Abstract
Objective
Pseudohypoparathyroidism (PHP) is a heterogeneous disorder characterized by hypocalcemia and hyperphosphatemia resulting from selective renal resistance to parathyroid hormone (PTH). One autosomal dominant form of PHP type 1b (PHP-Ib) is most frequently caused by a maternally inherited 3-kb deletion within STX16, the gene encoding syntaxin 16. To date, increased bone mineral density (BMD) has been described only in PHP type 1a, and there is a lack of detailed information on bone histomorphometry in PHP-Ib. The objective of this report was to present trans-iliac static and dynamic histomorphometry in two brothers with the 3-kb deletion in the STX16 region and elevated BMD.
Design
Observational study of two brothers (age 18.0 and 22.7 years) with the 3-kb STX16 deletion and increased BMD.
Results
The brothers had elevated PTH (146 pg/ml (15.6 pmol/l) and 102 pg/ml (10.9 pmol/l); normal: 10–64 pg/ml (1.1–6.8 pmol/l)) and striking osteosclerosis (lumbar spine areal BMD Z-scores: +5.4 and +4.9). Bone histomorphometry showed marked elevations in cortical width for both brothers (241 and 209% of the mean result expected for age), with elevations in the bone formation rate on the endocortical (119 and 260% of the healthy mean) and trabecular (220 and 190% of mean) surfaces.
Conclusion
Our findings suggest that PTH in this PHP-Ib genotype can increase cortical thickness due to its anabolic effect on endocortical bone, and underscore the heterogeneity in the skeletal phenotype among patients with PHP-Ib.
Introduction
The α-subunit of the stimulatory G-protein (Gsα) is an intracellular signaling protein essential for the actions of many hormones, including parathyroid hormone (PTH). Although Gsα is biallelically expressed in most tissues, it has predominantly maternal expression in renal proximal tubules. Pseudohypoparathyroidism (PHP) is characterized by proximal tubular resistance to PTH due to impaired Gsα expression and results in hypocalcemia and hyperphosphatemia. The two major forms of PHP are PHP type 1a (PHP-Ia) and PHP type 1b (PHP-Ib). PHP-Ia is associated with a distinctive phenotype termed Albright’s hereditary osteodystrophy (AHO), which includes short stature, obesity, round faces, brachydactyly, and subcutaneous ossifications. It is caused by maternally inherited inactivating mutations in the GNAS gene on chromosome 20q that encodes Gsα (1). Patients with PHP-Ib usually do not have features of AHO; renal resistance to PTH is the main feature of the condition, occurring with sporadic or autosomal dominant (AD) inheritance (2). Most patients with AD-PHP-Ib show loss of methylation at GNAS exon A/B involved in Gsα transcription regulation, which is associated with a heterozygous, maternally inherited 3-or 4.4-kb deletion within syntaxin 16 (STX16). As a result of biallelic A/B transcription, patients with AD-PHP-Ib are predicted to have impaired maternal Gsα expression in proximal renal tubules (1). PHP-Ib is heterogeneous at the clinical level; for example, the severity of hypocalcemia can vary greatly, and as many as 40% of individuals with maternally inherited STX16 deletions are asymptomatic (2).
While the renal proximal tubules are resistant to PTH in PHP-Ib, Gsα is derived in osteoblasts from both parental alleles, giving rise to PTH responsiveness at the bone tissue level. Observations of the skeletal phenotype in PHP have been diverse. Some reports have described accelerated subperiosteal resorption and osteitis fibrosa cystica on skeletal radiographs (3–5). However, osteosclerosis has also been described in non-specified PHP (5–7), and more recently, elevated total body bone mineral density (BMD) as measured by dual energy X-ray absorptiometry (DXA) was reported in PHP-Ia (8). Given these disparate findings by indirect assessment methods, bone histomorphometry can precisely define the effect of PHP on bone metabolism, but these studies are lacking. Therefore, the purpose of this report was to present trans-iliac static and dynamic histomorphometry in two brothers with the 3-kb deletion in the STX16 region and elevated BMD. In doing so, we provide insight into the mechanism of increased BMD observed in these cases.
