Abstract
Bariatric surgery is associated with near immediate remission of type 2 diabetes and hyperlipidemia. The mechanisms underlying restoration of normal glucose tolerance post-operatively are poorly understood. Herein, we examined the effect of Roux-en-Y gastric bypass surgery (RYGB) on weight loss, insulin sensitivity, plasma ceramides, pro-inflammatory markers, and cardiovascular risk factors before and at 3 and 6 months after surgery. Thirteen patients (10 female; age 48.5±2.7 yrs; BMI, 47.4±1.5 kg/m2) were included in the study, all of whom had undergone laparoscopic RYGB surgery. Insulin sensitivity, inflammatory mediators and fasting lipid profiles were measured at baseline, 3 and 6 months post-operatively, using enzymatic analysis. Plasma ceramide subspecies (C14:0, C16:0, C18:0, C18:1, C20:0, C24:0, and C24:1) were quantified using electrospray ionization tandem mass spectrometry after separation with HPLC. At 3 months post surgery, body weight was reduced by 25%, fasting total cholesterol, triglycerides, low density lipoproteins, and free fatty acids were decreased, and insulin sensitivity was increased compared to pre-surgery values. These changes were all sustained at 6 months. In addition, total plasma ceramide levels decreased significantly postoperatively (9.3±0.5 nmol/ml at baseline vs. 7.6±0.4 at 3 months, and 7.3±0.3 at 6 months, p<0.05). At 6 months, the improvement in insulin sensitivity correlated with the change in total ceramide levels (r= −0.68, p=0.02), and with plasma TNF-α (r= −0.62, P=0.04). We conclude that there is a potential role for ceramide lipids as mediators of the proinflammatory state and improved insulin sensitivity after gastric bypass surgery.
Keywords: Obesity, Ceramide, Free Fatty Acid, Lipids, Insulin Sensitivity, Diabetes, TNF-α
INTRODUCTION
In Western societies, obesity has reached epidemic proportions. In fact over 65% of the U.S population is now either overweight or obese (1). Obesity is a strong risk factor for the development of insulin resistance (IR), type 2 diabetes, and cardiovascular disease (CVD)(1). The cellular and molecular mechanisms underlying the development of obesity-induced comorbidities are not completely understood, although evidence has emerged to support critical roles of adipose tissue and abnormal lipid metabolism in the pathophysiology (2). In addition, it is postulated that a chronic state of low grade inflammation, induced by pro-inflammatory cytokines and peptides secreted by adipose tissue, mediates the link between obesity and both CVD and IR (3, 4).
Ceramides, a family of sphingolipid molecules with important structural and functional roles in cell signaling, cell differentiation, proliferation and apoptosis, are a major component of ectopic fat in obese individuals. It is well documented that ceramide accumulates within tissues of animals and humans, and inhibits insulin action and subsequent glucose uptake through inactivation of Akt (5). Ceramides are also known to induce inflammation through activation of the nuclear factor-κB–tumor necrosis factor-α (TNF-α) axis, and facilitate inflammatory signaling pathways which further contribute to the state of IR (5, 6). Plasma ceramide levels have been shown to correlate with coronary artery disease (7), and we have shown that plasma ceramides are also elevated in obese type 2 diabetes and the increase correlates with the degree of IR and inflammation (8).
In addition, levels of ceramide metabolites such as sphingosine correlate significantly with cardiovascular disease (9–12), and abnormalities in sphingolipid metabolism lead to adipose tissue-induced inflammation (mediated by cytokines such as TNF-α, interleukins and C-reactive protein), which is a hallmark of obesity, diabetes, and cardiovascular disease risk (13–18). TNF-α is a pleiotropic cytokine, and its role in inflammation and metabolism is particularly complex. TNF-α is released from adipocytes, mononuclear cells (MNC), and macrophages in response to stimuli such as lipopolysaccharides and interleukin-1. Increases in TNF-α are known to induce IR, through interference with insulin signaling by inhibiting insulin receptor tyrosine kinase activity and tyrosine phosphorylation of one of its substrates, IRS-1 (19, 20). TNF-α also activates the plasma membrane enzyme sphingomyelinase (SMase), which hydrolyzes sphingomyelin to ceramide thereby initiating a positive feedback mechanism and propagating the production of proinflammatory cytokines from adipose tissue, with resultant inhibition of insulin-stimulated glucose uptake (5). Both plasma TNF-α and ceramides have been shown to be simultaneously elevated in obese subjects with type 2 diabetes (21).
