Abstract
Circulating tumor cells (CTCs) represent a surrogate biomarker of hematogenous metastases. In recent years, their detection has gained increasing interest. There is ample evidence regarding the ability to detect CTCs and their prognostic relevance, but their demonstrated predictive value in therapeutic response monitoring is clinically even more meaningful. Many clinical trials in the early and metastatic cancer setting now include CTCs as a monitoring parameter, and numerous translational studies attempting their molecular characterization are under way. There has been great progress in defining the clinical importance of CTCs, and it now seems likely that we may expect wider implementation of CTCs as a diagnostic oncology tool to monitor therapeutic response in real time. Novel technologies may further facilitate molecular characterization of CTCs and development of novel therapeutic targets, possibly leading to more powerful treatment strategies for cancer patients. As the detection and evaluation of CTCs are becoming an increasingly important diagnostic and prognostic tool, the goal of this review is to communicate the knowledge obtained through analysis of primary tumors and CTCs to oncologists and medical specialists in managing patients with cancer.
Keywords: micrometastasis, minimal residual disease, disseminated tumor cells (DTCs), cancer stem cells (CSCs), therapeutic response
CLINICAL SIGNIFICANCE OF METASTASIS
During the past two decades, the introduction of novel and effective therapies targeting specific molecular processes of tumorigenesis has resulted in an increasingly individualized therapeutic approach to cancer management. The evolution of breast cancer treatment represents an early example of this approach. The use of targeted therapies, such as antihormonal treatment targeting steroid receptors and over-expression of the epidermal growth factor receptor 2 (Her2) with trastuzumab (Herceptin) and pertuzumab (1, 2), has vastly improved patient management. Evaluation of hormone receptor and Her2 expression in primary breast cancer tissues is employed as a companion diagnostic to identify patients who will benefit from such treatments and has been incorporated into the standard of care. The ability to specifically identify patients likely to respond to a given targeted treatment has had an impact in other cancers as well (3). However, even when targets are known and identified in primary tissues, not all patients will respond to a given therapy. In addition, the cost associated with new drug administration after first-line treatment is high. Thus, more improved methods of predicting which patients are most likely to respond, and which patients are responding or failing treatment despite the appropriate selection of drugs in real time, is essential to decrease recurrence of disease and improve patient management.
Further, there is a critical need to understand the mechanisms of metastasis in greater detail in order to define novel biomarkers and therapeutic targets. Primary tumor cells spread to distant sites through invasion into blood and lymphatic vessels. Depending on conditions in the microenvironment at secondary sites, a proportion of these single cells or cell aggregates may reinitiate tumor growth in distant organs. Such cells are collectively referred to as occult metastases, micrometastases, or minimal residual disease.
Differentiation Between DTCs and CTCs
We focus here on reviewing the literature on the hematological spread of cancer. In this review, we refer to tumor cells in bone marrow as disseminated tumor cells (DTCs) and those in the blood as circulating tumor cells (CTCs). Although this is a purely semantic distinction, it is in common usage in the field. Most early studies focused on the detection of DTCs. Initially, antibodies to epithelial cell surface antigens such as epithelial membrane antigen were used to identify DTCs by immunostaining of the bone marrow at the time of primary surgery in women diagnosed with breast cancer with no overt metastases (4). Later on, antibodies to cytokeratins were increasingly used immunohistochemically to identify DTCs in early-stage breast carcinoma (5, 6). Most studies demonstrating prognostic significance of the presence of DTCs in bone marrow aspirates relate to breast cancer (7). Whereas early studies demonstrated the presence of DTCs in 30% of breast cancer patients, the incidence of DTC detection is far lower in more contemporary studies, coincident with earlier detection of breast cancer overall due to increased use of screening mammography. Our recent report of the results of the American College of Surgeons Oncology Group (ACOSOG) study Z0010, one of the largest prospectively collected clinical trials in stage I and II breast cancer patients, analyzed the prognostic significance of DTCs and demonstrated a very low detection rate, with only 3% of bone marrow aspirates containing tumor cells (8). The presence of DTCs was a significant predictor of outcome, although the low incidence of DTCs in this highly selected group of patients called into question the value of its routine use in that setting. Nevertheless, the idea of identifying tumor cells that can be targeted with an adjuvant treatment seems appealing, and many attempts have been made to optimize the detection of tumor cells that circulate in peripheral blood, thus providing minimally invasive access to the early events in metastases.
Cancer patients undergoing treatment frequently undergo blood draws for various diagnostic and treatment-monitoring reasons. Thus, blood would be an ideal compartment for detection of metastatic tumor cells. Blood draws can easily be performed serially. Recent studies have demonstrated the potential value of a CTC assay to determine prognosis as well as to predict response to systemic therapy in patients with breast and other cancers (9–11). Despite a growing body of evidence, the detection and enumeration of CTCs have yet to achieve widespread use in the clinical management of cancer patients, primarily because of the limitations of the currently available technologies for obtaining and analyzing CTCs.
Nevertheless, recognizing the potential value of CTCs as biomarkers for prognosis, as predictors of therapeutic response, and as a companion diagnostic in the development of novel therapeutics, several new methods have evolved in the past decade for CTC enrichment and evaluation. Some have been reviewed previously (12, 13), including density-, affinity-, and sized-based methods. As technology in this growing field matures, a substantial impact on clinical practice could follow adoption of some of these novel methods. As of today, the only FDA-approved technology to clinically monitor CTCs in metastatic cancer patients is CellSearch (Veridex/J&J).
