Abstract
Rectally applied antiretroviral microbicides for preexposure prophylaxis (PrEP) of HIV infection are currently in development. Since enemas (rectal douches) are commonly used by men who have sex with men prior to receptive anal intercourse, a microbicide enema could enhance PrEP adherence by fitting seamlessly within the usual sexual practices. We assessed the distribution, safety, and acceptability of three enema types—hyperosmolar (Fleet), hypoosmolar (distilled water), and isoosmolar (Normosol-R)—in a crossover design. Nine men received each enema type in random order. Enemas were radiolabeled [99mTc-diethylene triamine pentaacetic acid (DTPA)] to assess enema distribution in the colon using single photon emission computed tomography/computed tomography (SPECT/CT) imaging. Plasma 99mTc-DTPA indicated mucosal permeability. Sigmoidoscopic colon tissue biopsies were taken to assess injury as well as tissue penetration of the 99mTc-DTPA. Acceptability was assessed after each product use and at the end of the study. SPECT/CT imaging showed that the isoosmolar enema had greater proximal colonic distribution (up to the splenic flexure) and greater luminal and colon tissue concentrations of 99mTc-DTPA when compared to the other enemas (p<0.01). Colon biopsies also showed that only the hyperosmolar enema caused sloughing of the colonic epithelium (p<0.05). In permeability testing, the hypoosmolar enema had higher plasma 99mTc-DTPA 24-h area under the concentration-time curve and peak concentration compared to the hyperosmolar and isoosmolar enemas, respectively. Acceptability was generally good with no clear preferences among the three enema types. The isoosmolar enema was superior or similar to the other enemas in all categories and is a good candidate for further development as a rectal microbicide vehicle.
Introduction
Receptive anal intercourse (RAI) is the principal risk factor for HIV transmission in men who have sex with men (MSM) and is a risk factor in women.1 Condom use, while prevalent, is inconsistent, and recent data suggest an increase in the incidence of sexually transmitted infections among MSM.2–5 Several preexposure prophylaxis (PrEP) strategies are being developed to address rectal HIV acquisition. In iPrEx, daily oral tenofovir/emtricitabine (Truvada) demonstrated modest 42% efficacy in MSM based on a modified intent-to-treat analysis. Adherence was suboptimal in many: only half of nonseroconverting participants had evidence of drug in their blood, indicating that the last three daily doses prior to the research clinic visit had been missed.6 In iPrEx, efficacy (relative risk reduction) jumped from 42% to 92% when drug was detectable in the blood, an indicator of at least a modest degree of recent adherence.6
Rectally applied antiretroviral (ARV) gel microbicides are in development as an alternative or an addition to oral PrEP. Using ex vivo HIV challenge of rectal biopsies taken from research participants on tenofovir- or UC781-containing rectal microbicide gels, HIV replication was reduced in rectal tissue, indicating biological activity of these ARV-containing gels. Clinical efficacy has not been evaluated in randomized controlled trials (RCTs).
Whatever the route of dosing, PrEP has to be used to be effective. In two large RCTs of tenofovir by both oral and vaginal routes, failure to demonstrate efficacy has been attributed to poor adherence to daily dosing regimens.7,8 Success in several other studies with similar dosing regimens9,10 was attributed largely to good product adherence, similar to the efficacy in the iPrEx subjects with higher levels of adherence.6 As noted, both oral tablet and rectal gel dosing require behavioral changes to incorporate PrEP dosing into one's routine. Since behavioral studies indicate a majority of MSM use a rectal enema (douche) prior to RAI, medication of an enema product with an ARV could greatly minimize the need for behavioral change for PrEP dosing.11–16 However, epidemiological studies early in the HIV epidemic implicated enema use as a risk factor for increased HIV transmission.17,18
The most commonly used commercial enema formulations rely on their hyperosmolarity to thoroughly cleanse the rectal vault. We have shown that a hyperosmolar sexual lubricant gel causes significant loss of single columnar epithelium minutes after a single dose; this loss was not observed when an isoosmolar lubricant was used.19 Other studies have also reported mucosal damage due to hyperosmolar solutions, though the osmolality is only indirectly implicated by association.20,21 A large proportion of users simply use tap water (a hypoosmolar formulation) due to easy availability; however, epithelial loss has also been reported after its use.22 Whether any of these types of enema are suitable for drug delivery as PrEP is unstudied.
To address the potential use of an enema as PrEP, either alone or as a complement to another PrEP method, we designed this study to assess the safety, distal gastrointestinal distribution, retention, and acceptability of three different types of enema. Given our concern for potential increased HIV acquisition posed by the more popular hyperosmolar enemas, we compared an isoosmolar enema to the commonly used Fleet (hyperosmolar) and distilled water (hypoosmolar) enemas in a crossover design allowing paired comparisons within each individual.
Materials and Methods
Study schema
The study and informed consent document was approved by the Johns Hopkins Institutional Review Board. The study was a randomized, blinded comparison of distribution, toxicity, and acceptability of three different types of enema (rectal douche) of varying osmolarity in healthy MSM. The hyperosmolar, hypoosmolar, and isoosmolar enemas consisted of a commercial Fleet Phosphate Enema of approximately 2,100 mOsmol/kg (Fleet Laboratories, Lynchburg, VA), 125 ml distilled water (0 mOsmol/kg), and 125 ml Normosol-R enema (294 mOsmol/kg) with pH 7.4 (Hospira, Inc., Lake Forest, IL), respectively; all were administered via an enema bottle. Nine HIV-negative healthy male research participants without a history of anorectal disease participated in the study. Each research participant provided written informed consent followed by eligibility screening, which included medical history, physical examination, and laboratory tests. Eligible research participants received each enema formulation four separate times with a washout period of 2 weeks between formulations. Before receiving the first enema, all subjects had a baseline sigmoidoscopy study at least 2 weeks before the first formulation. The first dose was administered in an inpatient setting followed by detailed analysis of distribution and toxicity via single photon emission computed tomography/computed tomography (SPECT/CT) imaging and histology of colon biopsies. Three more doses were self-administered by the research participants in outpatient settings in the context of RAI. Inpatient enemas contained a radioactive small molecule to simulate added drug, and enabled measurement of colon distribution using SPECT/CT and changes in rectal mucosal permeability by measuring radiolabel in blood. Toxicity was assessed by quantifying tissue damage in colonic biopsies collected after dosing. Acceptability was assessed using a series of web-based questionnaires and a structured interview at the end of the study. All research participants and all study personnel were blinded to enema product type, which was dispensed by the unblinded investigational research pharmacist according to a randomization sequence.
