Abstract
With the increased emergence of anti-infective resistance in recent years, much focus has recently been drawn to the development of new anti-infectives and the optimization of treatment regimens and combination therapies for established antimicrobials. In this context, the field of pharmacometrics using quantitative numerical modeling and simulation techniques has in recent years emerged as an invaluable tool in the pharmaceutical industry, academia and regulatory agencies to facilitate the integration of preclinical and clinical development data and to provide a scientifically based framework for rationale dosage regimen design and treatment optimization. This review highlights the usefulness of pharmacometric analyses in anti-infective drug development and applied pharmacotherapy with select examples.
Keywords: Modeling, simulation, pharmacokinetics, pharmacodynamics, anti-infectives, pharmacometrics, drug development
The increased emergence of multidrug resistant (MDR) microbial strains in recent years and the resulting complications in the clinical care for infected patients is a strong reminder for the urgent need to develop new anti-infective agents. As the battle against drug-resistant microorganisms rages on in clinical centers around the globe, the development of novel single agent and combination therapies against microbial infections has challenged research scientists in the pharmaceutical industry and academia as well as clinicians and other healthcare providers to seek a multi-disciplinary approach; one that encompasses microbiology, clinical expertise, and drug development sciences. Pharmacometrics has in this context arisen as a formidable tool to bridge and link between key concepts in these disciplines and to provide a rationale, scientific framework throughout the drug development continuum as well as in clinical applications [1,2]. There are case studies which suggest that basing optimization of treatment on pharmacometric assessments improves the risk-management of MDR pathogens in the clinical realm by minimizing the failures and mistakes associated with inappropriate or careless use of antibiotic therapies [3,4].
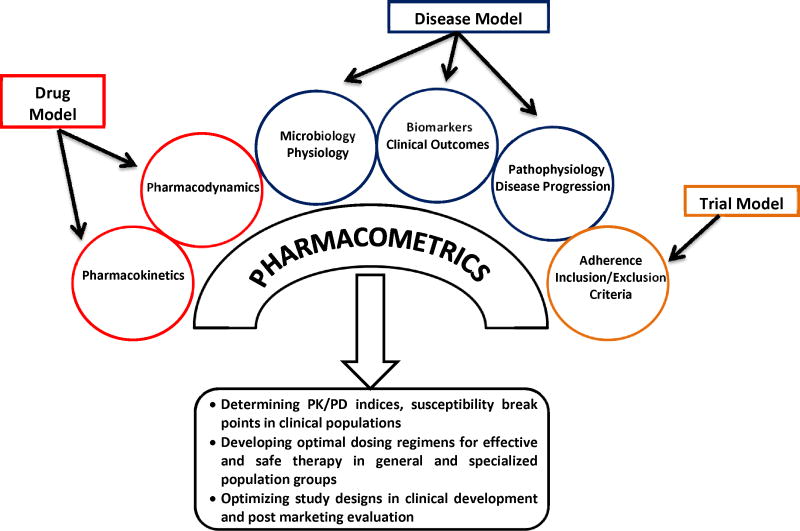
Pharmacometrics is the scientific discipline that uses mathematical models based on biology, pharmacology, physiology, and disease for quantifying the interactions between drugs and patients (Figure 1). Data and information from various sources are bridged together and quantitatively related to each other. Computer-based modeling and simulation of pharmacokinetic (PK) and pharmacodynamic (PD) data (denoted as PK/PD M&S) usually forms the basis for a pharmacometric analysis, but is frequently supplemented by models characterizing important other aspects of drug efficacy and/or safety in a given situation, such as disease progression, adherence to therapy or bacterial growth and infection [5]. PD measures used in pharmacometrics are frequently biomarkers, but can also be surrogate endpoints or clinical endpoints.
Figure 1.
Illustration of interplay of pharmacology, microbiology, physiology, disease and their association with pharmacometric evaluations in the clinical development and application of anti-infectives.
Pharmacometrics is aimed at establishing models that provide guidance and decision support in drug development such as trials design, efficacy comparisons, dosage regimen optimization and endpoint analysis, but also in supporting regulatory decisions and improving clinical care in specific patient populations [6,7]. Its purpose is thereby to reduce cost and shorten development time by optimizing the clinical assessment of efficacy and safety.
An increased application of pharmacometric methodologies in drug development and applied pharmacotherapy has in the last decade been strongly promoted by industry, academia and especially regulatory agencies, with specific emphasis from the U.S. Food and Drug Administration [8,9]. Pharmacometrics has been extensively used in the development and application of novel antibiotics [10–12]. In addition, increasing emphasis is more recently being placed on dose selection of approved antibiotics already in clinical use, with heavy reliance on quantitative benefit-risk evaluations [13].
This review aims to provide an overview on the usefulness and utility of pharmacometrics and in specific modeling and simulation approaches (M&S) in anti-infective drug development and clinical application (Table 1), and will highlight these in examples on the application of M&S approaches in three specific areas:
Table 1.
Examples of the application of pharmacometrics analyses for anti-infectives.
| Drug | Organism | Disease | Target Site | Objective for performing pharmacometrics analysis | Outcome | Population | Ref. |
|---|---|---|---|---|---|---|---|
| Biapenem | Gram positive and gram negative anaerobes | Continuous venovenous hemodiafiltration (CVVHDF) | Plasma and filtrate-dialysate | Determine appropriate dosage recommendation for patients on CVVHDF | 300 mg BID, intravenous (2 h infusion) | Adult Japanese (mean 65.1 yr), N=7 | [29] |
| Cefditoren | Streptococcus pneumoniae | Lower respiratory tract infections | Plasma and BAL | PD profiling & determining PTA (Dose: 400 mg QD, oral) | PTA is <80% (at T>MIC of 33%, MIC = 0.06mg/L) | Adult Caucasian (35–78 yr) N=24 | [35] |
| Cefepime | Streptococcus pneumoniae | Extracerebral infections | Serum and CSF | PD profiling & determining PTA (2g BID, 0.5h IV infusion) | PTA in CSF is 91.8% (at T>MIC of 50%) and 82% (at T>MIC of 100%) | Adult females (mean 58.9 yr) N=7 | [53] |
| Ceftobiprole | Staphylococcus aureus | Staphylococcal pneumonia | ELF, serum, BAL | PD profiling & determining PTA (500 mg, TID, 2h IV infusion) | PTA with T>MIC is 15% for 1-log10 CFU/g reduction; 25% for 2-log10 CFU/g reduction | Adults (>18 yr) N=25 | [54] |
| Staphylococcus aureus, Streptococcus pneumoniae | Nosocomial pneumonia | Skin, Plasma | PD profiling & determining PTA & renal dose adjustments (500 mg BID, 1h infusion; 500 mg TID, 2h infusion) | With 500 mg, BID: PTA of 30% and 50% T>MIC exceeded 90%. With 500 mg, TID: PTA of 40% and 60% T>MIC exceeded 90%. 500mg, BID is optimal dose for CLCr ≤ 50mL/min | Adults, N=150 | [55] | |
| Ceftriaxone | Streptococcus pneumoniae | Extracerebral infections | CSF | PD profiling & determining PTA (2g BID, 0.5h infusion) | PTA is 76% for 50% T>MIC in CSF; PTA is 65% for 100% T>MIC in CSF | Adult females (mean age 58.9 yr) N=7 | [53] |
| Garenoxacin | Streptococcus pneumoniae | Community-acquired pneumonia (CAP) | Serum, ELF | Evaluate exposure-response relationship by population PK/PD | fAUC0-24/MIC90 > 200 400 mg QD, Oral dosing is safe and adequate for efficacy | Adults (≥18 yr) N=580 | [56,57 ] |