This review focuses on the use of CTCs to better understand the process of metastasis and examines the clinical value of CTCs. Research in the coming decade will likely be focused on molecular characterization of CTCs and definition of these cells as novel biomarkers for defining treatment strategies. The mechanisms behind the epithelial-mesenchymal transition (EMT) and the transformation from cancer stem cells to differentiated cells are only two of many promising areas that could be better understood in the near future with the aid of CTC capture and characterization. Technological advances occurring simultaneously will enable examination of these phenomena at a single-cell level.
Numerous studies have provided a rationale to further intensify research in CTCs (9, 14–16). Although it is impossible to cover all the relevant studies of CTCs vis-à-vis their prognostic and predictive value, we begin by providing a summary of the most important findings.
Review of Prognostic Significance of CTCs
The prognostic significance of detection of CTCs in peripheral blood of cancer patients has been demonstrated for several types of cancers. Most of the evidence results from studies based on immunohistochemical detection of CTCs (9, 10, 14, 16), but several studies have successfully employed a variety of real-time polymerase chain reaction (RT-PCR) methods to detect epithelial or tumor-derived transcripts as surrogate reporters of CTCs (17, 18). Similar to DTCs, the prognostic significance of CTCs has mostly been demonstrated in breast cancer, particularly in metastatic disease.
Seminal studies on CTCs were performed employing the CellSearch method (19), an epithelial antigen-affinity-based system for enrichment and detection of CTCs, and their results have been reproducible across different independent testing sites. We have reviewed the CellSearch technology in detail elsewhere, along with alternative methods (20).
In a landmark study using CellSearch assay, Cristofanilli et al. established the threshold of 5 tumor cells/7.5 ml of blood to define the prognostic significance of CTCs in breast cancer patients (9). This threshold was later used in many studies. Cristofanilli et al. recognized the predictive significance of changes in CTC levels during the treatment. In a follow-up study, the authors demonstrated that the changes in CTC levels could predict response to therapy after only one cycle of treatment (21).
Several independent studies in metastatic breast cancer patients have confirmed prognostic significance of CTCs detected by CellSearch assay (19, 22). Analogous studies were performed in patients with other cancers, such as prostate and colorectal cancer (14, 23), which concluded that CTC detection was associated with unfavorable outcome. More importantly, serial CTC evaluation was capable of predicting response to treatment early in the course of the therapeutic intervention, and much sooner than serum biomarkers or radiographic examination. These studies led to FDA approval of the CellSearch method as a tool for CTC detection, prognostic evaluation, and therapeutic response monitoring in patients with metastatic breast, prostate, or colon cancer, and have set an important cornerstone for evaluating the clinical validity of CTCs.
Whereas the threshold of 5 tumor cells/7.5 ml of blood was used to determine prognosis in breast and prostate cancer, a threshold of 3 tumor cells was used for colorectal cancer patients, and the presence of >3 cells in 26% of patients was associated with unfavorable outcome (14). In castration-resistant prostate cancer, the threshold stratified patients in groups with favorable versus unfavorable prognosis, with >10 months difference in the overall survival (24). Other studies have confirmed the prognostic relevance of CTCs in prostate cancer patients (16, 23, 24).
Other methods besides the CellSearch assay have also demonstrated the prognostic significance of CTCs in peripheral blood of cancer patients and have shown similar association of higher CTC numbers with shorter overall survival and worse prognosis. These include microbead-based affinity enrichment of CTCs followed by identification by immunohistochemistry (10, 25), or RT-PCR approaches for detection of CTCs, where multiple epithelial-specific and tumor-specific transcripts are used as surrogate markers for the presence of CTCs (26, 27).
Evaluating CTCs in therapeutic decision making may improve cancer patient management. Giuliano et al. (28) suggested more aggressive treatment of patients with higher CTC levels versus a relatively modest treatment plan for patients with lower numbers of CTCs. Breast cancer patients with hormone receptor–positive tumors are frequently observed with progressive disease early on hormonal treatment. In such cases, detection of higher levels of CTCs may prove useful for identification of patients who would more likely benefit from a chemotherapy regimen.
Determination of prognosis may be even more beneficial in early-stage cancer patients. Overtreatment of patients diagnosed with primary breast cancer is often debated, and various biomarkers are being explored to help optimize the treatment of these patients. Several gene expression profiling platforms have been clinically evaluated, and prospective studies such as TailorX and Mindact (29, 30) will help to define the utility of these profiles to segregate patients for different treatment approaches. CTC evaluation in patients with early-stage cancer remains unexplored but could provide important decision-making information. Novel CTC detection methods with increased sensitivity may facilitate such studies. Recently, in a study where breast cancer patients were treated in the neoadjuvant setting (the Gepar-Quattro trial) (31), CTCs were detected with a relatively low frequency by CellSearch (>1 tumor cell/7.5 ml of blood) and had limited predictive value. Similarly, data from the Success clinical trial presented at the European Breast Cancer Conference in March 2012 in Vienna (32) demonstrated the adverse impact of the presence of >1 cell/22.5 ml of blood on recurrence-free survival in patients who underwent adjuvant treatment. Thus, it seems likely that higher CTC detection sensitivity, or capture of greater numbers of CTCs (for example, by testing larger samples of blood), may be necessary for the clinical use of CTC evaluation in patients with early-stage breast cancer (31).
