Abstract
(R)-3-Hydroxybutyl (R)-3-hydroxybutyrate (ketone monoester) has been developed as an oral source of ketones, which may be utilized for energy. In a 28-day toxicity study, Crl:WI (Wistar) rats received diets containing, as 30% of the calories, ketone monoester (12 and 15 g/kg body weight/day for male and female rats, respectively). Control groups received either carbohydrate- or fat-based diets. Rats in the test group consumed less feed and gained less weight than control animals; similar findings have been documented in studies of ketogenic diets. Between-group differences were noted in selected hematology, coagulation, and serum chemistry parameters; however, values were within normal physiological ranges and/or were not accompanied by other changes indicative of toxicity. Upon gross and microscopic evaluation, there were no findings associated with the ketone monoester. In a developmental toxicity study, pregnant Crl:WI (Han) rats were administered 2 g/kg body weight/day ketone monoester or water (control) via gavage on days 6 through 20 of gestation. No Caesarean-sectioning or litter parameters were affected by the test article. The overall incidence of fetal alterations was higher in the test group; however, there were no specific alterations attributable to the test substance. The results of these studies support the safety of ketone monoester.
Keywords: (R)-3-Hydroxybutyl (R)-3-hydroxybutyrate, Developmental toxicity, Ketones, Toxicity, Safety
1. Introduction
Although glucose and fatty acids are the primary energy sources for most cells, the body can utilize endogenously produced ketones under conditions in which glucose is limited, such as during prolonged starvation (Stanfield and Germann, 2008). During fasting, ketones (i.e., d-β-hydroxybutyrate, acetoacetate, and acetone) generated by the liver are transported to extrahepatic tissues, where they may be utilized for energy.
The combustion of d-β-hydroxybutyrate produces greater amounts of energy compared to that of the glycolytic substrate, pyruvate, increasing hydraulic efficiency in the working perfused rat heart by approximately 30% (Sato et al., 1995). The energy generated by ketones in the brain may have beneficial effects on cognition, as demonstrated by results of studies on experimental models of disease and patients with Alzheimer's and Parkinson's diseases (Kashiwaya et al., 2000), as well as in individuals with memory impairment (Reger et al., 2004).
While increasing the physiological levels of ketone bodies may have potential benefits in improving physical performance and cognitive function, inducing mild ketosis through the use of classic ketogenic diets containing high fat and low carbohydrate (CHO) is not desirable, as the diets are unpalatable and may present an atherogenic risk through the elevation of serum cholesterol and triglyceride levels (McPherson and McEneny, 2011). (R)-3-Hydroxybutyl (R)-3-hydroxybutyrate, referred to as ketone monoester, has been shown to elevate blood ketone levels in humans, as it is fully hydrolyzed to d-β-hydroxybutyrate and (R)-1,3-butanediol following consumption (Clarke et al., submitted for publication), with the latter being further metabolized to d-β-hydroxybutyrate and acetoacetate in the liver (Desrochers et al., 1992; Tate et al., 1971). These findings are corroborated by results of studies in which blood levels of d-β-hydroxybutyrate, acetoacetate, and (R)-1,3-butanediol were elevated in rats following gavage administration of ketone monoester (Carter et al., unpublished). Ketone monoester also can be delivered in a food form for energy purposes, thereby reducing the potential concerns associated with the administration of a ketogenic diet.
In preliminary studies, pair-feeding a diet containing ketone monoester (13.7 g/kg bw/day) to Wistar rats for 66 days did not significantly impact body weights in comparison to a Western diet or a CHO-based diet (Knight et al., unpublished). Plasma free fatty acid levels and lactate dehydrogenase (LDH) activity were also unaffected.
Results from a short-term study in humans also support the safety of ketone monoester (Clarke et al., submitted for publication). The ester was generally well tolerated when consumed (in a meal replacement beverage) at 420, 1071, and 2142 mg/kg body weight (bw)/day for 5 days and no abnormal changes in hematology, clinical biochemistry, or urinalysis parameters were observed. Moreover, blood ketone levels and glucose levels did not deviate from ranges deemed to be safe. Vital signs were stable throughout the course of the study, and no treatment-related abnormalities were reported upon physical examination.
As limited data are available on the safety of ketone monoester, a 28-day oral toxicity study and a developmental toxicity study in rats were undertaken. Results of these studies are reported herein.
2. Materials and methods
2.1. 28-Day oral toxicity study
This study was conducted in accordance with the Good Laboratory Practice (GLP) for Nonclinical Laboratory Studies by the United States Food & Drug Administration (US FDA), 21CFR, Part 58 and the Organisation for Economic Co-operation and Development (OECD) Principles of GLP (OECD, 1998; US FDA, 2011). The study design and sample sizes were based on the US FDA Redbook 2000: IV.C.3.a Short-Term Toxicity Studies with Rodents (US FDA, 2003).
2.1.1. Materials
(R)-3-Hydroxybutyl (R)-3-hydroxybutyrate was synthesized at the University of Oxford as a colorless oil comprising ethyl-(R)-3-hydroxybutyrate (~1%) and (R)-1,3-butanediol (~1%), which were the starting materials, (R)-3-hydroxybutyl (R)-3-hydroxybutyrate (94%), 3-betahydroxybutryl 1,3-butanediol monoester (~1%), and di-β-hydroxybutyrate 1,3-butanediol diester (~3%). Information on the synthetic pathway and methods used to characterize the material are considered proprietary and thus, are not described further. Unrefined palm oil (Pride Oils Plc, UK) and un-modified, regular corn starch (approximately 73% amylopectin and 27% amylase; Sigma–Aldrich) were used to supplement the fat and CHO diets, respectively. Palm oil was used as it is commonly employed to mimic a Western-style rat diet.
2.1.2. Preparation of diets
The test (ketone monoester) and control (CHO and fat) diets were prepared at the University of Oxford, frozen after preparation, and shipped to the test facility on dry ice. The diet base was prepared by mixing rodent chow (in powder form) with sugar-free jelly crystals. The test (ketone monoester) and control fat (palm-oil) and CHO (corn starch) were mixed in heated water and subsequently added to the chow/jelly base mixture. The jelly was used to bind the rodent chow so that it could be made into pellets. At the test facility, diets were kept frozen at –10 to –25 °C prior to use.
The compositions of the test and control diets, as well as the caloric content of each diet, are presented in Table 1. The ketone ester diet was formulated to contain 30% of calories from (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. The test and fat diets had the same calorie contribution from CHO (39% calories from CHO), whereas the test and CHO diets had the same calorie contribution from fat (4% calories from fat). All three diets were matched for protein.
Table 1.
Diet Compositions in the 28-day study.
| Component | Carbohydrate diet | Fat diet | Ketone ester diet |
|---|---|---|---|
| g/100 g | |||
| Rodent chow | 25.7 | 25.7 | 25.7 |
| Sugar-free jelly | 13.4 | 13.4 | 13.4 |
| Water | 46.3 | 55.1 | 49.6 |
| Palm oil | 0 | 5.8 | 0 |
| Cornflour | 14.5 | 0 | 0 |
| Ketone ester | 0 | 0 | 11.4 |
| Total | 100 | 100 | 100 |
| kcal (%) | |||
| CHO | 69 | 39 | 39 |
| Protein | 27 | 27 | 27 |
| Fat | 4 | 34 | 4 |
| Ketone ester | 0 | 0 | 30 |
| Total | 100 | 100 | 100 |
2.1.3. Analysis of dietary mixtures
Portions of the preparations of the test diet were analyzed for concentration validation and homogeneity. Chloroform extracts from each of four batches of ketone ester diets were analyzed in quadruplicate using a gas chromatography–mass spectrometry (GC–MS) analyzer.
2.1.4. Animals and feeding protocol
Male and female Crl:WI (Wistar) rats were obtained from Charles River Canada, Inc. (Montreal, Canada). Animals were housed individually in Nalgene® rat cages. The animal room environment was controlled (targeted ranges: temperature 18–26 °C, relative humidity 30–70%, 10–15 air changes/h) and monitored.
The animals were provided Teklad Certified Rodent diet and tap water ad libitum throughout the acclimatization period of 16 days. During the acclimatization period, rats were randomized into the test and control groups using randomization statistical software, with male and females rats randomized separately. Each group consisted of 10 animals per sex. At the start of the study, animals were approximately 9 weeks of age, and body weights were approximately 337–357 and 200–225 g for male and female rats, respectively.
During the test period, the ketone ester diet was offered to corresponding rats ad libitum. Originally, it was planned to provide the animals in the control groups an average of the food consumed by the ketone ester-fed rats. This approach was modified due to the wide range of daily food consumption by the ketone ester-fed animals (the difference in food consumption amongst the animals in ketone ester-fed diet group ranged between 5 and 15 g). All control animals received an amount of food approximately equal to the amount consumed by those ketone ester-fed rats that consumed most of their daily rations. Most of the ketone ester-fed rats had some food remaining by the next day, while most of the control rats had no food left the next day. This resulted in the control animals consuming more calories per day on average than the ketone ester-fed animals.
2.1.5. Observations
All animals were inspected twice daily during the course of the study. In addition, they were monitored closely during the morning weighing/feeding period. Animals were observed for reaction to treatment such as changes in skin, fur, eyes, and mucous membranes. Respiratory, circulatory, autonomic, central nervous system, somatomotor activity, and behavior patterns also were monitored along with any other signs of ill health.
Clinical signs were recorded once a day during the morning observation period. If the afternoon observations differed, they also were recorded. All animals received a weekly detailed clinical examination. These evaluations required handling and examination for general appearance, respiration, abnormalities for behavior and movement, and included external organs, skin, and any lesions.
