Abstract
Background
Despite there being a relatively large number of methods papers which detail specifically the development of stimulation devices, only a small number of reports involve the application of these devices in freely moving animals. To date multiple preclinical neural stimulators have been designed and described but have failed to make an impact on the methods employed by the majority of laboratories studying DBS. Thus, the overwhelming majority of DBS studies are still performed by tethering the subject to an external stimulator. We believe that the low adoption rate of previously described methods is a result of the complexity of replicating and implementing these methods.
New Method
Here were describe both the design and procurement of a simple and inexpensive stimulator designed to be compatible with commonly used, commercially available electrodes (Plastics 1).
Results
This system is initially programmable in frequency, pulsewidth and current amplitude, and delivers biphasic, charge-balanced output to two independent electrodes.
Comparison with Existing Method(s)
It is easy to implement requiring neither subcutaneous implantation or custom-made electrodes and has been optimized for either direct mounting to the head or for use with rodent jackets.
Conclusions
This device is inexpensive and universally accessible, facilitating high throughput, low cost, long-term rodent deep brain stimulation experiments.
Keywords: deep brain stimulation, rodent, programmable, chronic, bilateral, charge-balanced
1. Introduction
Several publications have reported the development and use of rodent stimulators for use in preclinical research (de Haas et al., 2012; Ewing et al., 2013; Forni et al., 2012; Harnack et al., 2008; Millard and Shepherd, 2007; Winter et al., 1998). Unfortunately, the description of these devices rarely contains sufficient detail to enable the faithful replication of them in independent laboratories. The detail necessary to reproduce such electronic designs requires (i) an accurate and complete circuit diagram, (ii) the PCB (printed circuit board) artwork, (iii) a complete BOM (bill of materials), (iv) placement plan and (v), in designs incorporating microprocessors, the firmware to control the microprocessor. Given such details, an individual with experience in electronics would be expected to succeed in replicating the design. In this contribution, we provide a full and detailed description of the design as well as all details necessary for the full procurement procedure and the subsequent final assembly of a simple microstimulator sufficient for many preclinical DBS applications. Further, we provide the necessary files (PCB, BOM and placement plan, see supplementary materials) necessary to obtain fully populated PCBs from an international supplier when transmitted to the fabrication house (Beta LAYOUT). The device is designed to be simple and inexpensive, reproducible without specialist skills, compatible with commonly used, commercially available electrodes (Plastics 1) and easy to use. What this approach lacks in sophistication it gains in simplicity, ease of reproduction, ease of implementation and cost. It is our belief that the majority of preclincal DBS research labs are likely to be interested in testing only a single set of specific stimulation parameters in any subject and that these parameters are determined in the experimental design stage. Thus the ability to adjust the stimulation parameters midway through an experiment may be unnecessary. Finally we believe that these laboratories will be interested in stimulation periods of no more than 2–3 weeks. The final device design is tempered by these reasonable limitations in order to achieve maximum efficiency at the lowest cost.
2. Materials and Methods
2.1. Circuit design
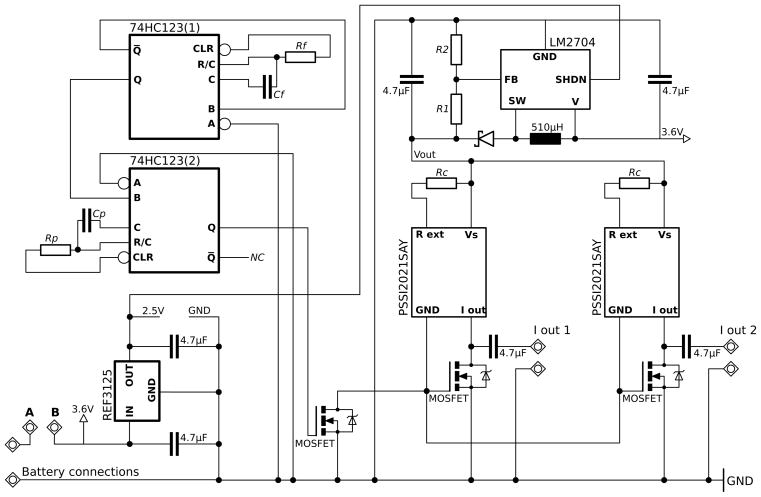
The stimulation circuit comprises three sub-assemblies, (i) pulse generator, (ii) two (bilateral) current sources and (iii) DC-DC voltage converter. These circuits are switched via high speed MOSFET switching transistors and the output is charge balanced via capacitive coupling of the electrode output and shorting to ground inbetween pulses (Fig. 1).