| Streptococcus pneumonia, Staphylococcus aureus, Klebsiella pneumonia, Moraxella catarrhalis, Haemophilus influenzae | Pneumonia, secondary infection of chronic respiratory diseases, bronchitis, sinusitis, otis media, laryngopharyngitis, tonsillitis | Determine PTA in serum and ELF | PTA > 95% for fAUC0-24/MIC90 is 100 (serum) and 120 (ELF) | Adult Japanese (≥18 yr) N=136 | [27] | ||
| Gatifloxacin | Streptococcus pneumoniae | Community-acquired pneumonia (CAP) | N/A | PD profiling & determining PTA at 400 mg QD, oral (young) and 200 mg QD, oral (elderly) | PTA for AUC0-24/MICall ≥ 30 is 92.3% in young; 91.4% in elderly | Adults Young (<65 yr) Elderly (>65 yr) N=183 | [58] |
| Gemifloxacin | Streptococcus pneumoniae | Community-acquired pneumonia (CAP) | Serum, ELF | PD profiling & PTA in serum and ELF (320 mg QD, oral) | PTA (>95%, or >99% [59]) for fAUC0-24/MIC90 is 100 (ELF) and 78.3–88 (Serum) | N/A | [57,59 ] |
| Levofloxacin | Eschirichia coli, Chlamydia | Prostatitis | Prostatic tissue | Determine penetration at site of action (500 mg QD) | AUCprostrate/AUCplasma is 2.96 | Adult (47–94 yr) N=22 | [60] |
| Organisms causing Pneumonia | Nocosomial pneumonia | Plasma, ELF | Determine penetration ratio in ELF (500 mg QD; 750 mg QD) | AUCELF/AUCplasma is 1.16 | Adult (> 18 yr) N=24 | [32] | |
| Metronidazole | Bacteroides fragilis | N/A | Plasma and urine | PD profiling & determining PTA at 500 mg, TID; 1000 mg QD; 1500 mg QD | PTA for AUC/MIC ≥70 is 99% | Adults males, N=18 (10 healthy, 8 patients) | [61] |
| Moxifloxacin | Streptococcus pneumoniae | Community-acquired pneumonia (CAP) Gram-positive | Serum, ELF | PD profiling & determining PTA (400 mg QD, 1h infusion) PD profiling & prediction of | PTA (>95%) for fAUC0-24/MIC90 is 120 (ELF) and 78.3–88 (Serum); Cmax/MIC90 >10, AUC/MIC90 ~ 100 | Adults (18–80 yr) N=16 | [57,62] |
| Norvancomycin | Staphylococcus aureus | bacterial infections | Serum | CL estimates in population (400mg QD, intravenous) | 95% cured clinical outcome with AUC0-24/MIC of 579.9 CL=2.54(CLCr/50) in patients with renal dysfunction CL=6.0(Body Weight/60)0.52 in healthy subjects |
Adult, N=166 | [38] |
| Oseltamivir and Oseltamivir carboxylate (OC) | Influenza A (H1N1 virus) | Influenza A and B | Serum | To determine dosing in neonates and infants | 3mg/kg, BID, Oral in infants 1.7mg/kg, BID, Oral in neonates |
Adult males N=6; Infants (<2 yr) N=43; Neonates (1.5–17.5 weeks) | [47,63] |
| Piperacillin/T azobactam (combination) | Escherichia coli, S. aureus, Klebsiella pneumonia, Pseudomonas aeruginosa, Bacteroides fragilis, Acinetobacter baumannii | Gram-negative bacterial infections | Serum | To determine PK/PD parameters & in vivo effectiveness with doses 3.375g, Q4h, Q6h; 4.5g, Q6h, Q8h (intravenous,0.5 h infusion) | T>MIC is >60% for all doses | N=20 Adult males N=12 | [64,65] |
| Rifampin | Mycobacterium tuberculosis | Tuberculosis | Plasma, ELF, BAL, Alveolar Cells (ACs) | Determine pulmonary PK/PD in lungs & evaluate recommended dose (600 mg, QD, oral) | Cmax/MIC ≥ 175 are 95% (ACs), 48.8% (plasma), 35.9% (ELF) AUC0-24/MIC ≥ 271 is 100% (plasma); AUC0-24/MIC ≥ 665 is 54.5%(ELF); Higher doses (1200 mg) were needed as pulmonary concentration is too low with recommended dosing | Adults, N=40 | [39] |
| Telavancin | Staphylococcus aureus | Health care associated pneumonia | Plasma and ELF | To determine penetration in ELF with 10 mg/kg QD (intravenous, 1h infusion) | AUCELF/AUCplasma is 0.73 | Adult Caucasians, N=20 | [36] |
| Vancomycin | Staphylococcus aureus | Ventilator-associated pneumonia | Plasma, Bronchoalveolar lavage fluid, ELF | To determine penetration in ELF with 1000 mg BID (intravenous) | AUCELF/AUCplasma is 0.675 AUC/MIC ≥ 400 | Adults (>18 yr) N=10 | [34] |
| Voriconazole | Candida strains | N/A | Plasma | To investigate the effect of concomitant fluconazole on Voriconazole PK | Coadministration is not recommended, Monitor for adverse events if voriconazole is sequentially administered after fluconazole | Adult males (21–55 yr) N=10 | [40] |
BAL bronchoalveolar lavage fluid; BID twice daily; CL clearance; CLCR creatinine clearance; CSF cerebrospinal fluid; ELF epithelial lining fluid; N/A not available; PD pharmacodynamic; PTA pharmacodynamic target attainment; QD once daily; Q6h every six hours; Q8h every 8 hours
The determination of PK/PD indices and susceptibility breakpoints indicative of antimicrobial activity in clinical populations.
The development of optimal dosing regimens for effective and safe anti-microbial therapy in both general and specialized population groups, such as pediatric patients.
The optimization of key attributes of study design in clinical development and postmarketing evaluations.
Prerequisites for the Application of Modeling and Simulation in Anti-infective Drug Development and Pharmacotherapy
One of the major prerequisites for the application of M&S in clinical drug development and applied pharmacotherapy is the availability of a mathematical framework that captures the dose-exposure relationship for the antibiotic in relevant tissues of interest (i.e. the plasma and tissue pharmacokinetics), the growth characteristics of the pathogen and its relevant subpopulations in the in vivo environment (microbiology), and the dynamic interplay between pathogen and antibiotic exposure with regard to pathogen survival, death, latency and resistance (pharmacodynamics). Relevant PK and PD parameters may be derived from a variety of sources, including preclinical and clinical pharmacokinetic studies, studies assessing tissue concentration-time courses with techniques such as imaging or microdialysis, in vitro MIC data, in vitro time-kill curves, disease pathology, and microbiologic characteristics of the pathogen/infective agent itself [14,15]. These data can be combined with knowledge about the between- and within-subject variability in relevant PK parameters as well as microbiologic differences among different pathogen strains. Since between-subject variability is oftentimes a major complicating factor in drug development and pharmacotherapy, the potential impact of M&S approaches is usually the higher the larger the between-subject variability is. The resulting models help identify optimal drug concentrations necessary to achieve at the target site to be efficacious in overcoming an infection (i.e. bacterial eradication) and minimizing bacterial resistance, and the corresponding dosing regimens that provide a high likelihood of therapeutic success in a specific target population [10,16]. Once this basic framework has been established, additional factors including human physiological, biochemical, and genetic variability and differences to preclinical species that complicate the translation of preclinical results to the clinic and of data from controlled clinical studies to clinical practice may be considered and implemented in the modeling framework.