The unfasted body weight of each rat was recorded once (for randomization) prior to its assignment to the relevant group. Each rat was weighed again (unfasted) before dosing on day 1 and on days 8, 15, and 22. Final body weights (unfasted) were taken on the day preceding necropsy (day 28). Finally, following an overnight period (approximately 12–18 h) of food deprivation, each rat was weighed terminally prior to necropsy (day 29). The food intake of each rat was recorded daily during the study period.
2.1.6. Hematology and clinical chemistry
On day 29 following an overnight period (approximately 12 h) of food (but not water) deprivation, each animal was weighed, then anesthetized with isoflurane. Blood samples were collected from the abdominal aorta of all animals, prior to exsanguination and necropsy. Blood samples were analyzed for hematology, coagulation, and serum chemistry parameters (Tables 3 and 4). Hematology parameters from whole blood were measured using an automated blood cell counter (Advia 120, Bayer Diagnostics Mfg. Ltd., Dublin, Ireland). Plasma was separated from blood, and coagulation parameters were assessed using an automated coagulation analyzer (Coag-A-Mate® RA4, Organon Teknika, Durham, North Carolina, USA). For serum chemistry analysis, the serum was separated from blood and examined using an automated biochemical analyzer (Vitros 250, Ortho Clinical Diagnostics, Rochester, New York, USA).
Table 3.
Hematology and coagulation measurements in the 28-day study.
| Parameter | Unit | CHO diet (n = 10/sex) | Fat diet (n = 10/sex) | Ketone ester diet (n = 10/sex) |
|---|---|---|---|---|
| Males | ||||
| RBC | ×1012/L | 7.62 ± 0.44 | 7.80 ± 0.32 | 8.41 ± 0.28*,† |
| Hb | g/L | 146 ± 8 | 146 ± 7 | 158 ± 8*,† |
| Hct | % | 39.3 ± 2.1 | 39.2 ± 1.8 | 42.1 ± 1.9*,† |
| MCV | fL | 51.5 ± 1.2 | 50.3 ± 1.0 | 50.0 ± 1.1* |
| MCH | pg | 19.1 ± 0.5 | 18.7 ± 0.4 | 18.8 ± 0.5 |
| MCHC | g/L | 371 ± 2 | 371 ± 5 | 375 ± 6 |
| Platelets | ×109/L | 1303 ± 145 | 1376 ± 149 | 1387 ± 218 |
| WBC | ×109/L | 4.69 ± 1.47 | 5.71 ± 2.26 | 5.44 ± 1.64 |
| Neutrophils | ×109/L | 0.92 ± 0.44 | 1.04 ± 0.37 | 0.88 ± 0.25 |
| Lymphocytes | ×109/L | 3.59 ± 1.21 | 4.43 ± 1.87 | 4.29 ± 1.35 |
| Monocytes | ×109/L | 0.10 ± 0.05 | 0.13 ± 0.07 | 0.16 ± 0.08 |
| Eosinophils | ×109/L | 0.06 ± 0.03 | 0.08 ± 0.02 | 0.09 ± 0.04 |
| Basophils | ×109/L | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| LUC | ×109/L | 0.02 ± 0.01 | 0.03 ± 0.02 | 0.02 ± 0.01 |
| Reticulocytes | ×109/L | 194 ± 30 | 193 ± 33 | 229 ± 36*,† |
| Prothrombin time | s | 18.0 ± 1.0 | 17.4 ± 0.8 | 17.1 ± 0.6 |
| APPT | s | 16.1 ± 1.7 | 16.7 ± 2.2 | 13.8 ± 2.2*,† |
| Fibrinogen | g/L | 2.60 ± 0.29 | 2.41 ± 0.19 | 2.57 ± 0.30 |
| Females | ||||
| RBC | ×1012/L | 7.73 ± 0.25 | 7.47 ± 0.20 | 8.36 ± 0.40*,† |
| Hb | g/L | 153 ± 4 | 143 ± 3 | 160 ± 6*,† |
| Hct | % | 40.0 ± 1.4 | 38.1 ± 1.0 | 41.8 ± 1.6*,† |
| MCV | fL | 51.7 ± 1.1 | 51.1 ± 1.4 | 50.1 ± 1.5* |
| MCH | pg | 19.8 ± 0.5 | 19.2 ± 0.5 | 19.1 ± 0.5* |
| MCHC | g/L | 382 ± 5 | 375 ± 6 | 382 ± 4† |
| Platelets | ×109/L | 1316 ± 125 | 1482 ± 85 | 1146 ± 252† |
| WBC | ×109/L | 3.90 ± 1.37 | 3.66 ± 0.73 | 4.13 ± 1.14 |
| Neutrophils | ×109/L | 0.61 ± 0.19 | 0.81 ± 0.36 | 0.67 ± 0.22 |
| Lymphocytes | ×109/L | 3.10 ± 1.21 | 2.66 ± 0.59 | 3.24 ± 1.09 |
| Monocytes | ×109/L | 0.10 ± 0.04 | 0.11 ± 0.06 | 0.14 ± 0.05 |
| Eosinophils | ×109/L | 0.07 ± 0.03 | 0.06 ± 0.02 | 0.06 ± 0.02 |
| Basophils | ×109/L | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.00 ± 0.01 |
| LUC | ×109/L | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 |
| Reticulocytes | ×109/L | 141 ± 25 | 158 ± 34 | 169 ± 42 |
| Prothrombin time | s | 18.2 ± 1.2 | 17.7 ± 0.8 | 17.5 ± 1.1 |
| APPT | s | 15.1 ± 1.9 | 15.3 ± 1.6 | 14.6 ± 1.9 |
| Fibrinogen | g/L | 2.18 ± 0.2 | 1.98 ± 0.15 | 2.21 ± 0.19† |
Abbreviations: CHO = carbohydrate; Hb = hemoglobin; Hct = hematocrit; LUC = large unstained cells; MCV = mean corpuscular volume; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; n = number; RBC = red blood cells; WBC = white blood cells; APPT = activated partial thromboplastin time.
All values are presented as mean ± standard deviation.
Significantly different than the CHO diet group (p < 0.05).
Significantly different than the fat diet group (p < 0.05).
Table 4.
Clinical chemistry measurements in the 28-day study.
| Parameter | Unit | CHO diet (n = 10/sex) | Fat diet (n = 10/sex) | Ketone ester diet (n = 10/sex) |
|---|---|---|---|---|
| Males | ||||
| A/G | – | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1† |
| ALB | g/L | 29 ± 1 | 29 ± 2 | 32 ± 2*,† |
| GLOB | g/L | 25 ± 1 | 25 ± 2 | 26 ± 2 |
| ALP | u/L | 140 ± 32 | 160 ± 41 | 145 ± 23 |
| Bil(T) | μmol/L | 3.4 ± 0.8 | 3.2 ± 0.9 | 5.0 ± 1.7*,† |
| BUN | mmol/L | 4.8 ± 0.5 | 4.6 ± 0.6 | 4.7 ± 0.7 |
| Ca | mmol/L | 2.51 ± 0.07 | 2.5 ± 0.11 | 2.56 ± 0.06 |
| Cl | mmol/L | 104 ± 2 | 104 ± 3 | 104 ± 1 |
| Creatinine | μmol/L | 39 ± 4 | 38 ± 3 | 40 ± 5 |
| Glucose | mmol/L | 9.0 ± 1.4 | 9.0 ± 1.5 | 8.2 ± 1.3 |
| LDH | u/L | 751 ± 381 | 636 ± 447 | 1205 ± 676† |
| P | mmol/L | 2.40 ± 0.16 | 2.45 ± 0.25 | 2.32 ± 0.20 |
| K | mmol/L | 4.5 ± 0.4 | 4.4 ± 0.4 | 4.9 ± 0.3† |
| Protein | g/L | 55 ± 2 | 54 ± 4 | 57 ± 2 |
| AST | u/L | 53 ± 6 | 55 ± 6 | 59 ± 7 |
| ALT | u/L | 39 ± 5 | 41 ± 6 | 47 ± 8* |
| Na | mmol/L | 142 ± 1 | 142 ± 1 | 141 ± 2 |
| Triglycerides | mmol/L | 0.72 ± 0.09 | 0.68 ± 0.10 | 0.75 ± 0.15 |
| CK | u/L | 109 ± 50 | 92 ± 63 | 175 ± 96† |
| Cholesterol | mmol/L | ±1.56 ± 0.23 | 1.95 ± 0.38 | 2.29 ± 0.49* |
| GGT | u/L | 6 ± 0 | 6 ± 0 | 6 ± 0 |
| Females | ||||
| A/G | – | 1.5 ± 0.2 | 1.4 ± 0.1 | 1.4 ± 0.1 |
| ALB | g/L | 36 ± 3 | 35 ± 3 | 34 ± 2 |
| GLOB | g/L | 25 ± 2 | 24 ± 1 | 24 ± 2 |
| ALP | u/L | 105 ± 24 | 148 ± 44 | 106 ± 23† |
| Bil(T) | μmol/L | 4.4 ± 0.4 | 6.2 ± 6.4 | 4.9 ± 0.8 |
| BUN | mmol/L | 5.0 ± 0.9 | 4.5 ± 0.5 | 4.7 ± 0.5 |
| Ca | mmol/L | 2.59 ± 0.09 | 2.52 ± 0.09 | 2.57 ± 0.06 |
| Cl | mmol/L | 106 ± 2 | 105 ± 2 | 104 ± 1 |
| Creatinine | μmol/L | 42 ± 5 | 37 ± 6 | 35 ± 5 |
| Glucose | mmol/L | 7.0 ± 0.6 | 7.8 ± 1.0 | 7.9 ± 2.0 |
| LDH | u/L | 1230 ± 581 | 1278 ± 376 | 1876 ± 662*,† |
| P | mmol/L | 2.29 ± 0.35 | 2.16 ± 0.29 | 2.21 ± 0.29 |
| K | mmol/L | 4.0 ± 0.2 | 4.1 ± 0.5 | 4.2 ± 0.4 |
| Protein | g/L | 61 ± 3 | 59 ± 4 | 58 ± 3 |
| AST | u/L | 57 ± 10 | 65 ± 14 | 61 ± 9 |
| ALT | u/L | 32 ± 5 | 34 ± 11 | 38 ± 7 |
| Na | mmol/L | 142 ± 1 | 141 ± 2 | 140 ± 1* |
| Triglycerides | mmol/L | 0.62 ± 0.17 | 0.51 ± 0.08 | 0.67 ± 0.13† |
| CK | u/L | 179 ± 92 | 167 ± 42 | 265 ± 115 |
| Cholesterol | mmol/L | 2.16 ± 0.39 | 2.03 ± 0.21 | 2.50 ± 0.52† |
| GGT | u/L | 6 ± 0 | 7 ± 3 | 6 ± 0 |
Abbreviations: A/G = albumin to globulin ratio; ALB = albumin; ALP = alkaline phosphatase; ALT = alanine amino transferase; AST = aspartate amino transferase; Bil(T) = bilirubin; BUN = blood urea nitrogen; CHO = carbohydrate; CK = creatine kinase; GGT = gamma-glutamyl transpeptidase; GLOB = globulin; LDH = lactate dehydrogenase.
