Abstract
Objectives
To determine how exercise influences penetrance of arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) among patients with desmosomal mutations.
Background
While animal models and anecdotal evidence suggest exercise is a risk factor for ARVD/C, there have been no systematic human studies.
Methods
Eighty-seven carriers (46 male, mean age 44±18) were interviewed about regular physical activity from age ten. The relationship of exercise with; 1) sustained ventricular arrhythmia (VT/VF), 2) stage C heart failure (HF), and 3) meeting diagnostic criteria for ARVD/C (TFC) was studied.
Results
Endurance athletes (n=56) developed symptoms at a younger age (30.1±13.0 vs. 40.6±21.1 years, p=0.05), were more likely to meet TFC at last follow-up (82% vs. 35%, p<0.001), and had a lower lifetime survival free from VT/VF (p=0.013) and HF (p=0.004). Compared to those who did the least (lowest quartile) exercise per year prior to presentation, those in the second (OR=6.64, p=0.013), third (OR=16.7, p=0.001), and top (OR=25.3, p<0.0001) quartiles were increasingly likely to meet TFC. Among 61 who did not present with VT/VF, the 13 subjects experiencing a first VT/VF event over a mean 8.4±6.7 year follow-up were all endurance athletes (p=0.002). Survival from first VT/VF was lowest among those who exercised most (top quartile) both prior to (p=0.036) and after (p=0.005) clinical presentation. Among individuals in the top quartile, a reduction in exercise decreased VT/VF risk (p=0.04).
Conclusions
Endurance exercise and frequent exercise increase risk of VT/VF, HF, and ARVD/C in desmosomal mutation carriers. These findings support exercise restriction for these patients.
Keywords: Arrhythmogenic right ventricular dysplasia / cardiomyopathy, exercise, heart failure, ventricular arrhythmias, penetrance
Introduction
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) is a heritable cardiomyopathy characterized by life-threatening ventricular arrhythmias, right ventricular (RV) dysfunction, and an elevated risk for sudden cardiac death(1, 2). Incomplete penetrance and variable phenotypic expression are characteristic of ARVD/C suggesting a potential role for environmental influences(3–5). Many ARVD/C patients are highly athletic and those who participate in competitive athletics have been found to have a five-fold increased risk of sudden death compared to non-athletes(6). It is currently recommended that ARVD/C patients participate in neither competitive(7) nor most recreational(8) sports, but no recommendations have been made for clinically unaffected mutation carriers.
Over the last decade, pathogenic ARVD/C associated mutations have been identified in five desmosomal genes (PKP2, DSG2, DSP, DSC2, and JUP) (9–13). Cardiac desmosome are specialized adhesion junctions composed of symmetrical group of proteins – the cadherins, the armadillo proteins, and the plakins – that provide a mechanical connection between cardiac myocytes. Desmosomal mutations can now be identified in up to 60% of ARVD/C patients(14, 15). The appreciation that ARVD/C is in many cases a “disease of the desmosome” suggests potential mechanisms through which exercise, particularly endurance exercise, could be a risk factor for ARVD/C(16, 17). Kirchhof and colleagues showed that endurance exercise accelerated the development of RV dysfunction and arrhythmias in a plakoglobin deficient mouse model(18), but there are no systematic human studies.
Clinical genetic testing for ARVD/C is now routinely performed(19). Therefore, the clinician is now confronted not only with ARVD/C patients seeking advice on physical activity, but also with making recommendations for a growing group of clinically unaffected mutation carriers. Because familial ARVD/C is a clinically heterogeneous disorder with incomplete penetrance and variable expressivity(20, 21) management of unaffected mutation carriers is challenging as individualized risk of developing disease is uncertain. Discerning whether lifestyle choices (exercise habits) can modify penetrance is critical. Therefore, we developed a study of ARVD/C-associated desmosomal mutation carriers to test the hypothesis that exercise influences age-related penetrance, arrhythmic risk, and progression to heart failure in ARVD/C.
Methods
Study population
The study population was recruited from the Johns Hopkins ARVD/C Registry (arvd.com) which prospectively enrolls ARVD/C patients and their family members. Participants are contacted and medical records are updated annually. Registry participants who 1) carried a single copy of a pathogenic ARVD/C associated desmosomal mutation, 2) were age ten or older, and 3) could speak English were invited to participate in a detailed interview to document exercise history. Parents were co-informants for children under age 18. All aspects of the study were approved by the Johns Hopkins School of Medicine Institutional Review Board.
Genotype
Genotype was derived from direct sequencing of the desmosomal genes PKP2, DSG2, DSC2, DSP, and JUP through either our research(14, 20) or commercially available testing. Family members are screened only for mutations identified in the proband. Individuals with digenic or compound heterozygous mutations were excluded.
