Abstract
Assembly and disassembly of Rad51 and Rad52 complexes were monitored by immunofluorescence during homologous recombination initiated by an HO endonuclease-induced double-strand break (DSB) at the MAT locus. DSB-induced Rad51 and Rad52 foci colocalize with a TetR–GFP focus at tetO sequences adjacent to MAT. In strains in which HO cleaves three sites on chromosome III, we observe three distinct foci that colocalize with adjacent GFP chromosome marks. We compared the kinetics of focus formation with recombination intermediates and products when HO-cleaved MATα recombines with the donor, MATa. Rad51 assembly occurs 1 h after HO cleavage. Rad51 disassembly occurs at the same time that new DNA synthesis is initiated after single-stranded (ss) MAT DNA invades MATa. We present evidence for three distinct roles for Rad52 in recombination: a presynaptic role necessary for Rad51 assembly, a synaptic role with Rad51 filaments, and a postsynaptic role after Rad51 dissociates. Additional biochemical studies suggest the presence of an ssDNA complex containing both Rad51 and Rad52.
Keywords: mating-type switching, Rad51, Rad52, recombination
Introduction
Homologous recombination repairs double-strand breaks (DSBs) both in mitotic and meiotic cells. Our knowledge of the mechanism of recombination has been obtained from biochemical and cytological analyses of the proteins involved in recombination, as well as physical analysis of recombination intermediates (Pâques and Haber, 1999; Symington, 2002). In eucaryotes, homologous recombination requires genes of the RAD52 epistasis group: RAD50, -51, -52, -54, -55, -57, -59, MRE11, XRS2, and TID1. These genes have originally been identified in the budding yeast Saccharomyces cerevisiae, and are evolutionarily conserved from yeast to humans.
Biochemical analysis has established the importance of the bacterial RecA homolog, Rad51, to carry out the search for homologous sequences and strand exchange (Shinohara et al, 1992). Rad51 forms helical filaments on single-stranded DNA (ssDNA) (Ogawa et al, 1993; Sung and Robberson, 1995). In vitro, formation of the Rad51 filament is promoted by ssDNA-binding protein, RPA (Sung and Robberson, 1995; Sugiyama et al, 1997). RPA removes secondary structure from ssDNA, which inhibits Rad51 function. However, Rad51 by itself cannot bind to RPA-coated ssDNA; assembly requires the so-called mediator proteins Rad52 and the Rad55–Rad57 complex. Rad52 forms a heptameric ring on the ssDNA (Shinohara et al, 1998; Passy et al, 1999; Kagawa et al, 2002; Singleton et al, 2002) and binds to both RPA and Rad51 (Shinohara et al, 1992, 1998). Rad52 apparently recruits Rad51 on ssDNA covered with RPA and promotes formation of the Rad51 filament (Sung, 1997a; Benson et al, 1998; New et al, 1998; Shinohara and Ogawa, 1998). The Rad55–57 heterodimer also facilitates formation of the functional Rad51 complex on RPA-coated ssDNA (Sung, 1997b). In vivo, the requirement for Rad55 and Rad57 in the repair of DSBs is less stringent than Rad52 (Pâques and Haber, 1999; Symington, 2002).
In addition to presynaptic roles in recombination, Rad52 protein exhibits a distinct activity: the pairing of two complementary DNA strands (Mortensen et al, 1996; Shinohara et al, 1998; Sugiyama et al, 1998). This strand-annealing activity is stimulated by an interaction with RPA (Shinohara et al, 1998; Sugiyama et al, 1998). Sugiyama and Kowalczykowski (2002) proposed a role for Rad52's annealing activity in the postsynaptic phase of recombination, allowing the second end of a DSB to anneal to a D-loop created by strand invasion of the other DSB end.
Immunostaining is one method that is used to detect the presence of specific recombination proteins bound to chromosomes. Proteins such as Rad51 and Rad52 form foci (punctate staining) on chromosomes and in the nuclei of both mitotic cells suffering from DNA damage and meiotic cells (Bishop, 1994; Gasior et al, 1998, 2001). Genetic analysis of these foci suggests that they may represent sites of ongoing recombination. Rad52 is required for Rad51 focus formation both in mitosis and meiosis. Rad52 foci form in the absence of Rad51 function, consistent with the mediator function of Rad52 in the assembly of Rad51 onto ssDNA. Recent analysis using a GFP–Rad52 fusion protein raises an intriguing possibility concerning the nature of these foci (Lisby et al, 2001, 2003). When more than 20 DSBs per cell are introduced by ionizing radiation, only one or two Rad52–GFP foci were observed, suggesting the presence of a recombination center, where multiple recombination reactions occur.
One of the best-analyzed homologous recombination reactions is mating-type (MAT) switching in S. cerevisiae (Haber, 1998; Pâques and Haber, 1999). MAT switching is a site-specific recombination event in which mating-type information is transferred to the MAT locus by gene conversion using as templates the heterochromatic and untranscribed donor sequences HMLα or HMRa. The event is initiated by the introduction of a DSB at the MAT locus by HO endonuclease. DSB formation is followed by 5′-to-3′ resection of DSB ends, forming ssDNA with a 3′-OH (White and Haber, 1990). Rad51 facilitates a search for homology and invasion of ssDNA of the MAT distal end into the donor duplex (HMLα or HMRa). Invasion of the 3′-OH end into the duplex triggers initiation of DNA synthesis. The mechanism of events following DNA synthesis is still largely unknown.
Using a strain in which the MAT locus is tagged with a GFP fusion protein bound to nearby sequences, we analyzed the formation of Rad51 and Rad52 foci in response to a single HO-induced DSB. Rad51 and Rad52 foci colocalize with the GFP-tagged DSB site. In strains bearing multiple HO cut sites, we find that the number of foci is proportional to the number of DSBs. This argues that each focus represents a site of recombination. Kinetic analysis of focus formation relative to the appearance of recombination intermediates and products reveals a new view on the assembly of recombination complexes. We propose that Rad52 carries out three distinct functions during MAT switching.
Results
Localization of recombination proteins to a single DSB site
In order to study the assembly and disassembly of Rad51 (and Rad52) foci at the site of a DSB, we used a strain in which the MAT locus is marked with tetracycline repressor protein (TetR) fused to GFP (Michaelis et al, 1997). An array of 112 tetracycline operators (tetO) was introduced 2 kb distal to the HO cleavage site at MAT (Figure 1A). The TetR–GFP fusion protein is expressed from a constitutive promoter and HO endonuclease from an inducible GAL10 promoter. Induction of HO creates a single DSB at the MAT locus. Exponentially growing yeast cells exposed to galactose were collected and chromosomes were surface spread. The spreads were stained simultaneously with anti-Rad51 (or anti-Rad52) and anti-GFP antibodies, followed by staining with chromophore-conjugated secondary antibodies, and were examined under an epifluorescent microscope.
Figure 1.
Experimental design. (A) Schematic diagram of the MAT locus visualized by GFP. A tetO array, able to bind TetR–GFP, is located 2 kb distal to the MAT locus. (B) Mating-type switching from MATα to MATa. The location of StyI sites used to monitor DSB formation and products by Southern analysis. (C) PCR analysis of the SIE recombination intermediate.
GFP foci on nuclear spreads
In cells subjected to surface spreading, nearly 90% of the nuclei contained one or two GFP spots (Figure 2). In cells from asynchronous cultures, approximately 60% of nuclei showed a single focus of GFP (Figure 2A), whereas the remainder had two closely positioned spots (Figure 2B). Cells with two GFP spots were likely to be in G2, since most cells show two closely positioned spots when arrested by nocodazole (data not shown). Thus, to simplify our analysis described below, we restricted our observations to cells containing only one GFP spot, which presumably correspond to cells in the G1 and S phases. Irrespective of cell cycle, a (DSB) is formed and repaired efficiently in asynchronous cultures (Supplementary Figure 1).
Figure 2.

