Abstract
A significant proportion of patients with burn injury have diabetes. Although hyperglycemia during critical illness has been associated with poor outcomes, patients with chronic hyperglycemia based on elevated hemoglobin A1c (HbA1c) measurements at admission have been shown to tolerate higher glucose levels during hospitalization. This relationship has not been evaluated in the burn population. The objective of this study was to examine the impact of chronic glucose control on outcomes in the acute period after burn. This is a retrospective analysis comparing outcomes in patients with chronic hyperglycemia (HbA1c ≥6.5%) and euglycemia (HbA1c <6.5%). Patients aged 18 to 89 years, admitted for initial burn care between January 1, 2009, and June 30, 2010, with an HbA1c measurement at admission were included. The primary endpoint was unplanned readmissions, with secondary endpoints of length of stay and mortality. We included 258 patients (32 with chronic hyperglycemia and 226 with euglycemia). Burn severity was similar between the groups. Patients with chronic hyperglycemia were significantly older and were more likely to have diabetes, respiratory disease, and hypertension. Chronic hyperglycemia was associated with significantly higher time-weighted glucose and glucose variability. Survival rates were similar, but the chronic hyperglycemia group had a significantly longer length of stay (13 vs 9 days; P = .038) and a higher rate of unplanned readmission (18.8 vs 3.6%; P = .001). Chronic hyperglycemia before burn injury is associated with altered glycemic response after burn injury and worse outcomes. Further research is needed to identify whether chronic hyperglycemia necessitates a modified approach to burn care or glycemic management.
Diabetes is present in 10% of all burn patients and 32% of all burn patients older than age 55.1 In the general population of patients 65 years or older, 18% have a diagnosis of diabetes, whereas about 85% of patients with burn injury who are older than 65 years have diabetes.2 Several complications associated with diabetes put this population at higher risk for incurring burn injuries, including peripheral neuropathy, retinopathy, and gait instability. Diabetic patients with burn injuries are more likely to incur injury from scalding or contact and have significantly delayed presentation compared with nondiabetics.1,2 In addition to their increased risk for suffering burn injuries, diabetes-associated comorbidities including atherosclerosis, peripheral neuropathy, and impaired wound healing also predispose these patients to complications such as increased requirements for burn-related surgeries, higher incidence of infection, and longer durations of hospital stay.1,2 A recent preliminary report of a prospective study by Schwartz et al3 confirmed that diabetic patients with burn injury have significant delays in burn wound healing, which may further complicate the care of the diabetic burn patient. These findings in burn-injured patients highlight the need for identification of best practices for management of diabetic patients in the postburn setting. Glycemic control with a goal glucose range of 100 to 140 mg/dl in the acute period after burn injury has been associated with improved outcomes including fewer infection-related complications.4 These interventions have not been assessed specifically in the diabetic burn population. The interaction among chronic hyperglycemia, acute glycemic control during hospitalization, and outcomes has been evaluated only in a few studies of the general critical care population, and there is currently no data on the burn population. Chronic elevations in glycemic control as defined by elevated hemoglobin A1c (HbA1c) at admission correlates with increased episodes of hyperglycemia during a stay in the intensive care unit (ICU). Critically injured patients with preexisting diabetes have an altered response to hyperglycemia in the acute setting, with a significantly lower risk of mortality at all levels of hyperglycemia during hospitalization when compared with patients without diabetes.5,6 Furthermore, the risk of mortality in diabetic critically injured patients with chronic hyperglycemia as indicated by higher HbA1c measurements has been shown to be significantly lower with hyperglycemia than with normoglycemia during hospitalization. These findings suggest that the current standards for tight glycemic control may not be as beneficial in patients with chronic hyperglycemia (ie, uncontrolled/ undiagnosed diabetes).7 In burn injury, glucose values during hospitalization are difficult to assess; both diabetic and nondiabetic patients frequently experience insulin resistance and hyperglycemia as a result of the physiologic stress response associated with burn injury. Severe stress related to burn injury is associated with a hypermetabolic response characterized by increases in catecholamine release, growth hormones, cortisol, and proinflammatory cytokines.8 This proinflammatory and hypermetabolic state results in stimulation of gluconeogenesis, glycogenolysis, inhibition of insulin release, and insulin resistance.9,10 Although present in all critically ill patients, the severity, duration, and magnitude of this response are increased in burn-injured patients and can persist for several years.10 On the basis of the current understanding of the detrimental effects of diabetes and hyperglycemia in burn injury and the challenges in glucose management after burn injury, there is a critical need to further describe the relationships between chronic glycemic control before injury, acute glycemic control after burn injury, and burn-related outcomes. Therefore, the objective of this study was to evaluate the impact of chronic hyperglycemia in burn-injured, hospitalized patients on outcomes and glycemic parameters in the acute period after burn.