Review of Predictive Significance of CTCs
Aside from their use in determining the prognosis of patients with metastatic cancer, the ability of CTCs to predict the response to an ongoing anticancer treatment at the earliest time point is especially attractive. Several studies have evaluated such predictive potential through monitoring change in levels of CTCs to determine either response or resistance to a therapy. Nole et al. (19) showed that the number of CTCs per patient, as well as the number of patients with ≥5 CTCs at baseline, can be reduced by 50% following therapy. A significant proportion of patients who demonstrate no decrease in the number of CTCs may benefit from earlier intervention than is standard in current clinical practice. Two aspects make this important: a potential to decrease costs through early detection and cessation of a failed treatment and, importantly, a potential to spare patients the adverse effects of an ineffective treatment. In a recent prospective trial of serial CTC measurements by CellSearch with side-by-side comparison of several potentially clinically useful serum markers, Bidard et al. demonstrated similar performance in terms of prediction of progression-free survival (33). The question of the clinical utility of CTCs for early decision making is being evaluated in the prospective collection of blood samples for the Southwest Oncology Group (SWOG) 0500 clinical trial. The trial has stopped recruiting patients, and the evaluation of the clinical utility of CTCs may be expected within a year (34).
Serial determination of CTC counts performed in addition to conventional imaging may be highly informative for patient prognosis and therapeutic response. Budd et al. performed a comparison of CTC detection and imaging for prediction of outcome in metastatic breast cancer and validated that CTC detection can provide clinically useful data much earlier during the course of treatment (35). A significant disadvantage of conventional imaging is great interreader variability for evaluation of radiographic scans, which may be overcome with automated CTC detection and counting.
MOLECULAR BIOLOGY OF THE METASTATIC PROCESS
The biology of the metastatic process is still largely unknown, with each step in dissemination representing a significant investigative challenge. The journey of a tumor cell from the primary tumor to a distant site begins with the local invasion of the tumor and intravasation into peripheral circulation. The ability of a tumor cell to undergo EMT has been hypothesized to play a crucial role during the early parts of the metastatic process. The plasticity of these tumor cells may also be an attribute of a subpopulation of putative cancer stem cells within the CTCs (36). We review in the following sections the evidence of these phenomena while metastatic cells are in circulation. Later components of the metastatic cascade, namely mechanisms of extravasation, establishment of a secondary deposit at a distant site, and dormancy, are beyond the scope of this review.
Local Tumor Invasion and Intravasation
In solid tissue tumors, it is hypothesized that the primary malignant cells undergo EMT, a transition in which epithelial tumor cells go through a specific set of molecular changes that results in detachment from their primary sites and assumption of a motile, invasive, mesenchymal cell–like phenotype. The EMT program is typically characterized by loss of E-cadherin expression and subsequent translocation of β-catenin from the membrane to the nucleus, increased expression of vimentin, secretion of matrix metalloproteinase enzymes, and upregulation of various EMT-inducing transcription factors such as Twist, Snail, Slug, Zeb-1, Zeb-2, and others (37). Thus, EMT likely provides a potential mechanistic basis for CTC intravasation from the primary tumor and extravasation to seed tumors at distant secondary sites.
Several groups have reported upregulation of the markers associated with the EMT phenotype on CTCs. In a study evaluating 226 blood samples of 39 metastatic breast cancer patients, Aktas et al. found 62% of CTC-positive patients were also positive for at least one of three multiplexed EMT markers (Akt2, PI3K, and Twist1) by RT-PCR. 62% of patients with EMT-positive CTCs were unresponsive to palliative chemo-, antibody, or hormonal therapy, and only 10% of patients with EMT-positive CTCs responded positively to a similar course of therapy. This study indicated that EMT markers in CTCs might serve as indicators for therapy-resistant tumors and, therefore, an inferior prognosis (38). Evaluating EMT markers Twist and Vimentin on CTCs in breast cancer patients by immunofluorescence, Kallergi et al. found Vimentin/Twist expressing CTCs in 100% of patients with metastatic disease in whom CTCs were detected compared to 77% of early-stage patients in whom CTCs were detected, strongly supporting the notion that EMT is involved in the metastatic potential of CTCs (39).
While the EMT hypothesis continues to gain acceptance in the research community, there remains some speculation about its true contribution to metastasis, as non-EMT modes of tumor cell dissemination have also been documented. For instance, Friedl & Wolf suggested that tumor cells disseminate as single cells through alternative non-EMT-related mechanisms such as ameboid movement or clusters of cells using collective migration (40). Hou et al. have demonstrated in a metastatic lung cancer pilot study the occurrence of circulating tumor microemboli (CTM), contiguous groups of tumor cells, in circulation (41). They further demonstrated that single CTCs expressed markers associated with apoptosis at a higher rate than CTM. These findings suggest that collective migration of tumor cells in circulation could confer a survival benefit through suppression of anoikis-mediated cell death, and thus CTM might contribute to the successful establishment of metastases.
Evidence of EMT has largely been described in vitro. To date, despite attempts by our group and others, there has been no convincing and reproducible demonstration of EMT in vivo, or in patients. The study of EMT has focused on the investigation of changes that take place at the beginning and at the end of the metastatic process, i.e., in primary tumors and in metastatic foci, respectively. Little work has been done to characterize the transition where it most likely occurs: in CTCs. If at least a proportion of CTCs are cells transitioning between epithelial and mesenchymal states with stem-like properties and the ability of reversible modulation (42), these processes have to be well characterized in circulation. Once again, the development of new technologies that improve the sensitivity and efficiency of CTC capture, while permitting the functional characterization of CTCs to assess invasiveness, aggressiveness, plasticity, and tumorigenic potential, will help to further elucidate the mechanisms governing tumor cell dissemination and colonization of distant sites.
