Summary
Synthetic N-acetyllactosamine (LacNAc) glycoside sequences coupled to BSA competitively inhibit enteropathogenic Escherichia coli (EPEC) localized adherence (LA) to human intestinal biopsy specimens and tissue culture cell monolayers. The LacNAc-specific adhesin appears to be associated with the bundle-forming pili (BFP) expressed by EPEC during the early stages of colonization. Herein, we report that recombinant bundlin inhibits EPEC LA to HEp-2 cells and binds to HEp-2 cells. Recombinant bundlin also binds, with millimolar association constants (Kassoc), to synthetic LacNAc-Benzene and LacNAc-O(CH2)8CONH2 glycosides as assessed in the gas phase by nanoelectrospray ionization mass spectrometry. Furthermore, LacNAc-BSA inhibits LA only of EPEC strains that express α bundlin alleles, suggesting putative locations for the LacNAc-binding pocket in the α bundlin monomer. Collectively, these results suggest that α bundlin possesses lectin-like properties that are responsible for LacNAc-specific initial adherence of α bundlin-expressing EPEC strains to host intestinal epithelial cells.
Introduction
Enteropathogenic Escherichia coli (EPEC) is a major cause of infant diarrhoea in developing nations (Nataro and Kaper, 1998). EPEC infect and colonize their hosts by way of a highly interactive process that is thought to involve three steps: initial non-intimate binding, signal transduction and then intimate adherence (Donnenberg and Kaper, 1992; Delahay et al., 2001; Donnenberg and Whittam, 2001; Kenny, 2002; Campellone and Leong, 2003). The initial binding stage of the EPEC colonization process is also distinct from that of other enterovirulent E. coli and is characterized in typical strains by the formation of discrete microcolonies of EPEC on the host cell surface, a process known as localized adherence (LA). The LA phenotype requires the expression of rope-like fimbriae, called bundle-forming pili (BFP) which are encoded by genes located on an EPEC adherence factor (EAF) plasmid (Donnenberg et al., 1992; Giron et al., 1993). EAF plasmid-cured EPEC strains bind to tissue culture cells as individual bacteria, rather than as microcolonies (Knutton et al., 1987).
While BFP are known to be required for microcolony formation in LA, there are conflicting reports regarding their role as host cell adhesins. Using a panel of EPEC strains mutated in three putative adhesins, intimin, EspA and bundlin, Hicks et al. (1998) reported that BFP are required for microcolony formation alone, but not for adherence to in vitro cultured paediatric intestinal tissue. Contrary to this, Cleary et al. (2004) demonstrated, using a panel including double and triple EPEC mutants, that BFP, while not essential, are required for the efficient colonization of cultured human paediatric intestinal biopsy specimens. Moreover, volunteer studies have revealed that BFP are required for full EPEC virulence in adults (Bieber et al., 1998), and the purified pre-bundlin protein has been shown to adhere to Caco-2 brush border epithelial cells (Tobe and Sasakawa, 2001).
Previous work in our laboratory demonstrated that N-acetyllactosamine (LacNAc) neo-glycoconjugates inhibit EPEC LA to tissue culture cells as well as adult intestinal biopsy specimens (Vanmaele et al., 1999; Hyland et al., 2006a, b). The LacNAc-specific lectin in this process was shown by glyco-gold electron microscopy to be present on the BFP filament (Hyland et al., 2006b), providing further evidence that BFP act as an early adhesin in EPEC colonization of the human intestine. Herein, we specifically investigated the role of purified bundlin, the structural subunit of BFP, as a putative LacNAc-specific lectin.
Results
Time-dependent inhibition of EPEC LA to HEp-2 cells by LacNAc-BSA
Bundle-forming pili are thought to be required for the efficient LA of EPEC strains to intestinal epithelial cells in the first hour of infection, whereas other adhesins, such as EspA and Intimin, might play a role at later times (Cleary et al., 2004). We therefore determined if LacNAc-BSA was capable of inhibiting LA to HEp-2 cells at both early (45 min) and later times (3 h) in the binding assay (Vanmaele et al., 1999; Hyland et al., 2006a, b). As expected from our previous work, LacNAc-BSA inhibited the LA of strain E2348/69 by 97.14 ± 1.08% (n = 3) in the 45 min LA assay (Fig. 1). However, LacNAc-BSA failed to inhibit E2348/69 LA to HEp-2 cells in the 3 h LA assay. This same result was obtained regardless of whether LacNAc-BSA was added to the HEp-2 cells only at the outset of the 3 h LA assay or when an additional 280 μM LacNAc-BSA was added after 2 h (Fig. 1) of the EPEC-HEp2 cell co-incubation period. LacNAc therefore only appeared to inhibit the early phase of EPEC LA to HEp-2 cells.
Fig. 1.
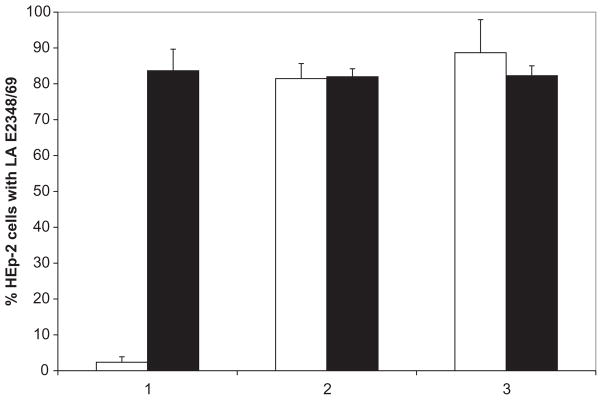
EPEC (strain E2348/69) LA to HEp-2 cells in the presence of 280 μM LacNAc-BSA (white bars) or unconjugated BSA (black bars). LA assays were performed by three protocols: (1) 45 min incubation on HEp-2 cells in the presence or absence of glycoconjugate, (2) 3 h incubation on HEp-2 cells in the presence or absence of glycoconjugate, and (3) 3 h incubation on HEp-2 cells in the presence or absence of 280 μM glycoconjugate with additional 280 μM glycoconjugate added after 2 h. For each experiment, 100 HEp-2 cells were observed in triplicate for LA EPEC, defined as microcolonies composed of at least four bacteria. Error bars represent the standard deviations from the means.
Effect of recombinant bundlin on HEp-2 cell LA of EPEC strain E2348/69
In the absence of purified recombinant bundlin, LA was observed on 70 ± 6% (n = 3) of HEp-2 cells that were exposed to wild-type EPEC strain E2348/69. When 6 μM bundlin was included in the binding assay, EPEC LA was observed on only 41 ± 6% (n = 3) of the HEp-2 cells. This represented a significant (P = 0.004, Student’s t-test) reduction in the LA of EPEC strain E2348/69 to HEp-2 cells in the presence of recombinant bundlin protein.