METHODS
Study Location and Patients
The study was conducted at a single American Burn Association–verified burn center at the Ohio State University Medical Center and was approved by the institutional review board. Patients aged 18 to 89 years admitted for initial management of burn injury between January 1, 2009, and June 30, 2010, with an HbA1c measurement at admission were included. Prisoners, pregnant patients, and patients who were readmissions and nonburn-related admissions (ie, chronic wound, Steven-Johnsons/toxic epidermal necrolysis, necrotizing fasciitis, etc.) were excluded.
Study Design
A retrospective cohort analysis, designed to compare burn patients with chronic hyperglycemia and euglycemia as indicated by HbA1c measurement at admission regardless of known diabetic status, was performed. The primary endpoint was readmission with secondary endpoints of mortality, admission to the ICU, infection, and hospital length of stay. Glycemic parameters evaluated included time-weighted glucose (GluTW), hypoglycemia, and glycemic variability.
Data Collection
Patients in the institutional burn database were identified electronically by admission to the burn service. Data were collected retrospectively from patients’ medical records at the Ohio State University Medical Center by the investigators. Pertinent demographic, laboratory, and clinical data collected included age, sex, race, preexisting diagnosis of diabetes per medical history, burn severity including TBSA burned and depth of burn, HbA1c level at admission, and all glucose measurements during hospitalization. In addition, clinical outcomes including hospital length of stay, requirement for admission to the ICU, survival, and burn-related procedures requirements were collected.
Definitions
According to standard institutional practice, burn patients have initial admission glucose measurement followed by glucose monitoring every 6 hours using point-of-care devices. All patients are started on a sliding scale of insulin for management of hyperglycemia. Subcutaneous insulin therapy is given for blood glucose values greater than 120 mg/dl. A continuous infusion of insulin titrated per protocol may be initiated if a patient has two consecutive blood glucose values greater than 200 mg/dl or at the discretion of the physician for continued hyperglycemia. Titration of the insulin infusion is based on current and previous blood glucose values to maintain the blood glucose between the target range of 110 and 150 mg/dl.
Chronic hyperglycemia was defined as an HbA1c level >6.5% at admission, based on the American Diabetes Association criteria for the diagnosis of diabetes.11 The HbA1c test measures the average glucose values for the preceding 2 to 3 months and, therefore, can be used to evaluate the severity of chronic hyperglycemia.12 In addition, the HbA1c test has been validated as a diagnostic test for diabetes and has been accepted by the American Diabetes Association as a standard method of diagnosis.11 The cutoff of 6.5% was chosen over the treatment goal for diabetics of 7% because of the assumption that our population included patients who had undiagnosed diabetes. By choosing a cutoff of 6.5% to represent chronic hyperglycemia, those patients who were undiagnosed before their admission for burn injury would be included in the chronic hyperglycemia group.