Dose preparation and administration
Inpatient doses were prepared by mixing 1 mCi 99mTc-diethylene triamine pentaacetic acid (DTPA) (Cardinal Health, Halethorpe, MD) with 125 ml test enema and administered to research participants. For outpatient dosing, the volunteers were provided with three individual doses of each enema type without radiolabel following hospital discharge. Volunteers self-administered these doses in the context of RAI during periods ranging from weeks to several months until completion.
Sigmoidoscopy and biopsy collection
Sigmoidoscopy by a gastroenterologist blinded to treatment assignment was performed approximately 1 h after the first dose of each of the three study products. The procedure was performed using a flexible sigmoidoscope (Evis Exera, Olympus America Corp., Center Valley, PA) with the subject in the left lateral decubitus position. Once the sigmoidoscope was inserted, 3.3-mm pinch-biopsy forceps (Microvasive no. 1599; Boston Scientific Corp., Natick, MA) were introduced through the sigmoidoscope and biopsies were acquired separately at a 5, 10, and 20 cm distance from the anus following this same sequence to prevent contamination due to scope movement. Each biopsy was placed in tissue culture RPMI 1640 medium containing l-glutamine, 25 mM HEPES (Mediatech, Herndon, VA) pen/strep antibiotic, and 10% fetal bovine serum (Invitrogen, Carlsbad, CA). Endoscopic brushes (Cytobrush model no. 60315; Ballard Medical Products, Draper, UT) were used separately to collect sample from the mucosal surface. Two biopsies and three brushes were taken at each designated location. From prior studies, we have determined that proximal distribution of rectally applied radiotracer is typically beyond the 20 cm distance, so sigmoidoscopy was not believed to alter the upper extent of enema distribution.23,24 Biopsies were fixed in formalin in preparation for histological examination.
Mucosal interferon-gamma
Levels of interferon-gamma (IFN-γ) mRNA from colonic brush samples were measured using qPCR as previously described.25 Standardization across samples was achieved by expressing cytokine copy number per 106 beta-actin copies per sample. All assays were performed in triplicate and reported as mean from the three measurements.
SPECT/CT imaging distribution
After sigmoidoscopy at 2, 4, and 24 h after dosing, SPECT/CT imaging was performed to assess colonic distribution of the radiolabel. Participants were imaged using a dual-head VG SPECT/CT series system (GE Medical Systems, Waukesha, WI) equipped with a CT (computed tomography) unit (Hawkeye) as previously described.23,25 In brief, CT images were acquired before SPECT acquisition for anatomical reference and to generate an attenuation correction map for SPECT image reconstruction. CT images were reconstructed with a filtered back projection algorithm onto a 256×256-matrix size. After SPECT acquisition, images were reconstructed using the OSEM algorithm26 and fused using the General Electric eNTEGRA workstation, software version 1.04 (GE Medical Systems, Waukesha, WI) into a 128×128×128 matrix size with each voxel representing 3.45 mm3. In images showing activity in the bladder or on the perianal or intergluteal skin, the signal was subtracted using in-house software.
Curve-fitting and concentration-by-distance calculations were done using R version 2.13.1 (The R Foundation for Statistical Computing, Vienna, Austria) according to previously described algorithms.25,27 In short, a flexible principal curve algorithm was used to construct a three-dimensional curve based on the colon images. After the centerline was constructed, a concentration-by-distance curve was estimated along the centerline using the orthogonal projections. The origin of the centerline was normalized to the coccygeal plane defined as the transverse plane crossing the coccyx in the SPECT/CT as previously described.28 The distance between the origin of the centerline and the coccygeal plane was recorded as Dmin (minimum distance associated with the closest point where a radiolabeled signal was detected within the luminal path of the colon). Previously defined imaging pharmacokinetic-distance parameters—Dmax (distance associated with the most proximal radiolabel signal within the colon), DCmax (distance at concentration maximum), and Dave (mean residence distance)—were calculated for further analysis.28
Mucosal permeability
Four milliliter blood samples were drawn at 2, 4, 8, 16, and 24 h postdose. Plasma was separated, gamma emissions in 1 ml plasma aliquots were measured on a gamma counter (Wizard2automatic gamma counter model 2480, PerkinElmer, Waltham, MA) within a 110- to 150-keV energy window, and data were corrected for decay relative to the time of dosing. Radioactivity in the plasma was expressed as a fraction of the dose administered to normalize readouts among subjects and products. Area under the concentration-time curve for 24 h (AUC0–24) and peak concentration (Cmax) were calculated using WinNonlin (Pharsight, 5.1, Cary, NC).
Colon histology
Six formalin-fixed biopsies were embedded, sectioned, stained with hematoxylin-eosin, and assessed by a pathologist blinded to sampling level and enema assignment. Epithelial surface denudation was measured as previously described19 using a categorical scale ranging from 0 to 3: 0, surface intact; 1, less than 1/3rd of the surface denuded; 2, 1/3rd to 2/3rd of the surface denuded; 3, more than 2/3rd denudation. Lamina propria hemorrhage was also scored on a scale of 0 to 3: 0, no hemorrhaging seen; 1, less than 1/3rd of fields with hemorrhaging; 2, between 1/3rd and 2/3rd of fields with hemorrhaging; 3, hemorrhaging detected in more than 2/3rd of the fields examined.
Acceptability
Acceptability of enemas was assessed through a series of web-based questionnaires completed in private settings by the research participants. Questionnaires were conducted (1) prior to receiving any product [Baseline Behavioral Questionnaire (BBQ)], (2) after each inpatient and outpatient product dose [Brief Acceptability Questionnaire (BAQ)], (3) at the end of each product phase [Product Acceptability Questionnaire (PAQ)], and (4) at the end of the study [Overall Product Preference Questionnaire (OPP)]. Subjects also participated in a semistructured interview at the end of the study.