Rad51 and Rad52 focus formation at the MAT locus. (A–J) wt (YDB057) and sir3 mutants (YDB058 and YDB236) were incubated with and without galactose. YDB057 (A–E) and YDB058 (F, G) contain a GFP-binding site near the MAT locus. YDB236 (H–J) contains three GFP-binding sites on chromosome III. Chromosome spreads were stained with anti-Rad51 (red; A–D, F, H–J) or anti-Rad52 (red; E, G) and anti-GFP (green) antibodies and examined under an epifluorescence microscope. DNA was stained with 4′, 6′-diamidino-2-phenylindole (DAPI; blue). Bars indicate 2 μm. (K, L) Colocalization of Rad51 and Rad52 with the GFP spot. Chromosome spreads from wt (K; YDB057) and the rad54 mutant (L; YTM132) were stained simultaneously with anti-GFP (green), anti-Rad51 (pink), anti-Rad52 (red), and DAPI (blue). Bars indicate 2 μm.
Rad51 and Rad52 foci colocalize with the site of a DSB
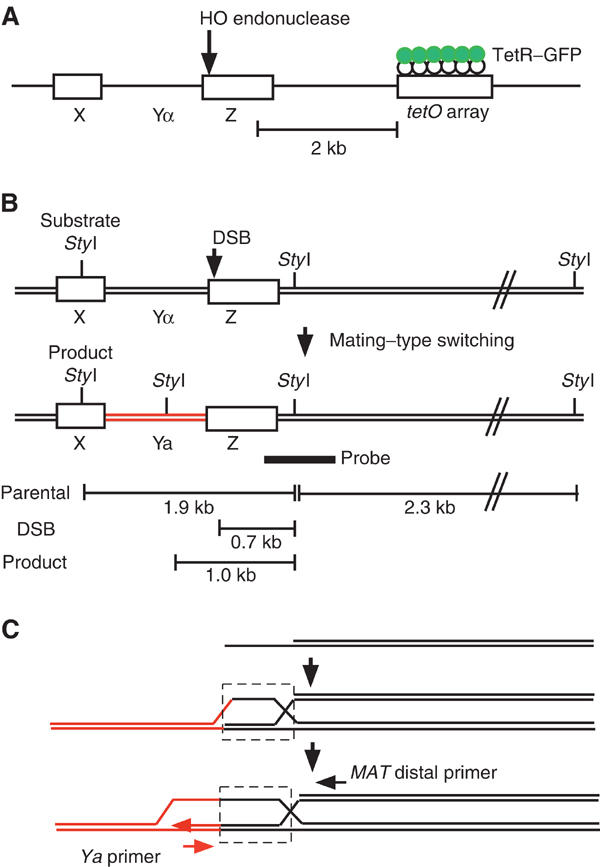
Nuclear Rad51 foci form specifically in response to the DSB created by the galactose-induced HO endonuclease, as fewer than 3% of uninduced cells exhibit such foci. The spontaneously induced focus is located randomly in respect of the GFP focus (Figure 2C). Upon continuous exposure of wild-type (wt) cells to galactose, nuclei containing a Rad51 focus appear at t=1 h (Figure 2D), with the number of Rad51 focus-positive nuclei increasing up to 50% during a 3-h incubation (Figure 3A). The number of nuclei with a single GFP spot gradually starts to decrease after 2 h. The loss of the GFP spot most likely reflects the 5′-to-3′ degradation of DSB ends (Lee et al, 1998; Vaze et al, 2002). The number of Rad51 focus-positive nuclei, if one includes GFP-negative nuclei, increases up to 80% at 5 h (data not shown). Importantly, in 90% of cases where there is a GFP spot, the Rad51 focus partly overlaps with the GFP focus, whereas the remainder show a side-by-side configuration (Figure 2D). This may reflect the degree of spreading between the two sites that are 2 kb apart. The average number of foci per Rad51 focus-positive nucleus is 1.1 at 3 h following DSB induction, consistent with the fact that a small proportion of cells have a focus that is independent of an HO-induced DSB.
Figure 3.
Kinetic analysis of Rad51 and Rad52 foci. (A–D) wt (YDB057; A, B) and the sir3 (YDB058; C, D) cells were exposed to galactose at t=0. At indicated times, chromosome spreads were prepared and stained with either anti-Rad51 (A, C) or anti-Rad52 (B, D) together with anti-GFP. Nuclei containing Rad51 focus (closed circles) or Rad52 focus (closed squares) were counted and percentages per focus-positive nuclei were plotted. Results for one experiment are shown. GFP-positive nuclei (closed rectangles) were also counted and plotted against total nuclei. At least 100 nuclei were analyzed at each time point. (E) The numbers of Rad51 (open bars) and Rad52 (closed bars) foci per focus-positive nuclei at a 3-h incubation were counted and the average numbers of each focus per focus-positive nuclei are shown.
When chromosome spreads were stained with anti-Rad52 antibodies, there was a pattern of focus formation similar to Rad51 (Figures 2E and 3B). Rad52 foci are induced specifically in response to a DSB and colocalize with the GFP spot. The average number of Rad52 foci per focus-positive nucleus at t=3 h of incubation is 1.2. These results indicate that a focus containing either Rad51 or Rad52 marks the site of the DSB.
The number of Rad51 foci is proportional to that of DSBs
We then determined if there is a one-to-one correspondence between the numbers of Rad51 foci and DSBs. We analyzed DSB formation in a sir3 derivative, which is defective in the silencing of the HML and HMR donor cassettes; consequently, HO can cleave HML and HMR as well as MAT. In this strain, there are no intact templates to complete recombination. This strain also contains a tetO array near the MAT locus as described above.
Following HO induction, nuclear spreads were prepared and stained with anti-Rad51 and anti-GFP antibodies. Rad51 foci are induced after 1 h, reach a plateau after 3 h (Figure 3C). At later time points, the Rad51 foci become much brighter. This could be due to longer ssDNA regions that would form longer Rad51 filaments. In nuclei with three Rad51 spots, the three foci often occupy a small portion of the spread chromatin, with one of them colocalized with the MAT adjacent GFP spot (Figure 2F). The mean number of Rad51 foci per focus-positive nucleus in the sir3 mutant increases up to 2.6 at t=3 h (Figure 3E). These data indicate a near one-to-one correspondence of the number of Rad51 foci with the number of DSBs, suggesting that each focus reflects a single recombination site. Similar results were obtained for Rad52 focus formation in sir3 cells (Figures 2G and 3D). The average number of Rad52 foci in sir3 mutant strains is 2.7 after a 3-h exposure to galactose (Figure 3E). The above experiments demonstrate that the nuclear foci containing either Rad51 or Rad52 each correspond to the site of a single DSB.
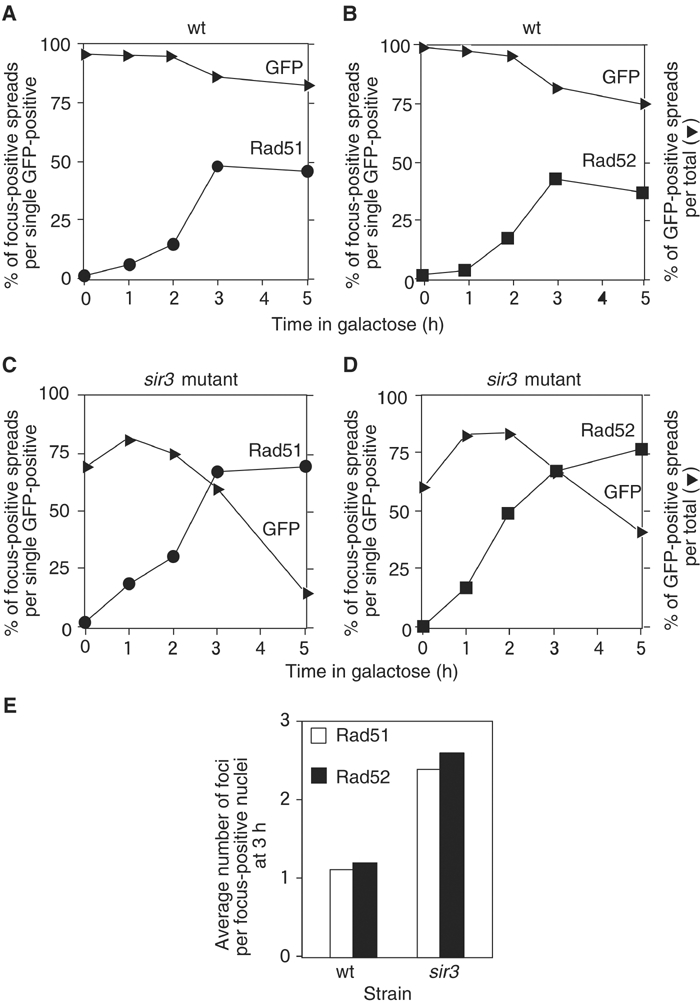
To support our conclusion that, in most cases, three DSBs produce three distinct foci, we constructed a sir3 mutant with lac operator (lacO)-binding sites (Robinett et al, 1996; Straight et al, 1996) near both HML and HMR as well as the tetO-binding site near MAT. This strain expresses not only TetR–GFP but also LacI–GFP (see Materials and methods). Nuclei often show three GFP spots on spreads. After the induction of DSBs, chromosome spreads were stained with anti-Rad51 and anti-GFP antibodies (Figures 2I and J). We found that 52% of chromatin spreads contained three Rad51 foci after a 3-h incubation with galactose, each of which overlaps or is closely adjacent to a single GFP spot.