Bariatric surgery is associated with near immediate remission of obesity-associated comorbidities, such as type 2 diabetes and hyperlipidemia (22, 23). Laparoscopic Roux-en-Y gastric bypass (RYGB) in particular has been associated with a decrease in IR, levels of inflammatory mediators, and functional markers of coronary atherosclerosis (24–26). Given their potential role as mediators of IR, inflammation, and weight loss, alterations in plasma ceramide levels after RYGB, may serve as a biomarker of IR and lipid-induced inflammation. The primary objective of this study was to quantify individual ceramide subspecies in the circulation of severely obese patients undergoing RYGB; at baseline, 3 months and 6 months postoperatively. In addition we examined the relationship between total plasma ceramide levels, weight loss, insulin sensitivity, and plasma TNF-α concentrations, at all time-points.
MATERIALS AND METHODS
Study cohort
All participants gave written informed consent. The study was approved by Institutional Review Board of the Cleveland Clinic Foundation. Our study cohort consisted of 13 morbidly obese patients undergoing laparoscopic RYGB, on the basis that they met the criteria for bariatric surgery as outlined by the National Institutes of Health Consensus Development Panel report of 1991 (27). All patients underwent an extensive preoperative evaluation including history and physical examination, nutritional and psychiatric assessments, consultation with internal medicine and anesthesiologists, in addition to mandatory attendance at a bariatric seminar and support group. Failure to comply with the required preoperative work-up disqualified a patient from participation in the study. Additional consultations with medical subspecialists (endocrinology, cardiology, pulmonary medicine) were obtained as clinically indicated. Individuals with known autoimmune disease, cancer, thrombotic disorders, and valvular heart disease were excluded, as were those who were unable or unwilling to cooperate with postoperative follow-up. Consenting participants were evaluated at three time-points for the purpose of this study; preoperatively, 3 and 6 months postoperatively. Each evaluation comprised a clinical and physical review, anthropometric measurements, cardiovascular risk assessment, and blood sampling for measurement of biochemical, metabolic, and inflammatory biomarkers. Blood samples were collected after a 12 hour fast using evacuated blood collection tubes containing EDTA.
Surgical Procedures
Laparoscopic RYGB was performed as described previously (23). Briefly, RYGB involves the following components: 1) Gastric restriction, by creating a 15–30 ml proximal gastric pouch that is separated from the distal stomach (gastric remnant); 2) Proximal intestinal bypass; a 150 cm roux limb (alimentary limb) is measured and anastomosed to the gastric pouch; 3) Exclusion of nutrient flow through the remnant stomach, duodenum and proximal jejunum, by connecting the biliopancreatic limb to the jejunum, 50 cm distal to the Ligament of Treitz. This is where food and digestive juices mix and continue distally in a common channel.
Analytical Measurements
Fasting blood samples were analyzed for concentrations of the following metabolites: plasma insulin (Diagnostic Products, Los Angeles, CA), plasma triglycerides and total cholesterol, which were assayed by enzymatic analysis (Roche Modular Diagnostics, Indianapolis, IN). Plasma free fatty acids were measured using a NEFA assay kit (Wako Chemical, Richmond, VA). An UtraSensitive ELISA (Biosourse International, Camarillo, CA) was used to measure plasma TNF-α concentrations. Insulin sensitivity was estimated using the quantitative insulin check index (ISQUICKI) (28). ISQUICKI is the inverse log sum of fasting insulin (I0) and fasting glucose (G0).
Ceramide Analysis by Liquid Chromotography and Mass Spectrometry
Calibration curves (0 to 1000 ng) for each ceramide standard (Avanti Polar Lipids, Alabaster, AL: purity >99%) were prepared in a 50 ul plasma matrix. C17:0 and C25:0 ceramides were used as non-naturally occurring internal standards. Plasma samples (50 ul), in parallel with standard solutions, were spiked with 10 ng of C17:0 and 20 ng of C25:0 ceramides, and were extracted with 2 ml of a chloroform/methanol (1:2) mixture according to the protocol of Bligh and Dyer (29). Ceramide species were quantified by HPLC on-line electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS)(30). Extracted samples (40 ul) were injected onto a Waters HPLC (2690 Separation Module, Waters, Corp., Franklin, MA) and separated through an Ascentis C18 column (2.1 × 50 mm, 5 um, SUPELCO, Bellefonte, PA) using a gradient starting from 15% mobile phase A (water containing 0.2% formic acid) at flow rate of 0.3 ml/min, to 100% mobile phase B (acetonitrile/2-propanol (60:40 v/v) containing 0.2% formic acid) over 3 min, and then with 100% B for 22 min. The HPLC column effluent was introduced onto a Micromass triple quadrupole mass spectrometer (Quattro Ultima, Waters Inc., Beverly, MA) and analyzed using electrospray ionization in positive mode. All the ceramides were quantified using multiple reaction monitoring. The MS/MS transitions (m/z) were 510 →264 for C14:0, 538→264 for C16:0, 552→264 for C17:0, 564→264 for C18:1, 566→264 for C18:0, 594→264 for C20:0, 648→264 for C24:1, 650→264 for C24:0, and 664→264 for C25:0. Ceramide subspecies were then quantified (nmol/ml) using calibration curves and the ratios of the integrated peak areas (MassLynx 3.5, Manchester, UK) of ceramide subspecies and internal standards. C17:0 ceramide was used as an internal standard for quantification of C14:0, C16:0, C18:0, and C20:0, subspecies. Concentrations of C24:0 and C24:1 were quantified using C25:0 as an internal standard. Total measured ceramide was calculated from the sum of C14:0, C16:0, C18:1, C18:0, C20:0, C24:0 and C24:1 ceramide subspecies.