Survival of CTCs Through Immunosuppression
It is believed that a critical factor influencing tumor progression and metastasis is the immune system. At the primary tumor site, different immune cells including macrophages, T cells, and dendritic cells infiltrate malignant tissue via inflammatory response signals. Importantly, myeloid-derived suppressor cells (MDSCs) are recruited by the primary tumor and have been shown to influence tumor pathogenesis. Whether the effect of the infiltration of immune cells within a tumor dictates a positive or negative clinical outcome depends on the subset of specific immune cells that infiltrates a given tumor (43). Specifically, MDSCs have been shown to be increased in a tumor-dependent manner in xenograft models of breast cancer (44) and have also been detected in peripheral blood of cancer patients (45), indicating their possible role in facilitating tumor growth and metastasis.
It is then logical to speculate that the systemic immune microenvironment could have a direct interaction with CTCs to exert an immunosuppressive effect while they are in transit, thus allowing CTCs to escape immuno-surveillance en route to colonization of distant organs. Interestingly, using a novel microfilter device for size-based isolation of CTCs developed in our lab (representative data shown in Figures 1 and 2), we have recently observed the occurrence of CTC clusters in direct association with nontumor, CK−/CD45+ cells in blood samples from metastatic prostate cancer patients (A Williams, RH Datar, RJ Cote, et al., unpublished data; Figure 2). To date, the detection of CTC/immune cell clusters has not been reported. In relation to their interaction with CTCs, immune cells may not only participate in immunosuppression but could also form cluster interactions with MDSCs and others to “hitchhike” through the circulatory system, exploiting immune cells for transportation, protection from immune cell attack, and shielding from destruction by systemic shear stress. Further, this suggests a potential mechanism for CTC/immune cell cluster extravasation from circulation via the interaction of immune cells with selectins and integrins to migrate across blood vessel barriers. However, although the role of MDSCs in tumor progression at the primary site has been explored, the immunosuppressive role that systemic MDSCs and other cells may play in protecting CTCs while in transit and facilitating extravasation into distant sites is poorly understood. Further investigation is warranted to fully elucidate this critical stage in the metastatic process, an area currently under study in our laboratory.
Figure 1.

Capture and immunofluorescent (IF) staining of CTCs by the microfilter device in model systems. Following filtration, microfilters are stained with a CK cocktail conjugated to Alexa Fluor 488 secondary fluorescent antibody (green), CD45 conjugated to Alexa Fluor 594 secondary fluorescent antibody (red), and DAPI (blue) for nuclear visualization. CTCs are identified as nucleated, CK+/CD45− cells with morphologic criteria consistent with malignancy. (a) Positive control cytospin preparation where MCF-7 breast cancer cells were mixed with nontumor blood cells from a healthy donor. Note the difference in size between CK+/CD45− tumor cells and CK−/CD45+ nontumor peripheral blood mononuclear cells. (b) T47D breast cancer cells were mixed with phosphate buffer saline, captured by the microfilter device, and identified by CK/CD45 IF staining using confocal microscopy.
Figure 2.
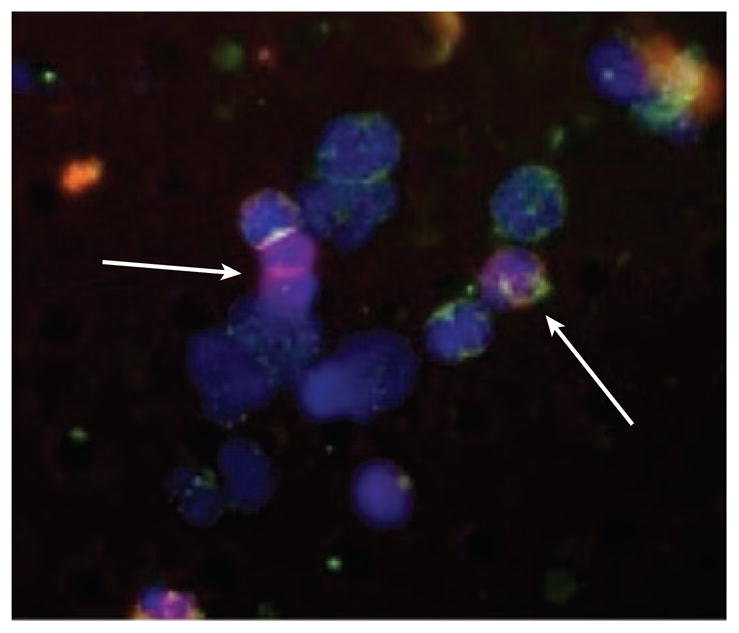
CTC/immune cell clusters captured by the microfilter device in clinical blood samples. Representative image from a metastatic prostate cancer blood sample demonstrating a CTC/immune cell cluster, where CK−/CD45− cells directly associated in the cluster are indicated with arrows.