Overall, glycemic control during hospitalization in the acute period after burn were assessed on the basis of GluTW measurements based on work of Finney et al.13 Acute hyperglycemia was defined as a GluTW greater than 150 mg/dl. Glycemic variability was defined by the standard deviation around the mean blood glucose value, as described by Krinsley.14 Hypoglycemia was defined as any blood glucose measurement less than 70 mg/dl, whereas severe hypoglycemia included only episodes less than 40 mg/dl.
Burn-related procedures included debridement and grafting based on the surgical diagnosis-related group codes and Current Procedureal Terminology codes from the American Burn Association Coding and Reimbursement Primer15
Infection-related complications included diagnosis of pneumonia, urinary tract infection, and blood-stream infections (both primary and secondary) and were defined based on the criteria of the Centers for Disease Control and Prevention.16
Unplanned readmissions were defined by the Medicare definition for related and unplanned hospitalizations in which a patient is readmitted to address issues or complications related to their original burn injury (ie, readmission for burn wound infection or graft failure associated with the burn injury). Only patients with survival as their initial discharge disposition were eligible for evaluation for an unplanned readmission.
Statistical Methods
Patients with chronic hyperglycemia were compared with those with euglycemia. Normally distributed continuous data are reported as the mean + standard deviation and analyzed using the Student’s t-test. Nonnormally distributed continuous data are reported as a median with interquartile range and were analyzed with the Mann–Whitney U test. Categorical data are expressed as frequency distributions, and the χ2 or Fisher’s exact test was used to determine whether differences existed between groups. All tests were two-tailed, and P < .05 was determined to represent statistical significance. Analyses were performed using SPSS version 19.0 for Windows (SPSS, Inc., Chicago, IL).
RESULTS
Patients
Two hundred fifty-eight patients were included for evaluation; 32 had chronic hyperglycemia and 226 had euglycemia. Burn characteristics were similar between the two groups, with a median TBSA of 3%, and the majority of patients experienced partial-thickness burn injury (Table 1). The primary cause of burn injury was thermal, followed by scald, for both groups. Patients with chronic hyperglycemia were significantly older (54.7 + 14.4 vs 44.3 + 16.1 years; P = .001) and were more likely to have diabetes, hypertension, and chronic respiratory disease.
Table 1.
Baseline characteristics of burn patients with chronic hyperglycemia and euglycemia
| Chronic Hyperglycemia (n = 32) |
Euglycemia (n = 226) |
P | |
|---|---|---|---|
| Age, years (mean ± SD) | 54.7 ± 14.4 | 44.3 ± 16.1 | .001 |
| Male sex, n (%) | 26 (81.3) | 173 (76.5) | .553 |
| Race, n (%) | .874 | ||
| Caucasian | 28 (87.5) | 194 (85.8) | |
| African American | 3 (9.4) | 27 (11.9) | |
| Other | 1 (3.1) | 5 (2.2) | |
| Diabetes mellitus, n (%) | 29 (90.6) | 17 (7.5) | <.001 |
| Hypertension, n (%) | 19 (59.4) | 59 (26.1) | <.001 |
| Respiratory disease, n (%) | 10 (31.3) | 37 (16.4) | .041 |
| Current smoker, n (%) | 14 (43.8) | 115 (50.9) | .450 |
| Laboratory values at admission | |||
| HbA1c, % (mean ± SD) | 8.0 ± 1.5 | 5.4 ± 0.4 | <.001 |
| Glucose >150 mg/dl, n (%) | 19 (59.4) | 27 (11.9) | <.001 |
| Glucose, mg/dl | 162 (129–201.75) | 106 (95–126) | <.001 |
| BUN, mg/dL | 14.5 (11–18.5) | 11 (8–14) | .002 |
| Serum creatinine, mg/dL | 0.97 (0.74–1.14) | 0.82 (0.69–0.98) | .015 |
| AST, mg/dl | 25.5 (18–36) | 25 (19–34) | .603 |
| ALT, mg/dl | 28 (17–36) | 22.5 (16–33) | .443 |
| Burn characteristics | |||
| TBSA (%) | 3 (1–7) | 3 (1.5–7) | .441 |
| Partial thickness, n (%) | 23 (71.9) | 200 (88.5) | .023 |
| Partial-thickness TBSA | 3 (1–6.5) | 3 (1.5–6.5) | .876 |
| Full thickness, n (%) | 12 (37.5) | 83 (36.7) | .932 |
| Full-thickness TBSA | 1.75 (0.75–5) | 2.5 (0.63–7) | .996 |
| Burn origin, n (%) | .546 | ||
| Thermal | 17 (53.1) | 144 (63.7) | |
| Scald | 9 (28.1) | 9 (4.0) | |
| Electrical | 1 (3.1) | 9 (4.0) | |
| Chemical | 3 (9.4) | 10(4.4) | |
| Other | 2 (6.3) | 5 (2.2) | |
| Inhalation injury, n (%) | 4 (12.5) | 15 (6.6) | .270 |
All data presented as median (interquartile range) unless otherwise specified.