Clinical outcomes
Clinical and demographic data were drawn from the Johns Hopkins ARVD/C Registry. Baseline data included sex, date of birth, date and type of clinical presentation, family history, and results of non-invasive and invasive studies (12-lead ECG, exercise testing, 24-hour Holter monitoring, 2-dimensional transthoracic echocardiography, cardiac MRI, signal averaged ECG, RV angiogram). Clinical presentation was defined as the first medical visit for a cardiac complaint related to ARVD/C. Primary outcome measures included: 1) first sustained ventricular arrhythmia (VT/VF), 2) onset of stage C heart failure (HF), and 3) diagnosis of ARVD/C at last follow-up (TFC). First sustained ventricular arrhythmia was a composite measure of the occurrence of spontaneous sustained VT, aborted sudden cardiac death, or appropriate implantable cardioverter-defibrillator (ICD) intervention. In patients without an ICD, VT/VF outcome was adjudicated based on reviewing ECGs and medical records; in patients with an ICD, the device stored ECGs were reviewed for appropriateness of ICD therapy. In patients with multiple endpoints, the first event was considered the censoring event. Stage C heart failure (HF) was defined using the American College of Cardiology/American Heart Association heart failure staging system(22), but required both evidence of structural heart disease including RV abnormalities and symptoms directly attributed to HF as done previously(23). Heart failure was adjudicated independently by two members of the study team blinded to exercise history. The diagnosis of ARVD/C was based on the presence of major and minor diagnostic criteria according to the 2010 revised Task force Criteria (TFC)(24).
Exercise interviews
Structured telephone or in-person interviews were conducted by three genetic counselors and one physician. During the interview, we prompted participants to list regular exercise done for leisure/recreation, for work, and for transportation since age ten. Two aspects of each regularly performed exercise were collected: intensity and duration. Intensity of each activity was rated as “light”, “moderate” or “vigorous” using language and definitions from the Multi-Ethnic Study of Atherosclerosis (MESA) Typical Week Physical Activity Survey (25). To determine duration, participants were asked between which ages they had participated in each exercise activity. Then the participant was asked on average how many months of the year, days of the month, and hours a day he/she did this activity at each relevant intensity level. Responses were transcribed on pre-prepared data collection sheets.
Exercise history analysis
In this study, we evaluated the influence of two aspects of exercise history: 1) aerobic intensity - defined as participation in vigorous intensity endurance (aerobic) athletics and 2) duration – defined as annual hours of all regular exercise. “Endurance athletics” were sports with a high dynamic demand (>70% Max O2), as defined by the 36th Bethesda Conference Classification of Sports (Task Force 8)(26), done for at least 50 hours/year at vigorous intensity. “Vigorous” intensity had been self-rated during the interview as described. Individuals meeting these criteria are referred to as endurance athletes throughout the manuscript. For exercise duration, average hours per year of all regular exercise was calculated for each participant both prior to and following clinical presentation.
Statistical analysis
Categorical variables are reported as frequency (%) and compared between groups by the chi-square or Fisher’s exact test. Continuous variables are summarized as either mean ± SD or median (interquartile range) and compared across groups using a t test or Mann-Whitney U test as appropriate. Median hours per year of exercise before and after presentation were compared by the Wilcoxon signed rank test. Likelihood of meeting TFC was estimated using multivariate logistic regression. Cumulative freedom from the composite arrhythmic outcome and heart failure outcome were determined by the Kaplan Meier method. Differences in survival among groups were evaluated with a log-rank test. A p-value of <0.05 was considered significant. SPSS (version20; SPSS Inc., Chicago, IL) statistical software was used.
Results
Population
Of 134 mutation carriers from 68 families invited to participate, 89 (66%) were interviewed. There was no difference in response rate by sex or whether an individual was a proband or family member. Two interviews were excluded from analysis – one in which a minor and her mother gave discordant responses and a second in which the participant was unable to attend to interview questions due to distress related to a change in her personal life.
The final study population included 87 individuals (46 male) from 51 families with a single copy of a pathogenic ARVD/C-associated desmosomal mutation (76 PKP2, 7 DSG2, 3 DSP, 1 DSC2, Supplementary Table 1). Age at interview ranged from 11–88 years (mean 44±18). Two individuals were of Asian ancestry. The remainder were Caucasian. Approximately two-thirds (57/87, 66%) met TFC and 45% (39/87) had experienced at least one life-threatening VT/VF episode at last clinical follow-up. Ten (12%) had developed HF at last follow-up and 2 (2%) had undergone cardiac transplant, both for VT. Participants had 14 first-degree relatives who had died from ARVD/C-associated arrhythmias (13) or heart failure (1). Additional clinical characteristics are shown in Table 1.
Table 1.