Statistical analysis
All statistical analyses were performed using StatView version 5.0.1 (SAS Institute). Data are presented as mean ± SE. Comparisons over time for the ceramide species and key outcome variables was performed using repeated measures analysis of variance (ANOVA), and associations between variables were determined using Spearman correlation analyses. In all tests, p<0.05 was considered statistically significant.
RESULTS
Metabolic Characteristics
Thirteen patients were enrolled in this study and all completed 3 months follow-up, one patient was lost to follow-up at 6 months. Baseline demographic, anthropometric and biochemical data from the study cohort are presented in Table 1. The mean BMI (kg/m2) was 48.3 ± 3.0, and 30.5% were ‘super-obese’ (BMI >50). All patients had at least one obesity-related comorbidity confirmed or newly diagnosed during their preoperative assessment, including hypertension, diabetes mellitus or impaired glucose tolerance, dyslipidemia, and obstructive sleep apnea. At 3 months post-operatively, the mean BMI had decreased significantly from 47.4 ± 1.5 to 37.9 ± 1.3, corresponding to an average excess weight loss of 38.5%. RYGB resulted in significant decreases in fasting plasma insulin, glucose, free fatty acids, LDL and cholesterol/HDL ratio (P<0.05). At 6 months post RYGB, these reductions were sustained, excess weight loss was 51.7%, and additionally fasting triglyceride levels had also decreased significantly at this time (P<0.05). Resolution or improvement of all comorbidities at 6 months was documented, as determined by review of clinical findings and medication usage. There were no postoperative complications in this group at the 6-month follow-up time point.
Table 1.
Patient Characteristics
| Pre-Surgery | 3 months Post-Surgery | 6 months Post-Surgery | |
|---|---|---|---|
| N | 13 | 13 | 12 |
| Age, y | 48.5 ± 3 | 48.5 ± 3 | 49.5 ± 3 |
| Gender | 10 women/ 3 men | 10 women/ 3 men | 9 women/ 3 men |
| Weight (kg) | 128.2 ± 7.6 | 105.1 ± 6.9 * | 103.9 ± 8.8 * |
| BMI (kg/m2) | 47.4 ± 1.5 | 37.9 ± 1.3 * | 35.4 ± 1.5 * |
| Comorbidities | |||
| Type 2 Diabetes Mellitus | 23% (n=3) | - | - |
| Hypertension | 77% (n=10) | - | - |
| Hyperlipidemia | 85% (n=11) | - | - |
| Obstructive Sleep Apnea | 46% (n=6) | - | - |
| HbA1c (%) | 5.97 ± 0.38 | 5.49 ± 0.26 | 5.4 ± 0.24 |
| FPG(mg/dl) | 104.3 ± 5.8 | 88.9 ± 1.9 * | 86.9 ± 3.3 * |
| FPI (pg/ml) | 635.3 ± 102.0 | 338.8 ± 49.3 * | 283.6 ± 41.0 * |
| ISquicki | 0.32 ± 0.001 | 0.36 ± 0.01 * | 0.37± 0.01 * |
| FFA (mmol/l) | 0.7 ± 0.1 | 0.4 ± 0.1 * | 0.3 ± 0.01 * |
| TG (mg/dl) | 140.1 ± 14.9 | 117.4 ± 13.5 | 105.5 ± 14.8 * |
| Total cholesterol (mg/dl) | 205.8 ± 13.3 | 171.6 ± 10.6 | 163.0 ± 13.9 * |
| LDL (mg/dl) | 128.8 ± 10.9 | 100.5 ± 7.5 * | 91.4 ± 8.1 * |
| HDL (mg/dl) | 48.9 ± 4.9 | 46.0 ± 4.3 | 56.1 ± 7.3 |
| Cholesterol/HDL | 4.5 ± 0.4 | 3.8 ± 0.2 * | 3.3 ± 0.3 * |
| TNF-α (pg/ml) | 4.00 ± 0.29 | 3.60 ± 0.27 | 3.07 ± 0.27 * |
Data represent mean ± SEM. BMI, Body mass index; FPG, fasting plasma glucose; FPI, fasting plasma insulin; ISquicki, insulin sensitivity; TG, triglycerides; FFA, fasting plasma free fatty acids; LDL, low density lipoproteins; HDL, high density lipoproteins; TNF-α, tumor necrosis factor alpha.
significantly different from the corresponding pre-surgery value, P<0.05.