AST, aspartate aminotransferase; BUN, blood urea nitrogen.
Inpatient Glycemic Parameters
Patients with chronic hyperglycemia had a mean HbA1c of 8 + 1.5% compared with 5.4 + 0.4% in the euglycemia group. Patients with chronic hyperglycemia were more likely to have glucose greater than 150 mg/dl at admission (59.4 vs 11.9%; P < .001; Table 1). During admission, the chronic hyperglycemia group had significantly higher glucose levels, as indicated by an increased GLUTW (Table 2). Although these patients had higher glucose levels overall, they also experienced significantly more glucose variability, as well as an increased incidence of both moderate and severe hypoglycemia.
Table 2.
Inpatient glycemic variables
| Chronic Hyperglycemia (n = 32) |
Euglycemia (n = 226) |
P | |
|---|---|---|---|
| GLUTW | 161.5 ± 36.7 | 117.7 ± 25.3 | <.001 |
| GV | 50.3 ± 19.6 | 22.3 ± 18.6 | <.001 |
| Hypoglycemia episodes, n (%) | |||
| Moderate (<70 mg/dl) | 12 (37.5) | 27 (12) | .001 |
| Severe (<40 mg/dl) | 3 (9.4) | 4 (1.8) | .043 |
Data presented as mean ± SD unless otherwise specified.
GLUTW, time-weighted glucose; GV, glucose variability.
Clinical Outcomes
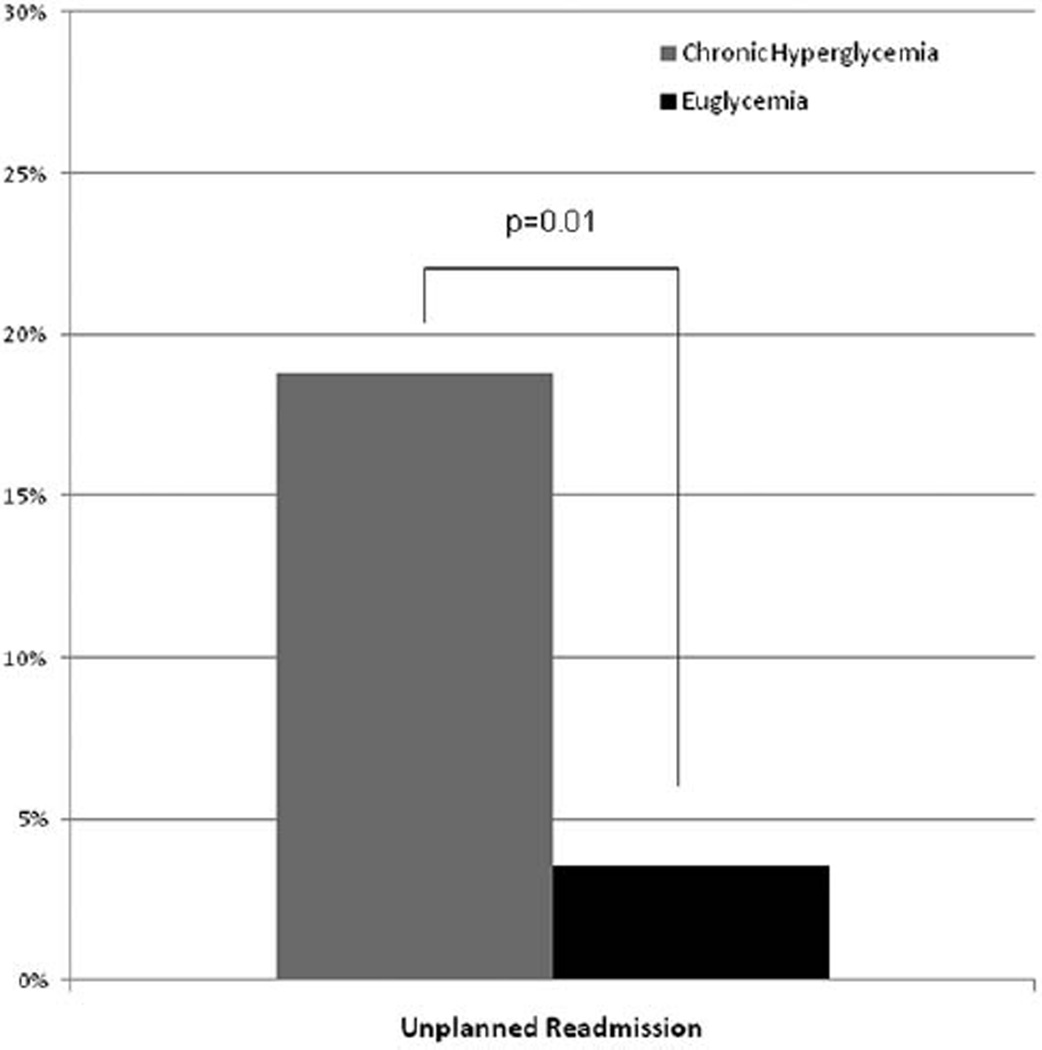
There was a significant difference in the primary endpoint of unplanned readmissions, with an increased rate of unplanned readmissions in patients with chronic hyperglycemia compared with patients with chronic euglycemia (18.8 vs 3.6%; P = .001; Figure 1). Of the 14 unplanned readmissions, eight were for uncontrolled pain and wound care, four for infection/sepsis, and