The determination of PK/PD indices and susceptibility breakpoints indicative of anti-microbial activity in clinical populations
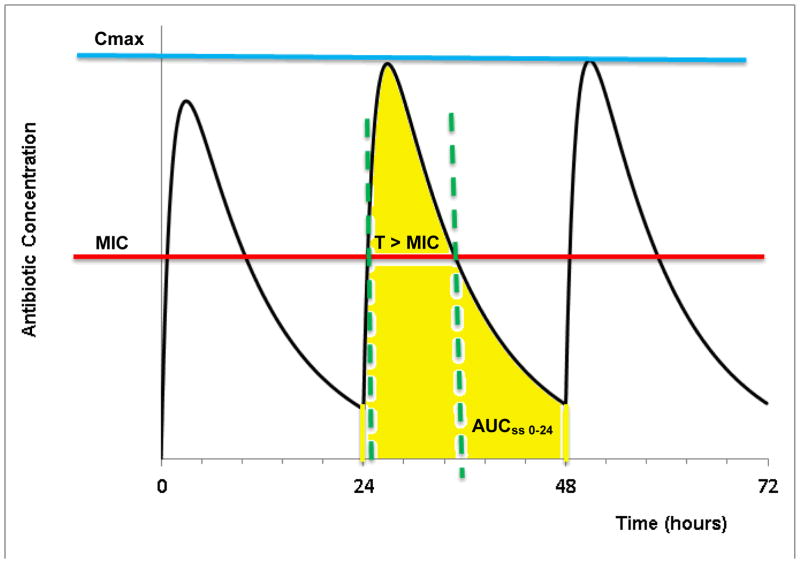
Susceptibility breakpoints, or critical antimicrobial concentrations, are valuable parameters in assessing the degree of drug susceptibility and/or resistance to an antimicrobial pharmacotherapy. Breakpoints refer to antibiotic concentrations or exposures that separate bacterial strains where there is a high likelihood of treatment success from those bacteria where treatment is more likely to fail. Historically, pharmacokinetic exposure parameters such as the area-under-the-plasma-concentration-time-curve (AUC) or the maximum or peak concentration (Cmax) have been the gold standard for monitoring drug action under the assumption that plasma concentration are indicative of target site concentrations and that a defined and fixed target site exposure is necessary for drug action. For antimicrobial pharmacotherapy, however, target site exposure is not sufficient to predict efficacy since the susceptibility of the microorganism to the target site concentration is variable among different microorganisms and strains and may change over time in the event of for example resistance development. Susceptibility of a microorganism to an antibiotic is frequently described by its minimum inhibitory concentration (MIC), an in vitro parameter that quantifies growth inhibition to a static antibiotic concentration. Combination of the pharmacodynamic parameter MIC with pharmacokinetic exposure parameters such as AUC or Cmax allows defining PK/PD indices that are hybrid parameters that integrate PK and PD information related to target exposure and microbial sensitivity, and can be used to derive PK/PD breakpoints. Dependent on the predominant mechanism of action of the antibiotic, the following three PK/PD indices (as shown in Figure 2) have been found useful in characterizing in vivo )
Figure 2.
Illustration of the PK/PD indices at steady state during multiple dosing: (i) T>MIC: Time above MIC (ii) Cmax/MIC (iii) AUC/MIC. Cmax: Maximum Plasma Concentration, AUCss 0-24: Area under the curve at steady state plasma concentration, MIC: Minimum Inhibitory Concentration
T > MIC: Time T of exposure of microorganisms to plasma concentrations exceeding their MIC.
Cmax/MIC: Ratio of peak plasma concentration to MIC.
AUC/MIC: Ratio of area under plasma concentration curve to MIC.
These indices are frequently used to classify the pattern of killing activity for antimicrobials, concentration-dependent vs. time-dependent. Antibiotics for which T > MIC best describes their antimicrobial activity follow time-dependent killing with minimal persistent effects that requires a maximum duration of exposure above MIC for maximum activity. Antibiotics for which Cmax/MIC best describes their antimicrobial activity follow concentration-dependent killing with prolonged persistent effects that require a maximum peak antibiotic concentration for maximum activity. Antibiotics for which AUC/MIC best describes their antimicrobial activity exhibit both time-dependent and concentration-dependent killing with moderately persistent effects that require a combination of high concentration and long duration above MIC for maximum activity. The AUC in these indices is calculated at pharmacokinetic steady state over 24 hours (AUCss,0-24) and is usually derived from Bayesian parameter estimates from population PK analysis approaches.
As indicated in these relationships, the MIC is consistently used as a denominator or ‘normalization factor’. The integration of drug exposure (PK) and the actual drug activity based on microbial sensitivity (PD) allows to develop generalized breakpoints with which multiple drugs can be evaluated for efficacy related to both their PK and PD parameters [18–20]. The efficacy is the desired clinical effect that a particular drug is producing and is the combined effect of PK and PD and other, for example patient-related, factors. The term ‘efficacy’ cannot be interchanged for PD. Thus, breakpoints are often used as a valuable tool in drug development and applied pharmacotherapy to optimize dosing regimens of an antibiotic for the treatment of a specific pathogen and to minimize the increase of resistant strains with optimal dosing.
It should be stressed, however, that many antibiotics are bound to variable degree to proteins in plasma and tissue, and that only the free, unbound concentration at the target site is pharmacologically active and exerts the antimicrobial activity [21]. Since MIC is frequently determined with no or limited binding proteins present, it usually represents an unbound concentration [21]. Thus, PK/PD indices also need to be based in free drug exposures rather than total concentrations to avoid any bias introduced by considering protein binding only in the PD parameter MIC, but not the PK parameters AUC and Cmax. Consequently, fT>MIC, fCmax/MIC, and fAUC/MIC based on free drug concentrations are now usually applied as appropriate PK/PD indices [18–20,22].
PK/PD indices assessed in M&S studies can be used to assess and compare the likelihood of achieving effective target exposure for different antibiotics and thus prioritize compounds for the treatment of specific infections. Noreddin et al. used this approach by applying simulation studies to determine the probability of target attainment in serum and epithelial lining fluid (ELF) defined as a fAUC0–24/MIC90 ratio of 30, with bacterial eradication as efficacy endpoint, for standard clinical regimens of azithromycin, clarithromycin, and telithromycin against S. pneumonia strains with different susceptibility [23].