Fig. 1.
Circuit diagram of the DBS device. NC indicates no connection. LM2704, charge pump; 74HC123, dual monostable multivibrator; PSSI2021SAY, constant current source. Shorting contacts A and B completes the power path switching the device on in design A (see section 2.3.1). In design B a dedicated switch is installed between these points (see section 2.3.2).
2.1.1. Power supply
Lithium Thionyl Chloride batteries provide high energy densities with extremely low self-discharge (less than 1% per year). The EVE EF651625 (Farnell Part No: 1973589) measuring 20.1×16.8×6.8mm and weighing only 6g provides an impressive battery capacity of 550mAh. Both design A and design B are designed to surmount this battery although for devices intended for use with rodent jackets design B may be powered by the larger (25.8×16.8×7.0mm, 8g) EVE EF651625 battery (Farnell Part No: 1973588) which has a capacity of 750mAh. In design A the power supply is switched on and off by shorting a break in the power supply path (see section 2.3.1). Design B has a dedicated on/off switch installed (see section 2.3.2).
2.1.2. Supply bypassing and regulation
The battery delivers a stable voltage of 3.6V when discharged at a constant rate. However, the DC-DC converter causes unusually large voltage fluctuations. Voltage ripple on power supply lines causes errors in timing in the pulse generator. Thus, the power supply for the pulse generator must be very well regulated. The operating voltage for the pulse generator may be selected between 2 and 6V. The MOSFET switches switch at a threshold of 2V so a low power 2.5V voltage regulator (Texas Instruments, REF3125, Farnell Part No: 1180185) is used to regulate the supply to the pulse generator. Minimizing the voltage supply in the pulse generator circuit minimizes the current draw in this subcircuit. Both the voltage regulator and the pulse-generator are then further bypassed with 4.7μF capacitors.
2.1.3. Pulse generator
Stimulation pulses are generated using a dual retriggerable monostable multivibrator (Texas Instruments 74HC123, Farnell Part No: 1601156). These can be wired as a pulse generator with independent frequency and duty cycle control. The output is a square wave with a frequency defined by the resistor-capacitor network connected to the first monostable multivibrator (Eqn. 1) and a pulse-width defined by the resistor-capacitor network connected to the second monostable multivibrator (Eqn. 2). The frequency (F) generated at the output of the first monostable multivibrator (Q1) is determined by the values of Rf and Cf which may be selected using equation 1.
| (1) |
K varies as a function of supply voltage: K = 0.46 for VCC = 2.5. The pulse-width (P) is a function of the duty cycle and is determined by the the values of Rp and Cp which may be selected using equation 2.
| (2) |
The minimum value for either of the external resistors is 5kΩ - choosing high values for these external resistors minimizes current draw and power dissipation.
2.1.4. DC-DC voltage converter
To be confident of constant current delivery to the stimulated region it is necessary to provide sufficient voltage at the input to the current sources that they will be capable of delivering current over a wide range of tissue impedances. To generate a large enough voltage from batteries small enough to be comfortably carried by a rat a charge pump (National Semiconductor, LM2704, Farnell Part No: 8207461) is used to generate a larger voltage from the 3.6V supply. The charge pump, by storing and accumulating charge, is capable of generating significantly larger output voltages than it receives on its input.
The DC-DC voltage converter requires careful component selection and attention paid to its layout recommendations to ensure efficient operation and minimal power consumption. Particularly the inductor must have a low DC resistance (Farnell Part No: 2112890, DCR = 0.5Ω) and the track lengths connecting the output diode and inductor to the device must be minimized. The maximum output voltage for this device is 20V. However a lower output voltage might be desirable in some applications, particularly those seeking even greater battery life. This can be set using equation 3:
| (3) |
2.1.5. Current sources and capacitive charge balancing
A current source supplies a fixed current to a load regardless of the load’s resistance. The current sources (NXP, PSSI2021SAY, Farnell Part No: 1758034) deliver a stable output current between 15μA and 50mA which is set by connecting an external resistor between pins 4 and 5 using equation 4.
| (4) |
Connecting no external resistor between these pins sets the output current to 15μA. The current sources are switched via a high speed N channel MOSFET transistor switch (NXP, 2N7002, Farnell Part No: 1510761) driven by the output from the pulse generator. Charge balancing is achieved by capacitor coupling and shorting the electrodes to ground immediately following the stimulation pulse using the same MOSFET switches. This series coupling capacitor prevents any net DC current flow on the stimulator output.