Plasma Ceramide Concentrations
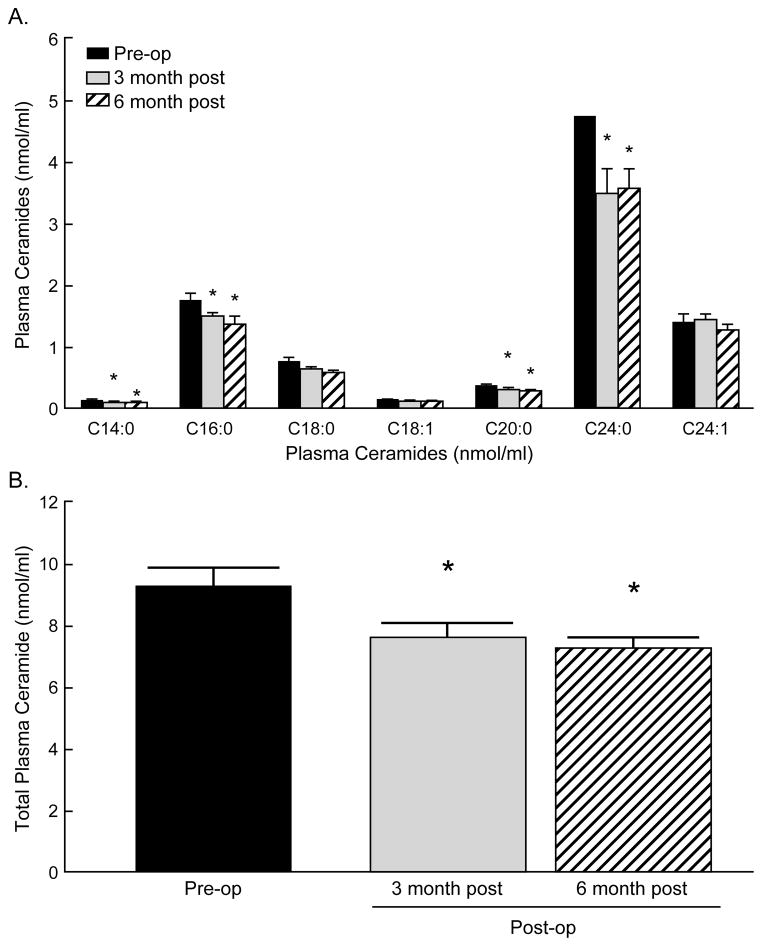
The distribution of ceramide subspecies in plasma of obese patients at baseline, 3 and 6 months post-operatively is illustrated in Figure 1. Consistent with previous reports for patients with atherosclerosis (31) and cardiovascular disease (32), the predominant ceramides in plasma of obese patients in this study were 24:1, 24:0 and 16:0. At 3 months post RYGB, total plasma ceramide levels (nmol/ml) had significantly decreased compared to baseline (P=0.02). Specifically, gastric bypass resulted in reduced levels of C14:0 (P=0.02), C16:0 (P=0.04), C20:0 (P=0.04), and C24:0 (P=0.04). In addition, C18:0 was reduced though this decrease did not meet statistical significance. After 6 months, total ceramide levels had decreased even further compared to baseline (P=0.03), with individual ceramide subspecies displaying similar reductions: C14:0 (P=0.04), C16:0 (P=0.005), C20:0 (P=0.03), and C24:0 (P=0.03).
Figure 1.
Gastric bypass surgery lowers fasting plasma ceramide concentrations. Plasma ceramide concentrations were measured before surgery (grey bars), 3 months (black bars) and 6 months (white bars) post-surgery. Panel (A) illustrates plasma concentration of ceramide subspecies in severely obese patients (N=13) before, 3 months (N=13) and 6 months (N=12) post-RYGB. Panel (B) illustrates total plasma ceramide concentrations at these time points. Data are expressed as means ± S.E.M. * P< 0.05 pre-surgery vs. 3 months and 6 months post surgery.