Data analysis and sample size
Nine research participants provided the ability to detect an effect size of 1.25 standard deviation units relative to the mean with 80% power using two-sided, 5% alpha error in a paired analysis. To account for multiple levels of variables in several of the analyses (e.g., product type, biopsy location, multiple biopsies) we used a multilevel analysis using the baseline assessment as reference where available (e.g., histology) and the isoosmolar enema as reference for drug distribution, concentration, and permeability assessments. Data were analyzed using the statistical package STATA/IC 11.2 for Windows software (StataCorp LP, College Station, TX). Statistical significance was defined as a p-value<0.05. To analyze the acceptability data, we used a generalized linear model to compare acceptability ratings among the three products controlling for past douching behavior and likelihood of using microbicidal enemas in the future as assessed at the baseline assessment. The normal distribution and identity link were used to analyze continuous variables, whereas the binomial distribution and logit link were chosen to analyze dichotomous outcomes. Generalized estimating equations (GEE) techniques were employed to analyze acceptability data for each outpatient product dose in order to correct for biased estimation of standard errors when correlated within-subject factors are present in time-series data sets. We selected an autoregressive working correlation matrix for all analyses as such a selection represented the best model fit according to the Quasi Likelihood under Independence Model Criterion.
Results
SPECT/CT evaluation of distribution and retention of enema
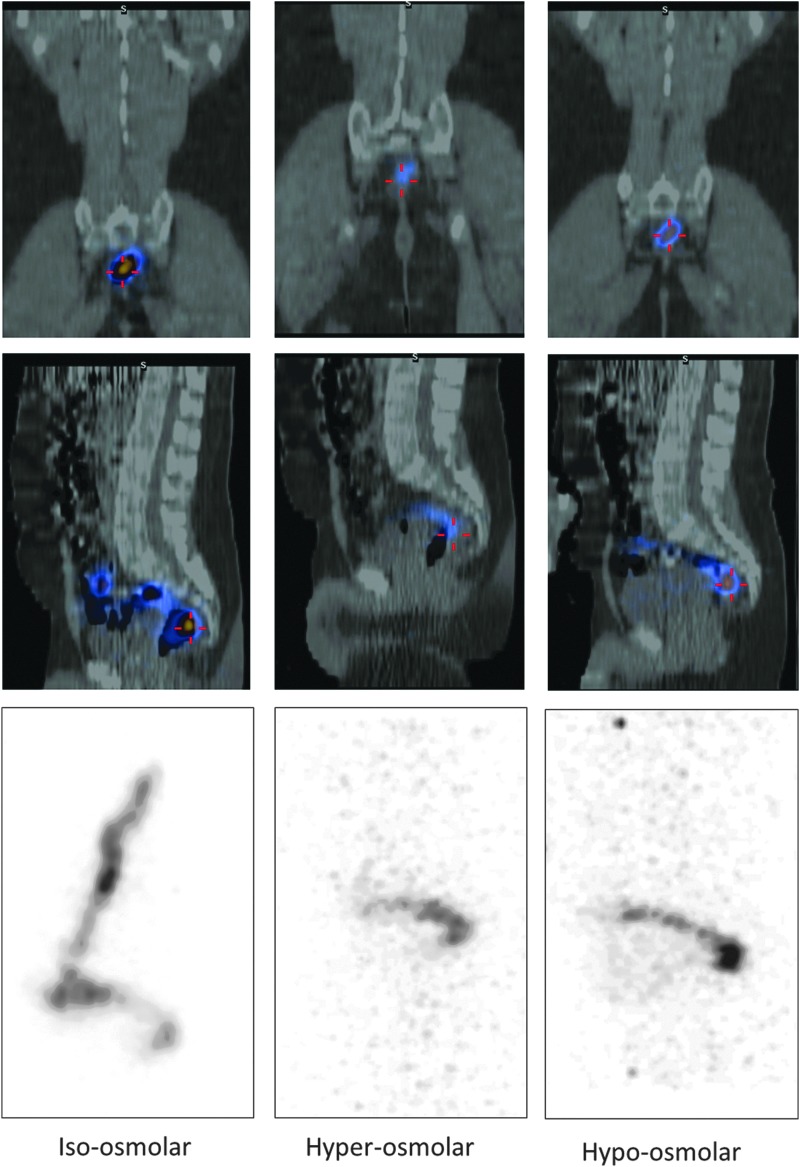
SPECT/CT imaging postdosing revealed consistent qualitative differences in colon distribution among the enema formulations (Fig. 1). Typically, 2-h postdose fused SPEC/CT images show that the isoosmolar enema typically distributed through the distal colon and, in some cases, up to the splenic flexure. By contrast, the hyperosmolar formulation was mostly confined to the rectosigmoid. In fact, no radiolabel was seen 2 h after the hyperosmolar enema dose in three of nine subjects. The hypoosmolar formulation showed a distribution intermediate between the hyperosmolar and isoosmolar products. The 24-h postdose SPEC/CT scans showed retained radiolabel in none of the hyperosmolar, three of the isoosmolar, and one of the hypoosmolar research participants.
FIG. 1.
Single photon emission computed tomography/computed tomography (SPECT/CT) imaging of a research participant 2 h following intrarectal dosing with each enema. The first and second row from the top show the coronal and left sagittal view of SPECT/CT, respectively. The CT image is in grayscale indicating bone and soft tissue. The SPECT image of the 99mTc-diethylene triamine pentaacetic acid (DTPA) signal is superimposed in color scale from high (yellow) to low (blue) intensity. [Note: this fused image includes only one sagittal plane (slice) that has the greatest intensity.] The third row shows SPECT maximal intensity projections (MIP) of 99mTc-DTPA activity in grayscale corresponding to the left sagittal view in the second row. [Note: The MIP images show the greatest signal intensity from among all sagittal planes (slices) projected onto a two-dimensional sagittal image. The MIP shows the full distribution more fully because it includes all, not only one sagittal plane, unlike the fused SPECT/CT images.] The isoosmolar enema (left panels) shows radiolabel throughout the rectum, sigmoid, and descending colon. The hyperosmolar and hypoosmolar enemas show distribution limited to the rectosigmoid and lower in intensity indicated by the upscaled background intensity.