Interaction between bundlin and HEp-2 cells
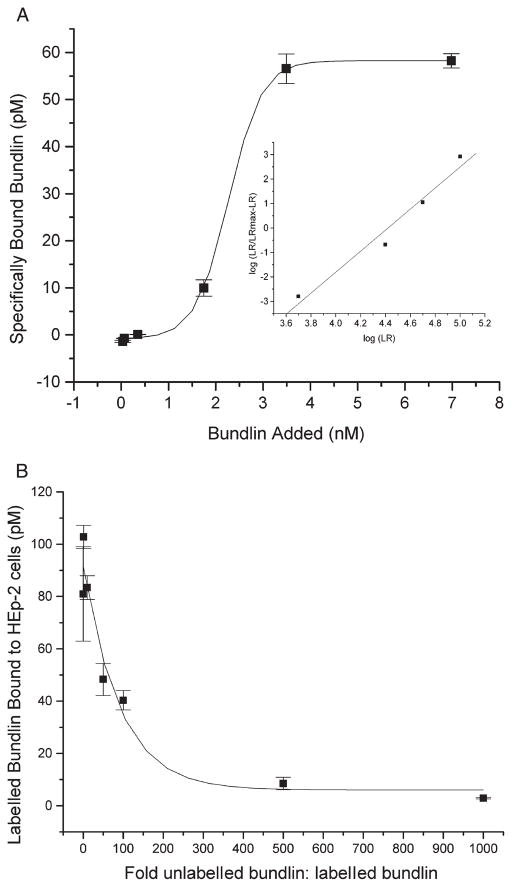
A radio-receptor equilibrium binding assay was performed to investigate the mechanism whereby soluble bundlin inhibited EPEC LA. The resulting binding isotherm (Fig. 2A) revealed that 125I-bundlin bound to HEp-2 cells by a concentration-dependant and saturable mechanism, suggestive of a receptor–ligand-type association. The data in Fig. 2A also suggested that the binding between recombinant bundlin and its complimentary receptors on HEp-2 cells might possess a positive-sense cooperative component. Analysis of these data by the Hill method (Fig. 2A, inset) (Schwarz, 1976) confirmed the cooperative nature of the bundlin–Hep-2 cell binding interaction. To ensure that the 125I-bundlin–Hep-2 cell interaction was a specific one, the binding of 125I-labelled bundlin to HEp-2 cells was competitively inhibited by adding increasing amounts of unlabelled bundlin to the binding mixtures (Fig. 2B). This exercise produced an inhibitory constant (IC50) of 0.75 nM of unlabelled bundlin which extrapolates to approximately 9.0 × 108 binding sites per HEp-2 cell. Taken together, these data are consistent with a process whereby EPEC adhere via BFP to LacNAc or LacNAc-related receptors on HEp-2 cells and this adherence is competitively inhibited by recombinant-soluble bundlin.
Fig. 2.
Radio-receptor binding isotherm (A) and competition binding assays (B) using recombinant bundlin.
A. Increasing amounts of radio-iodinated bundlin were applied to HEp-2 cell monolayers in the presence and absence of 500-fold excess unlabelled bundlin. Each of the data points represents specifically bound cpm, i.e. the difference between the cpm bound at each concentration in the absence of unlabelled bundlin minus the residual cpm bound in the presence of unlabelled bundlin. Data from the isotherm were converted into a linear (r2 = 0.9644) Hill plot, shown in the inset.
B. Radio-iodinated bundlin (3.4 nM) was added to HEp-2 cell monolayers in the presence of increasing concentrations of unlabelled bundlin.
In both panels, each data point represents the average of triplicate determinations and the error bars indicate the standard error of the individual means.
Bundlin is a LacNAc-specific lectin
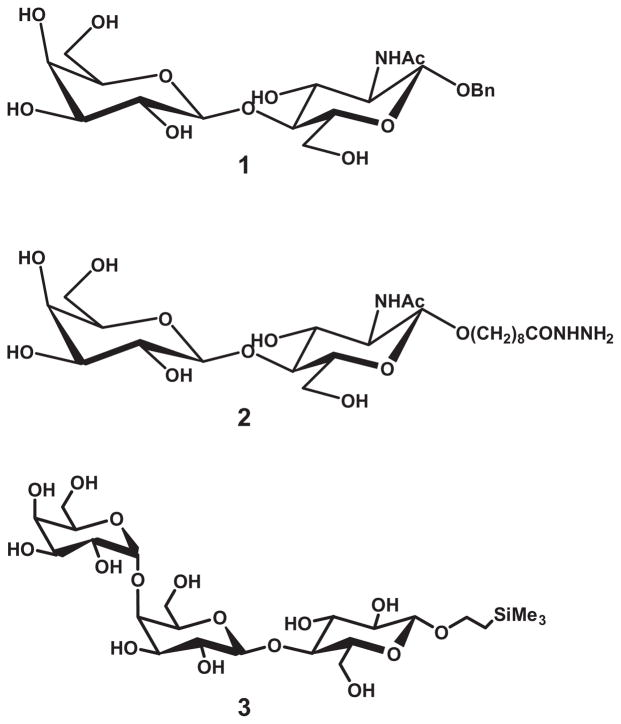
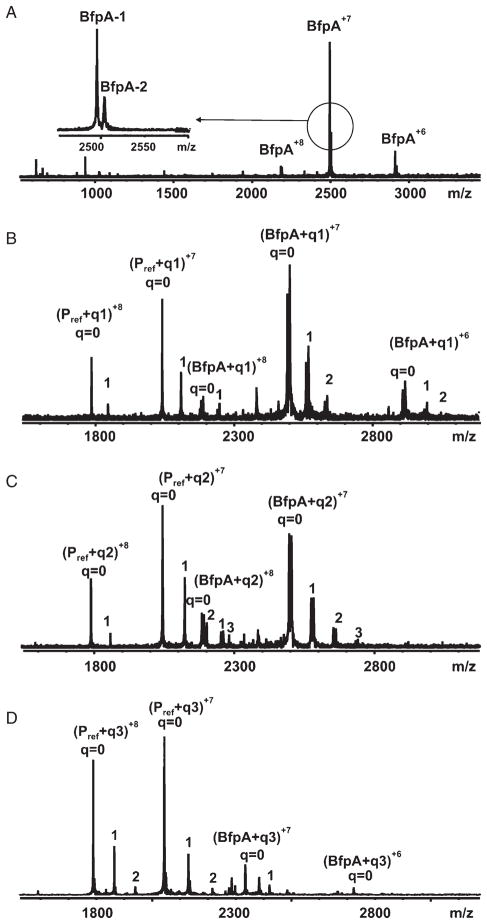
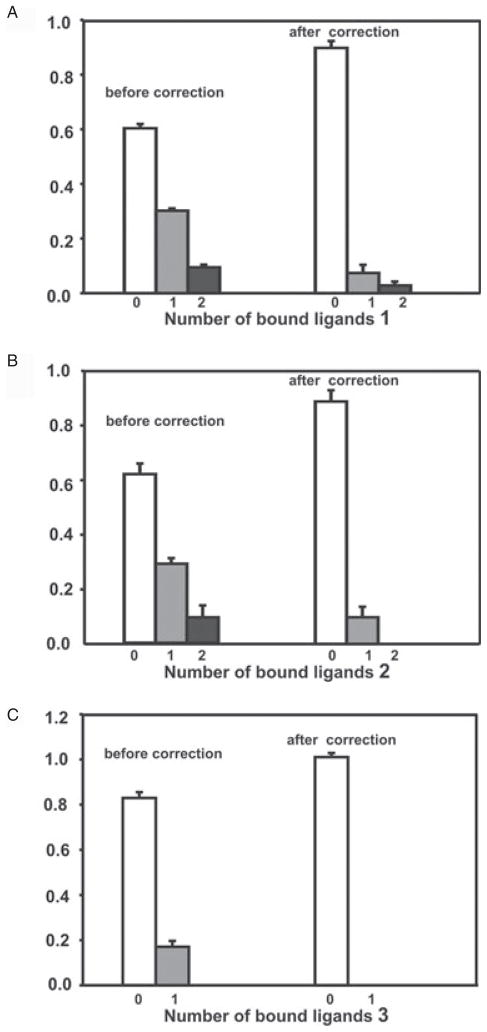
We further investigated the interaction between recombinant bundlin and LacNAc by the nanoelectrospray mass spectrometry (nanoES-MS) (Sun et al., 2006) technique. This ultra-sensitive assay has been shown to reveal affinities for protein–glycan complexes that agree with values obtained by other assays including isothermal titration microcalorimetry (Sun et al., 2006). Notably, the nanoES-MS method has distinguished itself in the analysis of very weak interactions, Kassoc = 103 M−1, which are often difficult to quantify by other techniques (Sun et al., 2006). The interactions between bundlin and synthetic LacNAc-Bn (1) and LacNAc-O(CH2)8CONH2 (2) glycosides, as well as the control synthetic Pk trisaccharide (3) (Fig. 3), were investigated. Shown in Fig. 