Plasma TNF-α Concentrations
Fasting levels of TNF-α (pg/ml), decreased significantly by 6 months post RYGB, compared to pre-operative levels (4.0 ± 0.29 vs. 3.07 ± 0.27, P=0.04).
Correlations Between Ceramides and IR, Inflammatory Mediators and Weight Loss
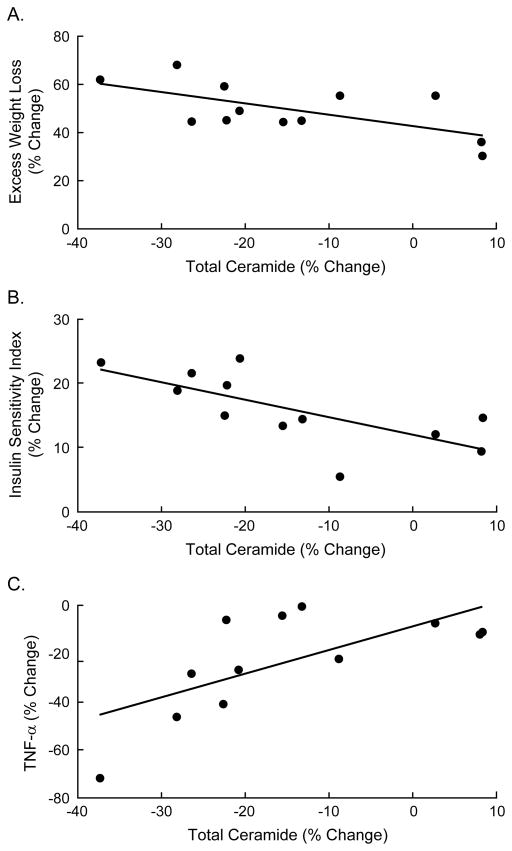
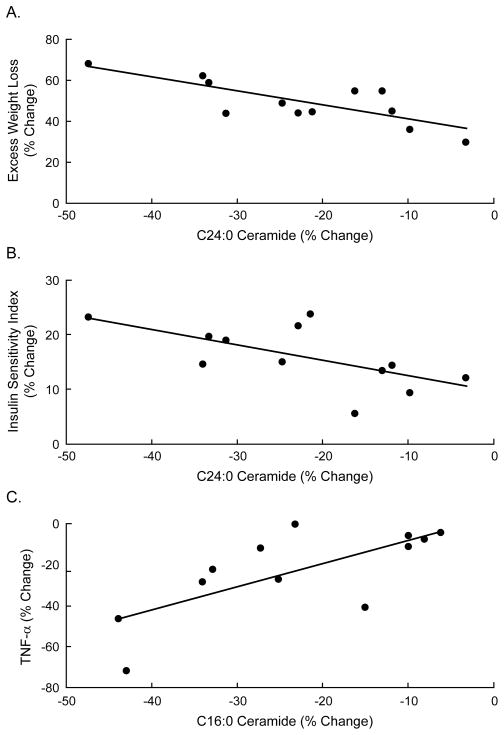
Spearman rank correlations were used to determine potential relationships between total plasma ceramide levels, degree of insulin sensitivity, TNF-α concentrations, and weight loss. Six months after surgery, the change in total ceramide levels correlated inversely with weight loss (r= −0.61, P=0.04, Fig 2a), such that individuals with the greatest weight loss also had the greatest decrease in ceramides. The decrease in total ceramide concentrations also correlated significantly with an increase in insulin sensitivity as measured by ISQUICKI (r= −0.65, P=0.02, Fig. 2b) and decrease in TNF-α concentrations (r=0.64 P=0.03, Fig. 2c). Examination of specific ceramide subspecies revealed that the decrease in C24:0 correlated with the amount of weight lost (r= −0.70, P=0.02, Fig. 3a), and the improvement in insulin sensitivity (r= −0.60, P=0.04, Fig. 3b). Further, the decrease in C16:0 correlated with the decrease in TNF-α (r=0.72, P=0.02, Fig. 3c) at 6 months.
Figure 2.
Correlations between total plasma ceramide and metabolic parameters at 6 months post-surgery. The decrease in total plasma ceramide correlated significantly with (A) excess weight loss (r= −0.61, P=0.04), (B) change in insulin sensitivity (r= −0.68, P=0.02), and (C) change in plasma TNF-α (r=0.64, P=0.03). Percent change was calculated as % change = (Post - Pre)/Post * 100.
Figure 3.