We also see some early spreads containing two Rad51 foci in the sir3 mutant, both of which are present in near vicinity to GFP spots (Figure 2H). These spreads are likely to come from cells with two DSBs, arguing against that TetR–GFP does not have an ability to bind Rad51 after HO induction.
To address the concern that the chromosome-spreading technique disrupts recombination centers, we examined intact sir3 mutant cells tagged with GFP-bound operator arrays after HO induction (Figure 4A). Under these conditions, efficient cutting by HO endonuclease is observed at MAT as well as at the unsilenced HML and HMR loci (Supplementary Figure 1). Consistent with the results obtained from spread nuclei, we found that 57% of intact cells contained three GFP foci following a 2-h induction of HO (Figure 4C). The average number of GFP foci in the sir3 mutant was 2.5 after a 2-h exposure to galactose, similar to that found in spread nuclei. These data support the conclusion that nuclear foci containing Rad51 or Rad52 represent individual DSB sites.
Figure 4.
Intact cell analysis of DSB sites marked with GFP and CFP. (A, B) GFP foci (A; YDB236) and GFP and CFP foci (B; YDB244) were observed in intact sir3 cells by 3D fluorescence microscopy. Cells were fixed following a 2-h incubation with galactose. Each image shown is a projection of a series of 16 sections through the cell spaced 0.2 μm apart. Bars indicate 2 μm. (C) The number of GFP foci per focus-positive cell was scored in sir3 cells (YDB236) at indicated times following exposure to galactose. At least 100 focus-positive cells were scored at each time point.
We noted that there was also an increase in the number of cells containing only one or two GFP foci following a 2-h HO induction in the sir3 strain. To determine if the increase in nuclei containing fewer than three foci reflects the formation of recombination centers (in which several DSBs are brought to one nuclear location), we replaced the TetR–GFP construct in the sir3 mutant strain above with a TetR–CFP fusion. This enabled us to distinguish between loss of foci due either to colocalization of the tagged DSB sites or to resection through the repressor binding arrays adjacent to the DSB sites. Consistent with the results described above, we found that 56% of intact cells contained three foci (Figure 4B; two GFP and one CFP) following a 2-h induction of HO, with an average of 2.5 foci per focus-positive nucleus (data not shown). We observed colocalization of the CFP and GFP foci in only 13% of focus-positive nuclei following a 2-h HO induction (5% contained a single GFP+CFP+ focus and 8% contained two distinct GFP foci, only one of which was colocalized with the CFP focus). Moreover, 22% of the focus-positive nuclei containing fewer than three foci did not contain a CFP focus. The loss of the CFP spot is likely the result of resection through the tetO arrays inserted adjacent to the DSB site at MAT. These results strongly suggest that the decrease in the number of nuclei containing three foci and the corresponding increase in the number of nuclei containing two foci following HO induction is due to hyper-resection of the DSB in the absence of a donor for repair, and not to colocalization of these foci into a recombination center.
Finally, we carried out immunostaining of chromosome spreads from wt and Rad52–YFP cells after induction of DNA damage (Supplementary Figure 3; T Miyazaki and A Shinohara, unpublished results). wt cells show several foci of Rad51 or Rad52 on spreads after the treatment with Zeocin, which causes DSBs. This is consistent with the previous observation that γ-rays induce Rad51 foci in a dose-dependent manner (Gasior et al, 1998, 2001). On the other hand, Rad52–YFP cells frequently formed only one Rad52 (or YFP) focus on spreads. Furthermore, Rad51 also shows aggregation, which is colocalized with the Rad52–YFP focus. These results suggest that Rad52–YFP forms one focus after the induction of exogeneous DNA damage, strongly suggesting that the fusion of Rad52 to YFP has an ability to induce the aggregation of recombination proteins.
Kinetic analysis of Rad51 and Rad52 focus formation during repair of a single DSB
To study the kinetics of assembly and disassembly of Rad51 and Rad52 at the MAT locus, we carried out experiments when the DSB is induced transiently. After a 1-h exposure to galactose, the cells were washed and resuspended in medium containing glucose to turn off HO expression. Chromosome spreads were prepared from cells taken at time intervals following induction, and stained with anti-Rad51 or anti-Rad52 antibodies. Nuclei containing a single Rad51 (or Rad52) focus near the GFP focus were scored and plotted versus time (Figure 5). As above, we confined our analysis to nuclear spreads with only one GFP spot. In these nuclei, Rad51 focus formation begins at t=0 (the time when the cells were shifted to glucose-containing medium), peaks at t=30 min and then disappears (Figure 5A). Rad52 foci begin to appear at t=0. However, in contrast to Rad51, Rad52 focus formation peaks at t=60 min, 30 min later than Rad51, and then disappears during further incubation. Thus, the kinetics of Rad52 focus dissociation is delayed relative to Rad51. These results were reproduced in three independent time-course analyses (see below).
Figure 5.
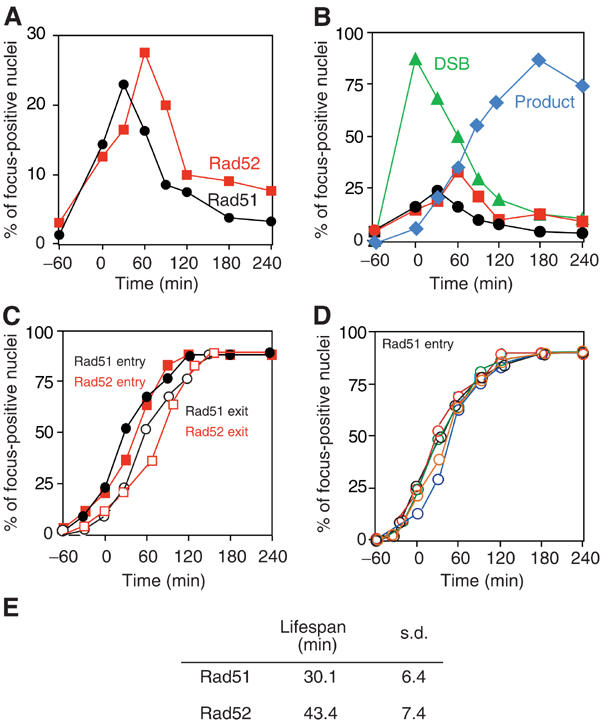
Cumulative analysis of Rad51 and Rad52 foci. (A) wt cells (YDB057) were transiently exposed to galactose for 1 h. At indicated times, chromosome spreads were prepared and stained for Rad51 or Rad52 and GFP. Time 0 is the time when the medium was exchanged. The nuclei containing Rad51 (closed circles) or Rad52 (closed squares) focus per single GFP-positive nuclei were counted. At least 100 nuclei were analyzed at each time point. (B) The kinetics of the foci are compared with that of DSBs and products. Results of experiment #6 are shown: DSBs (closed triangles) and products (closed diamonds). (C) Noncumulative curves of focus positive nuclei were converted into cumulative curves as described in Materials and methods. Rad51 assembly, closed circles; Rad51 disassembly, open circles; Rad52 assembly, closed squares; Rad52 disassembly, open squares. (D) Cumulative curves for Rad51 foci are from five independent experiments. (E) Lifespans of Rad51 and Rad52 foci were calculated from five independent noncumulative curves and the average time of each lifespan was obtained.