Association between clinical characteristics and participation in endurance athletics
| Overall (n=87) | Endurance athlete (n=56) | Not endurance athlete (n=31) | p-value | |
|---|---|---|---|---|
|
| ||||
| Male (%) | 46 (53) | 32 (57) | 14 (45) | NS |
|
| ||||
| Proband | 36 (41) | 28 (50) | 8 (26) | 0.028 |
|
| ||||
| Age at interview (mean ±SD) | 44±18 | 42±15 | 45±22 | NS |
|
| ||||
| Presentation | ||||
| Age at clinical presentation | 35±17 | 32±14 | 38±20 | NS |
| Type of presentation | ||||
| Symptomatic presentation | 44 (51) | 36 (64) | 8 (26) | 0.002 |
| Resuscitated SCD | 3 (3) | 2(4) | 1 (3) | |
| Asymptomatic | 40 (46) | 18 (45) | 22 (71) | |
| Sustained VT/VF at presentation | 26 (30) | 18 (32) | 8 (26) | NS |
| Stage CHF at presentation | 0 (0) | 0(0) | 0(0) | NS |
| Age first symptom | 32±15 | 30±13 | 41±21 | 0.05 |
|
| ||||
| Task Force Criteria at LFU (yes) | 56 (64) | 46 (82) | 11 (35) | <0.001 |
| Structural alterations | 30 (35) major | 24 (44) major | 6 (20) major | 0.021 |
| 10 (12) minor | 8 (15) minor | 2 (7) minor | ||
| Repolarization abnormalities* | 43 (50) major | 34 (62) major | 9 (29) major | <0.001 |
| 15 (17) minor | 12 (22) minor | 3 (10) minor | ||
| Depolarization abnormalities* | 5 (6) major | 5 (9) major | 0 (0) major | 0.003 |
| 35 (41) minor | 28 (51) minor | 7 (23) minor | ||
| Arrhythmias+ | 17 (21) major | 13 (24) major | 4 (14) major | 0.011 |
| 30 (36) minor | 24 (44) minor | 6 (21) minor | ||
| Family history/genetics | 87 (100) major | 56 (100) major | 31 (100) major | 1.000 |
|
| ||||
| VT/VF at LFU (yes) | 39 (45) | 31 (55) | 8 (26) | 0.008 |
| Type of arrhythmic event | ||||
| Sustained VT | 29 (74) | 22 (71) | 7 (88) | NS |
| Aborted SCD / resuscitated SCD | 3 (18) | 2 (6) | 1 (12) | |
| Appropriate ICD therapy | 7 (8) | 7 (23) | 0 (0) | |
| Activity at first VT/VF | ||||
| Exercise | 25 (64) | 21 (68) | 4 (50) | NS |
| Daily activity | 10 (26) | 6 (19) | 4 (50) | |
| At rest | 4 (10) | 4 (13) | 0 (0) | |
| Age first VT/VF | 33±12 | 32±11 | 37±16 | NS |
|
| ||||
| Stage C heart failure - LFU (yes) | 10 (12) | 10 (18) | 0 (0) | 0.012 |
|
| ||||
| Transplant at LFU | 2 (2) | 2 (4) | 0 (0) | NS |
One 11 year-old participant had no ECG available for review. Therefore only 86 individuals were characterized for repolarization and depolarization abnormalities
Three individuals had Holter monitoring performed noting PVCs were present, but no PVC count was provided so these individuals were not included in the arrhythmias category. Abbreviations: HF: heart failure, LFU: last clinical follow-up, NS: non-significant, SCD: sudden cardiac death, VT/VF: composite measure of the occurrence of spontaneous sustained ventricular tachycardia, aborted SCD, SCD, or appropriate implantable cardioverter-defibrillator intervention for a ventricular arrhythmia
Exercise history
Overall, 56/87 (64%) participants had been endurance athletes. The most common endurance sports were long and middle-distance running (37/56, 66%), basketball (27/56, 48%), soccer (14/56, 25%), and competitive swimming (8/56, 14%). Other qualifying sports included distance cycling (5), lacrosse/field hockey (5), tennis/squash (4), rowing (1), and cross-country skiing (1). Some endurance athletes competed in multiple endurance sports. Median age of starting a first endurance sport was 14 (range 10–45, IQR 10, 18). Males were not significantly more likely to have been endurance athletes. There was no significant difference in the age at interview between endurance athletes and non-athletes.
Interviewees participated in a median 284 hours/year (range 0–2657, IQR 135, 509) of regular exercise of all types prior to presentation and a median 155 hours/year (range 0–1658, IQR 17, 314) afterward (p<0.001). Most individuals (61/87, 70%) decreased exercise following presentation, with no difference between athletes and non-athletes. Endurance athletes did more annual exercise before presentation (median 404 hours/year, IQR 210, 684) than non-athletes (median 135 hours/year, IQR 38, 243) (p<0.001). There was no significant difference in annual exercise after clinical presentation between endurance athletes and non-athletes.
Influence of participation in endurance athletics on clinical course
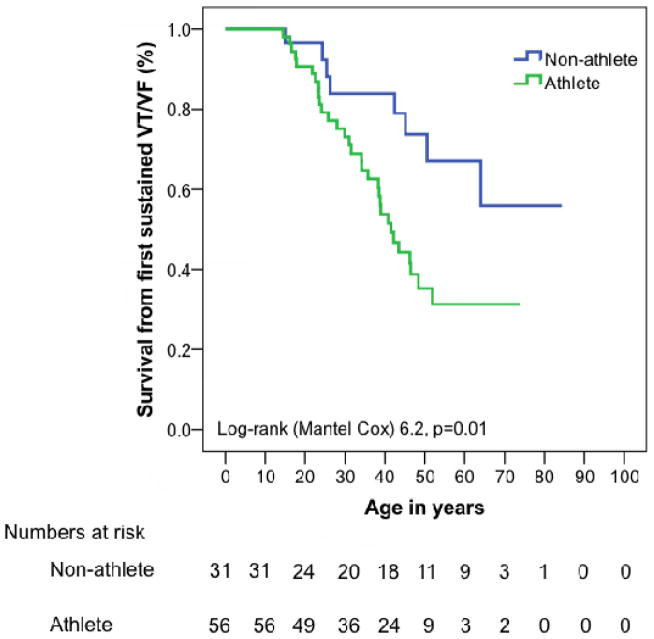
As shown in Table 1, mutation carriers who were endurance athletes had a different presentation and clinical course than non-athletes. Endurance athletes were more likely to be probands (50% athletes vs. 26% non-athletes, p=0.028) and to have cardiac symptoms (VT/VF, syncope, presyncope, palpitations, or chest pain) at presentation (68% athletes vs. 29% non-athletes, p=0.001) and during follow-up (75% athletes vs. 32% non-athletes, p<0.001). Endurance athletes developed symptoms at a younger age than non-athletes (30.1±13.0 vs. 40.6±21.1 years, p=0.05). Lifetime arrhythmic risk was higher among endurance athletes. Overall, 31 (55%) endurance athletes had experienced at least one sustained VT/VF at last follow up as compared to 8 (26%) non-athletes (p=0.008)(Table 1). As shown in Figure 1a, cumulative lifetime event free survival from VT/VF was significantly lower in endurance athletes (p=0.013).