Phenomenon of Cancer Stem Cells and Their Therapeutic Implications
It is hypothesized that tumor cells are heterogeneous and hierarchically ordered and that at the top of the hierarchy there is a small subpopulation of cancer cells with stem-like potential, known also as cancer stem cells or cancer-initiating cells (46). This cellular hierarchy is strongly related to the organization of normal tissues. There is increasing evidence suggesting that only this specific type of tumor cells harbors tumorigenic potential (47–49). Cancer stem cells have been identified for hematological malignancies (50), brain tumors (51), breast cancers (47), and other solid tumors. In recent studies, the putative breast cancer stem cell phenotype has been defined as CD44+CD24−/low (47, 52, 53). Primary tumors and metastatic lesions were disaggregated and processed in order to obtain single-cell suspensions from primary breast tumors and metastatic lesions. Different subclones were selected through flow cytometric sorting of the cells. These subclones were implanted into nude mice and their tumorigenicity measured. The CD44+CD24−/low subclone was found to be highly tumorigenic and capable of self-renewal and differentiation. Further studies performed by Wicha and his colleagues established another putative stem cell marker, aldehyde dehydrogenase 1 (ALDH1), for normal and breast cancer tissues (54, 55). ALDH1 is a detoxifying enzyme responsible for the oxidation of intracellular aldehydes and seems to have a role in early differentiation of stem cells (56). The expression of ALDH1 has been used in flow cytometry sorting by the commercially available Aldefluor assay. Cells enriched in the Aldefluor-positive fraction have been used to demonstrate their role in the putative breast cancer stem cell phenotype (57, 58). Analogous functional studies have been performed for other cancers. Whereas the presence of micrometastases (DTCs and CTCs) is the rationale behind the use of systemic adjuvant chemotherapy in patients who have undergone definitive local treatment of the primary tumor (59, 60), the presence of cancer stem cells in dissemination may represent an explanation for the failure of adjuvant chemotherapies in a proportion of early-stage breast cancer patients. In the recent in vitro studies, it was demonstrated that putative breast cancer stem cells were resistant to conventional treatment strategies, including radiation and chemotherapy (61, 62). Consequently, the identification of breast cancer stem cells among patients’ CTCs may represent a promising tool to assess the malignant potential of these cells and help in identification of novel therapeutic targets. The major limitation of this approach is the limited knowledge of the cancer stem cell phenotype, with knowledge that only a proportion of enriched cells may be breast cancer stem cells. It is still unclear if there are distinct stem cells for intrinsic subtypes of breast cancer, and if so, which markers define them. In a recent paper, Ricardo et al. evaluated these established phenotypes and their distribution among the molecular subtypes (63). The authors concluded that the methods and biomarkers identifying breast cancer stem cells within the distinct molecular subtypes need to be better explored in order to facilitate translation of the cancer stem cell concept to clinical practice.
Cancer Stem Cells in Dissemination
The enhanced risk of death from advanced breast cancer may be attributable to the potential impact of cancer stem cells among DTCs in patients (64, 65). Given that cancer stem cells are a minor population in primary tumors, we hypothesized they are present among cells metastasizing from primary breast cancer to distant locations. In addition, although the presence of DTCs/CTCs predicts a higher rate of recurrence in patients with early-stage cancer, not all patients with evidence of disseminated cancer cells will recur. It is possible that the differences in outcome may be due to biological distictions in the cell populations, and in particular related to the proportion of disseminated cells that have stem cell characteristics. To examine these ideas, we analyzed DTCs of patients treated in the Z0010 clinical trial from the American College of Surgeons Oncology Group (ACOSOG) for the putative breast cancer stem cell phenotype, using the CD44+CD24−/low tumor-initiating cell phenotype. This was a novel research effort examining the cancer stem cell phenotype among DTCs and, for the first time, showed that the large majority of the DTCs exhibited the expression phenotype criteria of putative cancer stem cells (52). Our finding has significantly impacted research on metastasis, as it suggests an enrichment of putative breast cancer cells in metastases; their percentage within the primary tumor was reported at <10% (66), whereas our study showed that 72% of DTCs showed the putative stem cell phenotype. This result is particularly significant because the analysis was performed in the cohort of stage I and II breast cancer patients, where only 3% of bone marrow samples are shown to be positive for DTCs (8). Also, it contributes significance to earlier findings that putative breast cancer stem cells play an important role in pathogenesis of breast cancer (67) and may offer critical therapeutic targets. Reuben et al. later performed prospective analysis of bone marrow aspirates with flow cytometry sorting and showed that patients with particularly high-risk clinicopathologic features had high percentages of putative breast cancer stem cells (68).
Analogous studies on CTCs in metastatic cancer patients have followed. Theodoropoulos et al. detected CTCs in 67% of patients, of which 35% of CTCs had the putative stem cell phenotype (69). Wang et al. used flow cytometry to evaluate peripheral blood of breast cancer patients at different stages and showed that the percentage of putative stem cells rose with increasing tumor stage, indicating that these cells can be used to guide therapy and predict prognosis (70). Again, these results indicate an emerging role of cancer stem cells as therapeutic targets.
POTENTIAL CLINICAL APPLICATIONS FOR CIRCULATING TUMOR CELLS
Utility of CTCs in Therapeutic Response Monitoring
While discussing the predictive value of CTCs above, we provided the evidence that CTCs may be useful in monitoring response and predicting resistance to treatment. Undoubtedly, such methods would offer patients a dependable, noninvasive, and economical tool. Most published studies have made use of serial CTC testing at the first follow-up visit or early in treatment (three to five weeks), demonstrating their clinical utility earlier than the conventional evaluation methods of response (9, 14).
As mentioned above, the Southwest Oncology Group (SWOG) 0500 clinical trial is currently investigating the benefit of early changes in cancer therapy based on CTC testing by CellSearch. The study closed to accrual in March 2012 and will soon present its first results. SWOG 0500 is a randomized phase III trial testing the strategy of changing therapy for metastatic breast cancer patients with elevated CTCs at the first follow-up visit. If this prospective clinical trial demonstrates the utility of CTCs as a basis for clinical decisions, it may profoundly influence the management of breast cancer patients in the near future. Although novel methods of CTC detection could be expected to have the same utility at decreased costs, their utility in monitoring treatment efficacy remains to be studied. Further, such an approach will have to be validated for other cancers as well before its clinical implementation.