Assessment of quantitative pharmacokinetic-distance parameters showed the isoosmolar enema had greater (more proximal) Dmax, DCmax, and Dave (Table 1) when compared to the hyperosmolar enema (mean difference of 21.2, 12.9, and 12.2 cm, respectively, p<0.05) and the hypoosmolar enema (mean difference of 12.7, 11, and 12.1 cm, respectively, p<0.05) adjusting by time from dose (2 or 4 h). In addition, the isoosmolar enema had a lower Dmin (more distal location) when compared to the hyperosmolar enema (mean difference of 1.6 cm, p<0.05) adjusting by time from dose.
Table 1.
Imaging Pharmacokinetic-Distance Parameters by Product at 2 and 4 h After Dosing, Median (25th Percentile, 75th Percentile)
| Isoosmolar | Hyperosmolar | Hypoosmolar | |
|---|---|---|---|
| Dmax (cm) | |||
| 2 h | 41* (34.7–45.3) | 19.3 (ND–29.9) | 25.5 (18.2–35.1) |
| 4 h | 38.6* (23.8–41.7) | ND (ND–32.4) | 15.3 (13.8–31.1) |
| DCmax (cm) | |||
| 2 h | 22.7* (12.6–29.1) | 6.9 (ND–7.9) | 5.3 (4.4–9.4) |
| 4 h | 17.5* (8.2–24.1) | ND (ND–7.9) | 6 (2.5–10.5) |
| Dave (cm) | |||
| 2 h | 20.6* (17.4–25.6) | 7.7 (ND–7.8) | 8.6 (6.8–11.8) |
| 4 h | 19.6* (9.8–23.6) | ND (ND–14.4) | 7.9 (5.1–10.3) |
| Dmin (cm) | |||
| 2 h | −0.001 (−0.3–3.4) | 1.5† (0.6–2) | 1.3 (0.6–1.7) |
| 4 h | 2 (−1.3–3.4) | 2.9† (1.8–5.1) | 1.7 (0.01–2) |
p<0.05, multilevel analysis showed the isoosmolar enema had greater Dmax, DCmax, and Dave when compared with the hyperosmolar and hypoosmolar enema adjusting by time from dose.
p<0.05, multilevel analysis showed the isoosmolar enema had lower Dmin when compared with the hyperosmolar enema adjusting by time from dose.
Dmax, most proximal point (furthest from anus) where radiosignal was detected; DCmax, distance at concentration maximum; Dave, mean residence distance; Dmin, distance associated with the most distal signal; ND, no signal detected.
Histology
Qualitative examination of postenema biopsy sections revealed that the isoosmolar and hypoosmolar enema had minimal or no effect on the colonic epithelium while the hyperosmolar enema caused sloughing of the epithelial layer (figure not shown). Quantitative scoring of biopsy sections supported this initial assessment. The epithelial surface denudation caused by isoosmolar and hypoosmolar enemas was indistinguishable from baseline (Table 2). However, biopsy sections taken after the hyperosmolar enema demonstrated significantly greater epithelial sloughing. The odds of having a higher epithelial denudation score in comparison with baseline were 4.2 [95% confidence interval (CI) 1.7–10.1; p<0.05]. Regarding lamina propria hemorrhage, the hyperosmolar enema caused the most damage with an odds of 2.2 (1.04–4.8) compared to baseline (p<0.05) (Table 2) after adjusting for biopsy site, while the isoosmolar and hypoosmolar treatments had no significant effect (p>0.05).
Table 2.
Histology, Interferon Gamma Induction, Tissue and Luminal Concentrations Among Products, Geometric Mean Ratios and 95% Confidence Interval
| Product | Baseline | Isoosmolar | Hyperosmolar | Hypoosmolar |
|---|---|---|---|---|
| Surface denudation | 1 | 0.5 (0.2–1.5) | 4.2* (1.7–10.1) | 0.6 (0.2–1.7) |
| Lamina propria hemorrhage | 1 | 0.8 (0.3–1.7) | 2.2* (1.04–4.8) | 0.8 (0.4–1.8) |
| Interferon gamma | 1 | 0.57* (0.3–0.8) | 0.57* (0.3–0.8) | 0.67 (0.4–1.02) |
| Concentration | ||||
| Biopsies | — | 1 | 0.1† (0.07–0.15) | 0.5† (0.3–0.8) |
| Brushes | — | 1 | 0.02† (0.13–0.03) | 0.09† (0.06–0.13) |
p<0.05 in comparison with baseline, predosing.
p<0.05 in comparison with isoosmolar.
Interferon-gamma
Colonic mucosa mRNA expression of IFN-γ was decreased 1 h after isoosmolar and hyperosmolar enemas in comparison with baseline (p<0.05) (Table 2). No change from baseline was seen after the hypoosmolar distilled water enema.
Tissue and luminal concentrations
Concentrations of the enema radiolabel in colonic biopsies after hyperosmolar and hypoosmolar products were lower compared to the isoosmolar enema, 9.4 and 1.8 times, respectively (p<0.01), after adjusting for the distance where colonic biopsies were taken (Table 2). Tissue concentrations at 10 cm were 1.6 times greater (95% CI 1.1–2.4; p<0.05) than at 5 cm and 1.5 times greater (95% CI 1.02–2.3; p<0.05) than at 20 cm after adjusting for enema type.
Similarly, luminal brush concentrations following hyperosmolar and hypoosmolar products were both lower (p<0.01) than with the isoosmolar enema, 48.5 and 11.1 times, respectively (p<0.01), adjusting for the distance where samples were taken. Luminal brush concentrations at 10 and 20 cm were 3.4 (95% CI 2.3–5.1; p<0.01) and 2.1 (95% CI 1.4–3.1; p<0.01) times greater, respectively, than at 5 cm after adjusting for enema type.