Comparison of the kinetics of focus formation with the appearance of recombination intermediates
To study the relationship between the assembly/disassembly of the recombination machinery and DNA transactions during DSB repair, we compared the kinetics of Rad51 and Rad52 focus formation with those of recombination intermediates and products. We analyzed the formation of a single DSB and the appearance of the recombination product (switching from MATα to MATa) by Southern blotting (Figure 1B). In addition, we measured the kinetics of joint molecule formation between the donor and recipient molecules indirectly by PCR (White and Haber, 1990). The sequence differences between the HMRa donor and the MATα recipient locus enable us to detect an intermediate molecule in which MAT distal ssDNA has invaded the donor sequence and has initiated synthesis of a short patch of DNA (30 bp) using the 3′-OH end of the invading strand as a primer (Figure 1C). We refer to this intermediate as a strand invasion-extension (SIE) structure. The kinetics of the formation of DSBs and products as well as SIE (Supplementary Figure 1) was similar to that reported previously (White and Haber, 1990; Sugawara et al, 1995).
We converted the kinetic data of Rad51 and Rad52 focus formation and of DSBs into cumulative curves as described (Padmore et al, 1991). In brief, we calculated the lifespan of a particular stage, for example, focus-positive nuclei. The average lifespan of Rad51 and Rad52 foci is 30 and 44 min, respectively (Figure 5E; the difference in lifespan between the proteins is statistically significant; Student's t-test, P=0.014), whereas that of the DSB is 110 min. Rad51 and Rad52 foci are shorter-lived intermediates than DSBs (Figure 5B). By this approach, the kinetics of entry into and exit out of the particular stage are obtained as a cumulative curve (Figure 5C). Cumulative curves of Rad51 focus formation data from five independent experiments were highly consistent, as shown in Figure 5D.
Using the cumulative curves, we compared cytological events with DNA events. Two typical results are presented in Figure 6. To simplify the results, the data are also presented in a schematic view (Figure 6G). There is about a 1-h lag between the introduction of the DSB and the formation of a detectable Rad51 focus. This gap may reflect the time that cells spend assembling a detectable amount of Rad51 and Rad52 on the ssDNA. In each of our experiments, the Rad51 focus appears at the same time or slightly earlier (10 min) than the Rad52 focus (Figures 6C and F). However, the Rad51 focus disappears 20 min earlier than Rad52. Thus, the Rad52 focus has a longer lifespan than Rad51, indicating that Rad52 associates with chromosomes after the disassembly of a detectable Rad51 focus. The timing of Rad51 disassembly correlates with the formation of the SIE intermediate at the MAT distal end (Figures 6A and D). In contrast, Rad52 disappears concomitantly with the formation of the product as well as disappearance of the DSB (Figures 6B and E). It is important to note that Rad51 and Rad52 are both present for a short time prior to the strand invasion/primer extension step.
Figure 6.
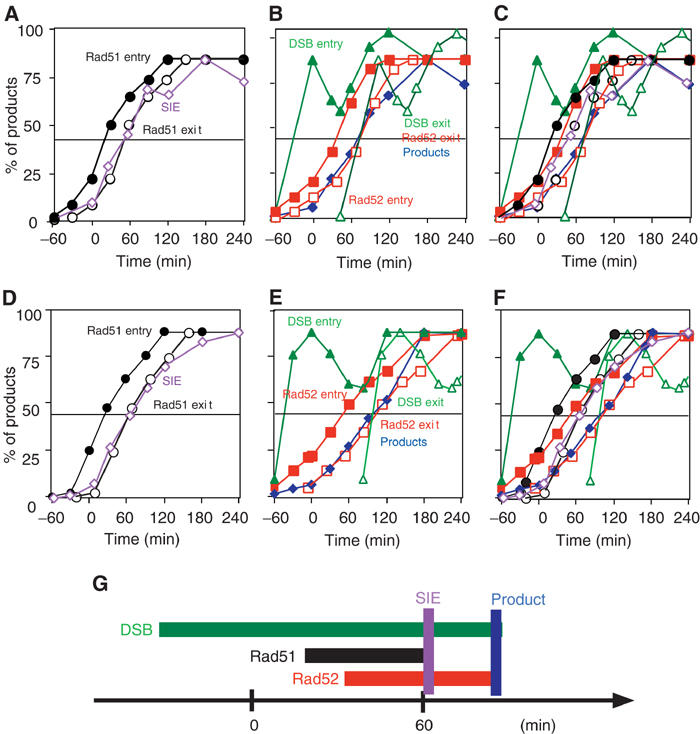
Comparison of focus formation with DNA events. Results from two independent time-course analyses (#6, A–C; #7, D–F) are presented. Assembly and disassembly of Rad51 foci are compared with the formation of SIE intermediates (A, D). Assembly and disassembly of Rad52 foci are compared with the formation of product and DSB appearance/disappearance (B, E). All curves of the foci and DNA events are indicated (C, F). The times when half of the cells enter into a particular stage are calculated. A schematic summary for #6 experiment is presented in (G). Rad51 assembly, closed circles; Rad51 disassembly, open circles (black); Rad52 assembly, closed squares; Rad52 disassembly, open squares (red); DSB entry, closed triangles; DSB exit, open triangles (green); SIE formation, open diamonds (purple); products, closed diamonds (blue).
Colocalization of Rad51 and Rad52 in wt MAT switching
The above kinetic analysis of the foci clearly indicates coexistence of Rad51 and Rad52 on chromosomes. We checked colocalization of the two proteins by quadruple staining with GFP and DAPI using rabbit anti-Rad51 and rat anti-Rad52 antibodies. As shown in Figure 2K, Rad51 and Rad52, which also coexist with the GFP spot, often colocalize on wt chromosomes after the introduction of the DSB. The frequency of nuclei with colocalized foci is low: around 10% at 30 min. This is not surprising given the short lifespan of the complex containing both proteins, compared to what is seen when the DSB cannot be repaired, as in a rad54 mutant (see below). This colocalization is not due to crossreaction of primary and secondary antibodies (data not shown).
Mutant analysis
Our results suggest that Rad51 assembles onto ssDNA simultaneously or just prior to the assembly of Rad52. This is unexpected, given that in vitro studies had suggested that Rad52 facilitates Rad51 assembly onto ssDNA and might bind prior to Rad51. We analyzed the role of Rad52 in Rad51–ssDNA assembly using rad51 and rad52 mutant strains as well as the role of Rad55 and Rad54 in assembly and disassembly of Rad51 and Rad52 foci. Cells from rad51, rad52, rad54, and rad55 mutant strains accumulate unusual recombination intermediates (a longer ssDNA, which is shown by the presence of larger restriction fragments detected by Southern hybridization; see Sugawara et al, 1995), which suggests that the substrate for Rad51 and Rad52 assembly is somewhat different between wt and the mutants. Therefore, we focused our analysis on early time points following DSB induction (Figure 7 and see also Supplementary Figure 2). At least three independent time-course analyses were carried out for each mutant strain, with reproducible results. One of each experiment is shown here.
Figure 7.