All values are presented as mean ± standard deviation.
Significantly different than the CHO diet group (p < 0.05).
Significantly different than the fat diet group (p < 0.05).
2.1.7. Urinalysis
Urine was collected prior to necropsy (over approximately a 12-h period) by placing rats in metabolic cages. Urine was evaluated for the following parameters: appearance (cloudiness), color, and volume (by observation); bilirubin, blood, glucose, ketones, leukocytes, nitrite, pH, protein, specific gravity, and urobilinogen [Bayer Multistix® 10 SG Reagent Strips for Urinalysis, Bayer HealthCare LLC, Elkhart, IN, USA]; and amorphous phosphates, amorphous urate, bacteria or yeast, calcium oxalate, fat, pus, spermatozoa, squamous cells, triple phosphates, casts, epithelial cells, red blood cells, white blood cells, and transitional epithelial cells (by sediment microscopy).
2.1.8. Clinical pathology and histopathology
Gross necropsy included an examination of the external surfaces of the body; all orifices; cranial cavity; external surfaces of the brain and spinal cord; nasal cavity and paranasal sinuses; joints; thoracic, abdominal, and pelvic cavities; and viscera.
For each animal euthanized as per schedule at the end of the study, the following organs were dissected, trimmed free of fat and weighed: adrenals, brain, pituitary gland, prostate, heart, spleen, kidneys, liver, thymus, testes, lungs, ovaries, uterus, and seminal vesicles. Paired organs were weighed together. Absolute and relative organ weights (relative to terminal body weight and brain weight) were calculated.
Liver, kidneys, stomach, duodenum, jejunum, ileum, colon, brain, heart, and skeletal muscles from all animals were retained, fixed in neutral buffered 10% formalin, and prepared for microscopic examination by embedding in paraffin wax, sectioning and staining with hematoxylin and eosin. Additional tissues and organs were retained from all animals and preserved but not analyzed.
2.1.9. Statistical analysis
The data for males and females were separately analyzed for homogeneity of variance and for normality. Homogenous data were analyzed using the Analysis of Variance (at p = 0.05) and the significance of intergroup differences was analyzed using Duncan's or other appropriate test. Heterogenous data were analyzed using the Kruskal–Wallis test and the significance of intergroup differences between the control and treated groups was assessed using Dunn's or other appropriate test. Data are presented as mean ± standard deviation.
2.2. Developmental toxicity study
This study was conducted in accordance with the following: US FDA GLP for Nonclinical Laboratory Studies (21CFR, Part 58), the OECD Principles on GLP [ENV/MC/CHEM(98)7], and the Japanese GLP Standards for Safety Studies on Drugs (Ordinance No. 21 of the Pharmaceutical Affairs Bureau, Ministry of Health, Labour and Welfare, Japan). The study design and sample sizes were based on the US FDA Redbook IV.C.9.b Guidelines for Developmental Toxicity Studies (US FDA, 2000).
2.2.1. Materials
(R)-3-Hydroxybutyl (R)-3-hydroxybutyrate was synthesized at the University of Oxford as a colorless oil comprising ethyl R 3-hydroxybutyrate (~1%) and (R)-1,3-butanediol (~1%), which were the starting materials, ketone monoester (94%), 3-betahydroxybutryl 1,3 butanediol monoester (~1%), and di-β-hydroxybutyrate-1,3 butanediol diesters (~3%) The test article was used without dilution. The control article was local water, processed by passage through a reverse osmosis membrane (RO water).
2.2.2. Animals and treatment
Virgin female Crl:WI (Han) rats were obtained from Charles River Laboratories (Portage, MI). Male rats of the same source and strain were used as breeders. Rats were acclimated for 13 days prior to initiation of cohabitation.
Rats were individually housed in stainless steel, wire-bottomed cages, except during the cohabitation period. During cohabitation, each pair of rats was housed in the male rat's nesting box. Cages of mated female rats assigned to the study were arranged to minimize possible effects due to cage placement. All cage sizes and housing conditions were in compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
The study room was maintained under conditions of positive airflow relative to a hallway and independently supplied with a minimum of 10 changes per hour of 100% fresh air that had been passed through 99.97% HEPA filters. Room temperature and humidity were monitored constantly throughout the study [targeted ranges: room temperature 64–79°F (18–26 °C), relative humidity 30–70%].
Rats were given Certified Rodent Diet® #5002 (Purina Mills Inc., Richmond, Indiana) available ad libitum from individual feeders. Local water that had been processed by passage through a reverse osmosis membrane (RO water) was available to the rats ad libitum from an automatic watering access system and/or individual water bottles attached to the cages. Chlorine was added to the processed water as a bacteriostat. Chewable Nylabones® were supplied to all rats during the course of the study.
After acclimatization, 69 virgin female rats were cohabited with 69 breeder male rats (one male per female). The cohabitation period consisted of a maximum of 5 days; mating was evaluated daily during this period. Female rats with spermatozoa observed in a smear of vaginal contents and/or a copulatory plug observed in situ were considered to be at day 0 of presumed gestation (DG 0). Healthy, mated females (25 per group) were assigned to the test or control group using a computer-generated randomization procedure, using body weight assessment from DG 0.
Female rats were administered the test substance (ketone monoester, at a dosage of 2000 mg/kg bw/day) or the control article (RO water, at a volume of 2 mL/kg bw/day), on DGs 6 through 20, by oral gavage once daily. Dosages were adjusted based on the most recently recorded body weight and administered at approximately the same time each day.
2.2.3. Clinical observations
Rats were observed for general appearance at least weekly during the acclimatization period, on DG 0 (pre-dosage period), daily before dosage administration, and once on the day of scheduled euthanasia (DG 21). During the dosage period (DG 6–20), post-dosage observations were recorded at approximately hourly intervals for the first four hours and at the end of the normal working day for the first 4 days of the dosage period. Subsequent post-dosage observations were recorded between 1–2 h after dosage administration and at the end of the normal working day. Body weights were recorded at least weekly during the acclimatization period, on DG 0 (pre-dosage day), and then once daily during the dosage and post-dosage periods. Food consumption values were recorded on DG 0 (pre-dosage period), and then once daily during the dosage period. A food left value was recorded on the day of scheduled euthanasia.
2.2.4. Maternal hematology and clinical chemistry
On the day of scheduled euthanasia (DG 21), blood was collected from the inferior vena cava from each rat while under isoflurane/oxygen anesthesia for hematology and clinical chemistry (Table 6). For hematology analysis, blood samples were transferred to K2EDTA-coated tubes and analyzed using an automated hematology analyzer (Bayer Advia 120®, Siemens Healthcare Diagnostics Inc., Deerfield, Illinois). For clinical chemistry analyses, blood samples were processed for serum and the serum was analyzed using an automated clinical chemistry analyzer (Olympus AU640c, Beckman Coulter Inc., Brea, California).
Table 6.
Maternal hematology and clinical chemistry results at day 21 of gestation in the developmental toxicity study.