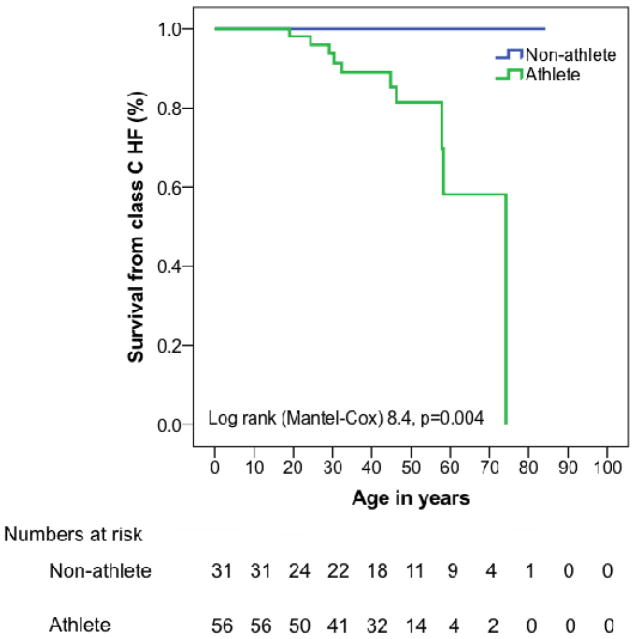
Figure 1. Cumulative lifetime survival from sustained ventricular arrhythmia and class C heart failure.
Cumulative lifetime survival free from (A) sustained ventricular arrhythmias and (B) stage C heart failure (B) stratified by participation in endurance athletics. Event free survival from sustained arrhythmias and stage C heart failure is significantly lower among endurance athletes. Abbreviations: HF - heart failure, VT/VF - sustained ventricular arrhythmia.
ARVD/C diagnosis
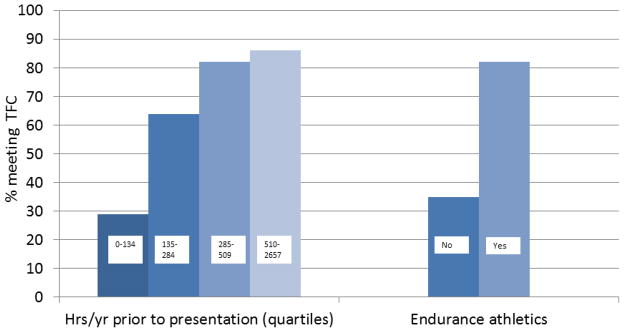
We evaluated the influence of both participation in endurance athletics and average annual duration of all exercise on likelihood of meeting diagnostic criteria (TFC) at last follow-up. Endurance athletes were more likely to meet both overall TFC (82% vs. 35%, p<0.001) (Figure 2) and criteria for each of the domains of the TFC (structural abnormality/dysfunction, repolarization abnormalities, depolarization abnormalities, arrhythmias) except family history/genetics. The absence of a relationship with genetics is consistent with the entry criteria for this study requiring that all subjects have a pathogenic desmosomal mutation.
Figure 2. Likelihood of ARVD/C diagnosis is associated with exercise history.
Likelihood of meeting ARVD/C diagnostic criteria at last follow-up is associated with increasing hours per year of exercise (<0.001) and participation in endurance athletics (p<0.001).
Abbreviations: Hrs/yr – hours per year, TFC – 2010 Task Force diagnostic Criteria.
Amount of annual exercise was also positively correlated with an increasing likelihood of meeting TFC at last follow-up. Among those who participated in the fewest (lowest quartile) hours/year of exercise at presentation, 6/21 (29%) met TFC as compared to 14 (64%) in the second quartile, 22 (82%) in the third quartile, and 19 (86%) among those in the top quartile (p<0.001) (Figure 2). Controlling for sex and age at last clinical follow-up, compared with those who did the least (lowest quartile) exercise, those in the second (OR=6.64, 95% CI=1.49 – 29.5), p=0.013), third (OR=16.7, 95%CI=3.21 – 86.9, p=0.001), and top (OR=25.3, 95%CI=4.21–153, p<0.0001) quartiles of exercise per year prior to presentation were increasingly likely to meet TFC at last follow-up.