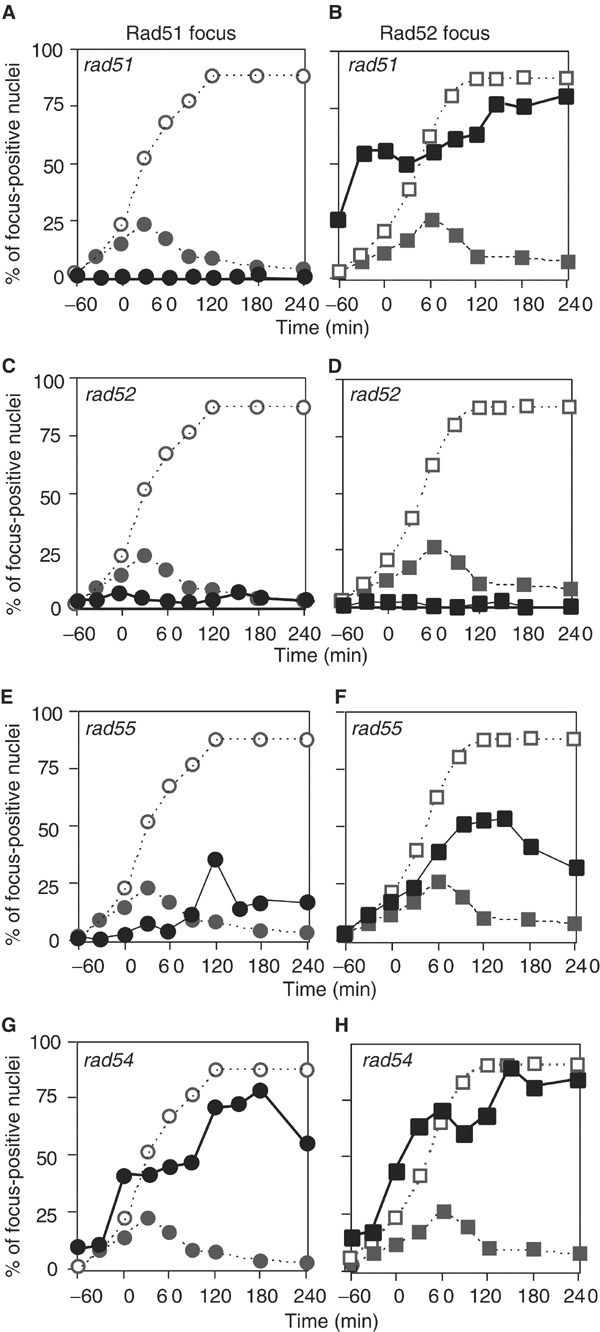
Rad51 and Rad52 focus formation in various mutants. The rad51 (A, B; YTM134), rad52 (C, D; YTM171), rad55 (E, F; YTM167), and rad54 (G, H; YTM132) mutants were analyzed for HO-induced Rad51 and Rad52 focus formation. The DSB was induced by incubating cells in medium containing galactose for 1 h. The percentages of Rad51 (closed circles in A, C, E, G) and Rad52 (closed squares in B, D, F, H) focus-positive nuclei were plotted against total nuclei. In each figure, noncumulative (closed circles or squares) and cumulative curves (open circles or squares) of the focus in wt are indicated as dotted lines.
rad51. No Rad51 focus is observed in the rad51 mutant, as expected (Figure 7A) and the SIE intermediate is not detected (data not shown). Interestingly, at 0 h, about 25% of cells show a Rad52 focus, which is not localized with GFP, probably a spontaneously induced focus. The rad51 strain does form an HO-induced Rad52 focus (Figure 7B), localized with GFP, indicating that Rad52 binds to the ssDNA in the absence of Rad51 (Gasior et al, 1998). However, the Rad52 focus does not disassemble in the mutant. Thus, assembly of a Rad52 focus does not require RAD51 function, whereas its disassembly is RAD51-dependent. The kinetics of Rad52 focus formation in the rad51 mutant are slightly slower than the cumulative curve of Rad52 assembly in wt cells. This does not simply mean that Rad52 assembles on chromosomes slower than in wt, since the cumulative curve of Rad52 in wt probably constitutes several assembly reactions. Moreover, the amount of Rad52 needed in the initial phase of recombination may be too low to be detected by immunocytology (see Discussion).
rad52. As expected from various studies, a rad52 mutant did not show any detectable HO-induced Rad51 foci (Figure 7C), indicating that Rad52 is required for detectable Rad51 focus formation. Neither Rad52 foci (Figure 7D) nor the SIE intermediates formed in the rad52 mutant strain following DSB induction.
rad55. Previous biochemical analyses have shown that the Rad55–Rad57 heterodimer has a mediator function that helps the assembly of a Rad51 filament onto RPA-coated ssDNA (Sung, 1997b). Although there are reports that the rad55 mutant is partially defective in mating-type switching (Schmuckli-Maurer and Heyer, 1999; Fortin and Symington, 2002), the defect of the rad55 mutant in the formation of SIE or products is comparable to those in the rad51 and rad52 mutants (data not shown), as shown previously (Sugawara et al, 2003). Although the maximum percentage of Rad51 focus-positive rad55 cells is comparable to that in wt strains, rad55 mutant cells exhibit a 90-min delay in Rad51 focus formation relative to wt (Figure 7E). This indicates that RAD55 is required for timely assembly of Rad51 on chromosomes. The rad55 mutant forms Rad52 foci, which accumulate to higher levels than in wt during further incubation (Figure 7F). Surprisingly, in contrast to the accumulation of foci observed in other mutants tested, the rad55 mutant exhibits disassembly of both Rad51 and Rad52 foci, even without repairing the DSB. In addition, the foci formed in rad55 cells are fainter than those in other mutants, particularly at late time points following DSB induction (compare with rad54 below; see Supplementary Figure 2). These suggest that Rad55 (and likely Rad57) plays a role in stabilizing both the Rad51 and Rad52 complexes formed on ssDNA, consistent with a previous report (Fortin and Symington, 2002).
rad54. Rad54 protein functions in a synaptic or postsynaptic phase of recombination (Petukhova et al, 1998; Mazin et al, 2000; Solinger and Heyer, 2001). A rad54 mutant forms neither SIE intermediates nor the product of DSB repair (data not shown). As expected, both Rad51 and Rad52 foci appear, but fail to disappear in the rad54 mutant (Figures 7G and H). This indicates that Rad54 is required for the disassembly of Rad51 and Rad52. In the rad54 mutant, at t=2 h, 73% of Rad51 foci are colocalized with Rad52 (Figure 2L). Thus, Rad51 and Rad52 seem to bind to the same recombination intermediates prior to strand invasion.
In vitro formation of an ssDNA complex containing both Rad51 and Rad52
To confirm formation of a DNA complex with both Rad51 and Rad52, we carried out in vitro experiments using a biotinylated-dT60 ssDNA (Figure 8A). This DNA was incubated first with a saturating amount of Rad52 and then various amounts of Rad51, and then purified using magnetic beads coated with streptavidin. Purified ssDNA complexes were analyzed for the presence of Rad51 or Rad52. Under these conditions, Rad51 cannot efficiently form a complex with dT60 (Figure 8B, lanes 1–4). However, the presence of Rad52 promotes the binding of Rad51 to the Rad52–ssDNA complex (Figure 8B, lanes 5–8), indicating that Rad52 promotes the formation of a complex containing both Rad51 and Rad52. Interestingly, the amounts of Rad52 in the complex increased by the addition of Rad51 (Figures 8B and E), indicating a cooperative effect between the two proteins. The ratio of Rad52 to Rad51 is around 1.1 (Figure 8E). Increased amounts of Rad52 promote the recruitment of Rad51 onto the ssDNA complex (Figure 8C). The presence of nucleotides does not affect the formation of complex (data not shown). These indicate that Rad51 and Rad52 bind to the same single ssDNA molecule simultaneously in vitro.
Figure 8.