Permeability
The hypoosmolar enema had a greater plasma 99mTc-DTPA AUC0–24 when compared to the hyperosmolar enema and a greater Cmax when compared to the isoosmolar enema (Table 3; both p<0.05). In addition, the isoosmolar enema had a greater Tmax when compared with the hyperosmolar and hypoosmolar enemas.
Table 3.
Permeability Parameters by Product, Median (25th Percentile, 75th Percentile)
| Isoosmolar | Hyperosmolar | Hypoosmolar | |
|---|---|---|---|
| AUC0–24 (×107 μCi·h/ml) | 19.7 (8.4–27.4) | 9.4 (8.7–13) | 21.4* (17.4–44.9) |
| Cmax (×107 μCi/ml) | 1.7 (0.7–2.5) | 2.1 (1.5–2.8) | 3.5† (2.8–8) |
| Tmax (h) | 3.7‡ (2.6–4) | 2 (1.7–3) | 2.2 (1.4–2.5) |
p<0.05, multilevel analysis showed the hypoosmolar enema to have greater AUC when compared to the hyperosmolar enema.
p<0.05, multilevel analysis showed the hypoosmolar enema to have greater Cmax when compared to the isoosmolar enema.
p<0.05, multilevel analysis showed the isoosmolar enema to have a greater Tmax when compared to the hyperosmolar and hypoosmolar enemas.
Acceptability of enema products
According to data from the baseline assessment, all nine participants had used enemas in preparation for sex, ranging from 2 to 24 times in the prior 6 months, six of whom reported using enemas always or frequently before sex. Only two participants used enemas following sex. At baseline, five of nine subjects (56%) indicated they were extremely likely to use a microbicidal enema in the future. In the BAQ administered after every enema dose, seven of nine participants indicated no change or increase in sexual satisfaction. A different subject for each product identified each of the three products as unacceptable. After all doses of a product had been used, when participants were asked to indicate overall, how much they liked the product in the PAQ using a Likert scale (10, “liked very much”), the three enemas were rated similarly: mean (SD) hyperosmolar 7.8 (2.4), isoosmolar 7.6 (2.6), and hypoosmolar 7.7 (2.8), with all three products having a range in scores from 2 to 10.
In specific questions related to sexual enjoyment of participant or partner, application of the product, and likelihood of future use of the product, the isoosmolar product tended to have higher scores than the other products. The GEE analysis (Table 4) indicates greater sexual enjoyment of the isoosmolar product compared to each of the other products (p<0.04) and greater liking of the application of the isoosmolar compared to the hyperosmolar product (p=0.04). In other categories, all products were scored comparably (Table 4). After participants had used all three products, they were asked to rank the product that they (1) liked the most and (2) liked the least. All three products were “liked the most” by two or three out of the nine subjects. However, the hyperosmolar product was “liked the least” by four of nine subjects.
Table 4.
Generalized Estimating Equations Models Comparing Enema Acceptability by Study Product
| Outcome | Comparison | β | SE | 95% CI | Wald | p |
|---|---|---|---|---|---|---|
| Brief Acceptability Questionnaire | ||||||
| Enema acceptabilitya | Hyper vs. iso | 0.17 | 0.41 | −0.63–0.97 | 0.17 | 0.68 |
| Hypo vs. iso | −0.16 | 0.55 | −1.30–0.91 | 0.09 | 0.77 | |
| Product Acceptability Questionnaire | ||||||
| Liking the enemab | Hyper vs. iso | 0.53 | 0.39 | −0.24–1.29 | 1.83 | 0.18 |
| Hypo vs. iso | −0.34 | 0.99 | −2.29–1.60 | 0.12 | 0.73 | |
| Sexual enjoyment after enema useb | Hyper vs. iso | −1.16 | 0.58 | −2.29–−0.03 | 4.06 | 0.04 |
| Hypo vs. iso | −0.96 | 0.35 | −1.65–−0.28 | 7.55 | 0.01 | |
| Sexual satisfaction after enema use (self)c | Hyper vs. iso | −0.75 | 0.61 | −1.96–0.45 | 1.78 | 0.18 |
| Hypo vs. iso | −0.33 | 0.44 | −1.19–0.54 | 0.55 | 0.46 | |
| Sexual satisfaction after enema use (partner)c | Hyper vs. iso | −0.37 | 0.44 | −1.23–0.49 | 0.72 | 0.40 |
| Hypo vs. iso | −0.42 | 0.52 | −1.43–0.59 | 0.66 | 0.42 | |
| Sexual satisfaction with partner in general when you have receptive anal intercourse not using this study productc | Hyper vs. iso | 0.44 | 0.38 | −0.30–1.18 | 1.35 | 0.25 |
| Hypo vs. iso | −0.94 | 0.77 | −2.45–0.56 | 1.50 | 0.22 | |
| Difference between sexual satisfaction after douching vs. sexual satisfaction in generald | Hyper vs. iso | −1.14 | 0.70 | −2.51–0.23 | 2.66 | 0.10 |
| Hypo vs. iso | 0.64 | 0.86 | −1.06–2.33 | 0.54 | 0.46 | |
| Liking the process of applying enemab | Hyper vs. iso | −1.70 | 0.84 | −3.34–−0.06 | 4.15 | 0.04 |
| Hypo vs. iso | −0.76 | 0.97 | −2.65–1.14 | 0.61 | 0.44 | |
| Likelihood of future usee | Hyper vs. iso | −0.62 | 0.34 | −1.27–0.04 | 3.36 | 0.07 |
| Hypo vs. iso | −0.32 | 0.37 | −1.04–0.40 | 0.75 | 0.39 | |
| Likelihood of use without condomse | Hyper vs. iso | −0.10 | 0.67 | −1.40–1.20 | 0.02 | 0.88 |
| Hypo vs. iso | −0.32 | 0.42 | −1.14–0.50 | 0.59 | 0.44 | |
1=“Completely unacceptable,” 2=“Somewhat unacceptable,” 3=“Somewhat acceptable,” and 4=“Highly acceptable.”
1=‘‘Disliked very much’’ to 10=‘‘Liked very much.’’
1=‘‘Not at all’’ to 10=‘‘Very much.’’
Scores were computed by summing the difference between the sexual satisfaction ratings after product use and ratings of how satisfied they felt in general. Positive scores reflect increased satisfaction and negative scores reflect decreased satisfaction after douching with the study product.