2.2. PCB Procurement
The device has been optimized for either direct mounting to the head (Design A) or for use with rodent jackets (Design B). Each one comprises the files necessary (PCB schematic, layout, BOM and placement files) for the procurement of each design. The design files may be inspected using the freeware version of the software Eagle (www.cadsoftusa.com). For either design, the file PCB_N.brd (N should be replaced with either A or B depending on the design) can be sent, along with the bill of materials file BOM_N.cvs and the polarity file POL_N.pdf, to Beta LAYOUT (www.pcb-pool.com) for assembly. The values and part numbers for the three parameter set resistors need to be inserted in the BOM file before transmission. Some common values and corresponding part numbers are included in table 1. It is essential that the package of any selected resistor is 0603 for it to fit the board. This fabrication house will deal with the full procurement of all the electronic components, the printing of the circuit boards and the assembly of the ciruits. The customer should then receive a fully populated microstimulator PCB requiring only the addition of the battery, the addition of the electrode connector and encapsulation.
Table 1.
Resistance values give parameter to within approximately 1%, a typical tolerance for commonly available resistors. Cf = 1μF, Cp = 0.01μF
| F (Hz) | Rf (kΩ) | Farnell No. | P (μs) | Rp (kΩ) | Farnell No. | C (μA | Rc(kΩ) | Farnell No. |
|---|---|---|---|---|---|---|---|---|
| 10 | 215 | 2138528 | 50 | 11 | 1527585 | 50 | 17.6 | 2138437 |
| 50 | 43.2 | 1469810 | 100 | 22 | 2078917 | 100 | 7.3 | 1527567 |
| 130 | 18 | 1750691 | 150 | 33 | 1738924 | 150 | 4.53 | 2059374 |
| 185 | 12 | 1652834 | 200 | 44.2 | 1527652 | 200 | 3.32 | 1469795 |
2.3. Final assembly
2.3.1. Design A
Final assembly of design A includes attaching the electrode connector (363 Plug with pins only, Plastics 1) and battery, and encapsulation. The electrode connector includes a threaded captive collar which screws to the electrode housing (363 plug, Plastics 1) providing an extremely secure connection between the device and the implanted electrodes. The electrode connector should point out from the component side of the board (Fig. 2). The circuit board is then encapsulated in epoxy resin taking care to avoid fouling the captive collar on the electrode connector (Fig. 2).
Fig. 2.
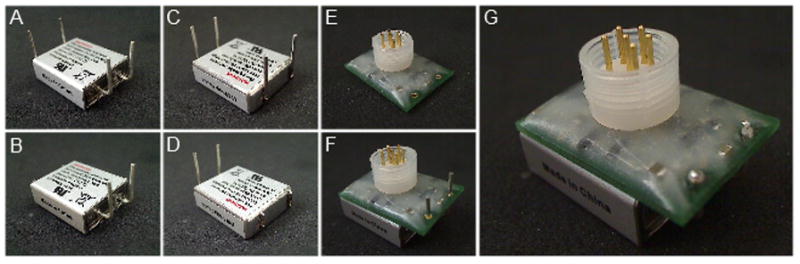
The PCB has a footprint area of 24×16.8mm. The electrode connector is centrally mounted perpendicular to the face of the PCB to minimize the vertical height of the device during operation. An edge mounted connector would greatly increase the device height. (A, C) View of the battery connections as shipped. (B,D) View of the battery with the additional case connections snipped off. (E) The assembled and encapsulated circuit. (F) The circuit slides directly onto the battery connections before soldering these connections and final encapsulation of the battery(G).
The battery comes supplied with 4 stiff wire terminations (Fig. 2). The two that are connected directly to the case can be snipped off leaving the positive and negative terminals which protrude from, and are bent at 90° to the case. The device is designed to connect to the battery by inserting these remaining wires through the battery connector holes which are positioned to mate specifically with this battery. The component side of the board should face away from the battery to ensure correct polarity. The battery and is encapsulated in silicon (Dow Corning 3140 RTV coating, Farnell Part No.: 101713) to make it possible to change the battery at the end of the device’s lifetime.