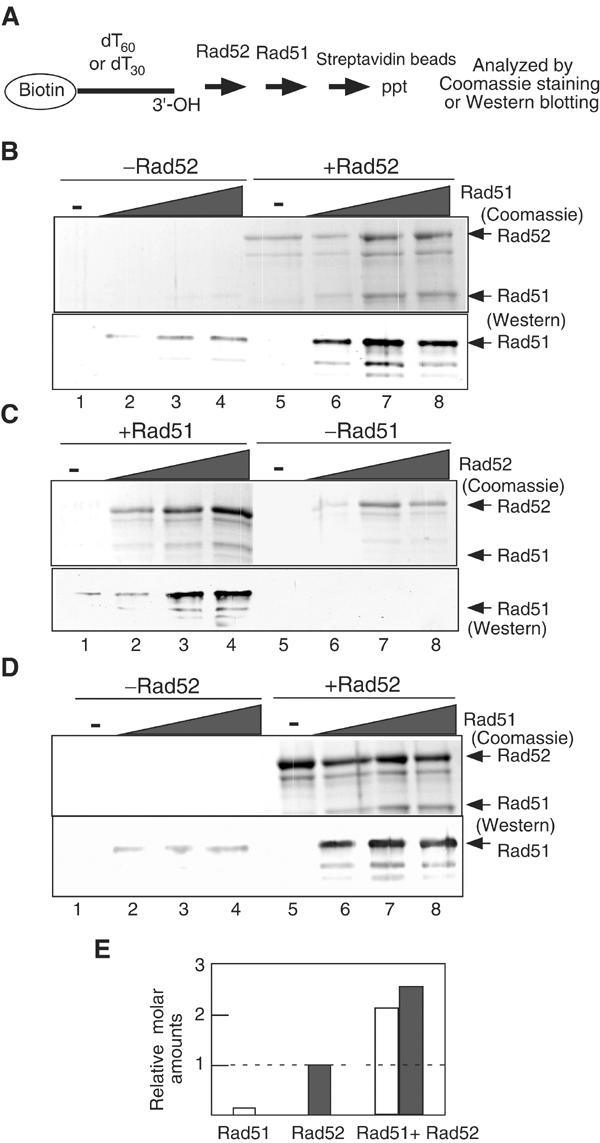
In vitro complex formation of Rad51–Rad52–ssDNA. (A) Schematic diagram of the assay. (B) Rad52 protein (1 μM) was incubated with a reaction mixture containing 3 μM (nucleotide) biotinylated-dT60 for 5 min, followed by the addition of various concentrations of Rad51 protein. After a 10-min incubation, the ssDNA complexes were purified and further processed as described in Materials and methods. One-tenth of the eluates were analyzed with Western blotting. Upper panel, Coomassie staining; lower panel, Western blotting using anti-Rad51 antibody. Lanes 1–4, in the absence of Rad52; lanes 5–8, in the presence of 1 μM Rad52. Lanes 1 and 5, no Rad51; lanes 2 and 6, 0.25 μM Rad51; lanes 3 and 7, 0.5 μM Rad51; lanes 4 and 8, 1.0 μM Rad51. (C) Various concentrations of Rad52 were incubated with 3 μM biotinated-dT60 for 5 min, followed by the addition of 1 μM Rad51. After a 10-min incubation, the mixtures were analyzed as described in Materials and methods. Upper panel, Coomassie staining; lower panel, Western blotting. Lanes 1–4, in the presence of 1 μM Rad51; lanes 5–8, in the absence of Rad51. Lanes 1 and 5, no Rad52; lanes 2 and 6, 0.25 μM Rad52; lanes 3 and 7, 0.5 μM Rad52; lanes 4 and 8, 1.0 μM Rad52. (D) Same as for (B) except 3 μM biotinated-dT30 was substituted for biotinated-dT60. Upper panel, Coomassie staining; lower panel, Western blotting. (E) The relative molar amounts of proteins bound to 3 μM biotinated-dT60 were quantified (B). Rad51 (1 μM) and/or Rad52 (1 μM) were used.
Although we used saturating amounts of the proteins in the above experiments, we cannot deny a possibility that a portion of the DNA is occupied by Rad51 and the remaining portion is by Rad52. We next used a dT30 ssDNA as a substrate. Rad52 forms a heptameric complex bound to ssDNA. Chemical footprint analysis suggests a periodicity of every four nucleotides upon Rad52 binding (Parsons et al, 2000), and X-ray crystallographic analysis supports this site size (Kagawa et al, 2002; Singleton et al, 2002). Thus, the footprint of a Rad52 heptameric ring should be 28 nucleotides. A single Rad52 ring would cover a dT30 oligonucleotide, possibly without a binding site for any other protein. Rad51 was also bound to the Rad52–dT30 complex (Figure 8D). Thus, it is likely that Rad51 and Rad52 can form a complex with ssDNA at least under the conditions described here.
Discussion
Recombination protein foci mark the site of DSB repair
We visualized the site of recombination by tagging an HO endonuclease cut site with GFP and showed that Rad51 and Rad52 foci colocalize with the GFP tag in response to an HO-induced DSB, demonstrating that the foci mark the site of recombination. Furthermore, the analysis of chromosome spreads described here shows that the number of foci per nucleus corresponds to the number of DSBs induced. On the other hand, recent analysis of a Rad52–YFP fusion protein in living yeast cells showed that the number of Rad52 foci did not correspond to the number of ionizing radiation-induced DSBs. Based on their results, Lisby et al (2001, 2003) proposed that a ‘recombination center' carries out multiple recombination events simultaneously, and that DSBs are recruited to this site. Recombination centers might be too fragile to survive during chromosome spreads. However, this is unlikely since we did not observe colocalization of DSB sites even in intact cells (Figure 4A). Although the types of DNA damage used in the current study are different from that by Lisby et al (2001, 2003), we believe that the difference could be due to the fusion of Rad52 to YFP. We showed that DNA damage-induced Rad51 or Rad52 foci do not form a center in wt, while Rad52–YFP does form a center even on chromosome spreads (Supplementary Figure 3; T Miyazaki and A Shinohara, unpublished results). Consistent with this, Gasior et al (2001) showed that γ-rays induce multiple Rad51 foci on spreads. These imply a potential danger in the use of GFP fusion protein in recombination proteins without a proper control: whole-cell immunostaining for a given protein.
Model of MAT switching: three functions of Rad52 during recombination
Based on the results described here, we propose a model of protein assembly during MAT switching. We could identify three distinct complexes of Rad52 at each stage of MAT switching: presynaptic, synaptic and postsynaptic roles. In the presynaptic stage, Rad52 promotes the assembly of a Rad51 nucleoprotein filament on ssDNA. This activity has already been inferred by various biochemical and cytological analyses. Purified Rad52 protein facilitates the formation of Rad51 complex on the RPA-coated ssDNA in vitro (Sung, 1997a; New et al, 1998; Shinohara and Ogawa, 1998). Furthermore, meiotic Rad51 focus formation is compromised in rad52 cells (Gasior et al, 1998). Chromatin immunoprecipitation (ChIP) analysis supports this finding as well (Sugawara et al, 2003; Wolner et al, 2003). Previous studies indicate a step-by-step assembly of Rad51 filaments (Gasior et al, 2001). First, RPA binds to the ssDNA and then recruits Rad52 to form a stoichiometric Rad52-RPA–ssDNA complex. Finally, Rad51 is recruited to the site through interaction with Rad52. Together with the Rad55–Rad57 complex, Rad52 helps in the formation of the Rad51 presynaptic filament. However, our analysis gives new insight into this process. Although we did not observe Rad52 foci prior to Rad51 foci, Rad51 focus formation is dependent on Rad52. One interpretation is that substoichiometric levels of Rad52, below our threshold of detection, are sufficient to promote the assembly of Rad51 on ssDNA. Alternatively, a Rad52 complex, which can assist in Rad51 binding to RPA-coated ssDNA, may be too unstable to detect by the current methods. Furthermore, it is possible that Rad52 and Rad51 assemble on chromosomes simultaneously as a complex, consistent with our biochemical results described here. Sung and his colleagues have identified a free stable complex of Rad51 and Rad52 in vivo by immunoprecipitation (Sung, 1997a; Song and Sung, 2000).
Once Rad51 assembles on the ssDNA, Rad52 binds to the Rad51 filament to form a complex containing both Rad51 and Rad52. We detected foci with both Rad51 and Rad52 prior to the strand invasion and initiation of DNA synthesis in wt cells, indicating that Rad51 and Rad52 bind to similar recombination intermediates. We note further that we could detect Rad51–Rad52 colocalized foci even in rad54 cells, which are completely deficient in strand invasion. These results argue that a complex containing Rad51 and Rad52 is an active species for homology search and strand invasion. This is in sharp contrast to the current models in which the Rad51–ssDNA complex is the active machinery for this process. The Rad51–Rad52 complex may carry out strand invasion more efficiently than the Rad51 filament. Consistent with this, we could detect a stable ssDNA complex containing both Rad51 and Rad52 in vitro. Previous biochemical analysis indicates that Rad52 alone promotes Rad51-mediated strand exchange even in the absence of RPA (Shinohara and Ogawa, 1998; Baumann and West, 1999). This cannot be simply explained by the mediator function of Rad52, but rather indicates an active role for Rad52 in Rad51-mediated recombination. A synaptic role of Rad52 was proposed previously (New and Kowalczykowski, 2002).