1=‘‘Extremely unlikely’’ to 10=‘‘Extremely likely.’’
The regression coefficients β represent the adjusted mean differences on acceptability scores between groups, controlling for douching frequency and likelihood of using microbicidal enemas in the future as reported at the baseline assessment.
Discussion
From a small number of intensively sampled research participants, we identified the isoosmolar candidate as superior for future microbicide enema development based on assessment of drug distribution and retention, toxicity, and acceptability. We used quantitative measures to enable this direct paired comparison and identified statistically significant differences in a fairly small number of healthy volunteers. Only in the domain of acceptability measures were there minor or no differences among products. Even for this critically important acceptability parameter with the potential for significant impact on adherence, the isoosmolar product was generally superior to the other products either by strict statistical criteria or trends in that direction. Given that the use of enemas is already common among MSM, we anticipate that an isoosmolar enema vehicle could be a favorable option as a rectal microbicide delivery device, especially for those accustomed to commercial enemas and/or tap water enemas. While we did not test it, an isoosmolar rectal microbicide enema might also be formulated as a powder in a small portable packet to be added to tap water for greater portability and convenience.
For a microbicide to be effective, it needs to both outdistance and outlast the HIV inoculum. SPECT/CT imaging after enema dosing and evacuation showed that at least a portion of the isoosmolar enema was retained with contiguity well up into the descending colon in most subjects. In contrast, the hyperosmolar enema did not remain at locations beyond the rectosigmoid. In fact, finding no residual radiolabel was a common result and not surprising given the purpose of the commercial enema. Hypoosmolar enemas demonstrated a distribution intermediate between the other enemas. In quantitative comparisons, the isoosmolar enema distributed both more proximally and more distally in the colon compared to the other enema types.
For context, using simulated RAI with cell-free and cell-associated HIV surrogates in autologous seminal plasma, we recently demonstrated that the location of HIV surrogates in the colon following RAI is confined to the rectosigmoid colon, with greatest concentrations between 15 and 20 cm.29 The isoosmolar enema following evacuation consistently covered both the lower and upper reaches of this distribution in our study and the peak concentration (DCmax) was largely coincident with this distribution at 2 and 4 h. By contrast, the hyperosmolar enema distribution following evacuation fell short of that HIV surrogate distribution in most research participants. The hypoosmolar enema covered that peak HIV distribution initially at 2 h, but fell short by 4 h. The highest concentrations (DCmax) of the hyperosmolar and hypoosmolar enemas were both far short of the peak HIV concentration.
Within the critical 15–20 cm colonic location, the concentration of radiolabel in tissue biopsies and luminal brushes was lower for both hyperosmolar and hypoosmolar enemas compared to a isoosmolar enema. For the hyperosmolar enema, this was not surprising given the bulk fluid shifts into the colonic lumen caused by a hyperosmolar enema. Considering both the luminal distribution and the tissue concentrations, it appears that the degree of dose retention after evacuation—lower for both hypoosmolar and hyperosmolar enemas compared to the isoosmolar enema—had the more dominant effect on the 1 h tissue and luminal concentrations compared to osmolality driven fluid shifts to or from tissue.
Despite its transient presence, the hyperosmolar enema reduced epithelial integrity and increased lamina propria hemorrhage whereas the other enemas were no different than baseline. This finding recapitulates the effect of hyperosmolarity seen in other studies.19,20,30,31 While the significance of these histological changes for HIV susceptibility remains unclear, it raises a cautionary flag with unclear clinical implications.
A comparative study of ex vivo infectibility of colorectal biopsies exposed to these three enema types in vivo, as tested in other studies,32,33 would provide useful insight. Previous studies have shown evidence of a correlation between increased epithelial IFN-γ in biopsies and ex vivo HIV infection susceptibility.24 Our study did not find any increase in IFN-γ among the three vehicles when compared with baseline untreated colonic tissue. In fact, all vehicles showed trends or significant decreases of IFN-γ compared to baseline.
With regard to permeability, the hyperosmolar enema had the lowest plasma 99mTc AUC0–24 and Cmax among enemas studied. This finding is consistent with a prior study of hyperosmolar gels in which histological damage was also seen with the hyperosmolar gel, but not the isoosmolar gel.19 We reasoned that even though there was a loss of epithelial integrity with the hyperosmolar products, both gel and enema, the net fluid flow into the hyperosmolar colonic lumen resulted in reduced permeability across the mucosal tissue into the blood where we measured the radioisotopes. This result is also consistent with the poor tissue penetration of the radiolabel in the hyperosmolar enema. Mucosal permeability was greatest with the hypoosmolar enema, measured by AUC0–24 and Cmax, compared to the hyperosmolar enema. This difference was anticipated due to the fluid shifts described above. We intended to use permeability as a toxicity measure, but this association is not at all clear. Increased permeability to drug, in contrast to HIV, could be a favorable microbicide vehicle trait if it also increases tissue drug concentrations to a greater extent than HIV penetration. However, this increased drug permeability could also increase the risk of systemic drug toxicity or increase the risk for HIV resistance if low, but nonsuppressive, concentrations are achieved in the blood.
Regarding acceptability assessments, the small sample size limits the statistical power to draw conclusive results in some categories. Nevertheless, trends seem to indicate that the isoosmolar enema has good acceptability and relatively better acceptability than the hyperosmolar enema. Participants appeared more neutral with respect to the hypoosmolar enema. Most participants felt that enemas increased or did not affect their sexual satisfaction and that of their partners. This is an important consideration that supports the finding of likely future use of microbicidal enemas if they are found to be effective in preventing HIV transmission. The fact that participants were experienced in using enemas in preparation for sex indicates that these participants had a frame of reference for what to expect from using a rectal enema. A larger sample would have allowed us to analyze associations between specific types of enema used prior to study participation and ratings of enemas used in the study. As expected from volunteers in a study like ours, participants already had a good disposition toward using microbicidal enemas in the future even before trying any of the products. Nevertheless, prior studies have observed that substantial proportions of MSM use enemas frequently in preparation for sex and that they are interested in using enemas that may have protective properties.13,16 The present study adds further evidence to support this point.