2.3.2. Design B
Three solder pads are positioned between the battery connection pads on the bottom surface of the PCB, two for the active electrode contacts and one for the ground connection (Fig. 2). The electrode connection cable (Fig. 2) is a modified 363 cable from Plastics 1 with only 3 conductors and no covering to maximize its flexibility (Plastics 1 product code: 363-SL/3, (€38.82), length = 12cm). The solder lug terminations must be soldered to the appropriate pads (colour, pad 1 (electrode 1); colour, pad 2 (electrode 2); colour, pad 3 (ground)). The device is designed to connect to the battery by snipping away the unnecessary ground wires and inserting the remaining wires through the battery connector holes which are positioned to mate specifically with this battery (Fig. 2). The component side of the board should face away from the battery to ensure correct polarity. The device can then be encapsulated in silicon (3140 RTV Coating, Dow Corning, Farnell Part No: 101713) taking care not to foul the switch.
2.4. Operation, implementation & testing
Design A has no moving parts and no external cabling making it virtually indestructible. It is designed to be plugged directly into a socket (363 plug, Plastics 1) cemented onto the subjects skull (Fig. 6). Thus it has no on/off switch. Switching the device on/off is achieved by connecting pins 1 and 2 together which completes the power line switching the device on. Two pins connected by a short wire must be inserted into the contact housing (pins 1 and 2, Fig. 4A) during implantation to provide this necessary connection. Thus the device switches on as soon as it is plugged into the socket and switches off again as soon as the device is unplugged. The contacts of the stimulating electrodes (E363/6/SP, Plastics 1) and the contacts for the current return path (“ground”) must be inserted into their respective positions in the contact housing (pins 5 and 6). Two contact positions (pins 3 and 4) connect with the battery ground for those using bipolar electrodes (E363/6/SP-2TW, Plastics 1). Only 1 (either will do) of these is necessary for monopolar stimulation referenced to a screw electrode (E363/20/SP, Plastics 1) implanted in the skull.
Fig. 6.
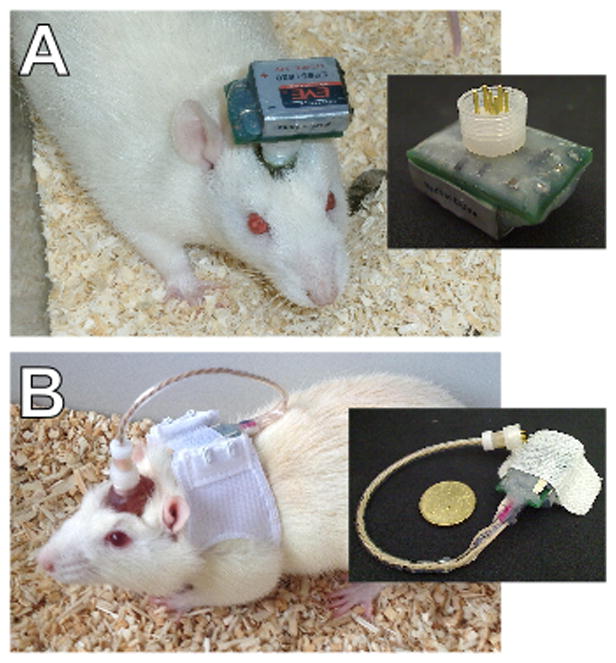
In design A the device connects directly to the headstage. In design B the device is design to be carried in a rodent jackets with secure attachment provided by the hook-and-loop fastener.
Fig. 4.