Heart failure
We also evaluated the influence of both intensity (participation in endurance athletics) and duration (hours/year of all exercise) on incidence of Stage C heart failure (HF). None of our population had HF at presentation, 12% developed HF in follow-up. Mean age of onset of HF was 42±18 years (range 19–74 years). Only endurance athletes developed HF (18% vs. 0%, p=0.012). As shown in Figure 1b cumulative lifetime survival from HF onset was significantly lower among endurance athletes (p=0.004).
Participants who did more average annual exercise prior to presentation (8/44, 18% more hours of exercise vs. 2/43, 5% fewer hours, p=0.048) were more likely to develop HF during follow-up. Hours of annual exercise following presentation, sex, and age at presentation had no association with development of HF.
Arrhythmic outcome
The relationship between exercise and development of a first sustained ventricular arrhythmia (VT/VF) during follow-up was evaluated in the subset of 61 (70%) subjects who did not present clinically with VT/VF. Thirty-eight of these 61 were endurance athletes (62%). This group of 61 decreased exercise from a median 242 hours/year prior to presentation (range 0–2657, IQR 119, 516) to 212 hours/year (range 0–1658, IQR 16, 425) afterwards (p=0.021).
Over a mean follow-up of 8.4±6.7 years, 13 of these 61 subjects (21%) experienced a first VT/VF, including a resuscitated sudden cardiac arrest in one individual. Mean age at first VT/VF was 32.3 ± 7.8 years (range 22–45 yrs). Mean VT cycle length was 282msec (range 240–394msec). Events disproportionately occurred in younger individuals (p=0.018). There was no difference by sex. Thirteen of the 38 (34%) endurance athletes experienced a first VT/VF during follow-up as compared with none of the 23 (0%) non-athletes (p=0.002).
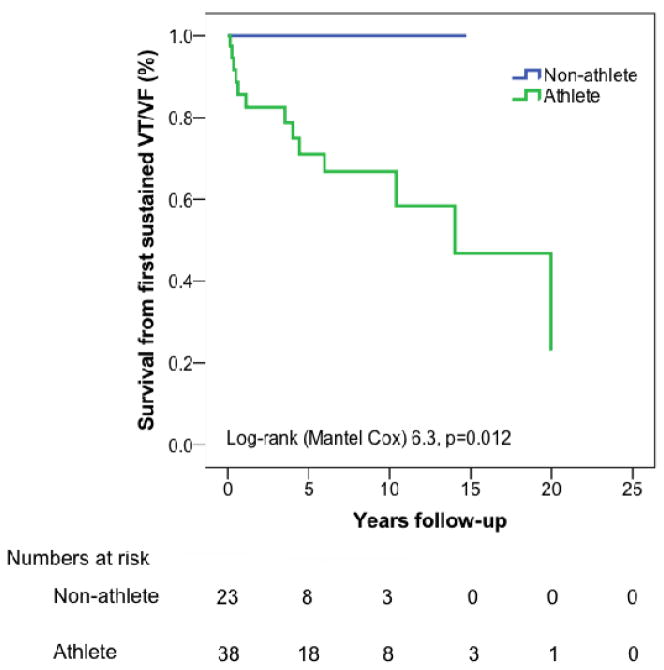
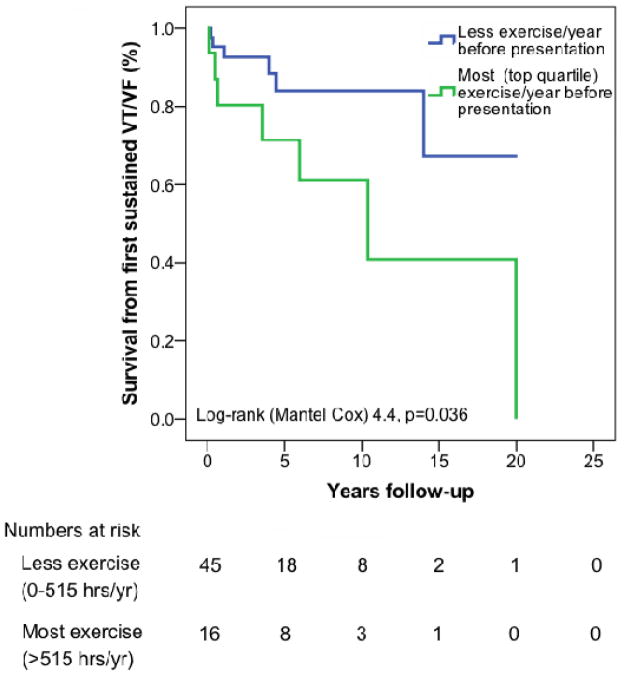
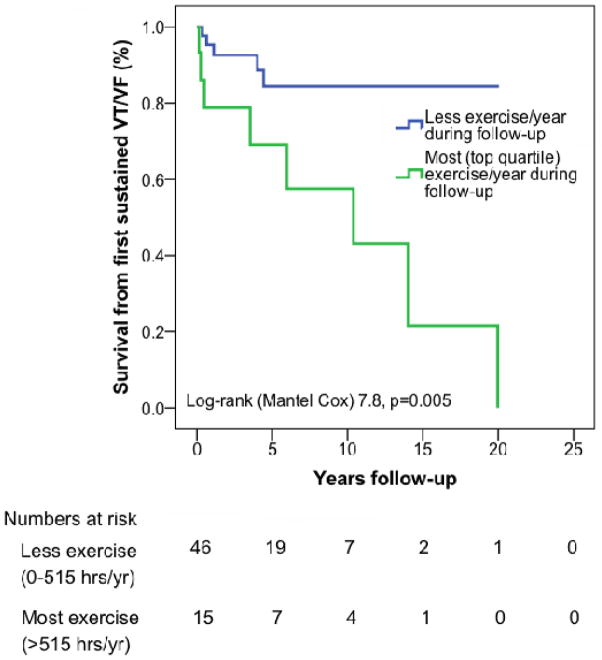
Duration of annual exercise (all types of regular exercise,) both prior to and following clinical presentation was also associated with a first VT/VF. Those who did the most (top quartile) hours of exercise annually prior to presentation (>516 hours/year) were disproportionately likely to develop VT/VF (7/16 (43%) top quartile vs. 6/45 (13%) in bottom three quartiles, p=0.01). Patients who did the most exercise (>425 hrs/yr) after clinical presentation were also more likely to develop VT/VF during follow-up (8/15, 53% top quartile vs. 5/46, 11% in bottom three quartiles, p<0.001). Overall, event free survival from a first VT/VF during follow-up was significantly lower among endurance athletes (Figure 3a, p=0.012) and among those who exercised most prior to (Figure 3b, p=0.036) and after (Figure 3c, p=0.005) clinical presentation.