| Parameter | Unit/measurement | Control (n = 25) | Test (n = 25) |
|---|---|---|---|
| Hematology | |||
| Erythrocytes | 106/CMM | 6.53 ± 0.671 | 6.47 ± 0.601 |
| Hemoglobin | g/DL | 11.9 ± 1.38 | 11.8 ± 1.07 |
| Hematocrit | % | 34.9 ± 3.66 | 34.5 ± 3.18 |
| MCH | PG | 18.3 ± 0.63 | 18.3 ± 0.62 |
| MCHC | g/DL | 34.2 ± 1.00 | 34.3 ± 0.76 |
| MCV | FL | 53.4 ± 1.57 | 53.3 ± 1.71 |
| Reticulocytes | 109/L | 151.6 ± 59.92 | 152.9 ± 56.88 |
| Platelets | 103/CMM | 995 ± 163.2 | 1078 ± 180.2 |
| Leukocytes | 103/CMM | 6.00 ± 1.430 | 5.95 ± 1.583 |
| Lymphocytes | 103/CMM | 3.64 ± 1.172 | 3.13 ± 0.974 |
| Monocytes | 103/CMM | 0.18 ± 0.060 | 0.20 ± 0.076 |
| SEG'D neutrophils | 103/CMM | 2.03 ± 0.654 | 2.49 ± 0.865* |
| Eosinophils | 103/CMM | 0.08 ± 0.030 | 0.07 ± 0.033 |
| Basophils | 103/CMM | 0.01 ± 0.006 | 0.01 ± 0.004* |
| LUC | 103/CMM | 0.060 ± 0.0341 | 0.054 ± 0.0314 |
| Clinical chemistry | |||
| AST | IU/L | 80 ± 21.6 | 79 ± 12.5 |
| ALT | IU/L | 44 ± 9.1 | 38 ± 7.5* |
| ALP | IU/L | 119 ± 47.0 | 91 ± 46.9* |
| GGT, serum | IU/L | 0.03 ± 0.069 | 0.07 ± 0.244 |
| Total bilirubin | mg/DL | 0.13 ± 0.023 | 0.13 ± 0.038 |
| Cholesterol | mg/DL | 70 ± 13.4 | 67 ± 10.3 |
| Triglyceride | mg/DL | 152 ± 150.4 | 165 ± 130.7 |
| Total protein | g/DL | 5.57 ± 0.567 | 5.41 ± 0.491 |
| Albumin | g/DL | 2.91 ± 0.410 | 2.82 ± 0.352 |
| Globulin | g/DL | 2.66 ± 0.239 | 2.60 ± 0.222 |
| A/G ratio | Ratio | 1.10 ± 0.140 | 1.09 ± 0.129 |
| Glucose | mg/DL | 106 ± 32.7 | 108 ± 25.9 |
| Urea nitrogen | mg/DL | 16 ± 4.4 | 14 ± 3.0 |
| Creatinine | mg/DL | 0.39 ± 0.042 | 0.39 ± 0.037 |
| Calcium | mg/DL | 9.35 ± 0.594 | 9.16 ± 0.579 |
| Phosphorus | mg/DL | 3.83 ± 0.973 | 3.85 ± 0.936 |
| Sodium | mmol/L | 139 ± 1.4 | 139 ± 1.8 |
| Potassium | mmol/L | 3.83 ± 0.270 | 3.97 ± 0.306 |
| Chloride | mmol/L | 100 ± 2.3 | 100 ± 2.6 |
Abbreviations: A/G = albumin/globulin; ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; GGT = gamma-glutamyl transferase; n = number; LUC = large unstained cell; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; MCV = mean corpuscular volume; SEG'D = segmented.
All values are presented as mean ± standard deviation.
Significantly different than the control group .
2.2.5. Clinical pathology
After blood sample collection, rats were euthanized by intravenous injection of sodium pentobarbital followed by exsanguination, Caesarean-sectioned, and examined for gross lesions. The reproductive tract was dissected from the abdominal cavity. The gravid uterus was weighed, the uterus was opened, and the contents were examined. Fetuses were removed. The ovaries and uterus were examined for number and distribution of corpora lutea, implantation sites, placentas (size, color, or shape), live and dead fetuses, and early and late resorptions. Dead fetuses and late resorptions were differentiated by the degree of autolysis present; “marked” to “extreme” autolysis indicated that the fetus was a late resorption. Uteri of apparently nonpregnant rats were stained with 10% aq (v/v) ammonium sulfide solution and examined for implantation sites.
A gross necropsy of the thoracic, abdominal, and pelvic viscera was performed for each rat. The cervix, ovaries, and uterus of all nonpregnant animals were collected and preserved in 10% neutral buffered formalin. At necropsy, a rat from the control group was randomly selected using a Table of Random Units and all tissues were retained to provide control tissues for any possible histopathological evaluations of gross lesions.
2.2.6. Fetal examinations
Fetuses were examined for sex and for external abnormalities. Late resorptions were examined for external abnormalities and/or sex when possible. The body weight of each fetus also was recorded.
Approximately one-half of the fetuses in each litter was examined for visceral abnormalities by using a modification of the micro-dissection technique of Staples (Staples, 1974; Wilson, 1965). Each fetus was fixed in Bouin's solution and heads were examined by free-hand sectioning. The remaining fetuses were examined for skeletal abnormalities after staining with alizarin red S (Staples and Schnell, 1964).
Fetal alterations were defined as: (1) malformations (irreversible changes that occur at low incidences in this species and strain); or (2) variations (common findings in this species and strain and reversible delays or accelerations in development). Litter averages were calculated for specific fetal ossification sites as part of the evaluation of the degree of fetal ossification.
2.2.7. Statistical analyses
Clinical observations and other proportional data were analyzed using the Variance Test for Homogeneity of the Binomial Distribution (Snedecor and Cochran, 1967a).
Continuous data (e.g., maternal body weights, body weight changes, food consumption values and litter averages for percent male fetuses, percent resorbed conceptuses, fetal body weights, and fetal anomaly data) were analyzed using Bartlett's Test of Homogeneity of Variances (Sokal and Rohlf, 1969a) and the Analysis of Variance (Snedecor and Cochran, 1967b), when appropriate [i.e., Bartlett's Test was not significant (p > 0.001)]. If the Analysis of Variance was significant (p ≤ 0.05), Dunnett's Test was used to identify the statistical significance of the individual groups (Dunnett, 1955). If the Analysis of Variance was not appropriate [i.e., Bartlett's Test was significant (p ≤ 0.001)], the Kruskal–Wallis Test was used (≤75% ties) (Sokal and Rohlf, 1969b). In cases where the Kruskal–Wallis Test was statistically significant (p ≤ 0.05), Dunn's Method of Multiple Comparisons was used to identify the statistical significance of the individual groups (Dunn, 1964). If there were greater than 75% ties, Fisher's Exact Test was used to analyze the data (Siegel, 1956). Count data were evaluated using the procedures described above for the Kruskal–Wallis Test (Sokal and Rohlf, 1969b). Data are presented as mean ± standard deviation.
3. Results
3.1. 28-Day oral toxicity study in rats
3.1.1. Diet analysis
The ketone ester diet was determined to be homogeneous (data not shown). The mean level of ketone monoester in the test diet was 12.3 g/100 g (higher than the target level of 11.4 g/100 g). Full compositional analysis is summarized in Table 1.
3.1.2. Observations
All animals survived to the scheduled necropsy date after the 28-day feeding period. Daily cage side observations and detailed weekly physical examinations showed no treatment-related toxicity in animals of either sex in all three diet groups. The exception was one male rat in the ketone ester-fed group which showed piloerection, decreased food consumption, and weight loss. Piloerection was not observed after the third week of dosing and, although this animal did not fully regain initial body weight, it was gaining weight and otherwise appeared normal by the end of the study.
Animals from all groups lost weight during the first 8–12 days of the study, but regained weight as the study progressed. These initial body weight losses were attributed to the change in diets (i.e., during the pre-study period, animals were not acclimated to the new diets).
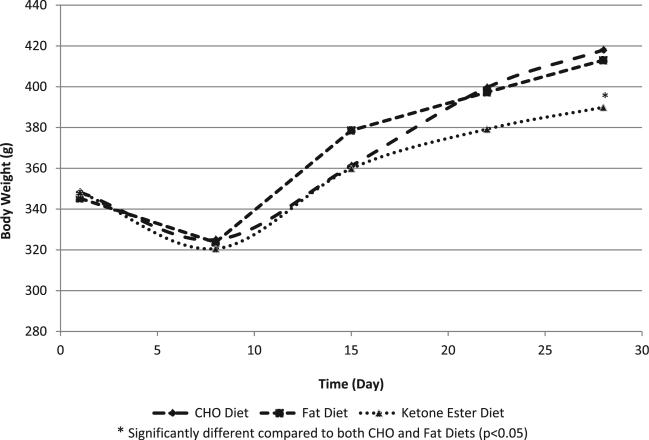
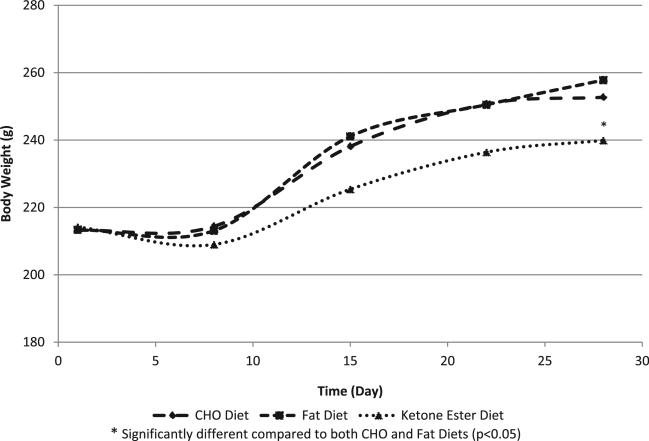
Rats from all groups (both sexes) gained weight during the 28-day feeding period; however, ketone ester-fed rats consumed significantly less food and gained significantly less weight than the animals fed CHO and fat diets (p < 0.05) (Figs. 1 and 2, Table 2). Male rats in the test group consumed 12.0 g ketone monoester/kg bw/day, while female rats in the test group consumed 15.1 g/kg bw/day over the study period.
Fig. 1.
Male rat body weights in the 28-day toxicity study.
Fig. 2.
Female rat body weights in the 28-day toxicity study.
Table 2.
Food consumption (g) in the 28-day study.
| Day | Carbohydrate diet (n = 10/sex) | Fat diet (n = 10/sex) | Ketone ester diet (n = 10/sex) |
|---|---|---|---|
| Males | |||
| 1–8 | 206 ± 7 | 211 ± 10 | 175 ± 33* |
| 8–15 | 312 ± 2 | 312 ± 1 | 292 ± 32* |
| 15–22 | 292 ± 1 | 293 ± 1 | 274 ± 20* |
| 22–29 | 269 ± 7 | 269 ± 7 | 239 ± 17* |
| Females | |||
| 1–8 | 205 ± 8 | 209 ± 4 | 177 ± 20* |
| 8–15 | 233 ± 2 | 234 ± 2 | 217 ± 8* |
| 15–22 | 222 ± 2 | 221 ± 3 | 201 ± 15* |
| 22–29 | 190 ± 5 | 194 ± 7 | 175 ± 12* |
All values are presented as mean ± standard deviation.