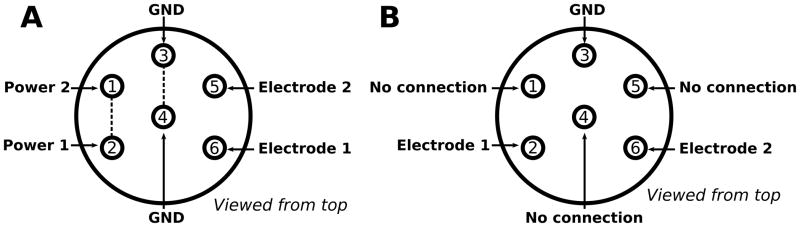
Design A is designed to be plugged directly into a socket (363 plug, Plastics 1) cemented onto the subjects skull, it has no on/off switch. Switching the device on/off is achieved by shorting the power line switching the device on. Two pins connected by a short wire must be inserted into the contact housing during implantation (pins 1 and 2). The contacts of the stimulating electrodes (E363/6/SP, Plastics 1, pins 5 and 6) and the contacts for the current return path (“ground”) must be inserted into their respective positions in the contact housing (pins 3 and 4). Two contact positions (pins 3 and 4) connect with the battery ground for those using bipolar electrodes (E363/6/SP-2TW, Plastics 1). Only 1 (either will do) of these is necessary for monopolar stimulation referenced to a screw electrode (E363/20/SP, Plastics 1) implanted in the skull. Design B is designed to be carried on a rodent jacket and includes a dedicated on/off switch. Thus fewer connections are required in the electrode pedestal. When ordering the cable the connection diagram must also be sent to Plastics 1 to guarantee the correct connections.
Design B is designed to be carried on a rodent jacket and includes a dedicated on/off switch (Fig. 6). Thus fewer connections are required in the electrode pedestal. When ordering the cable the connection diagram (connector_N.pdf, see supplementary materials) must also be sent to Plastics 1 to guarantee the correct connections. The devices are prepared for attaching to the small rodent jackets (Harvard Apparatus Ltd.) by sticking the hooked side of adhesive hook-and-loop tape to the top-most surface of the the device. The looped side of the hook-and-loop tape was stuck to the underside of the device to cushion the battery housings which would otherwise have been adjacent to the animals back (Fig. 3).
Fig. 3.
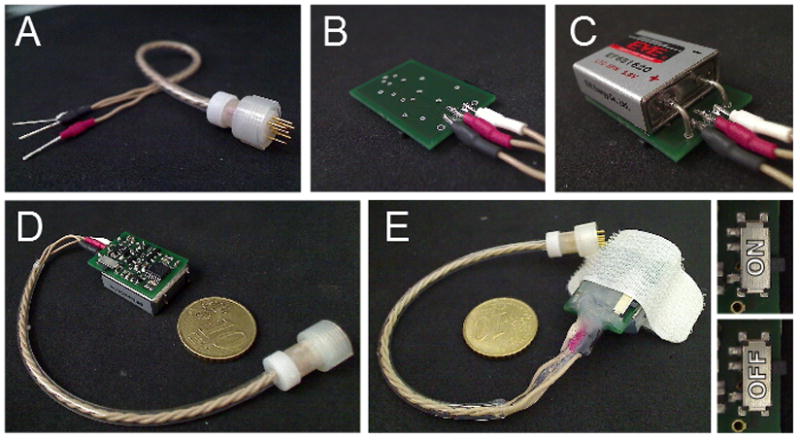
This PCB has a footprint identical the device presented in figure 2. The circuit switches on/off via a miniature slide switch rather than through shorting contacts in the power trace. Thus care must be taken not to encapsulate the switch. (A) 3 channel 363-SL/3 cable (Plastics 1). (B) Device output: the white termination must be connected to ground. The other terminations are the stimulation channels. (C) The output solder pads are nested between the battery connectors. (D) The complete (unencapsulated) device. (E) Devices are prepared for attachment to the small rodent jackets by sticking the hooked side of adhesive hook-and-loop tape to the topmost surface of the device. The looped side of the hook-and-loop tape was stuck to the underside of the device to cushion the battery housings which would otherwise have been adjacent to the animals back. The devices then stick easily to the hook-and-loop material stitched into the rodent jacket. A 10 cent (€) coin is included for scale. Inset: switch positions.
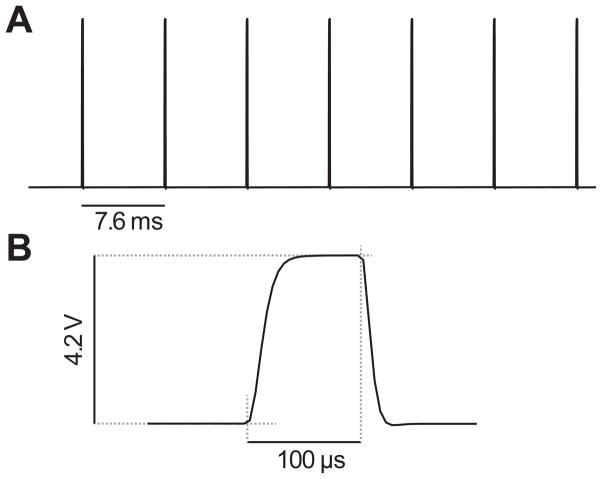
Devices are tested by making measurements from the voltage trace observed while stimulating a test load. Any resistor with an impedance similar to that of the electrode-tissue interface will suffice: here we have used a 22kΩ load (Fig. 5).