Value of CTC Molecular Characterization
Molecular characterization of CTCs and definition of biomarkers for therapeutic decision making may substantially facilitate individualization of treatment for cancer patients. Better knowledge of the mechanisms underlying cancer metastasis, such as EMT and transformation of tumor cells from cancer stem cells to differentiated cells, as well as technical progress in CTC detection and analysis, possibly at a single-cell level, will expedite the process. In the long term, this progress may lead to identification of novel therapeutic targets.
As it is believed that CTCs are constitutively shed from the primary tumors and metastatic lesions, they have potential for use as a “liquid biopsy.” This approach seems appealing and could influence clinical decisions in the near future. With the understanding that cancer changes its biology during its progression, such evaluation may enable selection of individuals most likely to benefit from a particular treatment based on real-time, dynamic assessment of biology of disease.
There are several examples indicating the utility of CTCs for such assessment. The Her2neu status of the primary tumor is not always concordant with the Her2neu status of CTCs; in fact, up to 30% discordance was demonstrated (71, 72). Another recent study demonstrated the ability to detect discordant estrogen receptor (ER) and Her2neu status between primary tumor or metastatic tumor sites and CTCs in metastatic breast cancer (73). Although preliminary, the data describing discrepancies between the expression status of drug targets in primary tumor and CTCs are critical and need further clinical validation. Similar studies were performed in non–small cell lung cancer and colorectal cancer patients. Other examples of biomarker variation between the primary tumor and the CTCs include EGFR status and KRAS mutations. EGFR status has been determined from CTCs enriched in patients with non–small cell lung cancer (74), and KRAS mutation status from CTCs in colorectal cancer patients (75, 76).
CTCs as a Companion Diagnostic for the Pharmaceutical Industry and Incorporation in Future Clinical Trial Design
The molecular characterization of CTCs is of great interest to the pharmaceutical industry, with the focus on development of novel anticancer compounds. Definition of novel biomarkers and therapeutic targets inevitably leads to further evaluation and validation. Novel upcoming technologies for detection and characterization of CTCs are promising as new platforms to define targets in identified cell subpopulations or single cells.
The concept of companion diagnostics includes using CTCs as surrogate endpoints for testing the efficacy of candidate drugs. Pharmaceutical companies invest millions of dollars in developing new drug candidates and testing them through various phases of clinical trials, which are labor-intensive, involve substantial costs (which are ultimately passed on to the consumer), and are associated with a high failure rate. Significant savings are possible if companies can assess efficacy or failure early in clinical trials. An increasing number of clinical trials involve CTCs as an endpoint. These trials will provide further evidence regarding the clinical relevance of CTCs. Early in drug development, pharmaceutical companies make all efforts to demonstrate the efficacy of the new compound, and modulation in the prometastatic activity of CTCs is an attractive marker.
The molecular characterization and identification of discrepancies between characteristics of the primary tumor and CTCs have led to early clinical studies, randomizing patients with Her2neu− tumors and Her2neu+ CTCs to treatment with trastuzumab. The TREAT CTC study is a phase II trial performed by the European Organization for Research and Treatment of Cancer (EORTC). Patients with primary tumor that tested negative for Her2neu, but with detectable CTCs in their peripheral blood and Her2neu-positive CTCs, are randomized after neoadjuvant treatment to receive adjuvant trastuzumab for a total of six intravenous administrations every three weeks (http://clinicaltrials.gov/ct2/show/NCT01548677). Controls receive no Her2neu-targeted treatment. Validation of CTCs as a tool for biological characterization of disease may lead to the design of novel and attractive clinical trials with CTCs as targets.
SUMMARY POINTS.
Blood represents an ideal compartment for detection of metastatic tumor cells in cancer patients, and efforts are being made to develop and optimize technologies for the detection and molecular characterization of CTCs.
Prognostic and predictive significance of detection of CTCs in peripheral blood of cancer patients has been demonstrated for several types of cancers.
The landmark studies performed with CellSearch provide a basis for further evaluation of clinical utility of CTCs as biomarkers in the management of cancer patients.
Molecular characterization of CTCs via improved enrichment methods is needed in order to better understand the biology of metastatic cancer and define novel biomarkers.
Use of CTCs as a “liquid biopsy” to study biomarkers of new targeted therapies could be a critical tool for monitoring therapeutic response and informing clinicians whether a change in the treatment course is indicated. Whether this tool can improve cancer patient management is the focus of current and future clinical trials.
Glossary
- EMT
epithelial-mesenchymal transition
Footnotes
DISCLOSURE STATEMENT
Drs. Cote, Datar, and Lin have patents and proprietary technology for CTC capture and evaluation, and are consultants to Filtini, Inc.
Contributor Information
Marija Balic, Email: marija.balic@medunigraz.at.
Anthony Williams, Email: Awilliams12@med.miami.edu.
Henry Lin, Email: Hklin@intopsys.com.
Ram Datar, Email: rdatar@med.miami.edu.
Richard J. Cote, Email: rcote@med.miami.edu.