two for other reasons. Overall, patients who required surgical interventions required a similar number of procedures (2.7 vs 2.8;P = .691). Although not statistically significant, there was a slightly higher probability of patients with chronic hyperglycemia requiring admission to the ICU during their initial hospitalization (Table 3). Infection-related complications were similar in both groups, with 15.6 and 12.9% of patients in the chronic hyperglycemia and euglycemia groups, respectively, experiencing any infection during admission (P = .588). Despite similar size and severity of burn injury and total number of procedures required during initial admission, the chronic hyperglycemia group had a significantly longer length of hospital stay (Table 3).
Figure 1.
Unplanned readmission rate for burn patients with chronic hyperglycemia compared with euglycemia.
Table 3.
Clinical outcomes associated with chronic hyperglycemia and euglycemia in burn patients
| Chronic Hyperglycemia (n = 32) |
Euglycemia (n = 226) |
P | |
|---|---|---|---|
| ICU admission, n (%) | 13 (40.6) | 57 (25.3) | .069 |
| Survival, n (%) | 32 (100) | 220 (97.3) | >.99 |
| Hospital length of stay, days (median [IQR]) | 13 (8–38) | 9 (3–33) | .038 |
| Hospital length of stay among survivors, days (median [IQR]) | 7 (2.5–13.5) | 3 (2–9) | .037 |
| Discharge to a facility, n (%) | 8 (25.0) | 32 (14.7) | .137 |
| Burn-related procedures (mean ± SD) | 2.7 ± 1.0 | 2.8 ± 1.3 | .691 |
| Patients with infection(s), n (%) | 5 (15.6) | 29 (12.9) | .588 |
IQR, interquartile range.