Fig. 5.
Devices are tested by making measurements from the voltage trace recorded while stimulating a dummy load, in this case a 22kΩ resistor. (A) Frequency is verified by measuring the inter-pulse interval (7.6 ms at 130Hz). B. Current amplitude can be calculated by measuring the voltage across the dummy load resistor (4.2V/22kΩ = 190μA). Pulsewidth (100μs) is measured directly. In this example the set parameters were F = 130Hz, P = 100μs and I = 200μA and realised with Rf = 18kΩ, Rp = 22kΩ, Rc = 3.32kΩ, with Cf = 1μF and Cp = 0.01μF.
Successful long-term implementation requires some necessary precautions. Repetitive agitation of externalised implants may over time lead to headcap failure requiring the subsequent removal of an animal from a study. Such attrition is common although steps can be taken to minimise its likelihood. The most critical component requires excellent surgical technique with the tight fitting of an appropriate number of anchoring screws. We recommend using at least 4 screws, each placed anterior and posterior to each electrode burr hole, bilaterally. In addition the skull must be clean and dry to ensure good adhesion. Attempting to apply dental cement to a wet skull will likely result in poor adhesion and possible infection leading to softening of the skull and almost certain headcap failure. Finally, housing animals in cages with a large vertical clearance following surgery minimises the likelihood of the device being repeatedly knocked against the top of the cage.
3. Results
The devices deliver charge balanced neural stimulation pulses to two independent stimulation channels with a frequency, pulsewidth and current determined by the user at the point of fabrication. Design A draws 1.1mA when stimulating a load of 33kΩ at a frequency of 130Hz with 150μA pulses 90μs in duration. It is designed to surmount a 550mAh EVE battery yielding a predicted device lifetime of approximately 21 days with these common stimulation parameters. The final mass of this device is 9.9g.
Design B draws slightly less current (0.95mA) and can be mounted on either the 550mAh EVE battery or the larger 750mAh battery which has a capacity of 750mAh. Thus using this version of the device with a larger battery affords the user an approximate runtime of 33 days using standard parameters. The final mass of this device using the 750mAh battery is 13.7g.
4. Discussion
We have presented the design of a neural stimulation device and detailed the steps required for its procurement and replication. The device delivers charge-balanced pulses at a frequency, pulsewidth and current amplitude determined by the selection of appropriate resistor values during procurement. The final assembly requires no specialist training, requiring competence in only very basic soldering techniques. Device implementation is greatly simplified since it is designed to be compatible with the full range of Plastics 1 electrodes, therefore custom-made electrodes and connectors are not necessary. Finally both designs are intended for externalized use, negating complications associated with subcutaneous implantation.
Design A is intended to be plugged directly into a socket affixed to the subject’s skull in much the same way as many of the stimulating and recording devices available from TBSI (Triangle Biosystems, Inc.). Its mass (approx. 10g) while much heavier than a similar commercial stimulation system (TBSI S-Series, 2 channel wireless neural stimulation system, 3.3g) is comparable with heavier recording devices (TBSI W-Series, 126 channel wireless neural recording system, 10g). This additional mass gives our device a lifetime of over 140 times that of the TBSI S-Series (21 days compared with 3.5 hours). Further, our device benefits from the use of a Plastics 1 connector which includes a threaded collar for an extremely secure and reliable connection and is a considerable improvement over the pin-header connections available in commercial systems. Such connections have no mechanical reinforcement and are easily dislodged by awake, freely behaving animals.
With high quality surgical technique we have not experienced any significant problems in the implementation of device A. Its design is similar to that of other headstage products such as the TBSI S-Series, 2 channel wireless neural stimulation system which is also encapsulated in epoxy. Epoxy has excellent mechanical properties and will certainly resist any abrasion incurred by rodent scratching for the lifetime of the device. Further device A was designed specifically to reduce its height and minimise its torque around the anchoring points. Again by comparison with the TBSI S-Series, 2 channel wireless neural stimulation system, design A has a vertical height of only 14.3mm whereas the S-Series has a vertical height of 18.4mm.