Another example for establishing PK/PD indices by M&S is the case of isoniazid which displays a multimodal elimination pattern in the population based on single nucleotide polymorphisms (SNPs) in the gene encoding for N-acetyl transferase-2 (NAT2). Since the allelic frequency of these genetic variants differs among different ethnic groups, these groups differ in the number of “slow” and “fast” acetylators based on NAT2 phenotype. Thus, standard recommended doses would obviously result in different bactericidal activities among different populations. In vitro models showed that AUC0-24/MIC is associated with both microbial death and emergence of resistance for isoniazid [24]. Based on this approach, M&S was used to predict the bactericidal effects of different doses of drug in various ethnic populations, accounting for the multimodal elimination of isoniazid. The model allowed to predict the dose with highest early bactericidal activity (EBA) and this dose was used in subsequent clinical studies [24].
Based on predefined susceptibility breakpoints, microorganisms are classified as susceptible, intermediate and resistant. The identification of susceptibility breakpoints is being performed using standard tests implemented by the Clinical Laboratory Standards Institute (CLSI) by examining MIC distributions across different strains of a bacterial species. MIC values are measured using Mueller-Hinton broth, as recommended by CLSI guidelines [17,18]. While these test methodologies achieve a precise estimate of breakpoints in vitro, the major challenge lies in the ability for clinical translation. Since target site exposure and microbial susceptibility will vary among different individuals with different infection sites and different pathogen populations, susceptibility breakpoints are inherently variable in a clinical population [19]. Thus, in vitro derived breakpoints have to be interpreted as point estimates in a breakpoint distribution with a confidence interval that presents itself in the clinic [25]. M&S approaches using clinical data may contribute to derive and increase the exactness or confidence in the derived breakpoints.
Multiple studies have indicated inconsistencies between the probability of PD target attainment (PTA) and susceptibility as per CLSI defined breakpoints. DeRyke et al. demonstrated that CLSI susceptibility breakpoints (%SCLSI) overestimated probability for achieving bactericidal exposure for antibiotics such as ciprofloxacin, levofloxacin and piperacillin-tazobactam against gram negative bacteria such as P.aeruginosa, A. baumannii, E. coli and Klebsiella spp [25]. To overcome this limitation, the authors used Monte-Carlo simulations based on published PK data and their between-subject variability to define PD derived breakpoints (%SPD). This %SPD accounts for between-subject variability in human pharmacokinetics as well as exposure differences resulting from different dosing regimens. In this approach, %SPD was defined as the highest MIC at which at least 90% of the population achieve the targeted bactericidal exposure, and bactericidal exposure was defined as fT>MIC (time of free concentration above MIC) of 40% for carbapenems, 50% for cephalosporins and the piperacillin component of the piperacillin-tazobactam combination, and 125 for the AUC/MIC ratio of fluoroquinolones. The analysis indicated that although there was a general lack of agreement between PTA and %SPD or %SCLSI, only %SCLSI overestimated the ability of the investigated dosing regimens to achieve bactericidal exposures. In contrast, %SPD often underestimated PTA, a deviation clinically far less concerning than overestimation. The underestimation of PTA using %SPD leads to an overestimation of dose in a particular subject (which is preferable), but the general overestimation of doses for a population can lead to an increase in bacterial resistance. Based on the performed simulations, the authors suggest pathogen AND dosing regimen specific breakpoints as a more appropriate way to classify bacteria as susceptible [25].
Development of Optimal Dosing Regimens
One of the top priorities in antibiotic pharmacotherapy is to optimize the duration of therapy with proper dosing regimens that are effective and safe, and minimize the occurrence of bacterial resistance. However, these goals are often requiring opposing measures and can often not be achieved simultaneously. The concept of clinical utility can help in optimizing these goals. Clinical utility allows to quantify and optimize “trade-offs” between multiple factors such as efficacy, safety, microbiology, and usage of drugs [26]. Pharmacometrics allows the integration of clinical utility with M&S, where “trade-offs” can be derived and explored mathematically.
The clinical efficacy (i.e. bacterial eradication) of drugs such as garenoxacin, telithromycin and doripenem is more than 90%. With such high efficacy it is challenging to design dosing regimens on the basis of efficacy and safety at varying doses. Population based pharmacokinetic M&S using Monte Carlo simulations helped in deciding on a dosage regimen by taking into account the between-subject variability in PK parameters and varying MIC distributions by various clinical isolates. This approach helped in determining the optimal dose for garenoxacin (400 mg) on the basis of (i) selecting AUC0-24/MIC as an appropriate PK/PD index derived from in vivo efficacy data, (ii) performing Monte Carlo Simulations using population PK parameters, MIC distributions and mutant prevention concentration (MPC), (iii) determining a recommended dose based on probability of target attainment, and (iv) validation of the recommended dose based on PK/PD data acquired from phase III studies for respiratory tract infection [27].
Design of dosing regimens is especially challenging for patients who are critically ill with severe bacterial infections, and who have renal failure and are on continuous venovenous hemodiafiltration (CVVHDF). In the event of renal failure, dosing regimen needs of individual patients can be adjusted by assessing the impact of kidney dysfunction on the disposition of drugs [28]. For example the PK and PD properties of biapenem change when a patient is on CVVHDF. Ikawa et al. performed PK M&S to determine PD exposure of T>MIC4μg/mL and suggested that for T>MIC4μg/mL for 30% of the dosing interval biapenem dosage should be 300 mg BID or 600 mg BID for dialysate flow rates of 1.4 L/h or 2.8 – 5.6 L/h, respectively [29]. As the dialysate flow rate increased, T>MIC4μg/mL decreased. The 600 mg BID dose was the optimum to reach T>MIC4μg/mL of 30% for both dialysate flow rates of 2.8 L/h and 5.6 L/h. These simulations illustrate that low doses or increased dosing intervals should not be recommended for patients who are on CVVHDF or a similar renal replacement technique.
Since critically ill patients often have altered drug distribution and elimination, they may experience inadequate dosing with antibiotics and may thus be at a much higher risk of infections with antibiotic resistant organisms [30]. For doripenem, a carbapenem, T>MIC for 35% of the dosing interval has been the recommendation in patients with normal kidney function [31]. Since doripenem is primarily excreted in unchanged form into the urine, PK-based Monte Carlo simulations were performed to develop dosing recommendations for patients with renal impairment. For mild-stage 2 chronic kidney disease (CKD) patients with a creatinine clearance (CLCr) >60 mL/min, 500 mg should be infused over 1 h every 8 h for bacteria with MIC ≤1 μg/mL; however, in the more severe stage 3 CKD patients with CLCr ~30–50 mL/min, an infusion of 250 mg of doripenem over 1 h every 8h for bacteria with MIC ≤2 μg/mL is recommended. For patients with severe renal impairment with a CLCr of 10–29 mL/min, 250 mg was recommended to be infused over longer durations, 4h instead of 1h, every 12 h for MIC ≤2 μg/mL (Figure 3). This is consistent with the time-dependent (t>MIC) killing pattern of carbapenems. Dosing strategies like this can only be achieved from PK/PD M&S where the creatinine clearance builds the parameter framework for achieving set exposure levels, as PK studies in critically ill patients would be extremely difficult to perform [31].