Correlations between different plasma ceramide subspecies and metabolic parameters at 6 months post-surgery. For individual ceramide species the decrease in C24:0 correlated significantly with (A) excess weight loss (r= −0.69, p=0.03), and (B) change in insulin sensitivity (r=−0.60, P=0.04), while the decrease in C16:0 ceramide correlated significantly with (C) the decrease in TNF-α (r= 0.72, P=0.02). Percent change was calculated as % change = (Post - Pre)/Post * 100.
Discussion
With respect to durable weight loss, bariatric surgery is the most effective long-term treatment for obesity and offers the greatest chances for amelioration and resolution of obesity-related comorbidities. The observation that acute phase reactants, such as TNF-α and plasma ceramides, decreased significantly 6 months after RYGB in conjunction with significant improvements in cardiovascular risk factors and insulin sensitivity, supports the hypothesis that inflammatory cytokines and ceramides at least in part mediate the link between obesity, cardiovascular comorbidities, and diabetes.
We observed significant reductions in total and specific plasma ceramide subspecies concentrations 3 and 6 months after RYGB, in parallel with substantial weight loss (Fig.1). Our results are consistent with previous reports documenting significant improvements in CVD risk factors in obese patients after surgically-induced weight loss, in association with improvements in insulin sensitivity, glucose homeostasis and systemic inflammation (4, 33). Recently, our group reported that RYGB improved insulin sensitivity and pancreatic β-cell function in severely obese humans with type 2 diabetes (26), and in an obese rat model (34). The present study demonstrates reproducible improvements in insulin sensitivity after RYGB in a separate cohort of patients, in addition to significant changes in inflammatory markers and ceramide levels. A plausible mechanism for this dramatic improvement in insulin sensitivity may be the interplay between altered gut peptide secretion secondary to exclusion of the foregut, and the reduced inflammatory environment observed following substantial loss of visceral adipose tissue. Excess adiposity, as occurs in the obese state, can cause lipotoxicity - a process by which excess fatty acids and associated triglyceride accumulation in non-adipose tissue (pancreatic islets, skeletal muscle, heart, hepatocytes) can cause cellular dysfunction. Such ectopic fat accumulation induces an inflammatory state and is considered to be a primary mechanism for rendering these tissues insulin resistant. Plasma sphingolipids and ceramides have also been shown to play a critical role in the pathogenesis of obesity-induced cardiovascular and metabolic disease (32). These lipid molecules are stimulated by inflammatory cytokines released from adipocytes, such as TNF-α, which is elevated in type 2 diabetic and obese patients (32, 35). Our previous study demonstrated that total and specific plasma ceramide subspecies were elevated in obese diabetic subjects, and that these elevated lipid moieties correlated with the severity of IR and elevated plasma TNF-α levels (8). We now demonstrate a weight loss-induced decrease in ceramide concentrations alongside improvements in insulin sensitivity, 6 months after RYGB. Our findings are consistent with results from in-vitro studies which have demonstrated increased intracellular ceramide concentrations in association with decreased insulin-stimulated glucose uptake, glycogen synthesis, and Akt serine phosphorylation in C2C12 myotubes incubated with a cell-permeable C2-ceramide analog (36), and in L6 muscle cells (37). Together these findings support the hypothesis that increased plasma ceramide levels in obese subjects may be an important mediator of IR and inflammation in peripheral tissues, and that the decrease in ceramide levels following surgically-induced weight loss is likely to contribute to the rapid amelioration of IR observed in diabetic bariatric patients post-operatively.
The effect of bariatric surgery on ceramide subspecies was most pronounced for the long chain C24:0 and shorter chain C16:0 species. The C24:0 ceramide is the most abundant species in plasma and higher levels are seen in moderately obese type 2 diabetics compared to lean healthy adults (8). In our previous study C24:0 correlated with insulin sensitivity as measured by glucose clamp (8). Herein, we extend these observations to show that improvements in insulin sensitivity after bariatric surgery are associated with a decrease in C24:0 in plasma. These data highlight the importance that this specific ceramide subtype may have in insulin resistant states, and from a mechanistic perspective, the role it may play in antagonizing insulin signaling through Akt, thus promoting insulin resistance in insulin targeted tissue. However, it is also possible that the relationship between C24:0 and insulin resistance is solely the result of weight loss, since we also saw an inverse correlation between the decrease in C24:0 and the amount of weight a patient lost. We recognize of course that such correlations do not rise to the level of cause/effect evidence, and that these data are from plasma rather than skeletal muscle or other insulin targeted tissue. Nevertheless, they do provide novel informative data and suggest that there may be a link between this long chain lipid and obesity-related insulin resistance.