Design B is intended to be carried on a rodent jacket in much the same way as the TBSI S-Series 32-channel wireless neural stimulator system and competes favorably on mass: our device weighs approximately 14g compared with TBSI’s 20g at the expense of the limited channel count. Design B can be mounted on a larger battery, thereby increasing the device lifetime. However, this additional lifetime comes at considerable additional expense: the cable is expensive and each rat requires a jacket with which to carry the device, effectively doubling the cost per subject for this design when compared with the device which mounts directly on the head (Fig. 2). Again, this device benefits from the use of a Plastics 1 connector which includes a threaded collar for an extremely reliable connection.
4.1. Limitations and extensions
Programming of the stimulation parameters (frequency, pulse-width and current amplitude) is done during fabrication via the appropriate selection of three resistors and is not adjustable post assembly. Although any resistance value is theoretically attainable either through combinations of series resistors or with trimming potentiometers such combinations of multiple components is undesirable both from a layout and construction perspective. The accuracy of typical resistors varies. 0.1% or 1% are easily available although the higher the accuracy the more expensive the component. Given the impracticality of setting the stimulation parameters to greater than 1% precision the additional expense of high accuracy components is unnecessary. The accuracy of the current sources is 0.5%. Thus, trying to achieve parameter accuracy to greater than 1% is extremely difficult and ultimately unnecessary in the context of DBS research.
The battery is hermetically sealed in a stainless steel case which is connected to the ground terminal, making it conceivable to use the case of the battery as an electrode contact in an implantable device. However, to achieve this requires some form of remote switching, presumably magnetic, and some means to provide assurance that the switching was successful.
A rechargeable alternative?
It would be simple to replace the battery with a rechargeable alternative. Renata supply a lithium-polymer battery which would fit the design (ICP641620PA, 3.7V, 165mAh ICP071622, 21.5 × 16 × 6.9mm). However, rechargeable cells store significantly less charge than primary cells. Thus with a capacity of 165mAh one might expect a device runtime of around 7 days using common stimulation parameters requiring regular recharge. Despite the obvious benefits we have elected not to pursue such an alternative. Lithium-polymer batteries are extremely volatile and great care must be exercised in their use. Over-charging, accidental shorting or incidental damage pose a very real fire risk. In devices designed specifically to be used externally the possibility that the device is removed from the jacket or the headcap is also very real leaving a potentially explosive battery in the custody of a laboratory rat. It goes without saying that rats, given their propensity to chew everything, wouldn’t hesitate to chew through an epoxy sealant and into a lithium-polymer battery resulting in the kind of damage that could easily result in a fire. In short, lithium-polymer batteries are unsuitable for use in environments in which the battery may become accessible to the rat.
5. Conclusion
This manuscript details for the first time the steps necessary to obtain an inexpensive, bilateral, charge-balanced, neural stimulator making chronic, freely moving DBS research accessible to all laboratories. What it lacks in the complexity of commercially available systems it gains in cost and simplicity of reproduction and compatibility with “off-the-shelf” electrodes. These devices have been procured and finally assembled by collaborators with no previous electronics experience following only those steps detailed in this manuscript. Thus we believe that this device is universally accessible, facilitating high throughput, low cost, long-term rodent deep brain stimulation experiments.
Highlights.
The device has an extremely long lifetime with typical stimulation parameters.
The device is initially fully programmable in frequency, pulse-width and current amplitude allowing the study of any common stimulation paradigm
Two independent outputs are charge-balanced ensuring zero net current delivery per period.
The device is inexpensive and easy to replicate
Acknowledgments
This work was partially supported by NIH grants MH086400 and MH57440 and by DFG KFO 247 and DLR/BMBF under the framework of Era-Net Neuron (01EW1103). Thanks to Jim Burhman (University of Pittsburgh) for recommending the replacement of resistor-biased PNP transistor switching with MOSFET transistors and additional discussion on electronic matters. Thanks to Tomek Banasikowski for additional photography and finally thanks to Mareike Voget for being the lab rat who did the dry run of the procurement and assembly of these lab rat stimulators.
Appendix A. Farnell Parts
Table A.2.