4 are mass spectra acquired for solutions of bundlin in the absence (Fig. 4A) and presence of ligand 1 (Fig. 4B), 2 (Fig. 4C) or 3 (Fig. 4D). From Fig. 4A it can be seen that ions corresponding to two distinct forms of bundlin, with molecular weights of 17 464 Da (BfpA-1) and 17 524 Da (BfpA-2), were detected. In the presence of ligand, at a concentration of > 115 μM, ions corresponding to bundlin bound to as many as three ligand molecules were detected (Fig. 4C). However, also evident in these mass spectra were ions of Pref bound to one or two ligand molecules. As Pref has no known affinity for these ligands in solution, these complexes must originate from non-specific interactions, and were subtracted from the final analysis. Shown in Fig. 5 are the distributions of ligand bound to bundlin and Pref, for 1–3, determined directly from the mass spectra. Also shown are the distributions of ligands bound to bundlin after correction for non-specific ligand binding. Following correction, it can be seen that bundlin binds specifically to ligand 1 and 2. The values of Kassoc for ligands 1 and 2, determined at two concentrations, are listed in Table 1. Notably, the binding affinities for these two ligands are weak. According to the nanoES-MS results, there is no specific interaction between bundlin and the Pk trisaccharide 3, a carbohydrate sequence that we previously demonstrated does not inhibit LA of EPEC strain E2348/69 or interact with BFP (Hyland et al., 2006b).
Fig. 3.
Structures of LacNAc-Bn (1), LacNAc-O(CH2)8CONH2 (2) and the αGal(1,4)βGal(1,4)βGlc-SiMe3 (Pk blood group trisaccharide) (3).
Fig. 4.
NanoES mass spectra of aqueous solutions of 20 μM bundlin in the absence (A) or presence of 115 μM 1 (B), 130 μM 2 (C) or 150 μM 3 (D).
Fig. 5.
Distribution of ligand 1 (A), 2 (B) and 3 (C) bound to bundlin and Pref, determined directly from the mass spectra. Also shown are the distributions of ligands bound to bundlin after correction for non-specific ligand binding based on the interaction between Pref and the ligands.
Table 1.
Association constants (Kassoc) for bundlin with the glycans (1–3) measured by ES-MS at 25°C.
| Ligand (μM) | Kassoc (M−1 ± standard deviation) |
|---|---|
| 1 (40) | 1170 ± 481 |
| 1 (115) | 739 ± 266 |
| 2 (45) | 980 ± 377 |
| 2 (130) | 871 ± 355 |
| 3 (150) | No binding |
Kassoc was obtained by taking the average of five replicate ES-MS measurements.
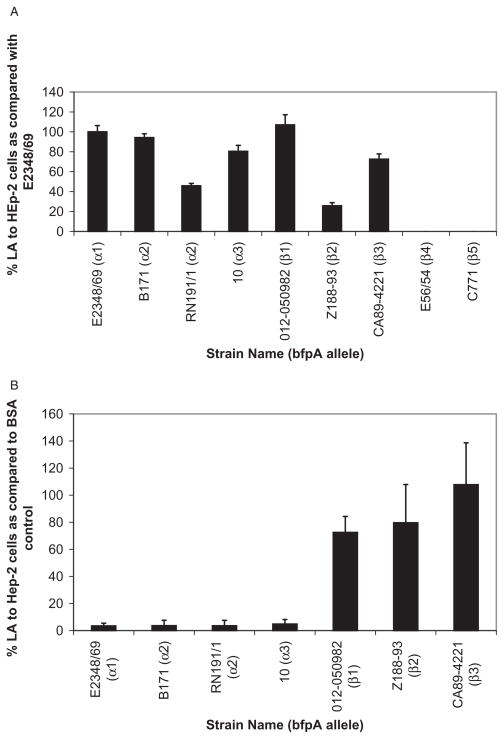
LA of EPEC strains that posses β-bfpA alleles is not inhibited by LacNAc-BSA
The interaction between bundlin and LacNAc was further investigated by assessing the ability of LacNAc-BSA to inhibit LA of EPEC strains that express bfpA alleles that differ from the α-1 allele of EPEC strain E2348/69. Representative EPEC strains expressing α-1, α-2, β-1, β-2, β-3, β-4 and β-5 bfpA alleles were used for this study (Table 2). All strains were previously shown to express the LA phenotype after 3 h of incubation with HEp-2 cells (Blank et al., 2000) and all but two strains, C771 (β-4 bfpA) and E56/54 (β-5 bfpA), also produced bundlin, as detected by the Western immunoblotting procedure (Blank et al., 2000).