In order to be efficacious, an antibiotic needs to reach the site of infection in therapeutically effective concentrations. In patients with severe lung infections, the concentration of antibiotics in ELF is an essential measure to decide on an optimal dosage regimen [32]. It has been reported recently that methicillin-resistant S. aureus (MRSA) is the likely culprit in causing more than 25% of all ventilator-associated pneumonias in intensive care unit patients [33]. A population PK model with Monte Carlo simulations was employed to determine the range of ELF exposure relative to that in plasma. It was determined that vancomycin penetrates ELF at approximately 50% of plasma concentrations [34]. Under the assumption that the PK/PD in ELF is similar to the AUC/MIC of ≥400 used in plasma, the M&S exercise showed that target attainment is expected to be suboptimal for MRSA with MICs in excess of 1 mg/L under current dosing regimens [34]. A similar approach was used for PD profiling of cefditoren and telavancin against penicillin-susceptible and penicillin-intermediate S. pneumonia [35] and methicillin-resistant S. aureus, respectively [36].
The MIC value for an antibiotic varies depending on which organism it acts upon. Carbapenems require higher MIC to be effective against P. aeruginosa as compared to other bacteria. The antibacterial efficacy of four commonly used dosage regimen for doripenem was determined using an integrated PK/PD M&S approach based on in vitro time-kill data and human PK data. The simulations indicated time-dependent antimicrobial activity of doripenem, in which the 250 mg TID regimen had a higher bactericidal effect than did 500 mg BID [37].
While dosage regimens for most antibiotics can be derived by PK/PD-based M&S as outlined in the presented examples, the therapeutic concentration obtained from those recommendations may be insufficient to safeguard optimum clinical efficacy and bacterial clearance from the site of infection, especially if they are based on studies in healthy individuals rather than patients. Hence, it is of utmost importance to take into account the disposition (PK) and target site accessibility of antibiotics in diseased individuals having abnormal physiological conditions such as renal dysfunction. Along these lines, it is often preferred to use in these PK/PD M&S evaluations drug resistant bacteria that constitute a worst case scenario. An example for such an approach is the dose optimization of norvancomycin against methicillin-resistant rather than non-resistant Staphylococcus isolates for patients with different physiological and pathological conditions in the Chinese population. The optimized norvancomycin dosing recommendations were 800–1000 mg BID for patients with normal renal function, 800 mg QD or BID for patients with mild renal dysfunction (CLCr >50 to ≤ 85 mL/min), 800 mg QD to 2.5 days for patients with modest renal dysfunction (CLCr >10 to ≤ 50 mL/min), and 600 mg over 5–11 days or 800 mg over 6–13 days for patients with severe renal dysfunction (CLCr >5 to ≤ 10 mL/min) [38].
Optimization of Clinical Studies and Clinical Applications
Over the last decade, it has become obvious in many examples that fine tuning of phase II/III clinical trials as well as post-marketing studies a priori is essential to reduce cost and optimize study performance and outcomes. PK/PD based M&S has played a major role in this process by integrating observations and data obtained from preclinical studies in animals and phase I studies in healthy volunteers as well as phase IIa studies in patients. The factors taken into consideration for mathematical modeling are often (a) MIC distribution patterns in clinical isolates, (b) the range of individual PK parameters in the target patient population, (c) identified PD targets from efficacy studies in animals, and (d) the degree of protein binding. The combination of microbiological, preclinical and clinical PK and PD data in mathematical models is essential in designing target antibiotic exposures. M&S with such data is not only beneficial for single drug therapy but also for the evaluation of multiple concurrently used antibiotics and their potential additive or synergistic effects [20].
There is a sustained need for monitoring the PK/PD of antibiotics even after their approval and marketing. Rifampin is widely used for treatment of tuberculosis since several decades, but there is only limited data on rifampin concentrations in the lungs. Using an M&S based simulation study, Goutelle et al. found that the existing dosage regimen (600 mg/day) for rifampin is suboptimal in most patients [39]. This could be a potential contributing factor for the increasing number of tuberculosis infections with multi drug-resistant and extremely drug-resistant strains. The authors suggest considering higher doses (1200 mg/day) of rifampin for an increase in pulmonary concentrations, thereby keeping in mind the toxicity and tolerability of such high doses in patients [39].
M&S may also be useful in developing strategies to minimize the effect of drug-drug interactions. Voriconazole and fluconazole are two widely used antifungal drugs with similar mechanism of action, but a strong drug-drug interaction when immediately switching between the two agents. Fluconazole is the most commonly used antifungal agent but unlike voriconazole it does not have anti-aspergillosis coverage sometimes necessitating a clinical switch. Fluconazole is a CYP2C19 inhibitor and its presence inhibits voriconazole metabolism causing substantial voriconazole-related adverse effects. Physiologically based PK modeling was used to determine the time lag required between the two therapies to avoid potential toxicity, but also minimize the time period without effective antifungal exposure which is essential in the event of serious fungal infections. The PK simulations demonstrated that a time lag of at least 24 hours is required to switch from fluconazole to voriconazole in order to avoid relevant interactions [40].
The use of M&S is not only restricted to optimizing dosing regimen and efficacy of treatment, but can also be used for building up clinical treatment guidelines. Goldie et al. constructed a population model of HIV infection to answer lifetime cost, life expectancy, quality-adjusted life expectancy, and quality-adjusted life years in patients receiving highly active antiretroviral therapy (HAART) and Pneumocystis carinii pneumonia (PCP) prophylaxis [41]. The simulation exercises were performed to compare different criteria of CD4 cell counts for discontinuing primary PCP prophylaxis in patients with CD4 cell count increases receiving HAART and second-line PCP prophylaxis (such as dapsone, atovaquone, and aerosolized pentamidine) due to intolerance to trimethoprim-sulfamethoxazole. The outcome of the exercise showed that treatment is cost-effective when PCP prophylaxis is discontinued with CD4 count >300/μL, and that in patients intolerant to trimethoprim-sulfamethoxazole dapsone therapy is more cost-effective than atovaquone [41].
Expert Commentary and Five-year view
The application of pharmacometric analyses using PK/PD based M&S approaches has in the recent decade developed into one of the most essential tools for interpreting and integrating large and diverse pools of preclinical and clinical data and translating them into an informative knowledgebase [5]. It is expedient not only for industry professionals to promote internal decision making at critical drug development steps, but is also increasingly utilized by regulatory authorities for compiling and analyzing data for approval and labeling. A recent survey shows that during the last decade, the drug approval and labeling decisions of more than 60% of submissions for New Drug Applications (NDA) to the US FDA were influenced by pharmacometric analyses. For 30% of the submissions during this period, FDA reviewers performed independent pharmacometric analysis [13]. For example, among anti-infectives, an appropriate dosing regimen of micafungin for the treatment of esophageal candidiasis was selected based on an exposure-response analysis by FDA personnel. The FDA reviewers utilized the phase II and III study data to model the relationship between dose and effectiveness, which led them to recommend approval of the 150 mg over the 100 mg micafungin dose due to similar maximum efficacy, but a 15% lower rate of relapse [9].