Component list and part numbers for a popular supplier (Farnell). Cost of components is based on a design run for 10+ devices configured to deliver approximately pulses 90μs in duration with a current amplitude of 150μA at 130Hz
| Part | Value | Package | Part No. | Price | Quantity |
|---|---|---|---|---|---|
| Battery | 3.6V | LTC-5PN | 1973589 | 7.99 | 1 |
| Switch | - | - | 9575146 | 0.97 | 1(B) |
| Regulator | REF3125 | SOT23 | 1470327 | 3.65 | 1 |
| Inductor | 10μH | 0805 | 1463501 | 0.146 | 1 |
| Capacitor | 4.7μF | 0603 | 1458898 | 0.43 | 3 |
| Capacitor | 4.7μF | 0805 | 1572631 | 0.49 | 3 |
| Charge pump | LM2704 | SOT23-5 | 8207461 | 1.38 | 1 |
| Diode | Schottky | SOD123 | 1175677 | 0.37 | 1 |
| R1 | 510kΩ | 0603 | 2059673 | 0.009 | 1 |
| R2 | 33kΩ | 0603 | 1469801 | 0.02 | 1 |
| Dual one-shot | 74HC123 | TSSOP16 | 1601156 | 0.45 | 1 |
| Freq cap | 1μF | 0603 | 1658868 | 0.41 | 1 |
| Freq res | 18kΩ | 0603 | 1750691 | 0.046 | 1 |
| Pulsewidth cap | 0.01μF | 0603 | 1833876 | 0.058 | 1 |
| Pulsewidth res | 20kΩ | 0603 | 1527615 | 0.047 | 1 |
| Current source | - | SOT-353 | 1758034 | 0.22 | 2 |
| Current res | 4.53kΩ | 0603 | 2059374 | 0.009 | 2 |
| Switching | MOSFET | SOT23 | 1510761 | 0.084 | 3 |
|
| |||||
| Total | 25 | ||||
Appendix B. PCB-pool specifications
Table B.3.
PCB and assembly specifications
| Layer count | 2 |
| Dimensions | 24×16.8mm |
| Base Material | FR4, 35μm Cu, 1mm |
| Soldermask | Yes, sides different |
| Silkscreen | No |
| Minimum gap size | >0.15mm |
| Minimum drill diameter | >0.3mm |
| Component No. top | (A) 24 (B) 25 |
| Component No. bottom | (A) 0 (B) 0 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- de Haas R, Struikmans R, van der Plasse G, van Kerkhof L, Brakkee JH, Kas MJH, Westenberg HGM. Wireless implantable micro-stimulation device for high frequency bilateral deep brain stimulation in freely moving mice. Journal of neuroscience methods. 2012 Jun;:1–7. doi: 10.1016/j.jneumeth.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Ewing S, Porr B, Riddell J. SaBer DBS: A fully programmable, rechargeable, bilateral, charge-balanced preclinical microstimulator for long-term neural stimulation. Journal of neuroscience …. 2013;213:228–235. doi: 10.1016/j.jneumeth.2012.12.008. URL http://www.sciencedirect.com/science/article/pii/S016502701200475X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni C, Mainard O, Melon C, Goguenheim D, Kerkerian-Le Goff L, Salin P. Portable microstimulator for chronic deep brain stimulation in freely moving rats. Journal of neuroscience methods. 2012 May;209 (1):50–57. doi: 10.1016/j.jneumeth.2012.05.027. URL http://www.ncbi.nlm.nih.gov/pubmed/22659685. [DOI] [PubMed] [Google Scholar]
- Harnack D, Meissner W, Paulat R, Hilgenfeld H, Mller WD, Winter C, Morgenstern R, Kupsch A. Continuous high-frequency stimulation in freely moving rats: Development of an implantable microstimulation system. Journal of Neuroscience Methods. 2008;167 (2):278–291. doi: 10.1016/j.jneumeth.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Millard RE, Shepherd RK. A fully implantable stimulator for use in small laboratory animals. Journal of neuroscience methods. 2007 Nov;166 (2):168–77. doi: 10.1016/j.jneumeth.2007.07.009. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2001238&tool=pmcentr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter KF, Hartmann R, Klinke R. A stimulator with wireless power and signal transmission for implantation in animal experiments and other applications. Journal of neuroscience methods. 1998 Jan;79 (1):79–85. doi: 10.1016/s0165-0270(97)00160-x. URL http://www.ncbi.nlm.nih.gov/pubmed/9531463. [DOI] [PubMed] [Google Scholar]