Figure 3. Cumulative survival free from sustained ventricular arrhythmia in follow-up.
Among 61 cases who did not present clinically with a sustained ventricular arrhythmia, survival from a first sustained ventricular arrhythmia during follow-up was significantly lower among (A) endurance athletes and those doing the most (top quartile hours per year) exercise prior to (B) and following (C) clinical presentation. Abbreviations: VT/VF – sustained ventricular arrhythmia.
Influence of change in exercise duration on arrhythmic outcome
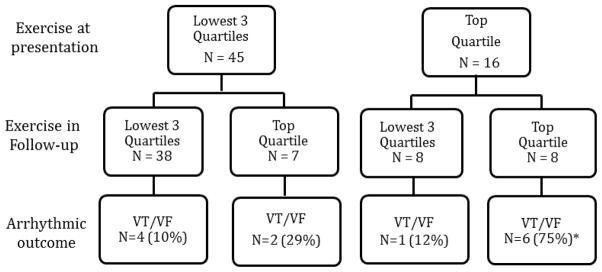
The impact of changing duration of annual exercise on the likelihood of developing a first VT/VF was also evaluated in these 61 participants. We examined whether patients were in the top quartile for hours of annual exercise (all types/intensity levels) prior to and following presentation. As shown in Figure 4, first VT/VF events occurred disproportionately more frequently in individuals who did the most (top quartile) exercise both prior to and following clinical presentation (p=0.007). Among 16 individuals who did the most (top quartile) exercise prior to presentation, 6/8 (75%) who continued do top quartile exercise after presentation had a first VT/VF in follow-up as compared to only 1/8 (12%) who reduced exercise after presentation (p=0.04) (Figure 4). Among 45 individuals who were in lowest 3 quartiles of hours of exercise prior to clinical presentation, 4/38 (10%) who continued lower levels of exercise developed a first arrhythmia as compared to 2/7 (29%) who increased exercise to the top quartile level (p=NS).
Figure 4. Change in exercise following clinical presentation influences likelihood of developing a first sustained ventricular arrhythmia.
Among 61 cases who did not present clinically with a sustained ventricular arrhythmia, those who did the most (top quartile) exercise both prior to and after clinical presentation were most likely to develop a sustained ventricular arrhythmia in follow-up (p=0.007). Among those doing the most (top quartile) exercise prior to presentation, those who continued to do top quartile exercise were more likely to develop a first sustained ventricular arrhythmia than those who reduced exercise (p=0.04). Abbreviations: VT/VF – sustained ventricular arrhythmia
Discussion
Main findings
The results of this study reveal for the first time in humans, that the amount and intensity of exercise increases the likelihood of diagnosis, of ventricular arrhythmias, and of developing heart failure among ARVD/C desmosomal mutation carriers. Furthermore, the results suggest reducing exercise duration may alter the clinical course of ARVD/C.
Relationship between exercise and ARVD/C
During the thirty years since publication of the first major reports describing ARVD/C(1, 27), threads of evidence have emerged suggesting that exercise influences both the development of ARVD/C and its associated arrhythmic risk. One of the first to highlight this important link was a study of sudden cardiac death in the Veneto region of Italy which found that young athletes had a 5-fold risk of dying of ARVD/C compared to non-athletes(6). Consistent with this observation, implementation of a pre-participation screening program resulted in a sharp decline in such deaths(28). The next major line of evidence supporting a link between ARVD/C and exercise was the landmark discovery that a mutation in the desmosomal protein plakoglobin (JUP) caused an unusual variant of ARVD/C(12) and subsequent discovery of ARVD/C-associated mutations in other desmosomal genes(9–11, 13). The cardiac desmosome provides a mechanical connection between myocytes. A third line of evidence concerns the body of research on the differential response of the RV and left ventricle (LV) to exercise(29, 30). Compared with rest, RV wall stress at peak exercise in athletes rises by 170%, compared with only a 23% increase in LV wall stress. Intense endurance exercise causes acute dysfunction of the RV, but not the LV(30). The RV of individuals with desmosomal mutations may be particularly vulnerable to pathologic remodelling in response to exercise. In these patients, endurance exercise may facilitate myocyte uncoupling at defective desmosomes leading to inflammation, fibrosis, adipocytosis and to direct impairment in electrical coupling(16). This sets the stage for arrhythmias, worsening structural involvement, and heart failure. A final thread of evidence supporting a causative link between exercise and ARVD/C was established by Kirchof and colleagues using a plakoglobin deficient mouse model(18). In this model, endurance exercise accelerated the development of RV dysfunction and ventricular arrhythmias.