Another prime example for use of M&S in regulatory decision making is the pharmacometrics-based dose selection of levofloxacin for postexposure treatment of inhalational anthrax in children. The available data from two PK studies on 47 adults at different doses (500 mg/kg and 750 mg/kg) and 90 pediatric patients who received a dose of 7 mg/kg were used to develop an integrated population PK model. The model was then utilized to derive dosing recommendations for pediatric patients that match adult exposure after a 500 mg/kg dose with regard to AUC, and maximum and minimum concentration during multiple dosing at steady state. Based solely on this M&S approach, a dose regimen of 8 mg/kg BID for children <50 kg and ≥ 6 months of age and 500 mg QD for children weighing ≥50 kg was recommended and included in the labeling information [42].
The US FDA and the European Medicines Agency (EMA) both acknowledge the impact of M&S on regulatory approvals, but assess the investigational plans and scientific information differently. FDA seems to prefer to conduct pharmacometric reviews including independent data analyses for all the submitted applications irrespective of whether the sponsor submitted an analysis or not. In contrast, EMA seems to consider a pharmacometric assessment only if it is included in the submission by the sponsor. EMA might ask for additional pharmacometric analyses, but these should be conducted by the sponsor. European regulatory bodies are especially encouraging and emphasizing the use of M&S for drugs in pediatrics and for anti-infectives [43–45]. Irrespective of these different philosophies in the review process, the emphasis and demand of M&S based pharmacometric analyses in drug approval will likely further increase the application of M&S throughout the drug development process [8,46,47].
More recently, physiologically based PK and PK/PD modeling (PBPK) is gaining more widespread application, predominantly because of improvements in the associated M&S methodologies and the availability of more robust and user-friendly software tools [48–50]. It is predicted that the use of PBPK will increase over the next years for identifying the effects of intrinsic and extrinsic factors modifying drug efficacy and safety in patients. PBPK will play a significant role in predicting and deciphering drug-drug interactions and will be the basis for M&S in special population such as pediatric patients or in critically-ill patients [50].
Evidently, pharmacometric analyses have proven their merits in myriad ways during the entire process of drug development. However, there is always room for improvements for more accurate and precise estimates resulting in more confident predictions. This fine-tuning can likely be accomplished by a more prospective integration of M&S approaches in each individual development phase, including goal-oriented and intelligent sample and data collection that specifically addresses and supports pharmacometric analyses. Some of the questions that need to be addressed relate to the transfer of knowledge from controlled studies to clinical practice: PK parameter estimates are generally derived from healthy subjects. How can we assure that PK/PD predictions are accurate in patients with variable physiologic function and variable sites and types of infection? How can we account for patients with multiple disease conditions? How will their confounding illnesses or polypharmacy affect drug exposure for the considered anti-infective? How will variability in the strains of microorganisms and their response to the survival stress elicited by the anti-infective such as latency and resistance development affect treatment outcome? A more systematic and intensive assessment of antibiotic concentrations at the site of action such as ELF over the time course of therapy might help to provide some of the needed answers. Techniques such as microdialysis have been proven of substantial value in assessing active drug concentrations in target tissues not as easily accessible as ELF [51,52].
In summary, more complex M&S models will likely be established in the future that integrate all therapeutic considerations when treating microbial infections. Using precise estimates of PK and PD parameters as well as their distributions in populations of patients and microorganisms, including their potential interdependencies, will help in facilitating more realistic and thus also even more clinically meaningful predictions for the in silico evaluation and development of optimal antibiotic dosing regimens, including combination therapies. To combat the growing bacterial resistance, we need to rapidly increase the available number of effective antibiotics in our armamentarium by relatively fast, safe, smart and inexpensive clinical development programs. We believe that M&S based pharmacometric approaches hold great promise in facilitating this process and thus may serve as a key technology for the development of future generations of anti-infectives.
Key Issues.
With respect to anti-infective drug development and applied pharmacotherapy, M&S has been shown as beneficial at multiple levels including the identification of PK/PD indices and susceptibility breakpoints, selection of dosage regimen, resistance development suppression and optimization of clinical trial designs.
The identification of covariates altering PK/PD parameters helps in fine tuning the design of clinical trials in healthy volunteers, patients with physiological impairments, and special populations.
The scope of M&S is not only restricted to clinical outcomes, but can also be implemented in setting up clinical guidelines and developing health care policies.
The regulatory bodies (FDA and EMA) encourage the use of pharmacometric approaches especially for anti-infectives to promote internal decision making, to optimize trial designs and to integrate and analyze data for drug approval and labeling.
Acknowledgments
This work was in part supported by research grants R01AI090810 and R01AI062415 by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Zhang L, Pfister M, Meibohm B. Concepts and challenges in quantitative pharmacology and model-based drug development. The AAPS journal. 2008;10(4):552–559. doi: 10.1208/s12248-008-9062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lalonde RL, Kowalski KG, Hutmacher MM, et al. Model-based drug development. Clinical pharmacology and therapeutics. 2007;82(1):21–32. doi: 10.1038/sj.clpt.6100235. [DOI] [PubMed] [Google Scholar]
- 3.Sotto A, Lavigne JP. A mathematical model to guide antibiotic treatment strategies. BMC medicine. 2012;10:90. doi: 10.1186/1741-7015-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joyner MLP, Manning CC, Canter BN. Modeling the effects of introducing a new antibiotic in a hospital setting: A case study. Mathematical biosciences and engineering : MBE. 2012;9(3):601–625. doi: 10.3934/mbe.2012.9.601. [DOI] [PubMed] [Google Scholar]
- 5.Suryawanshi S, Zhang L, Pfister M, Meibohm B. The current role of model-based drug development. Expert opinion on drug discovery. 2010;5(4):311–321. doi: 10.1517/17460441003713470. [DOI] [PubMed] [Google Scholar]
- 6.Meibohm B, Derendorf H. Pharmacokinetic/pharmacodynamic studies in drug product development. Journal of pharmaceutical sciences. 2002;91(1):18–31. doi: 10.1002/jps.1167. [DOI] [PubMed] [Google Scholar]
- 7.Romero K, Corrigan B, Tornoe CW, et al. Pharmacometrics as a discipline is entering the “industrialization” phase: standards, automation, knowledge sharing, and training are critical for future success. Journal of clinical pharmacology. 2010;50(9 Suppl):9S–19S. doi: 10.1177/0091270010377788. [DOI] [PubMed] [Google Scholar]