Table 2.
Strains and plasmids used in this study.
| Strain/plasmid | Phenotype/description | Reference |
|---|---|---|
| Strain | ||
| E2348/69 | α-1 bundlin expressing EPEC | |
| UMD901 | bfpA knockout of E2348/69 | Zhang and Donnenberg (1996) |
| B171 | α-2 bundlin expressing EPEC | Blank et al. (2000) |
| RN191/1 | α-2 bundlin expressing EPEC | Blank et al. (2000) |
| 10 | α-3 bundlin expressing EPEC | Blank et al. (2000) |
| 012-05982 | β-1 bundlin expressing EPEC | Blank et al. (2000) |
| Z188-93 | β-2 bundlin expressing EPEC | Blank et al. (2000) |
| CA89-4221 | β-3 bundlin expressing EPEC | Blank et al. (2000) |
| E56/54 | β-4 bundlin expressing EPEC | Blank et al. (2000) |
| C771 | β-5 bundlin expressing EPEC | Blank et al. (2000) |
| Plasmid | ||
| pRPA100 | α-1 bfpA under control of PerA | Anantha et al. (2000) |
| pXLW13 | β-1 bfpA under control of PerA | Fernandes (2007) |
| pXLW16 | β-2 bfpA under control of PerA | Fernandes (2007) |
| pXLW17 | β-3 bfpA under control of PerA | Fernandes (2007) |
With the exception of those strains (E56/54 and C771) containing the β-4 and β-5 bfpA alleles, all of the others displayed LA in our 45 min assay (Fig. 6A). In addition, 280 μM LacNAc-BSA significantly inhibited LA of the α-bfpA-positive EPEC strains (Fig. 6B), as we previously demonstrated for the E2348/69 strain (Vanmaele et al., 1999; Hyland et al., 2006b). However, the ability of LacNAc-BSA to inhibit LA of the β-bfpA-expressing strains (Fig. 6B) was greatly reduced. We observed a 27.5 ± 11.8% (n = 3) decrease of LA in the 012-050982 (β-1 allele) strain and a 20.4 ± 28% (n = 3) decrease in LA in the Z188-93 (β-2 allele) strain in the presence of LacNAc-BSA, relative to the BSA control. There was no difference in the number of LA CA89-4221 (β-3 allele) EPEC in the presence of LacNAc-BSA (107.7 ± 30.9%, n = 3) relative to the BSA control.
Fig. 6.
A. LA to HEp-2 cells of EPEC strains harbouring different bfpA alleles. BFP expression was induced by pre-incubating the EPEC strains in DMEM for 30 min in the CO2 incubator. Subsequently, EPEC cells were added to subconfluent HEp-2 cell monolayers and incubated for 45 min. LA was scored as described previously (Vanmaele et al., 1999), and the data are presented as the percentage of HEp-2 cells harbouring LA EPEC.
B. The effect of 280 μM LacNAc-BSA on EPEC LA. Subsequent to the induction of BFP expression, EPEC were incubated with either 280 μM LacNAc-BSA or BSA for 30 min and then applied to HEp-2 cell monolayers. Data were normalized to those obtained when underivatized BSA was used instead of LacNAc-BSA. Each experiment was performed in triplicate, and the error bars represent the standard deviations from the means.
Gene replacement of α-bfpA with β-bfpA alleles in strain E2348/69 eliminates early LA but maintains BFP expression and the autoaggregation phenotype
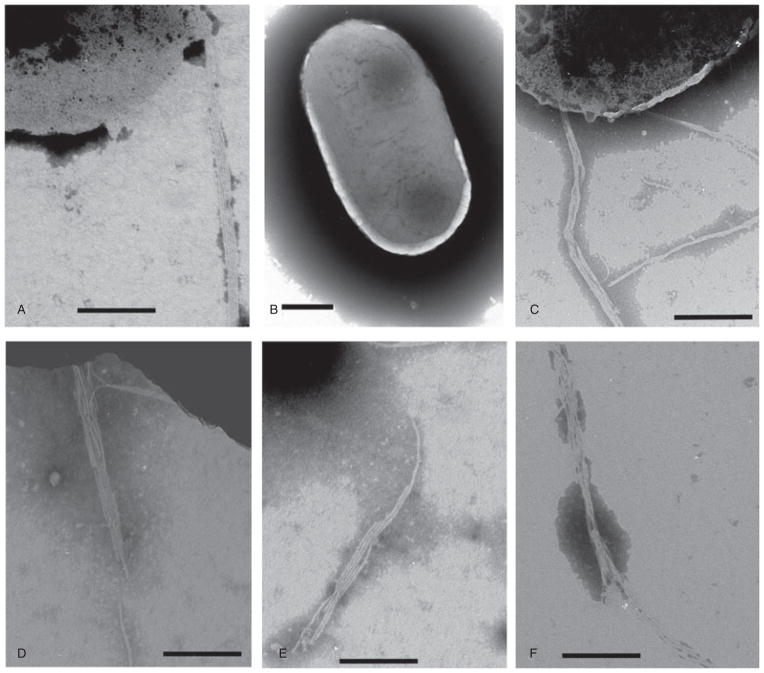
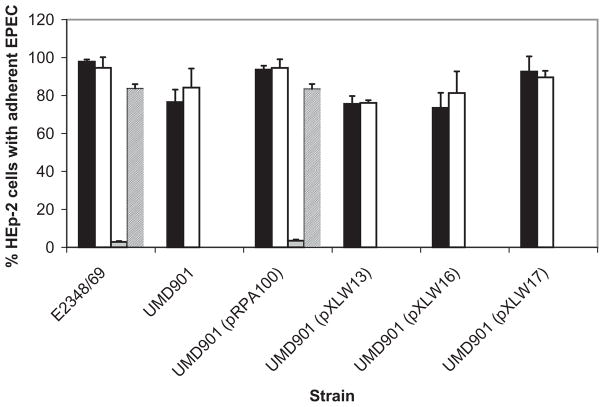
In order to confirm that the difference between the response to LacNAc-BSA in the different strains was due to the variation in the bfpA alleles, and not to unrelated differences, we complemented, in trans, UMD901, a bfpA null mutation in strain E2348/69 (Zhang and Donnenberg, 1996), with β-1, β-2 and β-3 bfpA. As a control, we used UMD901 complemented in trans with the wild-type α-1 bfpA allele from strain E2348/69. As expected, UMD901 expressed neither BFP (Fig. 7B) nor bundlin (Fig. 8, lane A). UMD901 did not exhibit autoaggregation (AA) (data not shown) or LA in the 45 min assay (Fig. 9), but did adhere in a non-LA phenotype as individual bacteria to HEp-2 cells in the 3 h assay (Fig. 9), confirming that at the 3 h time point, factors other than the BFP can mediate adherence to HEp-2 cells. UMD901 (pRPA100) expressed BFP (Fig. 7C), bundlin (Fig. 8, lane B) and exhibited both AA (data not shown) and LA in both the 45 min and 3 h LA assays (Fig. 9) similar to the wild-type E2348/69 strain. Furthermore, the LA of UMD901 (pRPA100) was inhibited by LacNAc-BSA in the 45 min LA assay (Fig. 9). In contrast, while UMD901 (pXLW13), UMD901 (pXLW15) and UMD901 (pXLW17) expressed bundlin (Fig. 8, lanes C, D and E), BFP (Fig. 7D, E and F) and AA (Fernandes, 2007), none of these strains displayed LA on HEp-2 cells in the 45 min LA assay (Fig. 9) but did express the LA phenotype after 3 h (Fig. 9).