After strand invasion followed by DNA synthesis, which is facilitated by Rad54, Rad51 dissociates from the recombination intermediate. In contrast, Rad52 foci remain visible on chromosomes following the disappearance of Rad51, indicating a postsynaptic function for Rad52. Rad52 might bind to the displaced strand in a D-loop. Alternatively, Rad52 is likely to associate with the ssDNA on the other end of the DSB, which is not engaged in the initial strand invasion event. In either case, Rad52 could play a role in the completion of the recombinational repair event by promoting the strand annealing.
In late times, we could only detect Rad52 foci that did not have Rad51. This indicates that Rad51 cannot bind to a Rad52–ssDNA complex that forms at a later stage in the recombination, suggesting a functional difference of Rad52 in the presynaptic and postsynaptic phases. The Rad52 complex at the late stage may involve a structure or form of Rad52 that does not bind to Rad51. Biochemical analysis has revealed that excess Rad52 protein may be inhibitory to Rad51-mediated strand exchange in the presence of RPA, and possibly the binding of Rad51 (Sung, 1997a; Baumann and West, 1999). Alternatively, structural hindrance might inhibit the binding of Rad51. We entertain the possibility that the nature of the Rad52 complex might give specificity for Rad51 binding. If Rad52 is located on the displaced strand, Rad51 might not form a stable complex with the DNA since it lacks a free end. During the initial assembly of Rad51 filament on DSB ends, the end might be critical for promotion of Rad51 assembly. For example, the Rad55–Rad57 complex might interact with the ends and facilitate the assembly of Rad51 with Rad52. The displaced strand might lack a binding site for the Rad55–Rad57 complex, and thus Rad51 cannot assemble on this strand. More provocatively, formation of a Rad52 complex on the displaced ssDNA might be coupled with the initial strand invasion event. This coupling of micromolecule assembly would ensure the possible transfer of Rad52 from the Rad51–Rad52-invading complex to the displaced ends and the dissociation of Rad51 from it.
Functions of Rad55/Rad57 and Rad54
Consistent with the results from the ChIP analysis (Sugawara et al, 2003), we found that Rad55, and possibly its partner Rad57, is required for the efficient focus formation of Rad51. Rad51 could form foci with ssDNA, but this complex is apparently nonfunctional, indicating that the complex formed in the absence of Rad55 is different from that formed in its presence. In addition, we found that Rad51 complex formed in the absence of Rad55 is very unstable. Therefore, Rad55 has two functions: to assist in the assembly of Rad51 and to stabilize the Rad51 filament.
Rad54 is required for the disassembly of Rad51 and Rad52 foci, consistent with biochemical and ChIP analyses, which demonstrate a postsynaptic role for Rad54 in recombination (Sugawara et al, 2003).
Both the rad55 and rad54 mutants accumulate hyper-resected ssDNA intermediates (Sugawara et al, 1995), but they differ in the nature of their protein assembly defects. By comparing these mutants, we can uncover the role of the other proteins in Rad52 assembly. The Rad52 focus in the rad55 mutant strain is fainter than that in the rad54 mutant, particularly at later time points following DSB induction. In addition, the kinetics of Rad52 assembly in the rad55 strain are slower than in rad54 cells. This may suggest that Rad55 or Rad51 assists or stabilizes the Rad52 complex.
Our results described here are consistent with one recent report using the ChIP analysis (Sugawara et al, 2003), but inconsistent with the other (Wolner et al, 2003). Particularly, the behavior of Rad52 is quite different between our and the latter study (Wolner et al, 2003). We found that Rad52 focus is formed in both the rad51 and rad54 mutants. On the other hand, Wolner et al showed that Rad52 binding to MAT is completely deficient in the rad51 and rad54 mutants. There are several differences to point out. First, we used a strain that can repair a DSB at the MAT locus and transiently expressed the DSBs. Second, we mainly examined early time points, for example, less than 2 h after the induction of the DSB, whereas Wolner et al mainly used a strain unable to repair the break and drew their conclusion based on the results at later time points. Furthermore, our cytological analysis could detect all protein complexes. However, the ChIP analysis could only analyze the local association of the proteins depending on the primers used in the experiment. Some recombination protein might be unevenly distributed on the recombination intermediates: near or far from DSB ends. Further studies are necessary to clarify these discrepancies.
Materials and methods
Strains
The tetracycline operator (tetO) array and Tet repressor–GFP fusion protein (TetR–GFP) have been described (Michaelis et al, 1997). YDB057 ho HML::HMRa-(URA3) MATα matdist::tetO(112)-(NAT1) HMRaade3::GAL10-HO ade1-110 trp1 leu2::TetR-GFP-(LEU2) and a sir3::TRP1 derivative, YDB058, were obtained by insertion of a tetO array 2 kb distal to MAT and a TetR–GFP fusion construct at the LEU2 locus. The lac operator (lacO) array and Lac repressor–GFP fusion protein (LacI–GFP) have been described (Robinett et al, 1996; Straight et al, 1996). YDB236 ho HMLα hmlprox:lacO(256)-(LEU2) MATα matdist::tetO(112)-(NAT1) HMRahmrdist::lacO(100)-(ADE1) ura3-52 ade3::GAL10-HO ade1-110 trp1 leu2::TetR-GFP-(LEU2) HIS3::URA3pro::lacI-GFP-(KanMX) trp1 sir3::TRP1, a derivative of YDB057, was obtained by insertion of lacO arrays 1.5 kb proximal to HML and 2 kb distal to HMR, and a URA3::LACI-GFP fusion construct at the HIS3 locus. YDB244 ho HMLα hmlprox:lacO(256)-(LEU2) MATα matdist::tetO(112)-(NAT1) HMRahmrdist::lacO(100)-(ADE1) ura3-52 ade3::GAL10-HO ade1-110 trp1 ade1::TetR-3X(CFP)-(HPH1) HIS3::URA3pro::lacI-GFP-(KanMX) trp1 sir3::URA3 was derived by insertion of a TetR–3X(CFP) fusion construct at the ADE1 locus. Details of the construction and integration of lacO/LacI and tetO/TetR constructs will be described elsewhere (Bressan et al, 2004).
Supplementary Material
Supplemental data on-line
Acknowledgments
We thank Drs H Masukata and T Nakagawa for helpful discussion. We are grateful to Ms I Yamamoto and Y Ohtsuka for their technical assistance. This work was supported by grants from Ministry of Education, Science and Culture of Japan, Priority area (to AS) and Human Frontier Science program (to AS and JEH). JEH is supported by NIH grant GM20056. DAB. is supported by the American Cancer Society–Virginia Cochary Award for Excellence in Breast Cancer Research.