Clinical report
Proband 1 presented at 11 years of age with a 1-year history of increasing leg pain with exercise. He was born to non-consanguineous French–Canadian parents, and his maternal uncle had been diagnosed with PHP at 13 years of age after a hypocalcemic seizure. At presentation, proband 1 measured 148.4 cm (75th percentile), weighed 44.8 kg (90th percentile), and had dental enamel hypoplasia, but no clinical features of AHO or tetany. The serum ionized calcium was low, whereas serum levels of phosphate, alkaline phosphatase activity (ALP) and PTH were elevated (Table 1). Serum creatinine, magnesium, TSH, and free thyroxine were normal. Based on these features, he was diagnosed with PHP-Ib and treated with calcitriol and elemental calcium.
Table 1.
Biochemistry.
| Proband 1 | Proband 2 | ||||
|---|---|---|---|---|---|
| Reference range |
Result at diagnosis |
Result at the time of biopsy |
Result at diagnosis |
Result at the time of biopsy |
|
| Ionized calcium (mg/dl; (mmol/l)) | 4.4–5.2 (1.10–1.30) | 2.6 (0.66) | 4.2 (1.05) | 3.2 (0.81) | 3.6 (0.91) |
| Phosphate (mg/dl; (mmol/l)) | 3.1–5.3 (1.0–1.7) | 8.7 (2.8) | 3.7 (1.2) | 7.1 (2.3) | 2.8 (0.9) |
| Alkaline phosphatase (IU/l) | 58–237 | 95 | 63 | 181 | 64 |
| PTH (pg/ml; (pmol/l)) | 10–64 (1.1–6.8) | 382 (40.8) | 146 (15.6) | 217 (23.2) | 102 (10.9) |
| 25-hydroxyvitamin D (ng/ml; (nmol/l)) | 8.0–36.1 (20–90) | ND | 39.7 (99) | ND | 33.7 (84) |
ND, not done.
When proband 1 was diagnosed with PHP-Ib, his older brother (proband 2) was examined for similar features and was found to be normocalcemic. However, a second assessment a few months later at 15.9 years of age revealed a decreased ionized calcium and elevated levels of serum phosphate, ALP, and PTH (Table 1), in the presence of normal serum creatinine and magnesium levels, consistent with a diagnosis of PHP-Ib (see also Jü ppner et al. (9) for the family pedigree where the probands were D-III/31 and D-III/32 respectively). He had no symptoms related to the hypocalcemia. He was clinically euthyroid, though thyroid function tests were not performed. Given the elevated PTH, he was treated with calcitriol and calcium supplementation. Neither boy reported a history of fractures nor dietary intakes of calcium that exceeded the dietary reference intake for age (10).
Skeletal radiography, bone densitometry, trans-iliac histomorphometry, and genetic testing were carried out in both patients to further characterize their clinical and genetic features.
Materials and methods
The study was approved by the ethics committee at the Children’s Hospital of Eastern Ontario. Height, weight, and pubertal status are reported at the time of the detailed skeletal evaluation. Pubertal status was evaluated according to the method of Marshall & Tanner (11). Height was measured with a Harpenden stadiometer and weight with a digital weight scale. Height and weight Z-scores were calculated using reference data provided by the National Center for Health Statistics (12).
Serum concentrations of calcium, phosphate, magnesium, creatinine, and ALP were measured using standard methods. Serum PTH was quantified by IRMA (N-tact*; Incstar Corp., Stillwater, MN, USA), and serum 25-hydroxyvitamin D was measured by RIA (Osteo SP; Incstar Corp.). Genetic testing had been detailed in a prior publication (9).
Radiographs were performed using standard methods. Lumbar spine (vertebrae L2 to L4) and total body areal BMD (aBMD) were measured by DXA in the anterior–posterior direction (Lunar Prodigy; General Electric, Madison, WI, USA). Results were transformed to age- and sex-specific Z-scores using reference data supplied by the manufacturer.
Trans-iliac bone biopsies were performed at a site 2 cm posterior to the anterior superior iliac spine on days 4 or 5 after dual labeling with demeclocycline (15–20 mg/kg per day taken orally for 2 days, and repeated for 2 more days after a 10-day free interval). Biopsy preparation and histomorphometric analyses were performed as described previously (13, 14). Measurements were carried out using a digitizing table with OSTEOMEASURE software (Osteometrics, Inc., Atlanta, GA, USA). Results were compared to reference data of healthy age-matched controls established in our laboratory, and expressed as percentages of the average value (13, 14).