Acute and chronic inflammation have been shown to affect the regulation of intracellular ceramide levels (38–40) which in turn propagate the production of inflammatory cytokines including TNF-α, interleukin (IL)-1, and IL-6. Wong et al. suggested that upregulation of S-SMase activity may contribute to the effects of inflammatory cytokines in atherosclerosis (40). Our prior observation that plasma TNF-α concentrations in obese type 2 diabetics correlated with several long chain plasma ceramides, and that there was an association postoperatively between weight loss and decrease in total ceramide and TNF-α levels, adds further credence to the view that ceramides are linked to insulin resistance through inflammatory mechanisms (8). Furthermore, our observation of a significant positive correlation between decreases in the shorter chain C16:0 ceramide, and decreased TNF-α, is important in light of recent cell data showing that TNF-α can induce S-SMase secretion and activity, as well as increase C16:0 ceramide levels (41). Thus, there is the possibility for a distinct metabolic role for C16:0 ceramide in inflammation-mediated disease processes. Whether this role extends to insulin resistance and type 2 diabetes remains to be determined. However, we did see a modest correlation between decreases in C16:0 and improvements in insulin sensitivity (r= −0.42, p=0.15). Based on these preliminary data, further examination of the link between ceramides, IR and weight loss, is warranted, including measurement of ceramides at the tissue level.
In conclusion, this study is the first to provide in-vivo evidence that a decrease in plasma ceramide levels following surgically-induced weight loss is associated with early improvement in insulin sensitivity and a decrease in pro-inflammatory cytokines. This improvement in insulin sensitivity and reduced inflammatory state, possibly secondary to decreased ceramide levels, highlight the major benefits of bariatric surgery in reducing or even eliminating comorbidities associated with severe obesity. It provides further evidence to support the development and application of surgical approaches for the treatment of obesity and diabetes.
Acknowledgments
We wish to thank the surgical nursing staff of the Bariatric and Metabolic Institute and the patients who volunteered for the advancement of research. This work was partially supported by grants from the Society of American Gastrointestinal and Endoscopic Surgeons (PRS), National Institutes of Health grants DK089547, AG12834 (JPK), and Multidisciplinary Clinical Research Career Development Programs Grant K12RR023264 (SRK).
Footnotes
Disclosure: The authors have no conflicts of interest.
References
- 1.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003 Feb 7;299(5608):853–5. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 2.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. Jun 26;375(9733):2267–77. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tretjakovs P, Jurka A, Bormane I, Mackevics V, Mikelsone I, Balode L, et al. Relation of inflammatory chemokines to insulin resistance and hypoadiponectinemia in coronary artery disease patients. Eur J Intern Med. 2009 Nov;20(7):712–7. doi: 10.1016/j.ejim.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Vazquez LA, Pazos F, Berrazueta JR, Fernandez-Escalante C, Garcia-Unzueta MT, Freijanes J, et al. Effects of changes in body weight and insulin resistance on inflammation and endothelial function in morbid obesity after bariatric surgery. J Clin Endocrinol Metab. 2005 Jan;90(1):316–22. doi: 10.1210/jc.2003-032059. [DOI] [PubMed] [Google Scholar]
- 5.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006 Jan;45(1):42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007 Mar;5(3):167–79. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, et al. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000 Dec;20(12):2614–8. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 8.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009 Feb;58(2):337–43. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auge N, Maupas-Schwalm F, Elbaz M, Thiers JC, Waysbort A, Itohara S, et al. Role for matrix metalloproteinase-2 in oxidized low-density lipoprotein-induced activation of the sphingomyelin/ceramide pathway and smooth muscle cell proliferation. Circulation. 2004 Aug 3;110(5):571–8. doi: 10.1161/01.CIR.0000136995.83451.1D. [DOI] [PubMed] [Google Scholar]
- 10.Auge N, Negre-Salvayre A, Salvayre R, Levade T. Sphingomyelin metabolites in vascular cell signaling and atherogenesis. Prog Lipid Res. 2000 May;39(3):207–29. doi: 10.1016/s0163-7827(00)00007-2. [DOI] [PubMed] [Google Scholar]
- 11.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, et al. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem. 2005 Mar 18;280(11):10284–9. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, et al. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation. 2002 Oct 22;106(17):2250–6. doi: 10.1161/01.cir.0000035650.05921.50. [DOI] [PubMed] [Google Scholar]