DISCUSSION
Although more than 23 million people in the United States have diabetes, the incidence is expected to increase to 29 million by 2050.17,18 This suggests that the number of diabetic burn injuries also will increase substantially over the coming decades, highlighting the importance of research focusing on glycemic control and outcomes in this population. Our study is the first to evaluate the impact of chronic glucose control defined by HbA1c level at admission on burn-related outcomes and glycemic control during hospitalization in the acute period after burn injury. Although previous studies have indicated worse burn-related outcomes in the diabetic population, including delayed wound healing, increased infection-related complications, and longer length of hospital stay, these studies are limited because of the definition of diabetes based on a preexisting diagnosis of diabetes, thereby excluding patients with undiagnosed diabetes.1–3 Our study showed that chronic hyperglycemia was associated with a higher probability of unplanned readmission, which may be a marker for increased complications in this population. In addition, patients with chronic hyperglycemia were more likely to demonstrate altered glycemic response during hospitalization with higher glucose measurement at admission and GluTW, increased glucose variability, and higher likelihood for hypoglycemic episodes. Schwartz et al3 previously demonstrated that burn-injured patients with diabetes had significant delays in wound closure of approximately 27 days, with more pronounced differences in patients with HbA1c greater than 8%. In the diabetic subgroup of their study, despite whether patients had been grafted, there was no difference in time to wound closure. The authors suggested that these results indicate that diabetic patients who do not show signs of wound healing within the first 2 weeks after injury may experience delays in wound healing, even with surgical intervention. Although the patients in our study had no differences in grafting requirements, there was a significantly higher rate of unplanned readmissions, which could indicate higher incidence of complications including wound infection, graft failure, and delayed wound healing.
Consistent with our study which included both critically ill patients in the ICU and stable patients in the burn unit, Dahagam et al19 found that diabetic burn patients admitted to the burn ICU were older, had higher glucose values at admission, higher mean glucose, and increased glucose variability. In the multivariable analysis, higher admission glucose and mean glucose were predictive of fewer ICU-free, ventilator-free, and hospital-free days. Increased glucose variability was predicted only with fewer hospital-free days. A preexisting diagnosis of diabetes by report before burn injury was not associated with these selected outcomes, although there was no evaluation of infection-related complications, wound healing, or grafting requirements.19 Although the alterations in inpatient glycemic variables among diabetic patients studied by Dahagam et al were similar to those demonstrated in our study, they found no association between diabetes and clinical outcomes. This could be because no burn-specific outcomes (eg, graft failure, wound healing, unplanned readmission) were evaluated. However, these results do imply that an intervention targeted toward improving glycemic control while minimizing glucose variability could potentially improve outcomes.
Although this is the first study to evaluate outcomes and glycemic parameters on the basis of admission HbA1c levels, there are limitations based on its retrospective design. In this study, we used unplanned readmission as the primary outcome as a marker for burn complications. This surrogate marker was chosen because of the lack of documentation of endpoints that would better represent burn complications, including graft failure, need for grafting to donor sites because of delayed or improper wound healing, burn wound infections, and burn related amputation. In addition, with the inclusion of all burn admissions, the majority of burns included were small in size, with a median of 3% TBSA, limiting applicability to more severe burns in critically injured patients. Many studies have demonstrated an association between diabetes or hyperglycemia with infection risk, but the lack of a difference observed in our study may also be due to the inclusion of primarily small burns with short durations of hospital stay. Finally, our study did not evaluate the interventions for glucose management during admission. Therefore, it is possible that the approach to glycemic control could influence the glycemic variables during admission (ie, glycemic variability).