The isoosmolar product we studied would be too expensive for practical use and not very portable. It was selected out of convenience as a test product, having passed the rigorous testing required of an intravenous product, having a reputation for cytological preservation, and having no deleterious effects in prior colon studies.34,35 Since our first report that a hyperosmolar gel produced mucosal damage, commercial isoosmolar enema products have been developed. The suitability of these products as rectal microbicide vehicles, however, will require rigorous testing to determine their safety with regard to HIV transmission; no such testing is currently required prior to marketing enema products or, for that matter, sexual lubricants. As noted above, to avoid the inconvenience of carrying about an enema bottle, if tap water was available, an ARV microbicide formulated as a small, portable powder or rapidly dissolving tablet that establishes isoosmolarity in solution at the time of use could be another viable option. This powder or tablet, of course, would require the same rigorous testing with the active ARV ingredient, in combination with suitable liquid vehicles, to demonstrate its safety and effectiveness in well-designed clinical studies.
This raises several important limitations of our study. First, the mucosal changes identified are of uncertain significance regarding HIV transmission, which remains to be assessed. Second, we sampled tissue only at 1 h after the dose and did not capture the rapid reconstitution of gut mucosa demonstrated previously.36,37 Similarly, we measured only gamma interferon among other inflammatory indicators (guided by the work of others24); therefore, later changes could not be assessed. The radioactive half-life of our radiolabel prevented observation beyond 24 h, but this time was sufficient to demonstrate substantial differences among our enema types and persistence of the isoosmolar enema in one-third of our subjects up to this time. One day of luminal product retention, however, may be inadequate or irrelevant as efficacy likely depends on tissue concentrations of both ARV and HIV over time. Finally, our radiolabel is only a surrogate small molecule and not an ARV. While we believe the luminal distribution will be similar to ARVs, the tissue kinetics of each ARV should be assessed separately and, critically, for coitally dependent PrEP suitability.
We present a composite picture favoring an isoosmolar enema assessed across multiple relevant domains, including distribution, retention, safety, and acceptability. The study demonstrates the feasibility of simultaneously testing multiple key domains in a small first-in-human study that includes an acceptability assessment of the product in the context of RAI with a partner. While an isoosmolar enema appears to be a suitable candidate vehicle for rectal PrEP delivery, individual candidate ARVs will need rigorous testing to demonstrate safety, adequate tissue persistence, and antiretroviral effect in order to advance an isoosmolar ARV enema as a rectal PrEP strategy.
Acknowledgments
The authors wish to thank our research participants for their volunteerism and for enduring the rigors of this intensive and invasive clinical study. We wish to thank the personnel of the Drug Development Unit and the Clinical Pharmacology Analytical Laboratory at Johns Hopkins University Division of Clinical Pharmacology and the Administrative and Regulatory Cores of the Microbicide Development Program at UCLA for their excellent technical support. We are grateful for the financial support of the NIH/NIAID Division of AIDS Integrated Pre-Clinical/Clinical Program for Microbicide Development (U19 AI060614) and the NIH/NIGMS Fellowship Training Program in Clinical Pharmacology (5T32GM066691-10).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.CDC. HIV and AIDS in the United States. 2010.
- 2.Franssens D. Hospers HJ. Kok G. Social-cognitive determinants of condom use in a cohort of young gay and bisexual men. AIDS Care. 2009;21(11):1471–1479. doi: 10.1080/09540120902883127. [DOI] [PubMed] [Google Scholar]
- 3.Hill SC. King G. Smith A. Condom use and prevalence of sexually transmitted infection among performers in the adult entertainment industry. Int J STD AIDS. 2009;20(11):809–810. doi: 10.1258/ijsa.2009.009381. [DOI] [PubMed] [Google Scholar]
- 4.Mendoza-Perez JC. Ortiz-Hernandez L. [Factors associated with infrequent condom use amongst men having sex with other men in Ciudad Juarez] Rev Salud Publica (Bogota) 2009;11(5):700–712. doi: 10.1590/s0124-00642009000500003. [DOI] [PubMed] [Google Scholar]
- 5.Stolte IG. de Wit JB. Kolader M. Fennema H. Coutinho RA. Dukers NH. Association between 'safer sex fatigue' and rectal gonorrhea is mediated by unsafe sex with casual partners among HIV-positive homosexual men. Sex Transm Dis. 2006;33(4):201–208. doi: 10.1097/01.olq.0000194596.78637.8e. [DOI] [PubMed] [Google Scholar]
- 6.Grant RM. Lama JR. Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Damme L. Ramjee G. Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: A randomised controlled trial. Lancet. 2002;360(9338):971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 8.Van der Straten A. Van Damme L. Haberer JE. Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26(7):F13–19. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 9.Baeten JM. Donnell D. Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thigpen MC. Kebaabetswe PM. Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 11.Baral S. Scheibe A. Sullivan P, et al. Assessing priorities for combination HIV prevention research for men who have sex with men (MSM) in Africa. AIDS Behav. 2012;17(Suppl 1):560–568. doi: 10.1007/s10461-012-0202-5. [DOI] [PubMed] [Google Scholar]