- 13.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA. 1998 Mar 3;95(5):2498–502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Summers SA, Nelson DH. A role for sphingolipids in producing the common features of type 2 diabetes, metabolic syndrome X, and Cushing’s syndrome. Diabetes. 2005 Mar;54(3):591–602. doi: 10.2337/diabetes.54.3.591. [DOI] [PubMed] [Google Scholar]
- 15.Samad F, Loskutoff DJ. Tissue distribution and regulation of plasminogen activator inhibitor-1 in obese mice. Mol Med. 1996 Sep;2(5):568–82. [PMC free article] [PubMed] [Google Scholar]
- 16.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003 Jun 10;100(12):7265–70. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006 Jan;116(1):115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003 Dec;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002 Jul;51(7):2207–13. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 20.Kirwan JP, del Aguila LF, Hernandez JM, Williamson DL, O’Gorman DJ, Lewis R, et al. Regular exercise enhances insulin activation of IRS-1-associated PI3-kinase in human skeletal muscle. J Appl Physiol. 2000 Feb;88(2):797–803. doi: 10.1152/jappl.2000.88.2.797. [DOI] [PubMed] [Google Scholar]
- 21.Adams JM, 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004 Jan;53(1):25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 22.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995 Sep;222(3):339–50. doi: 10.1097/00000658-199509000-00011. discussion 50–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003 Oct;238(4):467–84. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habib P, Scrocco JD, Terek M, Vanek V, Mikolich JR. Effects of bariatric surgery on inflammatory, functional and structural markers of coronary atherosclerosis. Am J Cardiol. 2009 Nov 1;104(9):1251–5. doi: 10.1016/j.amjcard.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 25.Batsis JA, Romero-Corral A, Collazo-Clavell ML, Sarr MG, Somers VK, Lopez-Jimenez F. Effect of bariatric surgery on the metabolic syndrome: a population-based, long-term controlled study. Mayo Clin Proc. 2008 Aug;83(8):897–907. doi: 10.4065/83.8.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2009 Dec 22; doi: 10.1038/ijo.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.conference N NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991 Dec 15;115(12):956–61. [PubMed] [Google Scholar]
- 28.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000 Jul;85(7):2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 29.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 30.Kasumov T, Huang H, Chung YM, Zhang R, McCullough AJ, Kirwan JP. Quantification of ceramide species in biological samples by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Biochem. 2010 Jun 1;401(1):154–61. doi: 10.1016/j.ab.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichi I, Nakahara K, Miyashita Y, Hidaka A, Kutsukake S, Inoue K, et al. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids. 2006 Sep;41(9):859–63. doi: 10.1007/s11745-006-5041-6. [DOI] [PubMed] [Google Scholar]
- 32.Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes. 2006 Sep;55(9):2579–87. doi: 10.2337/db06-0330. [DOI] [PubMed] [Google Scholar]
- 33.Jones KB., Jr The Effect of Gastric Bypass on Cholesterol, HDL, and the Risk of Coronary Heart Disease. Obes Surg. 1992 Feb;2(1):83–5. doi: 10.1381/096089292765560600. [DOI] [PubMed] [Google Scholar]
- 34.Gatmaitan P, Huang H, Talarico J, Moustarah F, Kashyap S, Kirwan JP, et al. Pancreatic islet isolation after gastric bypass in a rat model: technique and initial results for a promising research tool. Surg Obes Relat Dis. 2010 Sep-Oct;6(5):532–7. doi: 10.1016/j.soard.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008 Jun;29(4):381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. The Journal of biological chemistry. 1999 Aug 20;274(34):24202–10. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 37.Hajduch E, Balendran A, Batty IH, Litherland GJ, Blair AS, Downes CP, et al. Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia. 2001 Feb;44(2):173–83. doi: 10.1007/s001250051596. [DOI] [PubMed] [Google Scholar]
- 38.Lightle SA, Oakley JI, Nikolova-Karakashian MN. Activation of sphingolipid turnover and chronic generation of ceramide and sphingosine in liver during aging. Mech Ageing Dev. 2000 Dec 1;120(1–3):111–25. doi: 10.1016/s0047-6374(00)00191-3. [DOI] [PubMed] [Google Scholar]
- 39.Schissel SL, Schuchman EH, Williams KJ, Tabas I. Zn2+-stimulated sphingomyelinase is secreted by many cell types and is a product of the acid sphingomyelinase gene. The Journal of biological chemistry. 1996 Aug 2;271(31):18431–6. doi: 10.1074/jbc.271.31.18431. [DOI] [PubMed] [Google Scholar]
- 40.Wong ML, Xie B, Beatini N, Phu P, Marathe S, Johns A, et al. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2000 Jul 18;97(15):8681–6. doi: 10.1073/pnas.150098097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkins RW, Canals D, Idkowiak-Baldys J, Simbari F, Roddy P, Perry DM, et al. Regulated secretion of acid sphingomyelinase: implications for selectivity of ceramide formation. J Biol Chem. 2010 Nov 12;285(46):35706–18. doi: 10.1074/jbc.M110.125609. [DOI] [PMC free article] [PubMed] [Google Scholar]