CONCLUSION
This study confirmed that patients admitted for initial management of burn injury with elevated HbA1c levels at admission have higher glucose measurements at admission and throughout their hospital stay, as well as increased glucose variability. In addition, regardless of preexisting diabetic status, patients with chronic hyperglycemia are more likely to have an unplanned readmission after their initial admission for burn management. On the basis of the results from this study, there is a significant need to further evaluate interventions to improve burn-related outcomes in patients with chronic hyperglycemia. Further studies are warranted to determine the mechanisms for the disparity found between patients with chronic hyperglycemia and euglycemia with regard to glycemic variables during admission and clinical outcomes. Future research should also focus on the identification of alternative management strategies in this population, with emphasis on wound management and interventions for glucose control both during hospitalization and after discharge.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Memmel H, Kowal-Vern A, Latenser BA. Infections in diabetic burn patients. Diabetes Care. 2004;27:229–233. doi: 10.2337/diacare.27.1.229. [DOI] [PubMed] [Google Scholar]
- 2.McCampbell B, Wasif N, Rabbitts A, Staiano-Coico L, Yurt RW, Schwartz S. Diabetes and burns: retrospective cohort study. J Burn Care Rehabil. 2002;23:157–166. doi: 10.1097/00004630-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz SB, Rothrock M, Barron-Vaya Y, et al. Impact of diabetes on burn injury: preliminary results from prospective study. J Burn Care Res. 2011;32:435–441. doi: 10.1097/BCR.0b013e318217f954. [DOI] [PubMed] [Google Scholar]
- 4.Hemmila MR, Taddonio MA, Arbabi S, Maggio PM, Wahl WL. Intensive insulin therapy is associated with reduced infectious complications in burn patients. Surgery. 2008;144:629–635. doi: 10.1016/j.surg.2008.07.001. discussion 635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egi M, Bellomo R, Stachowski E, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36:2249–2255. doi: 10.1097/CCM.0b013e318181039a. [DOI] [PubMed] [Google Scholar]
- 6.Cely CM, Arora P, Quartin AA, Kett DH, Schein RM. Relationship of baseline glucose homeostasis to hyperglycemia during medical critical illness. Chest. 2004;126:879–887. doi: 10.1378/chest.126.3.879. [DOI] [PubMed] [Google Scholar]
- 7.Egi M, Bellomo R, Stachowski E, et al. The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit Care Med. 2011;39:105–111. doi: 10.1097/CCM.0b013e3181feb5ea. [DOI] [PubMed] [Google Scholar]
- 8.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373:1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control with insulin in the ICU: facts and controversies. Chest. 2007;132:268–278. doi: 10.1378/chest.06-3121. [DOI] [PubMed] [Google Scholar]
- 10.Gauglitz GG, Herndon DN, Jeschke MG. Insulin resistance postburn: underlying mechanisms and current therapeutic strategies. J Burn Care Res. 2008;29:683–694. doi: 10.1097/BCR.0b013e31818481ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Executive summary: standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Suppl 1):S4–S10. doi: 10.2337/dc10-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48:436–472. [PubMed] [Google Scholar]
- 13.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041–2047. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 14.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 15.American Burn Association, editor. ABA Coding and Reimbursement Primer: 2010 Edition. Chicago: American Burn Association; 2010. [Google Scholar]
- 16.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States 2007. Atlanta: US Department of Health and Human Services; 2008. [Accessed November 10, 2012]. Available at http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. [Google Scholar]
- 18.Boyle JP, Honeycutt AA, Narayan KM, et al. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care. 2001;24:1936–1940. doi: 10.2337/diacare.24.11.1936. [DOI] [PubMed] [Google Scholar]
- 19.Dahagam CK, Mora A, Wolf SE, Wade CE. Diabetes does not influence selected clinical outcomes in critically ill burn patients. J Burn Care Res. 2011;32:256–262. doi: 10.1097/BCR.0b013e31820aaf68. [DOI] [PMC free article] [PubMed] [Google Scholar]