Identity
Other names: LPGP1, Lpgp, MGC21659, S14, SPOT14
HGNC (Hugo): THRSP
Location: 11q14.1
Local order: According to NCBI Map Viewer, genes flanking THRSP in centromere to telomere direction on 11q13 are: PHCA (11q13.5), phytoceramidase, alkaline; GDPD4 (11q13.5), glycerophosphate phosphodiesterase domain containing 4; PAK1 (11q13–14), p21/cdc42/Rac1-activated kinase1 (STE20 homolog, yeast); DFKZp434E1119 (11q14.1), hypothetical protein DFKZp434e1119; AQP11 (11q14.1), aquaporin 11; THRSP; LOC646195 (11q14.1), ribosomal protein S28 pseudogene; LOC143543 (11q14.1), RNA binding motif protein X-linked pseudogene; RAB30 (11q12-q14), RAB30, member RAS oncogene family; PCF11 (11q13), PCF11, cleavage and polyadenylation factor subunit, homolog (S. cerevisiae).
DNA/RNA
Description
According to Entrez-Gene, THRSP maps to NC_000011.9 in the region between 77774907 and 77779307 on the plus strand and spans 5.6 kilobases. According to Spidey (mRNA to genomic sequence alignment tool), THRSP has two exons, the sizes being 481 and 603 bp. Only the smaller of these is translated.
Transcription
THRSP mRNA NM_003251.2 has 1084 nt. The coding region of human mRNA for THRSP has 438 nt. Transcription is regulated via thyroid hormone and the SREBP-1c binding sites. Expression can be induced by progestin, glucose, thyroid hormone, and insulin. Antisense RNA knocks down S14 expression in hepatocytes, and this abrogates the induction of genes concerned with fatty acid synthesis by triiodothyronine and glucose.
Pseudogene
The ancestral S14-related protein, also known as Strait 11499 and Mig12, may duplicate the function of S14 in hepatic, but not mammary, tissue.
Protein
Note
THRSP is primarily a nuclear protein which is important in the regulation of lipid metabolism. It is induced by thyroid hormone, carbohydrate intake, adipose tissue differentiation, and lactation, and is inhibited by glucagon and conjugated linoleic acid. Expression of THRSP (Spot14) parallels that of fatty acid synthase in adipose, liver, and mammary tissue in bovine and murine species. Elevated expression of THRSP in human breast tumors is correlated with poor prognosis, whereas absence of expression is associated with longer survival.
Description
A driver of de novo saturated fatty acid synthesis in normal and malignant tissues, Spot14 (S14, THRSP) was named for its position on two-dimensional gels of in vitro translation products. The gene is rapidly induced by thyroid hormone in rat liver, and it is strongly activated by glucose metabolism. An acidic protein of approximately 16 kD, it is localized primarily in the nucleus; three domains are conserved from its ancestral protein, Strait1499, also known as Mig12 and S14-related protein.
From immunohistochemical studies, the temporal and spatial expression patterns of murine Spot14 and fatty acid synthase (FASN) were regulated in parallel in mammary epithelium during pregnancy, lactation, and involution. In cattle, milk fat depression is associated with production of conjugated linoleic acid (CLA) isomers as intermediates of fatty acid synthesis by rumen bacteria. Ingestion of a low forage, high oil diet leads to increased production of CLA, and this results in low milk fat content, and decreased expression of S14, FASN, sterol response element binding protein (SREBP), and responsive genes INSIG1 and INSIG2 in a coordinate manner. Breast epithelium does not express detectable levels of Spot14 or FASN in the resting state; however, during pregnancy and lactation, Spot14 and enzymes of lipid biosynthesis are expressed at high levels. Spot14 and FASN are expressed in most breast cancers, and high levels of Spot14 expression portend an aggressive course and high risk of recurrence, regardless of nodal status at diagnosis. Thus Spot14 represents a potential target for therapeutic intervention in cancer.
Expression
Spot14 protein is expressed primarily in tissues which synthesize fatty acids. These tissues include white and brown adipose tissue, breast tissue, and liver. Expression is observed in a variety of malignancies, and it is a component of the lipogenic tumor phenotype, e.g., in human breast cancer.
Localisation
By immunohistochemistry, Spot14 is localized primarily in the nucleus of rat liver, human mammary gland, and breast cancer cells.
Function
Spot14 is involved in the regulation of lipid biosynthesis. Its precise function is not known. It exists as a heterodimer in human cells which are actively synthesizing lipids. Triggers for the induction of Spot14, such as hormones or refeeding after fasting, also trigger FASN activity. Furthermore, siRNAs and anti-sense RNAs directed against Spot14 inhibit expression of genes coding the lipid-synthesizing enzymes.