- 8.Manolis E, Herold R. Pharmacometrics for regulatory decision making: status and perspective. Clin Pharmacokinet. 2011;50(10):625–626. doi: 10.2165/11594340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Bhattaram VA, Bonapace C, Chilukuri DM, et al. Impact of pharmacometric reviews on new drug approval and labeling decisions--a survey of 31 new drug applications submitted between 2005 and 2006. Clinical pharmacology and therapeutics. 2007;81(2):213–221. doi: 10.1038/sj.clpt.6100051. [DOI] [PubMed] [Google Scholar]
- 10.Vaddady PK, Lee RE, Meibohm B. In vitro pharmacokinetic/pharmacodynamic models in anti-infective drug development: focus on TB. Future Med Chem. 2010;2(8):1355–1369. doi: 10.4155/fmc.10.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasipanodya J, Gumbo T. An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob Agents Chemother. 2011;55(1):24–34. doi: 10.1128/AAC.00749-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preston SL, Drusano GL, Berman AL, et al. Levofloxacin population pharmacokinetics and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection. Antimicrob Agents Chemother. 1998;42(5):1098–1104. doi: 10.1128/aac.42.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JY, Garnett CE, Gobburu JV, et al. Impact of pharmacometric analyses on new drug approval and labelling decisions: a review of 198 submissions between 2000 and 2008. Clin Pharmacokinet. 2011;50(10):627–635. doi: 10.2165/11593210-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Czock D, Keller F. Mechanism-based pharmacokinetic-pharmacodynamic modeling of antimicrobial drug effects. Journal of pharmacokinetics and pharmacodynamics. 2007;34(6):727–751. doi: 10.1007/s10928-007-9069-x. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt S, Barbour A, Sahre M, Rand KH, Derendorf H. PK/PD: new insights for antibacterial and antiviral applications. Current opinion in pharmacology. 2008;8(5):549–556. doi: 10.1016/j.coph.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Budha NR, Lee RB, Hurdle JG, Lee RE, Meibohm B. A simple in vitro PK/PD model system to determine time-kill curves of drugs against Mycobacteria. Tuberculosis (Edinb) 2009;89(5):378–385. doi: 10.1016/j.tube.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 18.Turnidge J, Paterson DL. Setting and revising antibacterial susceptibility breakpoints. Clinical microbiology reviews. 2007;20(3):391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stass H, Dalhoff A. The integrated use of pharmacokinetic and pharmacodynamic models for the definition of breakpoints. Infection. 2005;33 (Suppl 2):29–35. doi: 10.1007/s15010-005-8205-z. [DOI] [PubMed] [Google Scholar]
- 20.Drusano GL, Preston SL, Hardalo C, et al. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob Agents Chemother. 2001;45(1):13–22. doi: 10.1128/AAC.45.1.13-22.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeitlinger MA, Derendorf H, Mouton JW, et al. Protein binding: do we ever learn? Antimicrobial agents and chemotherapy. 2011;55(7):3067–3074. doi: 10.1128/AAC.01433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbour A, Scaglione F, Derendorf H. Class-dependent relevance of tissue distribution in the interpretation of anti-infective pharmacokinetic/pharmacodynamic indices. International journal of antimicrobial agents. 2010;35(5):431–438. doi: 10.1016/j.ijantimicag.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Noreddin AM, El-Khatib WF, Aolie J, Salem AH, Zhanel GG. Pharmacodynamic target attainment potential of azithromycin, clarithromycin, and telithromycin in serum and epithelial lining fluid of community-acquired pneumonia patients with penicillin-susceptible, intermediate, and resistant Streptococcus pneumoniae. Int J Infect Dis. 2009;13(4):483–487. doi: 10.1016/j.ijid.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Gumbo T, Louie A, Liu W, et al. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob Agents Chemother. 2007;51(7):2329–2336. doi: 10.1128/AAC.00185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deryke CA, Kuti JL, Nicolau DP. Reevaluation of current susceptibility breakpoints for Gram-negative rods based on pharmacodynamic assessment. Diagn Microbiol Infect Dis. 2007;58(3):337–344. doi: 10.1016/j.diagmicrobio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Korsan B. Transparent Trade-offs: A clinical utility index (CUI) openly evaluates a product’s attributes—and chance of success. Pharmaceutical Executive. 2005 [Google Scholar]
- 27.Tanigawara Y, Nozawa K, Tsuda H. Optimal dose finding of garenoxacin based on population pharmacokinetics/pharmacodynamics and Monte Carlo simulation. Eur J Clin Pharmacol. 2012;68(1):39–53. doi: 10.1007/s00228-011-1095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matzke GR, Aronoff GR, Atkinson AJ, Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease-a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80(11):1122–1137. doi: 10.1038/ki.2011.322. [DOI] [PubMed] [Google Scholar]
- 29.Ikawa K, Morikawa N, Ikeda K, Suyama H. Pharmacokinetic modeling and dosage adaptation of biapenem in Japanese patients during continuous venovenous hemodiafiltration. J Infect Chemother. 2008;14(1):35–39. doi: 10.1007/s10156-007-0572-1. [DOI] [PubMed] [Google Scholar]
- 30.Drusano GL. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug’. Nature reviews Microbiology. 2004;2(4):289–300. doi: 10.1038/nrmicro862. [DOI] [PubMed] [Google Scholar]
- 31.Samtani MN, Flamm R, Kaniga K, Nandy P. Pharmacokinetic-pharmacodynamic-model-guided doripenem dosing in critically ill patients. Antimicrob Agents Chemother. 2010;54(6):2360–2364. doi: 10.1128/AAC.01843-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drusano GL, Preston SL, Gotfried MH, Danziger LH, Rodvold KA. Levofloxacin penetration into epithelial lining fluid as determined by population pharmacokinetic modeling and monte carlo simulation. Antimicrob Agents Chemother. 2002;46(2):586–589. doi: 10.1128/AAC.46.2.586-589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Thoracic S, Infectious Diseases Society Of A. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. American journal of respiratory and critical care medicine. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 34.Lodise TP, Drusano GL, Butterfield JM, Scoville J, Gotfried M, Rodvold KA. Penetration of vancomycin into epithelial lining fluid in healthy volunteers. Antimicrob Agents Chemother. 2011;55(12):5507–5511. doi: 10.1128/AAC.00712-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodise TP, Kinzig-Schippers M, Drusano GL, et al. Use of population pharmacokinetic modeling and Monte Carlo simulation to describe the pharmacodynamic profile of cefditoren in plasma and epithelial lining fluid. Antimicrob Agents Chemother. 2008;52(6):1945–1951. doi: 10.1128/AAC.00736-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lodise TP, Jr, Gotfried M, Barriere S, Drusano GL. Telavancin penetration into human epithelial lining fluid determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob Agents Chemother. 2008;52(7):2300–2304. doi: 10.1128/AAC.01110-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katsube T, Yano Y, Wajima T, Yamano Y, Takano M. Pharmacokinetic/pharmacodynamic modeling and simulation to determine effective dosage regimens for doripenem. J Pharm Sci. 2010;99(5):2483–2491. doi: 10.1002/jps.21997. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Zhang Y, Shi Y, et al. Population pharmacokinetic and pharmacodynamic modeling of norvancomycin. Eur J Clin Microbiol Infect Dis. 2008;27(4):275–284. doi: 10.1007/s10096-007-0435-9. [DOI] [PubMed] [Google Scholar]