Fig. 7.
BFP expression by strain E2348/69 (A), UMD901 (B) and UMD901 complemented in trans with pRPA100 (C), pXLW13 (D), pXLW16 (E) and pXLW17 (F). Scale bars represent 200 nm.
Fig. 8.
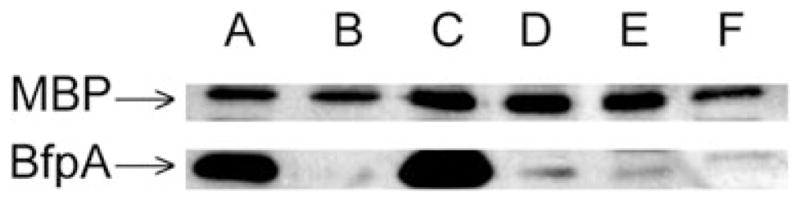
Bundlin expression by strain E2348/69 (A), UMD901 (B), UMD901 (pRPA100) (C), UMD901 (pXLW13) (D), UMD901 (pXLW16) (E) and UMD901 (pXLW17) (F) as detected by Western immunoblot analysis using polyclonal rabbit antisera prepared using bundlin from EPEC strain E2348/69. Sample loading on the gels was normalized using maltose-binding protein (MBP) as an internal standard as described previously (Hyland et al., 2006a). Blots are representative of three experiments.
Fig. 9.
LA of strain E2348/69, UMD901, UMD901 (pRPA100), UMD901 (pXLW13), UMD901 (pXLW16) and UMD901 (pXLW17) in the presence of 280 μM LacNAc-BSA (black bars) and BSA (white bars) in the 3 h LA assay, and in the presence of 280 μM LacNac-BSA (grey bars) or BSA (hatched bars) in the 45 min LA assay. Each experiment was performed in triplicate, and the error bars represent the standard deviations from the means. The reduction in LA in the presence of LacNAc-BSA is significant in the E2348/69 and UMD901 (pRPA100) in the 45 min experiments (P < 0.001, Student’s t-test). Strains UMD901 and UMD901 (pXLW13) did not display LA to HEp-2 cells after 3 h, but rather bound to the cells as individual bacteria, and data in these cases are given as percentage HEp-2 cells with adherent bacteria.
Discussion
In a previous article, we demonstrated that EPEC (E2348/69) LA to HEp-2 cells was almost completely abolished in the presence of synthetic LacNAc-BSA (Hyland et al., 2006b). This suggested that an interaction between EPEC strain E2348/69 and a LacNAc-containing receptor on the surface of HEp-2 cells was integral to the early stage of adherence to host cells. We subsequently demonstrated binding of LacNAc to BFP expressed by this strain of EPEC (Hyland et al., 2006b). In this present study, we demonstrated that recombinant 125I-labelled bundlin bound to HEp-2 cells in a concentration-dependant and saturable fashion. This binding appeared to be specific, as it could be competitively inhibited by adding excess unlabelled bundlin (Fig. 2A and B) to the reactions.
Analysis of the equilibrium binding data in Fig. 2A suggested a cooperative aspect to bundlin’s binding to HEp-2 cells. In particular, the coefficient calculated from the Hill plot suggests that a single bundlin monomer may have the capacity to bind up to four receptors on the HEp-2 cell surface. However, the nanoES-MS data presented in Fig. 4 indicate that each bundlin monomer possesses only two LacNAc-specific binding domains. The finding that only α, not β bundlin, binds to LacNAc receptors (Fig. 6) supports the nanoES-MS results as the amino acid sequence differences that define α and β bundlins are located in two discrete, surface-exposed regions of the monomers (Blank et al., 2000; Ramboarina et al., 2005), each of which might define a single LacNAc-specific binding domain.
The discrepancy between the magnitude of the Hill coefficient and the nanoES-MS data could result from bundlin oligomerization on the HEp-2 cell surface, a process that might be facilitated by conformational changes in the protein structure induced by sequential occupancy of the two potential LacNAc-specific binding sites in the bundlin monomer. Alternatively, Barnett Foster et al. (1999) previously reported that BFP bind to phosphatidyl-ethanolamine (PE) in host cell membranes. Consequently, it is possible that the cooperative nature of bundlin’s binding to HEp-2 cells may also derive from its ability to ligate both LacNAc or LacNAc-related receptors as well as PE, thereby influencing the magnitude of the Hill coefficient derived from the data presented in Fig. 2A. Regardless, the recombinant bundlin used in the present studies is missing the N-terminal α-helical domain which is required for the assembly of bundlin monomers into BFP filaments. It is possible that the cooperative nature of bundlin binding to its glycan receptors is not expressed when the intact monomers are assembled into BFP.
Purified recombinant bundlin also competitively inhibited the LA of strain E2348/69 to HEp-2 cells, a result that is consistent with the previous findings reported by Tobe and Sasakawa (2002), who demonstrated that recombinant pre-bundlin inhibited LA of strain E2348/69 to Caco-2 cells and also bound to Caco-2 cells in a concentration-dependant fashion. We extended these results using the nanoES-MS technique to demonstrate that LacNAc forms low-affinity (Table 1) complexes with bundlin. This finding is consistent with the low affinities typically observed for monovalent protein–carbohydrate interactions (Weis, 1997). Nature, however, compensates for this low affinity by expressing lectins in multimeric protein complexes, thereby enabling them to simultaneously engage multiple receptors and consequently achieving a high-avidity binding interaction (Weis and Drickamer, 1996; Vijayan and Chandra, 1999). BFP, as they consist of polymeric assemblies of bundlin (Ramboarina et al., 2005), would therefore appear to represent classical examples of this ‘Velcro’ principle.