Homology
Homologous proteins are found in cow, rat, mouse, chicken, dog, and chimpanzee, as well as other species. An acidic protein of approximately 16.4 kDa, human THRSP bears 99% homology to its counterpart in Pan troglodytes, 91% to that in Macaca mulatta, 82% to that in Mus musculus, and 80% to that in Rattus norvegicus (data from NCBI BLAST). Three domains are conserved from the ancestral S14-related peptide (Strait 11499, Mig12, S14-related protein).
Mutations
Note
Mutations have been characterized in the chicken. Single-nucleotide polymorphisms (SNPs) have been noted in cow, rat, mouse, and chimpanzee. The SNP database in NCBI lists 63 human SNPs for THRSP.
Implicated in
Breast tumors
Note
Along with cyclin D1, which shares the same amplicon at 11q13, S14 is amplified in about 20% of human breast cancers. Although cyclin D1 is a human and murine mammary oncogene, it was the concomitant overexpression of S14 and lipogenic enzymes in aggressive breast tumors that prompted investigation of the role of fatty acid metabolism in metastasis and recurrence of breast tumors. In an immunohistochemical study of invasive breast tumors, high levels of S14 expression correlated with reduced disease-free survival, irrespective of nodal status at diagnosis; there were no recurrences among those whose tumors expressed low levels of S14, even after prolonged follow-up. S14 expression levels did not segregate with cyclin D1, Her-2/neu amplification status, or hormone receptor status. Thus it appears that S14 promotes a virulent, lipogenic phenotype in breast tumors.
Aberrant hepatic lipogenesis and hepatic steatosis
Note
The relationship between lipid metabolism and disease is further corroborated by the finding in human hepatocytes that the pregnane X receptor (PXR), which is a nuclear receptor regulating xenobiotic and drug metabolism, upregulates lipogenesis via S14. Stimulation of PXR also enhances expression of the cd36 gene, which permits the uptake of exogenous fatty acids by cells, and also stimulates de novo lipogenesis as well as upregulation of the enzymes involved in lipid synthesis. Knockdown by short interfering RNAs to PXR, S14, or FASN abrogates lipid synthesis. S14-directed fatty acid synthesis has also been implicated in aberrant hepatic lipogenesis and hepatic steatosis.
Obesity
Note
It is possible that Spot14 plays a role in the regulation of lipid storage in humans. Whereas nonobese humans downregulate the level of Spot14 in response to fasting, obese subjects do not. Postfasting levels of glucose, insulin, and ketones did not differ between the two groups. The abnormal downregulation of Spot14 in adipose tissue of obese subjects implies that Spot14 may be important to the acquisition or maintenance of obesity in humans.
Figure 1.
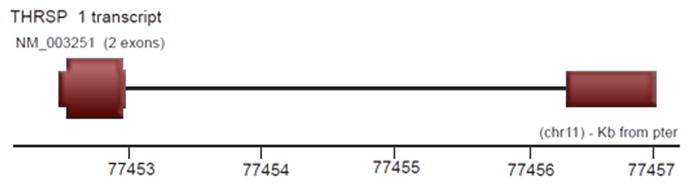
An intron (thin line) connects the two exons of THRSP. Most of the 5′ exon (thickest line) is translated.
Contributor Information
Nancy B Kuemmerle, Dept of Physiology, Dartmouth Medical School - Lebanon, New Hampshire, USA.
William B Kinlaw, Norris Cotton Cancer Center at Dartmouth Medical School - Lebanon, New Hampshire, USA.
