications, but further research is needed to determine the potential mechanism of ritonavir and/or protease inhibitors in this process.
While the strengths of this study include recruitment of subjects from two large diverse clinical cohorts and the care taken to exclude individuals with chronic inflammatory conditions or physiologic albuminuria, this study does have important potential limitations. First, our study design did not include an HIV-uninfected control group. As previously noted we used untimed urinary specimens and this may have contributed to variability among measurements. Additionally, with 182 subjects, this cohort is relatively small compared to other larger cohort studies reported previously. Finally, there is a possibility for a selection bias in that participants concerned with kidney function may have been more likely to agree to participate. We chose to exclude individuals with abnormal creatinine levels and to exclude diabetes in order to assess the prevalence of microalbuminuria in HIV-infected patients without evidence of kidney disease or known high risk co-morbidities.
In summary, these data demonstrate a period prevalence of microalbuminuria among an HIV-infected clinic population, carefully screened to exclude inflammatory conditions and physiologic albuminuria, which is similar to earlier reports. The present study is the first to demonstrate through serial determinations of ACR that a single normal ACR can largely exclude microalbuminuria, whereas an elevated ACR requires confirmation. In this cohort, microalbuminuria was associated with cardiovascular risk factors including hypertension and metabolic syndrome, and was independently associated with low CD4 lymphocyte count and current ritonavir exposure. Future investigation is needed to determine the natural history and progression of microalbuminuria in the context of HIV infection and to determine whether, how, and when to treat microalbuminuria.
Acknowledgments
The study is registered in ClinicalTrials.gov (NCT00524992). This project has been funded through the Intramural NIH Research Programs of National Institute of Allergy and Infectious Disease and National Institute of Diabetes and Digestive and Kidney Diseases. It was also supported through the National Cancer Institute under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
The authors have no conflicts of interest to report.
References
- 1.Bruggeman LA, Nelson PJ. Controversies in the pathogenesis of HIV-associated renal diseases. Nature Reviews Nephrology. 2009;5(10):575–82. doi: 10.1038/nrneph.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szczech LA, Grunfeld C, Scherzer R, Canchola JA, van der Horst C, Sidney S, et al. Microalbuminuria in HIV infection. Aids. 2007;21(8):1003–9. doi: 10.1097/QAD.0b013e3280d3587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baekken M, Os I, Sandvik L, Oektedalen O. Microalbuminuria associated with indicators of inflammatory activity in an HIV-positive population. Nephrology Dialysis Transplantation. 2008;23(10):3130–7. doi: 10.1093/ndt/gfn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szczech LA, Menezes P, Quinlivan EB, van der Horst C, Bartlett JA, Svetkey LP. Microalbuminuria predicts overt proteinuria among patients with HIV infection. Hiv Medicine. 2010;11(7):419–26. doi: 10.1111/j.1468-1293.2009.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyatt CM, Hoover DR, Shi Q, Tien PC, Karim R, Cohen MH, et al. Pre-existing albuminuria predicts AIDS and non-AIDS mortality in women initiating antiretroviral therapy. Antivir Ther. 2011;16(4):591–6. doi: 10.3851/IMP1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanagisawa N, Ando M, Ajisawa A, Imamura A, Suganuma A, Tsuchiya K, et al. Clinical characteristics of kidney disease in Japanese HIV-infected patients. Nephron Clin Pract. 2011;118(3):c285–91. doi: 10.1159/000322278. [DOI] [PubMed] [Google Scholar]
- 7.Overton ET, Patel P, Mondy K, Bush T, Conley L, Rhame F, et al. Cystatin C and baseline renal function among HIV-infected persons in the SUN Study. AIDS Res Hum Retroviruses. 2012;28(2):148–55. doi: 10.1089/AID.2011.0018. [DOI] [PubMed] [Google Scholar]
- 8.Plantinga LC, Crews DC, Coresh J, Miller ER, 3rd, Saran R, Yee J, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clinical journal of the American Society of Nephrology: CJASN. 2010;5(4):673–82. doi: 10.2215/CJN.07891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2009;54(2):205–26. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Pugliese G, Solini A, Fondelli C, Trevisan R, Vedovato M, Nicolucci A, et al. Reproducibility of albuminuria in type 2 diabetic subjects. Findings from the Renal Insufficiency And Cardiovascular Events (RIACE) study. Nephrol Dial Transplant. 2011;26(12):3950–4. doi: 10.1093/ndt/gfr140. [DOI] [PubMed] [Google Scholar]