- 39.Goutelle S, Bourguignon L, Maire PH, Van Guilder M, Conte JE, Jr, Jelliffe RW. Population modeling and Monte Carlo simulation study of the pharmacokinetics and antituberculosis pharmacodynamics of rifampin in lungs. Antimicrob Agents Chemother. 2009;53(7):2974–2981. doi: 10.1128/AAC.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damle B, Varma MV, Wood N. Pharmacokinetics of voriconazole administered concomitantly with fluconazole and population-based simulation for sequential use. Antimicrob Agents Chemother. 2011;55(11):5172–5177. doi: 10.1128/AAC.00423-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldie SJ, Kaplan JE, Losina E, et al. Prophylaxis for human immunodeficiency virus-related Pneumocystis carinii pneumonia: using simulation modeling to inform clinical guidelines. Arch Intern Med. 2002;162(8):921–928. doi: 10.1001/archinte.162.8.921. [DOI] [PubMed] [Google Scholar]
- 42.Li F, Nandy P, Chien S, Noel GJ, Tornoe CW. Pharmacometrics-based dose selection of levofloxacin as a treatment for postexposure inhalational anthrax in children. Antimicrob Agents Chemother. 2010;54(1):375–379. doi: 10.1128/AAC.00667-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.European Medicines Agency Committee for Proprietary Medicinal Products (Cpmp) Points to consider on pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. Points to consider on pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. 2009 [Google Scholar]
- 44.European Medicines Agency Committee for Proprietary Medicinal Products (Cpmp) Guideline on the clinical evaluation of medicinal products intended for treatment of hepatitis B. Guideline on the clinical evaluation of medicinal products intended for treatment of hepatitis B. 2009 [Google Scholar]
- 45.European Medicines Agency Committee for Proprietary Medicinal Products (Cpmp) Guideline on clinical investigation of medicinal products for the treatment of sepsis. Guideline on clinical investigation of medicinal products for the treatment of sepsis. 2009 [Google Scholar]
- 46.Jonsson S, Henningsson A, Edholm M, Salmonson T. Role of modelling and simulation: a European regulatory perspective. Clin Pharmacokinet. 2012;51(2):69–76. doi: 10.2165/11596650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 47.Parrott N, Davies B, Hoffmann G, et al. Development of a physiologically based model for oseltamivir and simulation of pharmacokinetics in neonates and infants. Clin Pharmacokinet. 2011;50(9):613–623. doi: 10.2165/11592640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annual review of pharmacology and toxicology. 2011;51:45–73. doi: 10.1146/annurev-pharmtox-010510-100540. [DOI] [PubMed] [Google Scholar]
- 49.Huang SM, Rowland M. The role of physiologically based pharmacokinetic modeling in regulatory review. Clinical pharmacology and therapeutics. 2012;91(3):542–549. doi: 10.1038/clpt.2011.320. [DOI] [PubMed] [Google Scholar]
- 50.Barrett JS, Della Casa Alberighi O, Laer S, Meibohm B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clinical pharmacology and therapeutics. 2012;92(1):40–49. doi: 10.1038/clpt.2012.64. [DOI] [PubMed] [Google Scholar]
- 51.Brunner M, Derendorf H, Muller M. Microdialysis for in vivo pharmacokinetic/pharmacodynamic characterization of anti-infective drugs. Current opinion in pharmacology. 2005;5(5):495–499. doi: 10.1016/j.coph.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Chaurasia CS, Muller M, Bashaw ED, et al. AAPS-FDA Workshop White Paper: microdialysis principles, application, and regulatory perspectives. Journal of clinical pharmacology. 2007;47(5):589–603. doi: 10.1177/0091270006299091. [DOI] [PubMed] [Google Scholar]
- 53.Lodise TP, Jr, Nau R, Kinzig M, Jones RN, Drusano GL, Sorgel F. Comparison of the probability of target attainment between ceftriaxone and cefepime in the cerebrospinal fluid and serum against Streptococcus pneumoniae. Diagn Microbiol Infect Dis. 2007;58(4):445–452. doi: 10.1016/j.diagmicrobio.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 54.Rodvold KA, Nicolau DP, Lodise TP, et al. Identifying exposure targets for treatment of staphylococcal pneumonia with ceftobiprole. Antimicrob Agents Chemother. 2009;53(8):3294–3301. doi: 10.1128/AAC.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lodise TP, Jr, Pypstra R, Kahn JB, et al. Probability of target attainment for ceftobiprole as derived from a population pharmacokinetic analysis of 150 subjects. Antimicrob Agents Chemother. 2007;51(7):2378–2387. doi: 10.1128/AAC.01181-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Wart S, Phillips L, Ludwig EA, et al. Population pharmacokinetics and pharmacodynamics of garenoxacin in patients with community-acquired respiratory tract infections. Antimicrob Agents Chemother. 2004;48(12):4766–4777. doi: 10.1128/AAC.48.12.4766-4777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noreddin AM, Reese AA, Ostroski M, Hoban DJ, Zhanel GG. Comparative pharmacodynamics of garenoxacin, gemifloxacin, and moxifloxacin in community-acquired pneumonia caused by Streptococcus pneumoniae: a Monte Carlo simulation analysis. Clin Ther. 2007;29(12):2685–2689. doi: 10.1016/j.clinthera.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 58.Noreddin AM, Hoban DJ, Zhanel GG. Comparison of gatifloxacin and levofloxacin administered at various dosing regimens to hospitalised patients with community-acquired pneumonia: pharmacodynamic target attainment study using North American surveillance data for Streptococcus pneumoniae. Int J Antimicrob Agents. 2005;26(2):120–125. doi: 10.1016/j.ijantimicag.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 59.Owens RC, Jr, Bhavnani SM, Ambrose PG. Assessment of pharmacokinetic-pharmacodynamic target attainment of gemifloxacin against Streptococcus pneumoniae. Diagn Microbiol Infect Dis. 2005;51(1):45–49. doi: 10.1016/j.diagmicrobio.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 60.Drusano GL, Preston SL, Van Guilder M, et al. A population pharmacokinetic analysis of the penetration of the prostate by levofloxacin. Antimicrob Agents Chemother. 2000;44(8):2046–2051. doi: 10.1128/aac.44.8.2046-2051.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sprandel KA, Drusano GL, Hecht DW, Rotschafer JC, Danziger LH, Rodvold KA. Population pharmacokinetic modeling and Monte Carlo simulation of varying doses of intravenous metronidazole. Diagn Microbiol Infect Dis. 2006;55(4):303–309. doi: 10.1016/j.diagmicrobio.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 62.Simon N, Sampol E, Albanese J, et al. Population pharmacokinetics of moxifloxacin in plasma and bronchial secretions in patients with severe bronchopneumonia. Clinical pharmacology and therapeutics. 2003;74(4):353–363. doi: 10.1016/S0009-9236(03)00201-7. [DOI] [PubMed] [Google Scholar]
- 63.Acosta EP, Jester P, Gal P, et al. Oseltamivir dosing for influenza infection in premature neonates. J Infect Dis. 2010;202(4):563–566. doi: 10.1086/654930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Occhipinti DJ, Pendland SL, Schoonover LL, Rypins EB, Danziger LH, Rodvold KA. Pharmacokinetics and pharmacodynamics of two multiple-dose piperacillin-tazobactam regimens. Antimicrob Agents Chemother. 1997;41(11):2511–2517. doi: 10.1128/aac.41.11.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frei CR, Burgess DS. Pharmacokinetic/pharmacodynamic modeling to predict in vivo effectiveness of various dosing regimens of piperacillin/tazobactam and piperacillin monotherapy against gram-negative pulmonary isolates from patients managed in intensive care units in 2002. Clin Ther. 2008;30(12):2335–2341. doi: 10.1016/j.clinthera.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 66.Nandy P, Samtani MN, Lin R. Population pharmacokinetics of doripenem based on data from phase 1 studies with healthy volunteers and phase 2 and 3 studies with critically ill patients. Antimicrob Agents Chemother. 2010;54(6):2354–2359. doi: 10.1128/AAC.01649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]