Significantly different compared to both CHO and Fat Diets.
Water consumption was not measured, but it did not appear that there was a difference in water consumption among groups.
3.1.3. Hematology, coagulation, and clinical chemistry
Hematological analysis revealed significantly higher values in red blood cells (RBCs), hemoglobin (Hb), and hematocrit (Hct) in ketone ester-fed male and female rats compared to animals in both control groups, as well as higher levels of reticulocytes in male rats fed the ketone ester diet in comparison to the two control diets (Table 3). Additionally, mean corpuscular volume (MCV) values were slightly lower in the male and female rats fed the ketone ester diet compared to the CHO-fed controls, mean corpuscular hemoglobin (MCH) values were decreased in female rats in the ketone ester diet in comparison to the CHO control group, and mean corpuscular hemoglobin concentration (MCHC) and platelet levels in female rats in the ketone ester group were increased and decreased, respectively, vs. female rats fed the fat diet. All values were within the normal physiological ranges.
Prothrombin time was not affected by treatments (Table 3). Activated partial thromboplastin time (APTT) was approximately three seconds and one second shorter in male and female rats on the ketone diet, respectively, compared to animals receiving the CHO and fat diets. APTT was, however, within the normal physiological range (APTT was 13.8 ± 2.2 s and 14.6 ± 1.9 s for male and female rats on the ketone diet; normal range is 12.9–29.3 s). Fibrinogen levels in female rats were slightly higher in ketone ester-fed female rats compared to fat-fed rats, but this difference was not observed in comparison to CHO-fed female rats or in male rats.
Clinical chemistry revealed that creatine kinase (CK) and albumin levels were higher in ketone ester-fed male rats compared to their controls (Table 4); however, the levels were within the normal ranges of 50–400 u/L for CK and 24–48 g/L for albumin. Albumin/globulin (A/G) ratios and potassium levels were 8% higher in males fed the ketone ester diet in comparison to rats in the fat group. In female rats, sodium levels were ~1 mM lower in the ketone ester group compared to the CHO diet controls, and triglyceride levels were higher in the ketone ester-fed animals versus fat-fed animals. Of the liver function parameters, alkaline phosphatase (ALP) was significantly higher in the female rats fed the fat diet and ALT and bilirubin were significantly higher in the male rats fed the ketone ester diet, yet all values were within normal physiological ranges and were not accompanied by gross or microscopic observations of liver toxicity. Cholesterol levels in control groups were lower than in the rats that received the ketone diet. All of the mean values in the above-listed parameters remained within normal ranges.
Serum LDH (u/L) was significantly increased in ketone ester-fed males (1205 ± 676) and females (1876 ± 662) compared with levels in animals fed the CHO diet (751 ± 381 males; 1230 ± 581 females) or the fat diet (636 ± 447 males; 1278 ± 376 females). Levels were slightly above the upper limit of the normal historical range in the testing facility.
No other significant differences in clinical chemistry parameters were observed.
3.1.4. Urinalysis
No significant differences among groups were observed in urinalysis parameters (data not shown).
3.1.5. Clinical pathology and histopathology
There were no statistically significant differences in the absolute weight and in the body/brain weight ratios among groups for the following organs: spleen, liver, adrenals, testes, kidneys, prostate, lungs, heart, thymus, brain, pituitary, seminal vesicles and ovaries (data not shown). The only difference observed was in the absolute weight of the uterus (uterine weight of ketone ester-fed rats was smaller than the uterine weight of female rats fed the CHO and fat diets). No difference was noted in the relative uterus weight, indicating that the difference in absolute uterine weight was a result of the smaller body weight of female rats fed the ketone ester diet, and not an adverse effect of the ketone ester. It also should be noted that uterine weights of rats in all three groups were within the laboratory's historical range for uterine weight.
At necropsy, two male rats and four female rats that received the fat diet, as well as one female rat given the ketone diet, had slight yellow discoloration of livers, which were presumed to be fat accumulation. No other findings that were considered to be related to the diets were noted.
No histopathological abnormalities of toxicological significance were observed in the heart, kidney, stomach, duodenum, ileum, colon, or brain.
Upon histopathological examination of the liver, several findings were noted. In comparison with what would be observed in rats fed a regular chow diet, increased numbers of round, clear, sharply demarcated cytoplasmic vacuoles (interpreted as lipid) were observed in some hepatocytes in all females of all three groups. Minimal vacuolation was observed in only two males on the fat diet. In the females, many hepatocytes contained small microvesicles distributed throughout otherwise normal cytoplasm. Some were greatly distended by single or multiple large vacuoles (macrovesicles) that sometimes displaced and compressed the nucleus. A minority of the highly vacuolated cells could not be identified as hepatocytes and were thought to be perisinusoidal stellate cells. This pattern of vacuolation of hepatocytes was consistent with a mild form of fatty liver (steatosis).
Minor necroinflammatory changes consisting of multiple microfocal non-hematopoietic clusters of macrophages and undifferentiated mononuclear cells, often associated with necrosis or apoptosis of one or more hepatocytes, were observed in the liver of some males and females in all three groups. In some females with lipid vacuolation, some of these necroinflammatory foci of necrosis involved hepatocytes with cytoplasmic fat accumulation. Such microfocal necroinflammatory lesions can occur as a background condition in healthy rats.
To determine whether the frequency or degree of fatty vacuolation and necroinflammatory responses were related to the diets, these changes were separately graded blindly on a 0–5 scale. Lipid vacuolation graded as minimal or higher occurred in all females in all groups. Lipid vacuolation graded as mild or greater (scores >2) was observed in 6/10 females in the CHO diet group, in 10/10 females in the fat diet group, and in 8/10 females in the ketone ester diet group. Lipid vacuolation graded as moderate or greater (scores >3) was observed in 1/10 females in the CHO-fed group, in 3/10 females in the fat-fed group and in 3/10 females in the ketone ester-fed group. As mentioned, minimal vacuolation in male rats was observed in only 2 animals in the fat-fed group. Necroinflammatory foci of minor clinical significance occurred at a similar low frequency (3/10 in the CHO-fed group, 1/10 in the fat-fed group, 1/10 in the ketone ester-fed group) and were graded all minimal in male rats in all three groups. In female rats, necroinflammatory foci were more frequent (10/10) in the CHO diet group than in the fat diet group (5/10) or the ketone diet group (7/10); however, this response was more frequently graded as mild in the fat diet (4/10) and ketone ester diet groups (5/10) compared with the CHO diet group (0/10).
With respect to muscle tissue, several animals in all three groups had occasional single degenerate or necrotic myofibers with hyaline sarcoplasm that was usually fragmented and invaded by macrophages. Some animals also had proliferation of local stromal cells or myocyte nuclei. These findings were diagnosed as myocyte necrosis and repair. Several animals also had microfocal interstitial clusters of basophilic macrophages sometimes conforming to the shape of a missing myofiber. These were diagnosed as focal histiocytosis. Muscle changes were graded as minimal with the exception of one male and one female in the ketone ester group; effects in these animals were graded as mild. There were no animals in which muscle changes were graded as moderate, marked, or severe.
In heart tissue, microfocal myocardial fibrosis was observed in two animals in the fat diet group and one in the ketone ester diet group. These myocardial lesions were typical of myocardial lesions that are sporadically detected in the early stages of murine progressive cardiomyopathy, a spontaneous background condition in rats (Ruben et al., 2000).
Two females in the ketone monoester group had focal necrosis and mineralization of the germinal centre of a Peyer's patch in the jejunum. These effects were graded as minimal in both animals. As mineralization of Peyer's patches has previously been reported in control animals (Hempenius et al., 2000) and the effects in the 28-day study were given a minimal grading by the study pathologist, the observed effects were considered to be inconsequential to safety. No other effects in the jejunum were observed.
Mild nephrocalcinosis, observed mainly in female rats, and tubular basophilia or interstitial inflammation, occurred in the kidney of some animals in all three groups. These findings were considered to be toxicologically insignificant because they are known background conditions in rats, and because the frequency and severity of these changes were not increased in animals on the test diet.
3.2. Developmental toxicity study
3.2.1. Maternal observations
In the test group, the number of rats with sparse hair coat on the head was significantly increased in comparison to the control group. This observation occurred in 6 of 25 rats, and was first observed after DG 9 and generally persisted in the affected rats until scheduled euthanasia. This finding was not considered to be toxicologically relevant. Additional clinical observations included rales, a scab on the head, urine-stained abdominal fur, chromorhinor-rhea, red perivaginal substance, moderate dehydration (based on skin turgor), labored breathing, and sparse hair coat on the back or underside. All clinical observations that occurred were considered unrelated to the test article because: (1) the observations were transient; or (2) the observations occurred in only one to three rats.
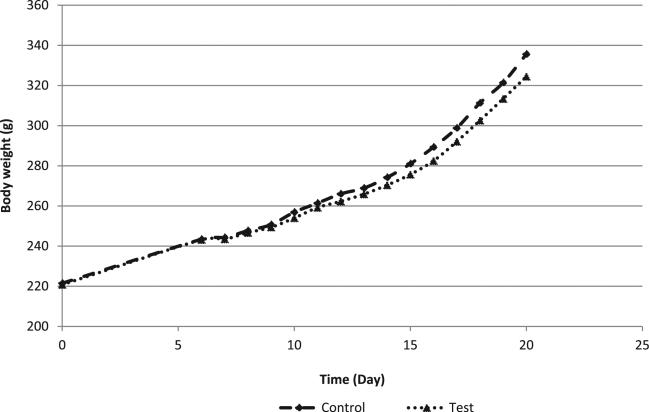
The average maternal body weight was comparable between groups throughout the dosage period (Fig. 3); gravid uterine weights also were generally comparable between groups (82 ± 28 g in control animals; 76 ± 25 g in test animals). There were no statistically significant changes in body weight gain during the dosage period; however, body weight gains in the test group were reduced by 14% for the cumulative dosage period (calculated as DGs 6–21), in comparison to the control group. Within the dosage period, the average body weight gains in the test group were 11–18% less than the corresponding control group values. After correction for gravid uterine weights, the average maternal body weight on DG 21 (DG 21C) was reduced (4% less than controls) and the average cumulative body weight gains (DGs 6–21C) were reduced (36% less than controls) in the test group, in comparison to the control group.