Impact of exercise on clinical course of ARVD/C-associated mutation carriers
This study tests the hypothesis that exercise is an important environmental factor in the development of ARVD/C among mutation carriers and influences outcomes of ARVD/C patients. The foundational work described earlier has provided circumstantial evidence that exercise influences penetrance, arrhythmic risk, and progression to heart failure but there have been no systematic studies in humans. Furthermore, the seminal work by Corrado and colleagues(6) assumed that “sports per se is not a cause of the increased mortality, rather, it acts as a trigger for cardiac arrest in the presence of underlying cardiovascular disease…” and therefore that the incidence of ARVD/C is the same in the athletic and non-athletic populations. With new appreciation of the pathogenesis of ARVD/C and the incomplete penetrance of disease, it is likely instead that the underlying proportion of athletes with disease may be substantially greater. Furthermore, Corrado’s study relies on autopsy cases, providing limited guidance to physicians treating mildly affected patients and unaffected mutation carriers. A strength of our study is the inclusion of not just ARVD/C patients, but also their at-risk relatives with desomsomal mutations. Furthermore, we found endurance athletes with desmosomal mutations not only were more likely be at risk of life-threatening arrhythmias, but also at increased risk of developing the full ARVD/C phenotype and heart failure. We also examined, for the first time, the relationship between quantity of total exercise and outcomes of patients. We found the duration of annual exercise is positively associated with increasing risk of meeting diagnostic criteria, of a first sustained arrhythmia, and of heart failure. Furthermore, among individuals doing the most average annual exercise at presentation, reducing exercise duration appeared to reduce arrhythmic risk. Our findings support the conclusion that exercise negatively influences cardiac structure and function in at least a proportion of ARVD/C associated mutation carriers.
Study limitations
Our retrospective interview-based exercise history collection may limit interpretation of study findings. Recall of athletic participation may have been inaccurate in a way that varied across subpopulations (recall bias). Additionally, participants may have deliberately altered their responses in a way they considered desirable (social desirability). We limited the influence of both by defining athletic history in broad categories (eg. endurance athletics participation vs. not, quartiles of average exercise/year). Additionally, a portion of our study assessed risk of developing events following clinical presentation (Figure 3) requiring a shorter period of recall.
Our study population also may limit findings. First, while one-third of ARVD/C cases present with sudden death, these individuals were not included in the study. The influence of exercise in this subpopulation may differ. Additionally, this study only included desmosomal mutation carriers, thus the results may have limited implications for ARVD/C patients who do not have a desmosomal mutation. Likewise, we anticipate that carriers of more than one copy of a desmosomal mutation may have a worse response to exercise, but our study cannot address that question. Third, those interviewed are all participants in a research registry run by a tertiary care center creating a potential selection bias. Additionally as our 87 interviewees were members of 51 families there was non-independence of observations within families. Finally, our sample size was limited with regard to our investigation of the influence of risk reduction following exercise reduction.
Conclusions and clinical recommendations
In conclusion, the results of this study, interpreted within the context of prior studies, provide strong evidence demonstrating an important link between exercise and the development and outcomes of ARVD/C. We have demonstrated for the first time in humans, that both participation in vigorous endurance (aerobic) athletics and greater duration of annual exercise of all types are associated with an increased likelihood of ARVD/C diagnosis, ventricular arrhythmias, and heart failure among a large cohort of patients with desmosomal mutations. These data, combined with our 15-year experience as a referral center for patients with ARVD/C, lead us to conclude that restriction from frequent and endurance exercise is important for these patients and modification of exercise at clinical presentation may change outcomes.
Supplementary Material
Acknowledgments
Sources of Funding: The authors wish to acknowledge the National Heart, Lung, and Blood Institute (K23HL093350 to HT), the St. Jude Medical Foundation, and Medtronic Inc. The Johns Hopkins ARVD/C Program (http://ARVD.com) is supported by the Bogle Foundation, the Healing Hearts Foundation, the Campanella family, and Wilmerding Endowments, and the Dr. Francis P. Chiaramonte Private Foundation.
The authors are grateful to the patients and families who have made this work possible.