LITERATURE CITED
- 1.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 2.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 4.Redding WH, Monaghan P, Imrie SF. Detection of micrometastases in patients with primary breast cancer. Lancet. 1983;2:1271–74. doi: 10.1016/s0140-6736(83)91150-9. [DOI] [PubMed] [Google Scholar]
- 5.Cote RJ, Rosen PP, Lesser ML, et al. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol. 1991;9:1749–56. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- 6.Balic M. Disseminated tumor cells as biomarkers for breast cancer. Biomark Med. 2009;3:215–17. doi: 10.2217/bmm.09.17. [DOI] [PubMed] [Google Scholar]
- 7.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano AE, Hawes D, Ballman KV, et al. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. JAMA. 2011;306:385–93. doi: 10.1001/jama.2011.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 10.Bauernhofer T, Zenahlik S, Hofmann G, et al. Association of disease progression and poor overall survival with detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer. Oncol Rep. 2005;13:179–84. [PubMed] [Google Scholar]
- 11.Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:617421. doi: 10.1155/2010/617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alix-Panabières C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annu Rev Med. 2012;63:199–215. doi: 10.1146/annurev-med-062310-094219. [DOI] [PubMed] [Google Scholar]
- 13.Balic M, Lin H, Williams A, et al. Progress in circulating tumor cell capture and analysis: implications for cancer management. Expert Rev Mol Diagnostics. 2012;12:303–12. doi: 10.1586/erm.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–21. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 15.Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–30. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 16.Danila DC, Heller G, Gignac GA, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053–58. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 17.Iinuma H, Watanabe T, Mimori K, et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J Clin Oncol. 2011;29:1547–55. doi: 10.1200/JCO.2010.30.5151. [DOI] [PubMed] [Google Scholar]
- 18.Reinholz MM, Kitzmann KA, Tenner KS, et al. Cytokeratin-19 and mammaglobin gene expression in circulating tumor cells from metastatic breast cancer patients enrolled in North Central Cancer Treatment Group (NCCTG) trials, N0234/336/436/437. Clin Cancer Res. 2011;17:7183–93. doi: 10.1158/1078-0432.CCR-11-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nole F, Munzone E, Zorzino L, et al. Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol. 2008;19:891–97. doi: 10.1093/annonc/mdm558. [DOI] [PubMed] [Google Scholar]
- 20.Balic M, Lin H, Williams A, et al. Progress in circulating tumor cell (CTC) capture and analysis: implications for cancer management. Expert Rev Mol Diagn. 2012;12:303–12. doi: 10.1586/erm.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–24. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 22.Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–28. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 23.Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–39. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 25.Gaforio JJ, Serrano MJ, Sanchez-Rovira P, et al. Detection of breast cancer cells in the peripheral blood is positively correlated with estrogen-receptor status and predicts for poor prognosis. Int J Cancer. 2003;107:984–90. doi: 10.1002/ijc.11479. [DOI] [PubMed] [Google Scholar]
- 26.Ignatiadis M, Kallergi G, Ntoulia M, et al. Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res. 2008;14:2593–600. doi: 10.1158/1078-0432.CCR-07-4758. [DOI] [PubMed] [Google Scholar]
- 27.Xenidis N, Ignatiadis M, Apostolaki S, et al. Cytokeratin-19 mRNA-positive circulating tumor cells after adjuvant chemotherapy in patients with early breast cancer. J Clin Oncol. 2009;27:2177–84. doi: 10.1200/JCO.2008.18.0497. [DOI] [PubMed] [Google Scholar]
- 28.Giuliano M, Giordano A, Jackson S, et al. Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast Cancer Res. 2011;13:R67. doi: 10.1186/bcr2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutgers E, Piccart-Gebhart MJ, Bogaerts J, et al. The EORTC 10041/BIG 03–04 MINDACT trial is feasible: results of the pilot phase. Eur J Cancer. 2011;47:2742–49. doi: 10.1016/j.ejca.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Zujewski JA, Kamin L. Trial assessing individualized options for treatment for breast cancer: the TAILORx trial. Future Oncol. 2008;4:603–10. doi: 10.2217/14796694.4.5.603. [DOI] [PubMed] [Google Scholar]
- 31.Riethdorf S, Muller V, Zhang L, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16:2634–45. doi: 10.1158/1078-0432.CCR-09-2042. [DOI] [PubMed] [Google Scholar]
- 32.Jäger B, Rack B, Schindlbeck C, et al. Survival in early breast cancer is influenced by circulating tumor cells—results from the SUCCESS A study. Presented at Eur. Breast Cancer Conf., 8th; Vienna. Mar. 21–24; 2012. p. Abstr. 301. [Google Scholar]
- 33.Bidard FC, Hajage D, Bachelot T, et al. Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: a prospective observational study. Breast Cancer Res. 2012;14:R29. doi: 10.1186/bcr3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes DF. A clinicians’ perspective on metastasic breast cancer: many diseases—we need to understand the biology. Presented at Eur. Breast Cancer Conf; Vienna. 8th, Mar. 21–24.2012. [Google Scholar]
- 35.Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging—predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12:6403–9. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 36.Scheel C, Weinberg RA. Phenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells? Int J Cancer. 2011;129:2310–14. doi: 10.1002/ijc.26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JM, Dedhar S, Kalluri R, et al. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–81. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aktas B, Tewes M, Fehm T, et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11:R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kallergi G, Papadaki MA, Politaki E, et al. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer. 2011;13:R59. doi: 10.1186/bcr2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 41.Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30:525–32. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 42.Scheel C, Eaton EN, Li SH, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–40. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 44.Younos I, Donkor M, Hoke T, et al. Tumor- and organ-dependent infiltration by myeloid-derived suppressor cells. Int Immunopharmacol. 2011;11:816–26. doi: 10.1016/j.intimp.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 45.Diaz-Montero CM, Salem ML, Nishimura MI, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–19. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 47.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–88. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawson JC, Blatch GL, Edkins AL. Cancer stem cells in breast cancer and metastasis. Breast Cancer Res Treat. 2009;118:241–54. doi: 10.1007/s10549-009-0524-9. [DOI] [PubMed] [Google Scholar]