We further investigated whether different EPEC strains expressing various bfpA alleles would provide insight into the location of the LacNAc binding site on the bundlin monomer. To accomplish this, we tested the ability of nine EPEC strains to display LA within 45 min of co-incubating the bacteria with HEp-2 cells, and whether this early binding could be inhibited by LacNAc-BSA. In these experiments, we demonstrated that neither the β-4 nor the β-5 bundlin-expressing strains could bind to HEp-2 cells (Fig. 6A), a phenotype that was not surprising given the lack of bundlin expression by these two strains (Blank et al., 2000). The β-1, -2 and -3 bundlin-expressing strains were capable of binding as microcolonies to HEp-2 cells after 45 min (Fig. 6A), similar to the E2348/69 strain; however, this binding was not inhibited by LacNAc-BSA (Fig. 6B). These observations imply that, unlike EPEC strain E2348/69, which expresses α-1 bundlin, the β bundlin-expressing strains bind to HEp-2 cells by a mechanism that does not involve LacNAc carbohydrate sequences. Regardless, the data presented in Fig. 9 suggest that rapid LA, within an hour, of the EPEC E2348/69 strain is mediated only by BFP composed of α bundlin subunits which recognize LacNAc or LacNAc-related receptors on HEp-2 cells. EPEC E2348/69 strains which express BFP composed of β bundlin subunits do not bind to LacNAc or LacNAc-related receptors on HEp-2 cells and can only form LA microcolonies on these cells after 3 h. The immunoblots presented in Fig. 8 suggest that β bundlin expression by EPEC strain E2348/69 is reduced relative to that of α bundlin. This, however, may simply be due to a reduced avidity of the α bundlin-specific polyclonal antibodies, towards the recombinant β bundling subunits.
The data presented herein suggest two distinct roles for BFP in EPEC, microcolony formation/autoaggregation and host cell adhesion. The morphology of BFP, whereby the individual pilus filaments intertwine to produce rope-like structures, suggests a physical mechanism for autoaggregation and microcolony formation, a phenotype that is shared by EPEC strains containing different bfpA alleles. However, it would appear that specific amino acids are necessary for the α-type bundlins to interact with LacNAc-containing receptors on the host cell surface. To our knowledge, these observations provide the first indication that BFP autoaggregation and adherence to the host cell may represent two distinct BFP functions. The fact that LacNAc inhibits only the early phase of LA is consistent with a model in which initial binding requires the interaction of BFP with LacNAc or LacNAc-like receptors in host cells. In contrast, subsequent development of microcolonies involves interactions among BFP produced by adjacent bacteria, a process that appears to be independent of LacNAc.
Experimental procedures
Bacterial strains, plasmids and recombinant bundlin production
The bacterial strains and plasmids used in this study are listed in Table 2. Bacteria were routinely cultured in tryptic soy broth (TSB) overnight from single colonies picked from an overnight tryptic soy agar (TSA) plate. Ampicillin and kanamycin selection was accomplished by supplementing all media with antibiotics at 50 μg ml−1. UMD901 contains a missense mutation in bfpA that results in the substitution of serine for cysteine at position 129 and leads to an unstable product that is rapidly degraded (Zhang and Donnenberg, 1996). UMD901 was complemented with α-1 bfpA (pRPA100), β-1 bfpA (pXLW13), β-2 bfpA (pXLW16) and β-3 (pXLW17). These plasmids each contain a bfpA allele cloned behind the native promoter from strain E2348/69 into the low-copy-number vector pWKS30 as described previously (Fernandes, 2007; Anantha et al., 2000). Soluble bundlin from strain E2348/69 lacking the first 24 hydrophobic residues that form an extended N-terminal helix responsible for BFP polymerization was purified as described previously (Ramboarina et al., 2004).
Preparation of tissue culture cell monolayers and LA assays
HEp-2 (CCL-23) cells were obtained from and propagated according to the American Type Culture Collection (Manassas, VA, USA) recommendations. Cell monolayers were prepared in 96-well plates containing polystyrene disks, as described previously (Vanmaele et al., 1999).
Forty-five-minute LA assays were performed by pre-inducing BFP expression by inoculating 10 μl of an overnight TSB bacterial culture into 1 ml of Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Burlington, ON, Canada) for 30 min prior to each experiment (Vanmaele et al., 1995; 1999). One hundred and fifty microlitres of the BFP-expressing EPEC cultures were then transferred to the wells of a 96-well microtitre plate containing subconfluent HEp-2 cell monolayers on polystyrene disks, as described previously (Vanmaele et al., 1999). After 45 min at 37°C in the CO2 incubator, the tissue culture cells were washed three times with phosphate-buffered (pH 7.2) physiological saline (PBS). The HEp-2 cells were then fixed with methanol for 10 min and stained with Giemsa for an additional 10 min. The polystyrene disks were removed from the microtitre plate wells, and EPEC LA was quantified microscopically by an observer blinded to the experimental conditions, as described previously (Vanmaele et al., 1999). The 3 h LA assays were performed as described elsewhere (Donnenberg and Nataro, 1995).
LA binding inhibition assays
The bundlin binding competition assays were performed exactly as described above, with the exception that either 6 μM of recombinant bundlin or BSA, as the negative control, was added to the tissue culture cells in addition to the BFP-expressing EPEC.
For the 45 min LacNAc-BSA LA binding inhibition assays, the DMEM-induced BFP-expressing EPEC cultures were transferred to a separate 96-well microtitre plate containing either LacNAc-BSA or BSA, as a negative control, to produce a final glycoconjugate concentration in DMEM of 280 μM. After 30 min at 37°C in the CO2 incubator, these mixtures were transferred to the wells of a 96-well microtitre plate containing subconfluent HEp-2 cells and subsequent steps in the assays were performed as described above.
For the 3 h LacNAc-BSA LA binding inhibition assays, LacNAc-BSA or BSA was admixed with the bacteria prior to adding to the HEp-2 cell monolayers, to obtain a final glycoconjugate concentration of 280 μM on the HEp-2 cells. In one assay, an additional 280 μM LacNAc-BSA or BSA was added to the HEp-2 cells at the 2 h interval to give a final concentration of 560 μM glycoconjugate. Subsequent steps were performed as described above.
Preparation of radio-iodinated bundlin and radio-receptor binding assays
Sixty micrograms of recombinant bundlin was labelled with 18.5 MBq of Na[125I] (GE HealthCare, Baie d’Urfe, QC, Canada) as described in a previous article (Armstrong et al., 2006), and the 125I-bundlin was immediately separated from the reaction by-products using a 0.7 × 15 cm Sephadex (GE HealthCare) G25 gel-filtration column. The protein concentration and specific activity (8.68 × 105 cpm μg−1) of the 125I-bundlin preparation were determined as described elsewhere (Armstrong and Peppler, 1987). Specificity of the label was confirmed by analysing the labelled protein on a 12.5% SDS-PAGE and subsequently exposing the dried gel to X-ray film (data not shown).