Results
For proband 1, height was 170.4 cm (Z-score −0.8) and weight was 70.4 kg (Z-score +0.3) at 18 years of age. Radiographs around the time of diagnosis showed thick diaphyseal cortices without medullary sclerosis. aBMD was markedly elevated both at the spine (1.8 g/cm2 (age- and gender-matched Z-score +5.4)) and for the total body (1.6 g/cm2 (Z-score +4.8)). Proband 2, aged 22 years, measured 177 cm (Z-score 0) and weighed 66 kg (Z-score −0.4). He also had an increased spine aBMD (1.8 g/cm2 (Z-score +4.9)) and total body BMD ((1.5 g/cm2 (Z-score +3.9)). Both brothers were Tanner stage V for pubertal development at the time of BMD testing.
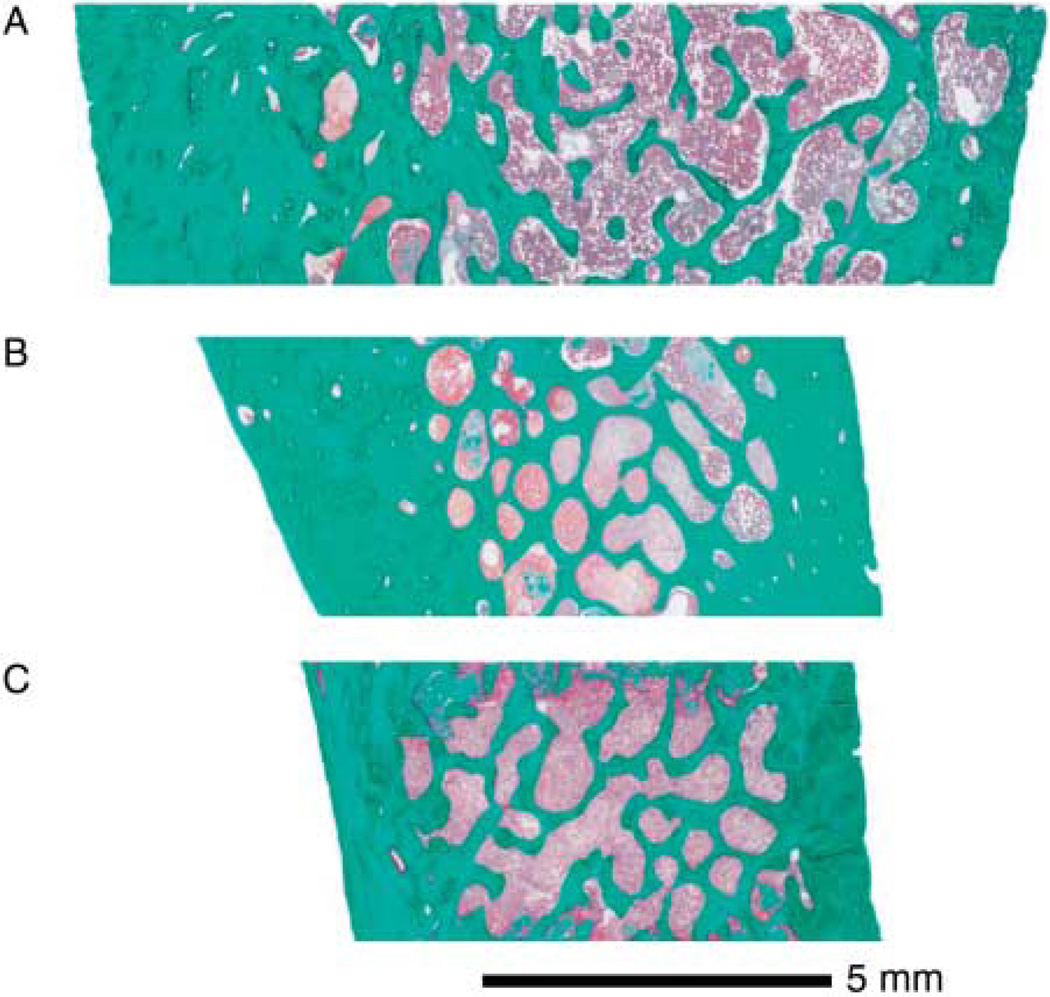
Results of trans-iliac histomorphometry are presented in Table 2. The outer size of the biopsy sample (core width) was above average for proband 1 and average for proband 2. Both brothers had extremely thick cortices (Fig. 1). Cortical thickness was ~7 and 6 standard deviation score respectively above the mean value of the reference range. The amount of trabecular bone was also somewhat elevated due to increased trabecular thickness, whereas the number of trabeculae was close to the age-appropriate average value.