References
- Seelig S, Liaw C, Towle HC, Oppenheimer JH. Thyroid hormone attenuates and augments hepatic gene expression at a pretranslational level. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4733–7. doi: 10.1073/pnas.78.8.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlaw WB, Tron P, Witters LA. Thyroid hormone and dietary carbohydrate induce different hepatic zonation of both “spot 14” and acetyl-coenzyme-A carboxylase: a novel mechanism of coregulation. Endocrinology. 1993 Aug;133(2):645–50. doi: 10.1210/endo.133.2.8102096. [DOI] [PubMed] [Google Scholar]
- Dickson C, Fantl V, Gillett C, Brookes S, Bartek J, Smith R, Fisher C, Barnes D, Peters G. Amplification of chromosome band 11q13 and a role for cyclin D1 in human breast cancer. Cancer Lett. 1995 Mar 23;90(1):43–50. doi: 10.1016/0304-3835(94)03676-a. [DOI] [PubMed] [Google Scholar]
- Kinlaw WB, Church JL, Harmon J, Mariash CN. Direct evidence for a role of the “spot 14” protein in the regulation of lipid synthesis. J Biol Chem. 1995 Jul 14;270(28):16615–8. doi: 10.1074/jbc.270.28.16615. [DOI] [PubMed] [Google Scholar]
- Brown SB, Maloney M, Kinlaw WB. “Spot 14” protein functions at the pretranslational level in the regulation of hepatic metabolism by thyroid hormone and glucose. J Biol Chem. 1997 Jan 24;272(4):2163–6. [PubMed] [Google Scholar]
- Moncur JT, Park JP, Maloney M, Mohandas TK, Kinlaw WB. Assignment of the “spot 14” gene (THRSP) to human chromosome band 11q13.5 by in situ hybridization. Cytogenet Cell Genet. 1997;78(2):131–2. doi: 10.1159/000134644. [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Mariash CN. Adipose S14 mRNA is abnormally regulated in obese subjects. Thyroid. 1999 Feb;9(2):143–8. doi: 10.1089/thy.1999.9.143. [DOI] [PubMed] [Google Scholar]
- Mater MK, Thelen AP, Pan DA, Jump DB. Sterol response element-binding protein 1c (SREBP1c) is involved in the polyunsaturated fatty acid suppression of hepatic S14 gene transcription. J Biol Chem. 1999 Nov 12;274(46):32725–32. doi: 10.1074/jbc.274.46.32725. [DOI] [PubMed] [Google Scholar]
- Compe E, de Sousa G, François K, Roche R, Rahmani R, Torresani J, Raymondjean M, Planells R. Spot 14 protein interacts and co-operates with chicken ovalbumin upstream promoter-transcription factor 1 in the transcription of the L-type pyruvate kinase gene through a specificity protein 1 (Sp1) binding site. Biochem J. 2001 Aug 15;358(Pt 1):175–83. doi: 10.1042/0264-6021:3580175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YA, Han WF, Morin PJ, Chrest FJ, Pizer ES. Activation of fatty acid synthesis during neoplastic transformation: role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Exp Cell Res. 2002 Sep 10;279(1):80–90. doi: 10.1006/excr.2002.5600. [DOI] [PubMed] [Google Scholar]
- Wang X, Carre W, Zhou H, Lamont SJ, Cogburn LA. Duplicated Spot 14 genes in the chicken: characterization and identification of polymorphisms associated with abdominal fat traits. Gene. 2004 May 12;332:79–88. doi: 10.1016/j.gene.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Harvatine KJ, Bauman DE. SREBP1 and thyroid hormone responsive spot 14 (S14) are involved in the regulation of bovine mammary lipid synthesis during diet-induced milk fat depression and treatment with CLA. J Nutr. 2006 Oct;136(10):2468–74. doi: 10.1093/jn/136.10.2468. [DOI] [PubMed] [Google Scholar]
- Kinlaw WB, Quinn JL, Wells WA, Roser-Jones C, Moncur JT. Spot 14: A marker of aggressive breast cancer and a potential therapeutic target. Endocrinology. 2006 Sep;147(9):4048–55. doi: 10.1210/en.2006-0463. [DOI] [PubMed] [Google Scholar]
- Martel PM, Bingham CM, McGraw CJ, Baker CL, Morganelli PM, Meng ML, Armstrong JM, Moncur JT, Kinlaw WB. S14 protein in breast cancer cells: direct evidence of regulation by SREBP-1c, superinduction with progestin, and effects on cell growth. Exp Cell Res. 2006 Feb 1;312(3):278–88. doi: 10.1016/j.yexcr.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Wells WA, Schwartz GN, Morganelli PM, Cole BF, Gibson JJ, Kinlaw WB. Expression of “Spot 14” (THRSP) predicts disease free survival in invasive breast cancer: immunohistochemical analysis of a new molecular marker. Breast Cancer Res Treat. 2006 Jul;98(2):231–40. doi: 10.1007/s10549-005-9154-z. [DOI] [PubMed] [Google Scholar]
- Tsatsos NG, Augustin LB, Anderson GW, Towle HC, Mariash CN. Hepatic expression of the SPOT 14 (S14) paralog S14-related (Mid1 interacting protein) is regulated by dietary carbohydrate. Endocrinology. 2008 Oct;149(10):5155–61. doi: 10.1210/en.2008-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau A, Téruel C, Beylot M, Albalea V, Tamasi V, Umbdenstock T, Parmentier Y, Sa-Cunha A, Suc B, Fabre JM, Navarro F, Ramos J, Meyer U, Maurel P, Vilarem MJ, Pascussi JM. A novel pregnane X receptor and S14-mediated lipogenic pathway in human hepatocyte. Hepatology. 2009 Jun;49(6):2068–79. doi: 10.1002/hep.22907. [DOI] [PubMed] [Google Scholar]