Fig. 3.
Maternal rat body weight in the developmental toxicity study.
Absolute (g/day) food consumption values were significantly reduced by 8–10% in the test group at the end of the dosage period, specifically on DGs 15–18 and DGs 18–21, in comparison to the control values (Table 5). Relative to body weight, food consumption values (g/kg bw/day) in the test group were significantly reduced on DGs 15–18 (6% less than controls). As a result, cumulative food consumption values (both absolute and relative) were significantly reduced in the test group on DGs 6–21, in comparison to the control article group values (6% and 5% less than controls, respectively).
Table 5.
Maternal absolute and relative food consumption in the developmental toxicity study.
| Day | Control – absolute (n = 22 pregnant rats) (g/day) | Test – absolute (n = 24 pregnant rats) (g/day) | Control – relativea (n = 22 pregnant rats) (g/kg bw/day) | Test – relativea (n = 24 pregnant rats) (g/kg bw/day) |
|---|---|---|---|---|
| 0–16 | 20.7 ± 1.8b | 21.8 ± 1.7 | 89.0 ± 7.2b | 93.8 ± 7.1* |
| 6–9 | 22.8 ± 2.4 | 22.0 ± 3.2 | 92.3 ± 8.6 | 89.6 ± 12.3 |
| 9–12 | 23.9 ± 2.4 | 22.7 ± 2.4 | 92.4 ± 7.1 | 88.4 ± 7.6 |
| 12–15 | 24.1 ± 2.3 | 23.0 ± 2.6 | 88.4 ± 6.4 | 85.5 ± 8.0 |
| 15–18 | 25.2 ± 2.7 | 23.2 ± 2.6** | 85.6 ± 8.0 | 80.4 ± 7.0* |
| 18–21 | 23.7 ± 3.4 | 21.4 ± 3.3c,* | 71.7 ± 8.1 | 66.9 ± 8.8c |
| 6–21 | 24.0 ± 2.0 | 22.5 ± 2.2c,* | 85.1 ± 5.3 | 81.1 ± 6.4c,* |
| 0–21 | 23.1 ± 2.0 | 22.3 ± 1.9c | 83.2 ± 5.4 | 81.5 ± 5.3c |
Abbreviations: n = number.
All values are presented as mean ± standard deviation.
Significantly different than the control group .
Significantly different than the control group .
Relative to body weight.
Value is average of 20 litters, values that were incorrectly recorded or those associated with spillage are excluded.
Value is average of 23 litters, values that were incorrectly recorded or those associated with spillage are excluded.
3.2.2. Maternal hematology, clinical chemistry, and gross pathology
There were no effects on hematologic parameters attributed to the ketone ester (Table 6). Statistically significant differences (i.e., higher segmented neutrophil levels and lower basophil levels in rats administered the test substance versus controls) were considered normal biological variations.
There were no changes in serum chemistry that were attributed to oral administration of ketone ester (Table 6). Changes in serum chemistry included a significant reduction in ALT and ALP levels in the test group, relative to the control group. All other values were comparable and did not differ significantly from the control group values.
No gross lesions were observed at necropsy examination.
3.2.3. Caesarean sectioning and litter observations
Pregnancy occurred in 22 rats in the control group and 24 rats in test group.
No Caesarean-sectioning or litter parameters were affected by the test article (Table 7). There were no dead fetuses. All placentas appeared normal. One dam in the test group had a litter that consisted of two early resorptions (i.e., resorption occurred prior to exposure to the test substance).
Table 7.
Caesarean-sectioning and litter observations at day 21 of gestation in the developmental toxicity study.
| Parameter | Unit/measurement | Control (n = 22 pregnant rats; 22 rats with viable fetuses) | Test (n = 24 pregnant rats; 23 rats with viable fetuses) |
|---|---|---|---|
| Caesarean-sectioning observations | |||
| Corpora lutea | Mean | 14.1 ± 2.6 | 13.5 ± 3.1 |
| Implantations | Mean | 12.8 ± 3.5 | 12.2 ± 3.7 |
| Pre-implantation loss | % Mean | 11.0 ± 18.2 | 10.4 ± 18.2 |
| Litter sizes | Mean | 11.6 ± 3.9 | 11.6 ± 4.1 |
| Live fetuses | N | 256 | 279 |
| Mean | 11.6 ± 3.9 | 11.6 ± 4.1 | |
| Dead fetuses | N | 0 | 0 |
| Resorptions | Mean | 1.2 ± 2.0 | 0.5 ± 0.6 |
| Early resorptions | N | 25 | 12 |
| Mean | 1.1 ± 2.0 | 0.5 ± 0.6 | |
| Late resorptions | N | 1 | 1 |
| Mean | 0.0 ± 0.2 | 0.0 ± 0.2 | |
| Post-implantation loss | % Mean | 11.4 ± 17.8 | 9.8 ± 21.9 |
| Dams with any resorptions | n (%) | 12 (54.5) | 11 (45.8) |
| Dams with all conceptuses resorbed | n (%) | 0 (0.0) | 1 (4.2) |
| Dams with viable fetuses | n (%) | 22 (100.0) | 23 (95.8) |
| Placentas appeared normal | n (%) | 22 (100.0) | 23 (100.0)a |
| Litter observations (Caesarean-delivered fetuses)a | |||
| Implantations | Mean | 12.8 ± 3.5 | 12.6 ± 3.0 |
| Live fetuses | N | 256 | 279 |
| Mean | 11.6 ± 3.9 | 12.1 ± 3.3a | |
| Live male Fetuses/litter | % Mean | 47.2 ± 18.6 | 49.2 ± 17.9 |
| Live fetal body weights/litter | g Mean | 5.05 ± 0.38 | 4.99 ± 0.75 |
| Live fetal body weights/litter – male fetuses | g Mean | 5.30 ± 0.20b | 5.09 ± 0.75* |
| Live fetal body weights/litter – female fetuses | g Mean | 4.90 ± 0.36 | 4.78 ± 0.49c |
| Resorbed conseptuses/litter | % Mean | 11.4 ± 17.8 | 5.8 ± 10.8 |
Abbreviations: n = number.
Values are presented as mean ± standard deviation.
Significantly different than the control group (p < 0.05).
Results for are based on 23 litters as total resorption occurred in 1 of the 24 pregnant rats.
Value is average of 20 litters, litters 7102 and 7107 had no male fetuses
Value is average of 22 litters, litter 7103 had no female fetuses.
Male fetal body weights in the test group were significantly lower compared to controls; however, combined fetal weights did not significantly differ, the percent difference from the control group was less than 5%, and the average value was within historical ranges at the test facility.
3.2.4. Fetal alterations
Fetal evaluations were based on 256 and 279 live DG 21 Caesarean-delivered fetuses in 22 and 23 litters in the control and test groups, respectively. Each of these fetuses was examined for gross external alterations. Of these respective fetuses, 122 and 132 fetuses in the control and test groups, respectively, were examined for soft tissue alterations, and 134 and 147 fetuses in the control and test groups, respectively, were examined for skeletal alterations and fetal ossification site averages.
Fetuses with alterations were identified in 7 (32%) and 14 (61%) litters in the control and test groups, respectively (Table 8). The numbers of fetuses with any alteration observed were 9 (4%) and 23 (8%), and the percentages of fetuses with any alteration per litter were 3% and 9% in these same respective groups. The fetal incidences of abnormalities (i.e., fetuses with any abnormality observed and the percentage of fetuses with any abnormality per litter) were significantly increased in the test group in comparison to the control group values.
Table 8.
Summary of fetal alterations of caesarean delivered live fetuses in the developmental toxicity study.
| Parameter | Unit/measurement | Control (n = 22 rats with viable fetuses) | Test (n = 23 rats with viable fetuses) |
|---|---|---|---|
| Litters with fetuses with any alterations observed | n (%) | 7 (32) | 14 (61) |
| Fetuses with any alterations observed | n (%) | 9 (4) | 23 (8)** |
| Fetuses with any alteration/litter | % Mean ± SD | 3 ± 6 | 9 ± 11* |
Abbreviations: n = number; SD = standard deviation.
Significantly different than the control group .
Significantly different than the control group .
There were no significant between-group differences in gross external abnormalities. One fetus in the test group had a medial cleft palate; this observation was confirmed at skeletal examination (as an incompletely ossified palate). No other abnormalities occurred in this fetus. Two fetuses from two litters in the test group had medial rotation of one or both hindlimbs. No other abnormalities were noted in these fetuses. No other gross external abnormalities were observed.
Examination of soft tissue revealed no soft tissue fetal alterations. No skeletal malformations were observed, with the exception of the incompletely ossified palate in one fetus from the test group (discussed above). A number of skeletal variations were observed but there were no significant differences in the incidence of skeletal variation between the test and control groups (data not shown). The two groups were comparable in the average numbers of ossification sites per fetus for the hyoid, vertebrae (cervical, thoracic, lumbar, sacral, and caudal), ribs, sternum (manubrium, sterna centers, and xiphoid), forelimbs (carpals, metacarpals, digits, and phalanges), or hindlimbs (tarsals, metatarsals, digits, and phalanges) (data not shown).