Table 2.
Histomorphometric results in trans-iliac bone samples: structural parameters.
| Proband 1 | Proband 2 | ||||
|---|---|---|---|---|---|
| Reference rangea |
Raw result |
Percentage of age- and gender-matched mean |
Raw result |
Percentage of age- and gender-matched mean |
|
| Core width (µm) | 8.2 ± 1.6 | 13.3 | 162 | 8.4 | 102 |
| Cortical width (µm) | 1006 ± 195 | 2429 | 241 | 2106 | 209 |
| Cortical porosity (%) | 6.6 ± 5 | 7.2 | 128 | 10.8 | 220 |
| Bone volume/tissue volume (%) | 27.8 ± 4.5 | 35.5 | 128 | 45.6 | 164 |
| Trabecular number (/mm) | 1.8 ± 0.3 | 1.8 | 96 | 2 | 108 |
| Trabecular thickness (µm) | 153 ± 24 | 203 | 132 | 230 | 150 |
Mean ± s.d. for age 17.0–22.9 years in a reference population (Glorieux 2000).
Figure 1.
(A) Iliac bone sample from proband 1. Cortical width is 2429 µm. (B) Sample from proband 2. Cortical width is 2106 µm. Note the difference in their cortical width when compared to the control (mean value based on the healthy average = 1006 µm) (C).
Histomorphometric parameters of bone metabolism for trabecular, intracortical, endocortical and periosteal surfaces are shown in Table 3. There was no evidence of a mineralization defect, as osteoid thickness was within normal limits on all surfaces. Bone formation rate on trabecular surfaces was above average, and was also elevated on the endocortical surface, especially for proband 2. Trabecular bone formation rate was extremely elevated as demonstrated by the elevation in mineralizing surface/bone surface (data not shown).
Table 3.
Histomorphometric results on trans-iliac bone samples: bone formation and resorption parameters on trabecular, intracortical, endocortical and periosteal surfaces.
| Proband 1 | Proband 2 | ||||
|---|---|---|---|---|---|
| Reference rangea |
Raw result |
Percentage of age- and gender- matched mean |
Raw result |
Percentage of age- and gender- matched mean |
|
| Trabecular surface | |||||
| Osteoid thickness (µm) | 6.9 ± 1.2 | 8.4 | 122 | 5.8 | 84 |
| Osteoid surface/bone surface (%) | 17 ± 5 | 19 | 117 | 17 | 103 |
| Mineralizing surface/bone surface (%) | 7.9 ± 2.7 | 13.5 | 172 | 14.8 | 188 |
| Mineral apposition rate (µm/day) | 0.8 ± 0.1 | 1 | 131 | 0.8 | 104 |
| Bone formation rate/bone surface (µm3/µm2 per year) | 22 ± 9 | 48.8 | 220 | 42.1 | 190 |
| Eroded surface/bone surface (%) | 18 ± 6 | 24 | 132 | 21 | 116 |
| Intracortical surface | |||||
| Osteoid thickness (µm) | 7.7 ± 3.3 | 9.6 | 125 | 6.9 | 90 |
| Osteoid surface/bone surface (%) | 22.5 ± 14 | 15.5 | 69 | 16.5 | 73 |
| Mineralizing surface/bone surface (%) | 14.5 ± 8 | 14.1 | 97 | 14.1 | 97 |
| Mineral apposition rate (µm/day) | 0.8 ± 0.3 | 0.9 | 115 | 1.0 | 125 |
| Bone formation rate/bone surface (µm3/µm2 per year) | 41.5 ± 23.5 | 48.1 | 116 | 53.2 | 128 |
| Eroded surface/bone surface (%) | 14 ± 9 | 21.5 | 154 | 16.5 | 118 |
| Endocortical surface | |||||
| Osteoid thickness (µm) | 6.3 ± 4.4 | 5.6 | 90 | 6 | 96 |
| Osteoid surface/bone surface (%) | 16 ± 14 | 15 | 94 | 24 | 150 |
| Mineralizing surface/bone surface (%) | 9.5 ± 7.5 | 11.1 | 116 | 18.4 | 193 |
| Mineral apposition rate (µm/day) | 0.5 ± 0.2 | 0.6 | 114 | 0.8 | 159 |
| Bone formation rate/bone surface (µm3/µm2 per year) | 20 ± 15.5 | 23.8 | 119 | 52.1 | 260 |
| Eroded surface/bone surface (%) | 14.5 ± 10.5 | 30.5 | 210 | 10 | 68 |
| Periosteal surface | |||||
| Osteoid thickness (µm) | 4.1 ± 1.5 | 3.9 | 96 | 3.5 | 86 |