Abbreviations list
- ARVD/C
Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy
- HF
Stage C heart failure
- ICD
Implantable cardioverter-defibrillator
- IQR
Inter-quartile range
- OR
Odds ratio
- LV
Left ventricle
- RV
Right ventricle
- TFC
2010 Task Force Criteria
- VT
Ventricular tachycardia
- VT/VF
First sustained ventricular arrhythmia
Footnotes
Disclosures: Dr. Calkins receives research support from Medtronic and St. Jude Medical. The other authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marcus F, Fontaine G, Guiraudon G, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–98. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 2.Fontaine G. Arrhythmogenic right ventricular dysplasia. Curr Opin Cardiol. 1995;10:16–20. doi: 10.1097/00001573-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 4.Dalal D, Nasir K, Bomma C, et al. Arrhythmogenic right ventricular dysplasia. Circulation. 2005;112:3823–32. doi: 10.1161/CIRCULATIONAHA.105.542266. [DOI] [PubMed] [Google Scholar]
- 5.Sen-Chowdhry S, Morgan RD, Chambers JC, McKenna WJ. Arrhythmogenic cardiomyopathy: etiology, diagnosis, and treatment. Annu Rev Med. 2010;61:233–53. doi: 10.1146/annurev.med.052208.130419. [DOI] [PubMed] [Google Scholar]
- 6.Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42:1959–63. doi: 10.1016/j.jacc.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Maron BJ, Ackerman MJ, Nishimura RA, Pyeritz RE, Towbin JA, Udelson JE. Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and Marfan syndrome. J Am Coll Cardiol. 2005;45:1340–5. doi: 10.1016/j.jacc.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, Chaitman BR, Ackerman MJ, et al. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation. 2004;109:2807–16. doi: 10.1161/01.CIR.0000128363.85581.E1. [DOI] [PubMed] [Google Scholar]
- 9.Gerull B, Heuser A, Wichter T, et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–4. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 10.Awad MM, Dalal D, Cho E, et al. DSG2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Hum Genet. 2006;79:136–42. doi: 10.1086/504393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rampazzo A, Nava A, Malacrida S, et al. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2002;71:1200–6. doi: 10.1086/344208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKoy G, Protonotarios N, Crosby A, et al. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–24. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- 13.Syrris P, Ward D, Evans A, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am J Hum Genet. 2006;79:978–84. doi: 10.1086/509122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.den Haan AD, Tan BY, Zikusoka MN, et al. Comprehensive desmosome mutation analysis in North Americans with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Cardiovasc Genet. 2009;2(5):428–35. doi: 10.1161/CIRCGENETICS.109.858217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox MGPJ, van der Zwaag PA, van der Werf C, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: pathogenic desmosome mutations in index-patients predict outcome of family screening. Circulation. 2011;123:2690–700. doi: 10.1161/CIRCULATIONAHA.110.988287. [DOI] [PubMed] [Google Scholar]
- 16.Saffitz JE, Asimaki A, Huang H. Arrhythmogenic right ventricular cardiomyopathy: new insights into mechanisms of disease. Cardiovascular Pathology. 2010;19:166–70. doi: 10.1016/j.carpath.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Delmar M, McKenna WJ. The cardiac desmosome and arrhythmogenic cardiomyopathies from gene to disease. Circ Res. 2010;107:700–14. doi: 10.1161/CIRCRESAHA.110.223412. [DOI] [PubMed] [Google Scholar]
- 18.Kirchhof P, Fabritz L, Zwiener M, et al. Age-and training-dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin-deficient mice. Circulation. 2006;114:1799–806. doi: 10.1161/CIRCULATIONAHA.106.624502. [DOI] [PubMed] [Google Scholar]
- 19.Ackerman MJ, Priori SG, Willems S, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies. Europace. 2011;13:1077–109. doi: 10.1093/europace/eur245. [DOI] [PubMed] [Google Scholar]
- 20.Dalal D, James C, Devanagondi R, et al. Penetrance of mutations in plakophilin-2 among families with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2006;48:1416–24. doi: 10.1016/j.jacc.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 21.Quarta G, Muir A, Pantazis A, et al. Familial evaluation in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2011;123:2701–9. doi: 10.1161/CIRCULATIONAHA.110.976936. [DOI] [PubMed] [Google Scholar]
- 22.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult. J Am Coll Cardiol. 2001;38:2101–13. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 23.Tedford RJ, James C, Judge DP, et al. Cardiac transplantation in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Col Cardiol. 2012;59:289–290. doi: 10.1016/j.jacc.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia proposed modification of the task force criteria. Eu Heart J. 2010;31:806–14. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turkbey EB, Jorgensen NW, Johnson W, et al. Physical activity and physiological cardiac remodelling in a community setting: the Multi-Ethnic Study of Atherosclerosis (MESA) Heart. 2010;96:42–8. doi: 10.1136/hrt.2009.178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell JH, Haskell W, Snell P, Van Camp SP. Task Force 8: Classification of sports. J Am Coll Cardiol. 2005;45:1364–7. doi: 10.1016/j.jacc.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318:129–33. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 28.Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006;296:1593–601. doi: 10.1001/jama.296.13.1593. [DOI] [PubMed] [Google Scholar]
- 29.Douglas PS, O’Toole ML, Miller WDB, Reichek N. Different effects of prolonged exercise on the right and left ventricles. J Am Coll Cardiol. 1990;15:64–9. doi: 10.1016/0735-1097(90)90176-p. [DOI] [PubMed] [Google Scholar]
- 30.La Gerche A, Burns AT, Mooney DJ, et al. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eu Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.