4. Discussion
(R)-3-Hydroxybutyl (R)-3-hydroxybutyrate has been proposed for use as a source of energy through the elevation of circulating ketone levels. Potential benefits of the ketone monoester include the improvement of physical performance in athletes, as well as enhancing cognitive function. The current 28-day and developmental toxicity studies were undertaken to assess the safety of ketone monoester when administered orally.
It has recently been shown that the consumption of ketone monoester in healthy adult volunteers resulted in elevated plasma levels of the ketones β-hydroxybutyrate and acetoacetate, while the intact ester was not detected (Clarke et al., submitted for publication). These observations are consistent with results of a pharmaco-kinetic study in which, following the oral administration of ketone monoester to Wistar rats, very low levels of the ester were detected in the plasma, and the small amount detected was rapidly degraded (Veech et al., unpublished). Additionally, the ester was not detected in the blood of rats administered ketone monoester in the diet for 66 days (Knight et al., unpublished). In an in vitro study, hydrolysis of ketone monoester was rapid and complete upon incubation with fresh human plasma (Carter et al., unpublished). These findings indicate that orally consumed ketone monoester is readily hydrolyzed, resulting in increased circulating levels of ketones.
In the 28-day study, ketone ester-fed rats consumed less food and gained less weight than animals in the control groups, as has been described previously (Kashiwaya, 2010). Decreased food consumption may have resulted from the palatability of the diet containing ketone monoester. Additionally, the observed reduced food consumption and weight gain in this study are consistent with reports of decreased hunger, reduced energy intakes, and increased weight loss in subjects consuming low-CHO ketogenic diets compared to low-fat diets or medium-CHO non-ketogenic diets (Johnstone et al., 2008; McClernon et al., 2007).
Hematological and clinical chemistry analyses revealed significant between-group differences in several hematology parameters (i.e., increased RBC, Hb, and Hct, and decreased MCV values in ketone ester-fed rats compared to controls) with increased cholesterol in ketone ester-fed rats; though values were within normal physiological ranges. LDH levels were significantly higher in ketone ester-fed rats (both sexes compared to control animals); however, the increases were small in magnitude and were not associated with changes in hemolytic or histological findings. LDH levels vary greatly in rats, as demonstrated by the historical control ranges for Sprague Dawley rats in the testing facility of 100–7201 u/L in males and 20–5236 u/L in females. LDH levels in the ketone ester-fed rats were within these ranges and thus were not considered to be of toxicological relevance. The observed elevated levels may be related to the release of LDH during handling of rats, such as grasping, dosing, etc. (Yerrourn et al., 1999); blood collection procedures (Friedel et al., 1974); or enzyme release from blood cells during clotting (Friedel and Mattenheimer, 1970). Furthermore, LDH levels were unaffected by ketone monoester consumption in the 66-day rat study (Knight et al., unpublished). Similarly, no subjects had LDH levels above the normal range in the 5-day clinical study (Clarke et al., submitted for publication).
Upon histological examination, liver vacuolation was observed in all female rats in all three groups; likewise, necroinflammatory foci were observed in some animals (males and females) in all groups. Given that these findings were present in all groups and that liver function enzyme levels were within normal ranges, it is unlikely that they were related to consumption of the ketone monoester. The diets were formulated by the separate addition of macronutrients (fat, CHO, ketone ester) to the rat chow; the vitamin and mineral compositions of each diet were diluted, which may explain the observed findings.
In addition to the results of studies on ketone monoester, safety was further corroborated by animal feeding trials of a similar ketone ester, namely (R,S)-1,3-butanediol mono- and diacetoacetate. A bolus intragastric dose of 1.3 g/kg body weight administered to pigs did not alter standard clinical chemistry parameters or have deleterious side effects (Desrochers et al., 1995). Likewise, the repeated administration of oral doses of (R,S)-1,3-butanediol diacetoacetate over a 300-min period (equivalent to 1.0–1.1 mg/kg bw) or a single bolus dose of 439–477 mg/kg bw (R,S)-1,3-butane-diol diacetoacetate to dogs did not alter clinical chemistry; moreover, no signs of distress in any of the animals were observed following the experiment (Puchowicz et al., 2000).
Studies conducted on the metabolites of d-β-hydroxybutyrate ester also support the safety of the compound. These include a 2-year study in which Sprague Dawley rats were administered diets comprising up to 10% (R)-1,3-butanediol (approximately 5 g/kg bw/day) with no adverse effects reported (Scala and Paynter, 1967). Similarly, no adverse effects were observed with the consumption of diets containing (R)-1,3-butanediol at levels up to 3.0% (approximately 0.75 g/kg bw/day) in purebred beagle dogs for 2 years (Scala and Paynter, 1967). Data related to the safety of (R)-1,3-butanediol and d-β-hydroxybutyrate in humans are limited; however, d,L-sodium-β-hydroxybutyrate was reported to be tolerated with no side effects in two 6-month-old infants with persistent hyperinsulinemic hypoglycemia when administered at levels of 0.9–1.0 g/kg bw/day for 5 and 7 months (Plecko et al., 2002). Kies et al. (1973) reported no adverse effects on several hematology or blood chemistry parameters in adult volunteers following the consumption of 15 g 1,3-butanediol (enantiomer not specified) incorporated into bread for 7 days. A significant decrease in blood glucose was observed following consumption of the 1,3-butanediol-containing bread; such effects on glucose levels were not observed in a study in which subjects consumed up to 2.1 g ketone monoester/kg bw/day for 5 days (Clarke et al., submitted for publication).
In the developmental toxicity study, decreased body weight gains and body weight corrected for gravid uterine weight were observed in dams administered ketone monoester compared to controls; food consumption was similarly reduced. These findings were expected, given that decreased hunger and energy intakes have been reported in subjects consuming ketogenic diets (Johnstone et al., 2008; McClernon et al., 2007). Maternal ALT and ALP values were lower in the test group relative to controls; however, in the 28-day study, no significant effects on ALT were noted in female rats fed the ketone ester diet and ALP levels were only lower in comparison to the fat control group, but the same as the CHO control group. Moreover, no gross or histopathological changes, suggesting liver toxicity due to the test substance, were noted in the 28-day study.
There were no significant between-group differences in the litter or fetal incidences of any gross external, soft tissue, or skeletal abnormalities (malformations or variations), nor were there differences in fetal ossification site averages. The number of fetuses and the percentage of fetuses within a litter with alterations were significantly higher in the ketone group compared to controls (Table 8). These findings were driven by skeletal variations; however, it should be noted that the incidence of skeletal variations did not significantly differ between groups (data not shown). Consequently, the skeletal variations were not considered to be of toxicological concern.
The lack of toxicologically relevant effects in the developmental toxicity study is consistent with the findings of an in vivo reproductive toxicity study with the related metabolite, 1,3-butanediol. In a study reported by Hess et al. (1981), it was shown that, in five successive breedings of Wistar rats, consumption of 5–24 g/kg bw/day of 1,3-butanediol (approximately 2500–12,000 mg/kg bw/day) (isomer not specified) did not result in teratological effects. The control and test animals were comparable with respect to gestation, viability, and lactation indices.
With respect to the metabolite, β-hydroxybutyrate, no traditional in vivo reproductive or developmental toxicity studies have been conducted; however, results of in vitro studies (with isolated embryos) suggest that physiologically relevant levels of β-hydroxybutyrate, particularly, the l-isomer, may disrupt normal embryogenesis (Hunter et al., 1987; Moley et al., 1994; Sheehan et al., 1985). Given that most (R)-1,3-butanediol is metabolized to the ketones, (R)-3-hydroxybutyrate and acetoacetate, while only approximately one-third of (S)-1,3-butanediol is converted to ketone bodies (Desrochers et al., 1992), data from reproductive and developmental toxicity studies on 1,3-butanediol provide indirect evidence of the safety of β-hydroxybutyrate. As the administration of 1,3-butanediol in the study by Hess et al. (1981) did not result in teratogenic effects, nor did the current developmental toxicity study conducted on the ketone ester, the relevance of the results observed in the in vitro studies conducted with β-hydroxybutyrate in isolated embryos is questionable.
In summary, results of a 28-day oral toxicity study in rats indicated that consumption of ketone monoester at a level of 11.4% (12.0 g/kg bw/day and 15.1 g/kg bw/day in male and female rats, respectively) in the diet did not cause adverse effects. Ketone monoester also did not adversely affect the development of rats exposed to the ingredient in utero at a level of 2 g/kg bw/day. Taken together, these data support the safety of ketone monoester for potential use as a source of ketones.
Acknowledgments
The authors thank Dr. Joseph Bielitsky, the late Dr. Catherine Golden, Dr. Brett Giroir and Dr. Kerrie DeMarco (Defense Advanced Research Projects Agency (DARPA) of the United States), whose constant support and advice made this study possible.
Funding sources statement
The authors thank the Defense Advanced Research Projects Agency (DARPA) of the United States for funding this work.
Abbreviations
- A/G
albumin/globulin
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- APTT
activated partial thromboplastin time
- bw
body weight
- CHO
carbohydrate
- CK
creatine kinase
- GC–MS
gas chromatography–mass spec-trometry
- GLP
good laboratory practice
- Hb
hemoglobin
- Hct
hematocrit
- LDH
lactase dehydrogenase
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentration
- MCV
mean corpuscular volume
- OECD
Organisation for Economic Co-operation and Development
- RO
reverse osmosis membrane
- RBC
red blood cell
- US FDA
United States Food & Drug Administration
Footnotes
Conflict of interest
The intellectual property covering the uses of ketone bodies and ketone esters are owned by BTG Ltd., the University of Oxford, and the National Institutes of Health. Should royalties ever accrue from these patents, Dr. Richard L. Veech, Professor Kieran Clarke, Dr. Andrew Murray and Mr. Todd King, as inventors, will receive a share of the royalties under the terms prescribed by each institution. Professor Kieran Clarke is a non-executive director of TdeltaS Ltd., a company spun out of the University of Oxford to develop products based on the science of ketone bodies in human nutrition. Drs. Andrea Wong and Ashley Roberts received financial support from DARPA and TdeltaS Limited for consulting services and manuscript preparation.