| Osteoid surface/bone surface (%) | 38.5 ± 26 | 22 | 58 | 30.5 | 79 |
| Mineralizing surface/bone surface (%) | 2.9 ± 6.4 | 0 | 0 | 0 | 0 |
| Mineral apposition rate (µm/day) | 0.2 ± 0.2 | 0 | 0 | 0 | 0 |
| Bone formation rate/bone surface (µm3/µm2 per year) | 4.9 ± 2.0 | 0 | 0 | 0 | 0 |
| Eroded surface/bone surface (%) | 32.5 ± 30.5 | 52.5 | 162 | 17 | 52 |
Biochemistry obtained on the same day as the biopsy included elevated PTH and normal 25-hydroxyvitamin D levels (Table 1). When the pattern of serum PTH secretion was reviewed, it was found that proband 1’s PTH was normal (12 pg/ml (1.3 pmol/l)) 1 year before his bone biopsy, and proband 2 also had a normal PTH value (37 pg/ml (4.0 pmol/l)) 3 years preceding his biopsy. Taken together, these findings provided evidence for fluctuating serum PTH levels in both patients. Magnesium and creatinine were normal. Genetic testing had previously revealed (for both patients) a 3-kb STX16 deletion upstream of GNAS on chromosome 20q13.3 (9, 15). Their mother and a maternal cousin had the 3-kb deletion when previously investigated (by maternal inheritance for their mother and paternal inheritance for their maternal cousin) (9). The mother’s lumbar spine aBMD T-score was below average (−0.5) while her PTH was normal at 63 pg/ml (6.7 pmol/l); N: 15–65 pg/ml (1.6–6.9 pmol/l), and their cousin had a mildly increased spine aBMD Z-score (+1.0) at age 11 years with a concomitant normal PTH of 49 pg/ml (5.2 pmol/l); N: 11–68 pg/ml (1.2–7.3 pmol/l). Neither the mother nor cousin required treatment with calcitriol to maintain eucalcemia.
Discussion
We present two brothers with PHP-Ib caused by a 3-kb deletion within STX16 associated with marked osteosclerosis due to thickened cortices. Although elevated BMD in PHP-Ia (8) and osteosclerosis in genetically undefined PHP have been previously described (5–7), this is the first report linking a mutation known to cause AD-PHP-Ib with marked increases in DXA-derived BMD parameters and elevations in cortical width on trans-iliac histomorphometry. The increase in cortical width could have resulted from skeletal resistance to PTH had bone resorption been impaired, since it has recently been demonstrated that a predominance of maternally inherited Gsα can exist in PHP-Ib skeletal tissue (16). However, the patients’ core width values combined with increased cortical width implicate elevated endosteal bone formation, which was confirmed on dynamic histomorphometry. Dynamic histomorphometric analysis showed elevated endosteal bone formation that was particularly evident in proband 2 (endocortical bone formation rate 260% of the mean). The best dynamic parameter for measuring the effect of hyperparathyroidism on bone turnover is bone formation rate per bone surface (17). Our data show that the bone formation rates per intracortical, endocortical, and trabecular bone surfaces were increased in both probands. A high bone formation rate does not necessarily lead to a net gain of bone if the remodeling balance is zero or negative (18), and the index case did have an elevated eroded intracortical surface. However, bone resorption is the least informative aspect of histomorphometric analysis since it is quantified only with static parameters (17), and the augmented cortical width and trabecular thickness in our cases suggest that the high bone formation rates do, in fact, indicate net bone gain.