- 12.Carballo-Dieguez A. Bauermeister J. Ventuneac A. Dolezal C. Mayer K. Why rectal douches may be acceptable rectal-microbicide delivery vehicles for men who have sex with men. Sex Transm Dis. 2010;37(4):228–233. doi: 10.1097/OLQ.0b013e3181bf9b2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carballo-Dieguez A. Bauermeister JA. Ventuneac A. Dolezal C. Balan I. Remien RH. The use of rectal douches among HIV-uninfected and infected men who have unprotected receptive anal intercourse: Implications for rectal microbicides. AIDS Behav. 2008;12(6):860–866. doi: 10.1007/s10461-007-9301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hylton J. Fuchs EJ. Hendrix CW. Microbicides; London: 2004. An assessment of sexual practices affecting the feasibility of microbicide development among MSM. [Google Scholar]
- 15.Kinsler JJ. Galea JT. Lama JR, et al. Rectal douching among Peruvian men who have sex with men, and acceptability of a douche-formulated rectal microbicide to prevent HIV infection. Sex Transm Infect. 2013;89(1):62. doi: 10.1136/sextrans-2012-050630. [DOI] [PubMed] [Google Scholar]
- 16.Schilder AJ. Orchard TR. Buchner CS. Strathdee SA. Hogg RS. Insert discourse: Rectal douching among young HIV-positive and HIV-negative men who have sex with men in Vancouver, Canada. Sex Cult. 2010;14(4):327. [Google Scholar]
- 17.Coates RA. Calzavara LM. Read SE, et al. Risk factors for HIV infection in male sexual contacts of men with AIDS or an AIDS-related condition. Am J Epidemiol. 1988;128(4):729–739. doi: 10.1093/oxfordjournals.aje.a115026. [DOI] [PubMed] [Google Scholar]
- 18.Moss AR. Osmond D. Bacchetti P. Chermann JC. Barre-Sinoussi F. Carlson J. Risk factors for AIDS and HIV seropositivity in homosexual men. Am J Epidemiol. 1987;125(6):1035–1047. doi: 10.1093/oxfordjournals.aje.a114619. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs EJ. Lee LA. Torbenson MS, et al. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: Potential implication for HIV transmission. J Infect Dis. 2007;195(5):703–710. doi: 10.1086/511279. [DOI] [PubMed] [Google Scholar]
- 20.Kameda H. Abei T. Nasrallah S. Iber FL. Functional and histological injury to intestinal mucosa produced by hypertonicity. Am J Physiol. 1968;214(5):1090–1095. doi: 10.1152/ajplegacy.1968.214.5.1090. [DOI] [PubMed] [Google Scholar]
- 21.Leriche M. Devroede G. Sanchez G. Rossano J. Changes in the rectal mucosa induced by hypertonic enemas. Dis Colon Rectum. 1978;21(4):227–236. doi: 10.1007/BF02586697. [DOI] [PubMed] [Google Scholar]
- 22.Schmelzer M. Schiller LR. Meyer R. Rugari SM. Case P. Safety and effectiveness of large-volume enema solutions. Appl Nurs Res. 2004;17(4):265–274. [PubMed] [Google Scholar]
- 23.Hendrix CW. Fuchs EJ. Macura KJ, et al. Quantitative imaging and sigmoidoscopy to assess distribution of rectal microbicide surrogates. Clin Pharmacol Ther. 2008;83(1):97–105. doi: 10.1038/sj.clpt.6100236. [DOI] [PubMed] [Google Scholar]
- 24.McGowan I. Elliott J. Fuerst M, et al. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J Acquir Immune Defic Syndr. 2004;37(2):1228–1236. doi: 10.1097/01.qai.0000131846.12453.29. [DOI] [PubMed] [Google Scholar]
- 25.Caffo BS. Crainiceanu CM. Deng L. Hendrix CW. A case study in pharmacologic colon imaging using principal curves in single photon emission computed tomography. J Am Stat Assoc. 2008;103(484):1470–1480. doi: 10.1198/016214508000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hudson HM. Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging. 1994;13(4):601–609. doi: 10.1109/42.363108. [DOI] [PubMed] [Google Scholar]
- 27.Goldsmith J. Caffo B. Crainiceanu C. Reich D. Du Y. Hendrix C. Nonlinear tube-fitting for the analysis of anatomical and functional structures. Ann Appl Stat. 2011;5(1):337–363. doi: 10.1214/10-aoas384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao YJ. Caffo BS. Fuchs EJ, et al. Quantification of the spatial distribution of rectally applied surrogates for microbicide and semen in colon with SPECT and magnetic resonance imaging. Br J Clin Pharmacol. 2012;74(6):1013–1022. doi: 10.1111/j.1365-2125.2012.04267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louissaint NA. Nimmagadda S. Fuchs EJ, et al. Distribution of cell-free and cell-associated HIV surrogates in the colon after simulated receptive anal intercourse in men who have sex with men. J Acquir Immune Defic Syndr. 2012;59(1):10–17. doi: 10.1097/QAI.0b013e3182373b5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billich CO. Levitan R. Effects of sodium concentration and osmolality on water and electrolyte absorption form the intact human colon. J Clin Invest. 1969;48(7):1336–1347. doi: 10.1172/JCI106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubsamen K. Hornicke H. Influence of osmolality, short chain fatty acids and deoxycholic acid on mucus secretion in the rat colon. Pflugers Arch. 1982;395(4):306–311. doi: 10.1007/BF00580794. [DOI] [PubMed] [Google Scholar]
- 32.Anton PA. Saunders T. Elliott J, et al. First phase 1 double-blind, placebo-controlled, randomized rectal microbicide trial using UC781 gel with a novel index of ex vivo efficacy. PLoS One. 2011;6(9):e23243. doi: 10.1371/journal.pone.0023243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anton PA. Cranston RD. Kashuba A, et al. RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2012;28(11):1412–1421. doi: 10.1089/aid.2012.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasuti JF. Fleisher SR. Cobbs BC. Gupta PK. Semiquantitative analysis of the cellular preservation quality of Normosol and Carbowax solutions for thyroid fine-needle aspiration specimens. Diagn Cytopathol. 2000;22(5):319–322. doi: 10.1002/(sici)1097-0339(200005)22:5<319::aid-dc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Brown WJ. Kim BS. Weeks DB. Parkin CE. Physiologic saline solution, Normosol R pH 7.4, and Plasmanate as reconstituents of packed human erythrocytes. Anesthesiology. 1978;49(2):99–101. doi: 10.1097/00000542-197808000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Feil W. Lacy ER. Wong YM, et al. Rapid epithelial restitution of human and rabbit colonic mucosa. Gastroenterology. 1989;97(3):685–701. doi: 10.1016/0016-5085(89)90640-9. [DOI] [PubMed] [Google Scholar]
- 37.Phillips DM. Taylor CL. Zacharopoulos VR. Maguire RA. Nonoxynol-9 causes rapid exfoliation of sheets of rectal epithelium. Contraception. 2000;62(3):149–154. doi: 10.1016/s0010-7824(00)00156-6. [DOI] [PubMed] [Google Scholar]