References
- Carter E, et al. Human Plasma Esterase Activity Study Report. unpublished.
- Clarke K, et al. Kinetics, safety and tolerability of d-β-hydroxybutyrate-(R)-1,3-butanediol monoester in healthy adult subjects. Regul. Toxicol. Pharmacol.: RTP. doi: 10.1016/j.yrtph.2012.04.008. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers S, et al. Metabolism of R- and S-1,3-butanediol in perfused livers from meal-fed and starved rats. Biochem. J. 1992;285:647–653. doi: 10.1042/bj2850647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers S, et al. Metabolism of (R,S)-1,3-butanediol acetoacetate esters, potential parenteral and enteral nutrients in conscious pigs. Am. J. Physiol. 1995;268:E660–E667. doi: 10.1152/ajpendo.1995.268.4.E660. [DOI] [PubMed] [Google Scholar]
- Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;6:241–252. [Google Scholar]
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 1955;50:1096–1121. [Google Scholar]
- Friedel R, Mattenheimer H. Release of metabolic enzymes from platelets during blood clotting of man, dog, rabbit and rat. Clin. Chim. Acta. 1970;30:37–46. doi: 10.1016/0009-8981(70)90190-7. [DOI] [PubMed] [Google Scholar]
- Friedel R, et al. Effects of blood sampling on enzyme activities in the serum of laboratory animals. Z. Klin. Chem. Klin. Biochem. 1974;12:229. [PubMed] [Google Scholar]
- Hempenius RA, Lina BAR, Haggitt RC. Evaluation of a subchronic (13-week) oral toxicity study, preceded by an in utero exposure phase, with arachidonic acid oil derived from Mortierella alpina in rats. Food Chem. Toxicol. 2000;38:127–139. doi: 10.1016/s0278-6915(99)00144-1. [DOI] [PubMed] [Google Scholar]
- Hess FG, Jr., et al. Reproduction and teratology study of 1,3-butanediol in rats. J. Appl. Toxicol. 1981;1:202–209. doi: 10.1002/jat.2550010404. [DOI] [PubMed] [Google Scholar]
- Hunter ES, III, Sadler TW, Wynn RE. A potential mechanism of d,l-β-hydroxybutyrate-induced malformations in mouse embryos. Am. J. Physiol. 1987;253:E72–E80. doi: 10.1152/ajpendo.1987.253.1.E72. [DOI] [PubMed] [Google Scholar]
- Johnstone AM, et al. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am. J. Clin. Nutr. 2008;87:44–55. doi: 10.1093/ajcn/87.1.44. [DOI] [PubMed] [Google Scholar]
- Kashiwaya Y, et al. d-Beta-hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease. Proc. Natl. Acad. Sci. USA. 2000;97:5440–5444. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, et al. A ketone ester diet increases brain malonyl-CoA and uncoupling proteins 4 and 5 while decreasing food intake in the normal Wistar rat. J. Biol. Chem. 2010;285:25950–25956. doi: 10.1074/jbc.M110.138198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kies C, et al. Utilization of 1,3-butanediol and nonspecific nitrogen in human adults. J. Nutr. 1973;103:1155–1163. doi: 10.1093/jn/103.8.1155. [DOI] [PubMed] [Google Scholar]
- Knight NS, et al. A (R)-1,3-Butanediol-d-β-hydroxybutyrate ester diet improves physical and mental performance in rats. unpublished.
- McClernon FJ, et al. The effects of a low-carbohydrate ketogenic diet and a low-fat diet on mood, hunger, and other self-reported symptoms. Obesity (Silver Spring) 2007;15:182–187. doi: 10.1038/oby.2007.516. [DOI] [PubMed] [Google Scholar]
- McPherson PA, McEneny J. The biochemistry of ketogenesis and its role in weight management, neurological disease and oxidative stress. J. Physiol. Biochem. 2011 doi: 10.1007/s13105-011-0112-4. (Advance, Publication – Oct. 8, 2011) [DOI] [PubMed] [Google Scholar]
- Moley KH, Vaughn WK, Diamond MP. Manifestations of diabetes mellitus on mouse preimplantation development: effect of elevated concentration of metabolic intermediates. Hum. Reprod. 1994;9:113–121. doi: 10.1093/oxfordjournals.humrep.a138298. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- OECD . OECD Principles of Good Laboratory Practice (as Revised in 1997), OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring, No. 1 – ENV/MC/CHEM(98)17) Organisation for Economic Co-operation and Development (OECD), Environment Directorate, Chemicals Group and Management Committee, OECD Environmental Health and Safety Publications; 1998. Available at: < http://www.oecd.org/officialdocuments/displaydocumentpdf/?cote=env/mc/chem(98)17&doclanguage=en>. [Google Scholar]
- Plecko B, et al. Oral β-hydroxybutyrate supplementation in two patients with hyperinsulinemic hypoglycemia: monitoring of β-hydroxybutyrate levels in blood and cerebrospinal fluid, and in the brain by in vivo magnetic resonance spectroscopy. Pediatr. Res. 2002;52:301–306. doi: 10.1203/00006450-200208000-00025. [DOI] [PubMed] [Google Scholar]
- Puchowicz MA, et al. Dog model of therapeutic ketosis induced by oral administration of R,S-1,3-butanediol diacetoacetate. J. Nutr. Biochem. 2000;11:281–287. doi: 10.1016/s0955-2863(00)00079-6. [DOI] [PubMed] [Google Scholar]
- Reger MA, et al. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging. 2004;2:311–314. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- Ruben Z, et al. Guides for Toxicologic Pathology. STP/ARP/AFIP; Washington, DC: 2000. Non-proliferative lesions of the heart and vasculature in rats. Available at: < http://www.toxpath.org/ssdnc/CardiovascularNonprolifRat.pdf>. [Google Scholar]
- Sato K, et al. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- Scala RA, Paynter OE. Chronic oral toxicity of 1,3-butanediol. Toxicol. Appl. Pharmacol. 1967;10:160–164. doi: 10.1016/0041-008x(67)90137-8. [DOI] [PubMed] [Google Scholar]
- Sheehan EA, et al. Effects of beta-hydroxybutyrate on rat embryos grown in culture. Experientia. 1985;41:273–275. doi: 10.1007/BF02002633. [DOI] [PubMed] [Google Scholar]
- Siegel S. Nonparametric Statistics for the Behavioral Sciences. McGraw-Hill Co.; New York, NY: 1956. The Fisher's exact probability test. pp. 96–105. [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. sixth ed. Iowa State University Press; Ames: 1967a. Variance test for homogeneity of the binomial distribution. pp. 240–241. [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. sixth ed. Iowa State University Press; Ames: 1967b. Analysis of variance. pp. 258–298. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. Freeman & Co.; San Francisco: 1969a. Bartlett's test of homogeneity of variances. pp. 370–371. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. Freeman & Co.; San Francisco: 1969b. Kruskal–Wallis test. pp. 388–391. [Google Scholar]
- Stanfield CL, Germann WJ. Principles of Human Physiology. third ed. Pearson Benjamin Cummings; San Francisco: 2008. Cell metabolism. pp. 58–93. [Google Scholar]
- Staples RE. Detection of visceral alterations in mammalian fetuses. Teratology. 1974;9:A37–A38. [Google Scholar]
- Staples RE, Schnell VL. Refinements in rapid clearing technic in the KOH-alizarin red S method for fetal bone. Stain Technol. 1964;39:61–63. [PubMed] [Google Scholar]
- Tate RL, Mehlman MA, Tobin RB. Metabolic fate of 1,3-butanediol in the rat: conversion to -hydroxybutyrate. J. Nutr. 1971;101:1719–1726. doi: 10.1093/jn/101.12.1719. [DOI] [PubMed] [Google Scholar]
- US FDA . Toxicological principles for the safety assessment of food ingredients: FDA Redbook 2000 [Updated to July, 2007] US Food and Drug Administration (US FDA), Center for Food Safety and Applied Nutrition (CFSAN); Silver Spring, MD: 2000. IV.C.9.b Guidelines for developmental toxicity studies. Available at: < http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/FoodIngredientsandPackaging/Redbook/ucm078399.htm>. [Google Scholar]
- US FDA . Toxicological principles for the safety assessment of food ingredients: FDA Redbook 2000 (Updated to July, 2007) US Food and Drug Administration (US FDA), Center for Food Safety and Applied Nutrition (CFSAN); Silver Spring, MD: 2003. IV.C.3.a Short-term toxicity studies with rodents. Available at: < http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/FoodIngredientsandPackaging/Redbook/ucm078339.htm>. [Google Scholar]
- US FDA . US Code of Federal Regulations (CFR). Title 21 – Food and Drugs (Food and Drug Administration) US Government Printing Office (GPO); Washington, DC: 2011. Part 58 – Good laboratory practice for nonclinical laboratory studies. Available at: http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CFR. [Google Scholar]
- Wilson JG. Methods for administering agents and detecting malformations in experimental animals. In: Wilson JG, Warkany J, editors. Teratology: Principles and Techniques. University of Chicago Press; Chicago: 1965. pp. 262–277. [Google Scholar]
- Yerrourn M, Braconnier F, Chariot P. Influence of handling procedures on rat plasma creatine kinase activity. Muscle Nerve. 1999;22:1119–1121. doi: 10.1002/(sici)1097-4598(199908)22:8<1119::aid-mus16>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]