Confluent HEp-2 cell monolayers were prepared in 24-well microtitre plates. For binding isotherm experiments, 34–697 pM (100–100 000 cpm) labelled bundlin was added to HEp-2 cells in triplicate in the presence or absence of 500-fold unlabelled bundlin. The plates were then incubated on ice for 30 min and then the monolayers were gently but thoroughly washed using ice-cold PBS to remove unbound radio-iodinated bundlin. The HEp-2 cells were subsequently trypsinized using 0.25% trypsin (Invitrogen, Burlington, ON, Canada) in PBS at 37°C for 15 min and the amount of radioactivity (counts per minute) present in these samples was determined using an LKB Wallac 1270 Rack-gamma II gamma counter. Each well was counted in duplicate and non-specific binding (radioactivity remaining in the samples co-incubated with unlabelled bundlin) was subtracted from the readings to give the specifically bound CPM.
Radiolabelled bundlin competition assays with unlabelled bundlin were performed essentially as described above. Approximately 3.4 nM (100 000 cpm) of radio-iodinated bundlin was added, in triplicate, to the HEp-2 cell monolayers and these were incubated on ice for 30 min in the presence of 0–3.4 μM unlabelled bundlin. The HEp-2 cells were then thoroughly washed, trypsinized and the counts were recorded as described above.
Binding isotherm and competition assays were plotted and the lines were fitted using Microcal Origin (version 5, Northampton, MA). The IC50 for unlabelled bundlin was also calculated using Microcal Origin (v. 5)
Nanoelectrospray ionization mass spectrometry binding assays
Association constants, Kassoc, for bundlin binding to the ligands LacNAc-Bn (1), LacNAc-O(CH2)8CONH2 (2) and αGal(1,4)βGal(1,4)βGlc-SiMe3 (3) (Pk blood group trisaccharide) glycosides (Fig. 3) were evaluated using the direct nanoES-MS technique essentially as described previously (Sun et al., 2006). Pref, lysozyme, was used as a control to distinguish specific from non-specific interactions with the ligands in the experiment. Prior to analysis, the bundlin solution was dialysed against 50 mM ammonium acetate using Amicon Ultra-4 centrifugal filters (Millipore) with a molecular weight cut-off of 10 000 Da. The concentration of protein was then measured by UV absorption. The synthetic glycan ligands (Fig. 3) were generously provided by D. Bundle (University of Alberta). Any adsorbed water was removed from the ligands prior to the preparation of stock solutions by drying the samples in a vacuum chamber maintained at ~5 torr and 56°C. Each of the nanoES solutions was prepared from aqueous stock solutions of bundlin and a known concentration of ligand. The electric field required to spray the solution was established by applying a voltage of 900 V to a platinum wire inserted inside the glass tip. The solution flow rate was typically 20 nl min−1. The ion/gas jet sampled by the capillary (50 V) was transmitted through a skimmer (−2 V) and stored electrodynamically in an rf hexapole. A hexapole accumulation time of 2 s was used for all experiments. The trapping plates of the cell were maintained at a constant potential of 1.6 V throughout the experiment. The time domain signal, consisting of the sum of 50–100 transients containing 128 K data points per transient, was subjected to one zero-fill prior to Fourier transformation.
Electron microscopy
EPEC were grown at 37°C overnight on TSA plates supplemented with 5% (v/v) defimbrinated sheep blood to induce BFP expression (Giron et al., 1991). A single bacterial colony was gently suspended in 200 μl of ice-cold monoethanolamine (Sigma-Aldrich) buffer (pH 10.0), 10 μl of which was applied onto Formvar and carbon-coated copper grids (Cederlane, Hornby, ON, Canada). These were incubated for 10 min at 4°C after which time excess fluid was gently wicked from the grids. The grids were stained with 1.0% (w/v) phosphotungstic acid (PTA) for 5 min, gently wicked dry and examined using a Hitachi S-7000 transmission electron microscope (Hyland et al., 2006b).
Immunoblotting
Whole-cell lysates were separated by 12.5% SDS-PAGE and transferred to Immobilon P (Millipore) membranes as described elsewhere (Hyland et al., 2006b). The membranes were blocked in 20 mM Tris-buffered saline (pH 7.5) + 0.1% (v/v) Tween 20 (TBST) plus 5% (w/v) skim milk overnight and probed with polyclonal rabbit antiserum raised against the recombinant bundlin protein. Blots were thoroughly washed in TBST and subsequently incubated with polyclonal goat anti-rabbit IgG serum (Sigma). These blots were then developed using the ECL Plus Western Blotting Detection reagents (GE HealthCare).
Acknowledgments
Financial support was from the Alberta Ingenuity Centre for Carbohydrate Science (J.S., J.S.K. and G.D.A.) and by Public Health Service award R01 AI-37606 (M.S.D.) from the National Institutes of Health. R.M.H. and T.P.G. were the recipients of a Canadian Graduate Scholarship from NSERC. We thank Dr David Bundle for supplying the synthetic ligands used in the nanoES-MS experiments. G.D.A. is a co-inventor on US patent 6 291 435 for the treatment of diarrhoea caused by EPEC.