- 49.Oliveira LR, Jeffrey SS, Ribeiro-Silva A. Stem cells in human breast cancer. Histol Histopathol. 2010;25:371–85. doi: 10.14670/HH-25.371. [DOI] [PubMed] [Google Scholar]
- 50.Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev. 2005;5:311–21. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 51.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 52.Balic M, Lin H, Young L, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–21. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 53.Buess M, Rajski M, Vogel-Durrer BM, et al. Tumor-endothelial interaction links the CD44+/CD24− phenotype with poor prognosis in early-stage breast cancer. Neoplasia. 2009;11:987–1002. doi: 10.1593/neo.09670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Resetkova E, Reis-Filho JS, Jain RK, et al. Prognostic impact of ALDH1 in breast cancer: a story of stem cells and tumor microenvironment. Breast Cancer Res Treat. 2009;123:97–108. doi: 10.1007/s10549-009-0619-3. [DOI] [PubMed] [Google Scholar]
- 55.Charafe-Jauffret E, Ginestier C, Iovino F, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–13. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chute JP, Muramoto GG, Whitesides J, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci USA. 2006;103:11707–12. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charafe-Jauffret E, Ginestier C, Iovino F, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–56. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 60.Schabel FM., Jr Rationale for adjuvant chemotherapy. Cancer. 1977;39:2875–82. doi: 10.1002/1097-0142(197706)39:6<2875::aid-cncr2820390675>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 61.Phillips TM, McBride WH, Pajonk F. The response of CD24−/low/CD44+ breast cancer–initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–85. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 62.Croker AK, Allan AL. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44+ human breast cancer cells. Breast Cancer Res Treat. 2012;133:75–87. doi: 10.1007/s10549-011-1692-y. [DOI] [PubMed] [Google Scholar]
- 63.Ricardo S, Vieira AF, Gerhard R, et al. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol. 2011;64:937–46. doi: 10.1136/jcp.2011.090456. [DOI] [PubMed] [Google Scholar]
- 64.Dearnaley DP, Ormerod MG, Sloane JP. Micrometastases in breast cancer: long-term follow-up of the first patient cohort. Eur J Cancer. 1991;27:236–39. doi: 10.1016/0277-5379(91)90504-7. [DOI] [PubMed] [Google Scholar]
- 65.Naume B, Wiedswang G, Borgen E, et al. The prognostic value of isolated tumor cells in bone marrow in breast cancer patients: evaluation of morphological categories and the number of clinically significant cells. Clin Cancer Res. 2004;10:3091–97. doi: 10.1158/1078-0432.ccr-03-0373. [DOI] [PubMed] [Google Scholar]
- 66.Abraham BK, Fritz P, McClellan M, et al. Prevalence of CD44+/CD24-/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–59. [PubMed] [Google Scholar]
- 67.Wicha MS. Cancer stem cells and metastasis: lethal seeds. Clin Cancer Res. 2006;12:5606–7. doi: 10.1158/1078-0432.CCR-06-1537. [DOI] [PubMed] [Google Scholar]
- 68.Reuben JM, Lee BN, Gao H, et al. Primary breast cancer patients with high risk clinicopathologic features have high percentages of bone marrow epithelial cells with ALDH activity and CD44CD24lo cancer stem cell phenotype. Eur J Cancer. 2011;47:1527–36. doi: 10.1016/j.ejca.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Theodoropoulos PA, Polioudaki H, Agelaki S, et al. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 2010;288:99–106. doi: 10.1016/j.canlet.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 70.Wang N, Shi L, Li H, et al. Detection of circulating tumor cells and tumor stem cells in patients with breast cancer by using flow cytometry: a valuable tool for diagnosis and prognosis evaluation. Tumour Biol J Int Soc Oncodev Biol Med. 2012;33:561–69. doi: 10.1007/s13277-011-0303-1. [DOI] [PubMed] [Google Scholar]
- 71.Fehm T, Becker S, Duerr-Stoerzer S, et al. Determination of HER2 status using both serum HER2 levels and circulating tumor cells in patients with recurrent breast cancer whose primary tumor was HER2 negative or of unknown HER2 status. Breast Cancer Res. 2007;9:R74. doi: 10.1186/bcr1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Munzone E, Nole F, Goldhirsch A, et al. Changes of HER2 status in circulating tumor cells compared with the primary tumor during treatment for advanced breast cancer. Clin Breast Cancer. 2010;10:392–97. doi: 10.3816/CBC.2010.n.052. [DOI] [PubMed] [Google Scholar]
- 73.Somlo G, Lau SK, Frankel P, et al. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary and metastatic sites in patients with locally advanced/inflammatory, and stage IV breast cancer, using a novel detection technology. Breast Cancer Res Treat. 2011;128:155–63. doi: 10.1007/s10549-011-1508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–77. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dharmasiri U, Njoroge SK, Witek MA, et al. High-throughput selection, enumeration, electrokinetic manipulation, and molecular profiling of low-abundance circulating tumor cells using a microfluidic system. Anal Chem. 2011;83:2301–9. doi: 10.1021/ac103172y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang MJ, Chiu HH, Wang HM, et al. Enhancing detection of circulating tumor cells with activating KRAS oncogene in patients with colorectal cancer by weighted chemiluminescent membrane array method. Ann Surg Oncol. 2010;17:624–33. doi: 10.1245/s10434-009-0831-8. [DOI] [PubMed] [Google Scholar]