- 11.Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009;20(2):436–43. doi: 10.1681/ASN.2008030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McHardy KC, Gann ME, Ross IS, Pearson DW. A simple approach to screening for microalbuminuria in a type 1 (insulin-dependent) diabetic population. Ann Clin Biochem. 1991;28 ( Pt 5):450–5. doi: 10.1177/000456329102800505. [DOI] [PubMed] [Google Scholar]
- 13.Wyatt CM, Hoover DR, Shi Q, Seaberg E, Wei C, Tien PC, et al. Microalbuminuria is associated with all-cause and AIDS mortality in women with HIV infection. J Acquir Immune Defic Syndr. 2010;55(1):73–7. doi: 10.1097/QAI.0b013e3181cc1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta SK, Eustace JA, Winston JA, Boydstun, Ahuja TS, Rodriguez RA, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005;40(11):1559–85. doi: 10.1086/430257. [DOI] [PubMed] [Google Scholar]
- 15.Taal MW. Screening for chronic kidney disease: preventing harm or harming the healthy? PLoS Med. 2012;9(11):e1001345. doi: 10.1371/journal.pmed.1001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalayjian RC. Renal issues in HIV infection. Curr HIV/AIDS Rep. 2011;8(3):164–71. doi: 10.1007/s11904-011-0080-x. [DOI] [PubMed] [Google Scholar]
- 17.Ando M, Yanagisawa N, Ajisawa A, Tsuchiya K, Nitta K. Urinary albumin excretion within the normal range is an independent risk for near-term development of kidney disease in HIV-infected patients. Nephrol Dial Transplant. 2011;26(12):3923–9. doi: 10.1093/ndt/gfr129. [DOI] [PubMed] [Google Scholar]
- 18.de Jong PE, Gansevoort RT. Focus on microalbuminuria to improve cardiac and renal protection. Nephron Clin Pract. 2009;111(3):c204–10. doi: 10.1159/000201568. discussion c11. [DOI] [PubMed] [Google Scholar]
- 19.Szczech LA, Gange SJ, van der Horst C, Bartlett JA, Young M, Cohen MH, et al. Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int. 2002;61(1):195–202. doi: 10.1046/j.1523-1755.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 20.Martins D, Tareen N, Zadshir A, Pan D, Vargas R, Nissenson A, et al. The association of poverty with the prevalence of albuminuria: data from the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2006;47(6):965–71. doi: 10.1053/j.ajkd.2006.02.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ucciferri C, Falasca K, Mancino P, Di Iorio A, Vecchiet J. Microalbuminuria and hypertension in HIV-infected patients: a preliminary study of telmisartan. Eur Rev Med Pharmacol Sci. 2012;16(4):491–8. [PubMed] [Google Scholar]
- 22.Vecchiet J, Ucciferri C, Falasca K, Mancino P, Di Iorio A, De Caterina R. Antihypertensive and metabolic effects of telmisartan in hypertensive HIV-positive patients. Antivir Ther. 2011;16(5):639–45. doi: 10.3851/IMP1809. [DOI] [PubMed] [Google Scholar]
- 23.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6(10):2364–73. doi: 10.2215/CJN.02180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worm SW, Friis-Moller N, Bruyand M, D’Arminio Monforte A, Rickenbach M, Reiss P, et al. High prevalence of the metabolic syndrome in HIV-infected patients: impact of different definitions of the metabolic syndrome. AIDS. 2010;24(3):427–35. doi: 10.1097/QAD.0b013e328334344e. [DOI] [PubMed] [Google Scholar]
- 25.Atta MG, Lucas GM, Fine DM. HIV-associated nephropathy: epidemiology, pathogenesis, diagnosis and management. Expert Rev Anti Infect Ther. 2008;6(3):365–71. doi: 10.1586/14787210.6.3.365. [DOI] [PubMed] [Google Scholar]
- 26.Izzedine H, Isnard-Bagnis C, Hulot JS, Vittecoq D, Cheng A, Jais CK, et al. Renal safety of tenofovir in HIV treatment-experienced patients. AIDS. 2004;18(7):1074–6. doi: 10.1097/00002030-200404300-00019. [DOI] [PubMed] [Google Scholar]
- 27.Monteagudo-Chu MO, Chang MH, Fung HB, Brau N. Renal Toxicity of Long-Term Therapy With Tenofovir in HIV-Infected Patients. J Pharm Pract. 2012 doi: 10.1177/0897190012442718. [DOI] [PubMed] [Google Scholar]
- 28.Izzedine H, Hulot JS, Vittecoq D, Gallant JE, Staszewski S, Launay-Vacher V, et al. Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients. Data from a double-blind randomized active-controlled multicentre study. Nephrol Dial Transplant. 2005;20(4):743–6. doi: 10.1093/ndt/gfh658. [DOI] [PubMed] [Google Scholar]
- 29.Gallant JE, Parish MA, Keruly JC, Moore RD. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis. 2005;40(8):1194–8. doi: 10.1086/428840. [DOI] [PubMed] [Google Scholar]
- 30.Hall AM, Edwards SG, Lapsley M, Connolly JO, Chetty K, O’Farrell S, et al. Subclinical tubular injury in HIV-infected individuals on antiretroviral therapy: a cross-sectional analysis. Am J Kidney Dis. 2009;54(6):1034–42. doi: 10.1053/j.ajkd.2009.07.012. [DOI] [PubMed] [Google Scholar]