References
- Anantha RP, Stone KD, Donnenberg MS. Effects of bfp mutations on biogenesis of functional enteropathogenic Escherichia coli type IV pili. J Bacteriol. 2000;182:2498–2506. doi: 10.1128/jb.182.9.2498-2506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong GD, Peppler MS. Maintenance of biological activity of pertussis toxin radioiodinated while bound to fetuin-agarose. Infect Immun. 1987;55:1294–1299. doi: 10.1128/iai.55.5.1294-1299.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong GD, Mulvey GL, Marcato P, Griener TP, Kahan MC, Tennent GA, et al. Human serum amyloid P component protects against Escherichia coli O157:H7 Shiga toxin 2 in vivo: therapeutic implications for hemolytic-uremic syndrome. J Infect Dis. 2006;193:1120–1124. doi: 10.1086/501472. [DOI] [PubMed] [Google Scholar]
- Barnett Foster D, Philpott D, Abul-Milh M, Huesca M, Sherman PM, Lingwood CA. Phosphatidylethanolamine recognition promotes enteropathogenic E. coli and enterohemorrhagic E. coli host cell attachment. Microb Pathog. 1999;27:289–301. doi: 10.1006/mpat.1999.0305. [DOI] [PubMed] [Google Scholar]
- Bieber D, Ramer SW, Wu CY, Murray WJ, Tobe T, Fernandez R, Schoolnik GK. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- Blank TE, Zhong H, Bell AL, Whittam TS, Donnenberg MS. Molecular variation among type IV pilin (bfpA) genes from diverse enteropathogenic Escherichia coli strains. Infect Immun. 2000;68:7028–7038. doi: 10.1128/iai.68.12.7028-7038.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone KG, Leong JM. Tails of two Tirs: actin pedestal formation by enteropathogenic E. coli and enterohemorrhagic E. coli O157:H7. Curr Opin Microbiol. 2003;6:82–90. doi: 10.1016/s1369-5274(03)00005-5. [DOI] [PubMed] [Google Scholar]
- Cleary J, Lai LC, Shaw RK, Straatman-Iwanowska A, Donnenberg MS, Frankel G, Knutton S. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology. 2004;150:527–538. doi: 10.1099/mic.0.26740-0. [DOI] [PubMed] [Google Scholar]
- Delahay RM, Frankel G, Knutton S. Intimate interactions of enteropathogenic Escherichia coli at the host cell surface. Curr Opin Infect Dis. 2001;14:559–565. doi: 10.1097/00001432-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Donnenberg MS, Kaper JB. Enteropathogenic Escherichia coli. Infect Immun. 1992;60:3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg MS, Nataro JP. Methods for studying adhesion of diarrheagenic Escherichia coli. Methods Enzymol. 1995;253:324–336. doi: 10.1016/s0076-6879(95)53028-2. [DOI] [PubMed] [Google Scholar]
- Donnenberg MS, Whittam TS. Pathogenesis and evolution of virulence in enteropathogenic and enterohemorrhagic Escherichia coli. J Clin Invest. 2001;107:539–548. doi: 10.1172/JCI12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg MS, Giron JA, Nataro JP, Kaper JB. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol Microbiol. 1992;6:3427–3437. doi: 10.1111/j.1365-2958.1992.tb02210.x. [DOI] [PubMed] [Google Scholar]
- Fernandes PL, Guo Q, Donnenberg MS. Functional consequences of sequence variation in bundlin, the enteropathogenic Escherichia coli Type IV pilin protein. Infect Immun. 2007 doi: 10.1128/IAI.00009-07. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron JA, Ho AS, Schoolnik GK. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991;254:710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- Giron JA, Ho AS, Schoolnik GK. Characterization of fimbriae produced by enteropathogenic Escherichia coli. J Bacteriol. 1993;175:7391–7403. doi: 10.1128/jb.175.22.7391-7403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks S, Frankel G, Kaper JB, Dougan G, Phillips AD. Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect Immun. 1998;66:1570–1578. doi: 10.1128/iai.66.4.1570-1578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland RM, Beck P, Mulvey GL, Kitov PI, Armstrong GD. N-acetyllactosamine conjugated to gold nanoparticles inhibits enteropathogenic Escherichia coli colonization of the epithelium in human intestinal biopsy specimens. Infect Immun. 2006a;74:5419–5421. doi: 10.1128/IAI.00739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland RM, Griener TP, Mulvey GL, Kitov PI, Srivastava OP, Marcato P, Armstrong GD. Basis for N-acetyllactosamine-mediated inhibition of enteropathogenic Escherichia coli localized adherence. J Med Microbiol. 2006b;55:669–675. doi: 10.1099/jmm.0.46344-0. [DOI] [PubMed] [Google Scholar]
- Kenny B. Mechanism of action of EPEC type III effector molecules. Int J Med Microbiol. 2002;291:469–477. doi: 10.1078/1438-4221-00155. [DOI] [PubMed] [Google Scholar]
- Knutton S, Baldini MM, Kaper JB, McNeish AS. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect Immun. 1987;55:78–85. doi: 10.1128/iai.55.1.78-85.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboarina S, Fernandes P, Simpson P, Frankel G, Donnenberg M, Matthews S. Complete resonance assignments of bundlin (BfpA) from the bundle-forming pilus of enteropathogenic Escherichia coli. J Biomol NMR. 2004;29:427–428. doi: 10.1023/B:JNMR.0000032511.89525.64. [DOI] [PubMed] [Google Scholar]
- Ramboarina S, Fernandes PJ, Daniell S, Islam S, Simpson P, Frankel G, et al. Structure of the bundle-forming pilus from enteropathogenic Escherichia coli. J Biol Chem. 2005;280:40252–40260. doi: 10.1074/jbc.M508099200. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Some general aspects regarding the interpretation of binding data by means of a Scatchard plot. Biophys Struct Mech. 1976;2:1–12. doi: 10.1007/BF00535648. [DOI] [PubMed] [Google Scholar]
- Sun J, Kitova EN, Wang W, Klassen JS. Method for distinguishing specific from nonspecific protein-ligand complexes in nanoelectrospray ionization mass spectrometry. Anal Chem. 2006;78:3010–3018. doi: 10.1021/ac0522005. [DOI] [PubMed] [Google Scholar]
- Tobe T, Sasakawa C. Role of bundle-forming pilus of enteropathogenic Escherichia coli in host cell adherence and in microcolony development. Cell Microbiol. 2001;3:579–585. doi: 10.1046/j.1462-5822.2001.00136.x. [DOI] [PubMed] [Google Scholar]
- Tobe T, Sasakawa C. Species-specific cell adhesion of enteropathogenic Escherichia coli is mediated by type IV bundle-forming pili. Cell Microbiol. 2002;4:29–42. doi: 10.1046/j.1462-5822.2002.00167.x. [DOI] [PubMed] [Google Scholar]
- Vanmaele RP, Finlayson MC, Armstrong GD. Effect of enteropathogenic Escherichia coli on adherent properties of Chinese hamster ovary cells. Infect Immun. 1995;63:191–198. doi: 10.1128/iai.63.1.191-198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanmaele RP, Heerze LD, Armstrong GD. Role of lactosyl glycan sequences in inhibiting enteropathogenic Escherichia coli attachment. Infect Immun. 1999;67:3302–3307. doi: 10.1128/iai.67.7.3302-3307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan M, Chandra N. Lectins. Curr Opin Struct Biol. 1999;9:707–714. doi: 10.1016/s0959-440x(99)00034-2. [DOI] [PubMed] [Google Scholar]
- Weis WI. Cell-surface carbohydrate recognition by animal and viral lectins. Curr Opin Struct Biol. 1997;7:624–630. doi: 10.1016/s0959-440x(97)80070-x. [DOI] [PubMed] [Google Scholar]
- Weis WI, Drickamer K. Structural basis of lectin-carbohydrate recognition. Annu Rev Biochem. 1996;65:441–473. doi: 10.1146/annurev.bi.65.070196.002301. [DOI] [PubMed] [Google Scholar]
- Zhang HZ, Donnenberg MS. DsbA is required for stability of the type IV pilin of enteropathogenic Escherichia coli. Mol Microbiol. 1996;21:787–797. doi: 10.1046/j.1365-2958.1996.431403.x. [DOI] [PubMed] [Google Scholar]