References
- Baumann P, West SC (1999) Heteroduplex formation by human Rad51 protein: effects of DNA end-structure, hRP-A and hRad52. J Mol Biol 291: 363–374 [DOI] [PubMed] [Google Scholar]
- Benson FE, Baumann P, West SC (1998) Synergistic actions of Rad51 and Rad52 in recombination and DNA repair. Nature 391: 401–404 [DOI] [PubMed] [Google Scholar]
- Bishop DK (1994) RecA homologs Dmc1 and Rad51 interact to form multiple nuclear complexes prior to meiotic chromosome synapsis. Cell 79: 1081–1092 [DOI] [PubMed] [Google Scholar]
- Bressan DA, Vazquez J, Haber JE (2004) Mating type-dependent constraints on the mobility of the left arm of yeast chromosome III. J Cell Biol 164: in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin GS, Symington LS (2002) Mutations in yeast Rad51 that partially bypass the requirement for Rad55 and Rad57 in DNA repair by increasing the stability of Rad51–DNA complexes. EMBO J 21: 3160–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior SL, Olivares H, Ear U, Hari DM, Weichselbaum R, Bishop DK (2001) Assembly of RecA-like recombinases: distinct roles for mediator proteins in mitosis and meiosis. Proc Natl Acad Sci USA 98: 8411–8418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior SL, Wong AK, Kora Y, Shinohara A, Bishop DK (1998) Rad52 associates with RPA and functions with Rad55 and Rad57 to assemble meiotic recombination complexes. Genes Dev 12: 2208–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber JE (1998) Mating-type gene switching in Saccharomyces cerevisiae. Annu Rev Genet 32: 561–599 [DOI] [PubMed] [Google Scholar]
- Kagawa W, Kurumizaka H, Ishitani R, Fukai S, Nureki O, Shibata T, Yokoyama S (2002) Crystal structure of the homologous-pairing domain from the human Rad52 recombinase in the undecameric form. Mol Cell 10: 359–371 [DOI] [PubMed] [Google Scholar]
- Lee SE, Moore JK, Holmes A, Umezu K, Kolodner RD, Haber JE (1998) Saccharomyces Ku70, Mre11/Rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell 94: 399–409 [DOI] [PubMed] [Google Scholar]
- Lisby M, Mortensen UH, Rothstein R (2003) Colocalization of multiple DNA double-strand breaks at a single Rad52 repair centre. Nat Cell Biol 5: 572–577 [DOI] [PubMed] [Google Scholar]
- Lisby M, Rothstein R, Mortensen UH (2001) Rad52 forms DNA repair and recombination centers during S phase. Proc Natl Acad Sci USA 98: 8276–8282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazin AV, Bornarth CJ, Solinger JA, Heyer WD, Kowalczykowski SC (2000) Rad54 protein is targeted to pairing loci by the Rad51 nucleoprotein filament. Mol Cell 6: 583–592 [DOI] [PubMed] [Google Scholar]
- Michaelis C, Ciosk R, Nasmyth K (1997) Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 91: 35–45 [DOI] [PubMed] [Google Scholar]
- Mortensen UH, Bendixen C, Sunjevaric I, Rothstein R (1996) DNA strand annealing is promoted by the yeast Rad52 protein. Proc Natl Acad Sci USA 93: 10729–10734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- New JH, Kowalczykowski SC (2002) Rad52 protein has a second stimulatory role in DNA strand exchange that complements replication protein-A function. J Biol Chem 277: 26171–26176 [DOI] [PubMed] [Google Scholar]
- New JH, Sugiyama T, Zaitseva E, Kowalczykowski SC (1998) Rad52 protein stimulates DNA strand exchange by Rad51 and replication protein A. Nature 391: 407–410 [DOI] [PubMed] [Google Scholar]
- Ogawa T, Yu X, Shinohara A, Egelman EH (1993) Similarity of the yeast RAD51 filament to the bacterial RecA filament. Science 259: 1896–1899 [DOI] [PubMed] [Google Scholar]
- Padmore R, Cao L, Kleckner N (1991) Temporal comparison of recombination and synaptonemal complex formation during meiosis in S. cerevisiae. Cell 66: 1239–1256 [DOI] [PubMed] [Google Scholar]
- Pâques F, Haber JE (1999) Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 63: 349–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CA, Baumann P, Van Dyck E, West SC (2000) Precise binding of single-stranded DNA termini by human RAD52 protein. EMBO J 19: 4175–4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passy SI, Yu X, Li Z, Radding CM, Egelman EH (1999) Rings and filaments of beta protein from bacteriophage lambda suggest a superfamily of recombination proteins. Proc Natl Acad Sci USA 96: 4279–4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petukhova G, Stratton S, Sung P (1998) Catalysis of homologous DNA pairing by yeast Rad51 and Rad54 proteins. Nature 393: 91–94 [DOI] [PubMed] [Google Scholar]
- Robinett CC, Straight A, Li G, Willhelm C, Sudlow G, Murray A, Belmont AS (1996) In vivo localization of DNA sequences and visualization of large-scale chromatin organization using lac operator/repressor recognition. J Cell Biol 135: 1685–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuckli-Maurer J, Heyer WD (1999) The Saccharomyces cerevisiae RAD54 gene is important but not essential for natural homothallic mating-type switching. Mol Gen Genet 260: 551–558 [DOI] [PubMed] [Google Scholar]
- Shinohara A, Ogawa T (1998) Stimulation by Rad52 of yeast Rad51-mediated recombination. Nature 391: 404–407 [DOI] [PubMed] [Google Scholar]
- Shinohara A, Ogawa H, Ogawa T (1992) Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell 69: 457–470 [DOI] [PubMed] [Google Scholar]
- Shinohara A, Shinohara M, Ohta T, Matsuda S, Ogawa T (1998) Rad52 forms ring structures and co-operates with RPA in single-strand DNA annealing. Genes Cells 3: 145–156 [DOI] [PubMed] [Google Scholar]
- Singleton MR, Wentzell LM, Liu Y, West SC, Wigley DB (2002) Structure of the single-strand annealing domain of human RAD52 protein. Proc Natl Acad Sci USA 99: 13492–13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinger JA, Heyer WD (2001) Rad54 protein stimulates the postsynaptic phase of Rad51 protein-mediated DNA strand exchange. Proc Natl Acad Sci USA 98: 8447–8453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Sung P (2000) Functional interactions among yeast Rad51 recombinase, Rad52 mediator, and replication protein A in DNA strand exchange. J Biol Chem 275: 15895–15904 [DOI] [PubMed] [Google Scholar]
- Straight AF, Belmont AS, Robinett CC, Murray AW (1996) GFP tagging of budding yeast chromosomes reveals that protein–protein interactions can mediate sister chromatid cohesion. Curr Biol 6: 1599–1608 [DOI] [PubMed] [Google Scholar]
- Sugawara N, Ivanov EL, Fishman-Lobell J, Ray BL, Wu X, Haber JE (1995) DNA structure-dependent requirements for yeast RAD genes in gene conversion. Nature 373: 84–86 [DOI] [PubMed] [Google Scholar]
- Sugawara N, Wang X, Haber JE (2003) In vivo roles of Rad52, Rad54 and Rad55 proteins in Rad51-mediated recombination. Mol Cell 12: 209–217 [DOI] [PubMed] [Google Scholar]
- Sugiyama T, New JH, Kowalczykowski SC (1998) DNA annealing by RAD52 protein is stimulated by specific interaction with the complex of replication protein A and single-stranded DNA. Proc Natl Acad Sci USA 95: 6049–6054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Zaitseva EM, Kowalczykowski SC (1997) A single-stranded DNA-binding protein is needed for efficient presynaptic complex formation by the Saccharomyces cerevisiae Rad51 protein. J Biol Chem 272: 7940–7945 [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kowalczykowski SC (2002) Rad52 protein associates with replication protein A8RPA-single stranded DNA to accelerate Rad51-mediated displacement of RPA and presynaptic complex c formation. J Biol Chem 277: 31663–31672 [DOI] [PubMed] [Google Scholar]
- Sung P (1997a) Function of yeast Rad52 protein as a mediator between replication protein A and the Rad51 recombinase. J Biol Chem 272: 28194–28197 [DOI] [PubMed] [Google Scholar]
- Sung P (1997b) Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev 11: 1111–1121 [DOI] [PubMed] [Google Scholar]
- Sung P, Robberson DL (1995) DNA strand exchange mediated by a RAD51–ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell 82: 453–461 [DOI] [PubMed] [Google Scholar]
- Symington LS (2002) Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol Mol Biol Rev 66: 630–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaze MB, Pellicioli A, Lee SE, Ira G, Liberi G, Arbel-Eden A, Foiani M, Haber JE (2002) Recovery from checkpoint-mediated arrest after repair of a double-strand break requires Srs2 helicase. Mol Cell 10: 373–385 [DOI] [PubMed] [Google Scholar]
- White CI, Haber JE (1990) Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J 9: 663–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolner B, van Komen S, Sung P, Peterson CL (2003) Recruitment of the recombinational repair machinery to a DNA double-strand break in yeast. Mol Cell 12: 221–232 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data on-line