Our findings contrast with other reports describing catabolic effects of PTH on bone biopsy in children with PHP, particularly cortical bone (3, 4, 19). However, it is important to take the age dependency of histomorphometric parameters into account (18), and the only case study on histomorphometric data in PHP by Duquesnoy et al. (19) was published before established reference data for pediatric quantitative histomorphometry were available. Furthermore, there is a paucity of information on dynamic parameters such as bone formation rate per bone surface in the study by Duquesnoy et al. (19). In our report, the elevated PTH in the brothers concurrent with their bone histomorphometric results demonstrates that PTH can have a positive effect on cortical bone formation, consistent with studies showing that subcutaneous PTH administration increases the cortical thickness of the ilium when used for osteoporosis treatment in men and women (20, 21).
Our report highlights the heterogeneity in the skeletal phenotype among patients with PHP-Ib. While there are other reports of osteosclerosis on skeletal radiographs (5–7) suggesting increased bone formation as in our patients, there are also reports of accelerated subperiosteal resorption, osteitis fibrosa cystica, and elevated bone turnover markers in PHP (3–5), specifically PHP-Ib (22). DXA-derived BMD has been reported to be normal in a patient with PHP-Ib (22), whereas another case report of patients with PHP-Ib caused by a 3-kb deletion in STX16 showed mild osteopenia (23).
These conflicting descriptions of the bone density phenotype in patients with PHP suggest differential effects of PTH on bone metabolism. One possibility to explain these findings may relate to the magnitude of PTH elevation. Markedly high PTH values that are persistently elevated can cause accelerated skeletal resorption and osteitis fibrosa cystica, but a chronically mild increase in PTH secretion has been associated with an increased net bone formation in humans (24), as observed in our patients with extremely elevated spine and total body BMD. In contrast, Laspa et al. (23) reported that patients with PHP-Ib caused by the 3-kb deletion had DXA-derived spine BMD indices that were −0.9 to −1.35 standard deviation scores below the mean. The most striking difference between our patients and those of Laspa et al. (23) was the magnitude of the PTH elevation (PTH was 9- and 6-fold higher than the upper limit of normal compared to 2- and 1.6-fold higher in our patients), suggesting that the higher PTH levels in the Laspa study (23) may have exerted a catabolic effect on bone. Along the same lines, the mother and maternal cousin in our report had normal PTH levels that were associated with relatively normal spine aBMD Z-scores of −0.5 and +1 respectively. Taken together, these observations further support that the magnitude of the PTH elevation may modulate the skeletal phenotype.
Besides the degree of PTH elevation, the duration of the PTH increase may also impact bone tissue responsiveness. Intermittent PTH administration can induce large increases in cortical thickness and spine BMD in postmenopausal women (20, 21, 25). Additionally, PTH stimulates endosteal and periosteal bone formation when intermittently released in growing mice and adult humans (26, 27), resulting in both increased bone modeling and remodeling. Specifically, Lindsay et al. (27) reported increased bone formation on the endocortical surface in postmenopausal women, consistent with our cases. The increase in bone formation in this report was largely due to an increase in osteoblast number. PTH appears to increase osteoblast numbers by exerting pro-differentiating and prosurvival effects on pre-osteoblasts in periosteal bone (26), resulting in an increased cortical diameter similar to our cases. It is possible that periosteal bone formation was also elevated while the boys were growing, though we were not able to fully evaluate this aspect since linear growth was complete at the time of the biopsy, and periosteal bone growth is minimal following cessation of longitudinal growth (28, 29).
In addition to increased cortical bone, our patients had elevated trabecular thickness. Animal models show that daily PTH injections attenuate the rate of osteoblast apoptosis in trabecular bone, which has a higher rate of apoptosis, resulting in increased trabecular thickness (30). While our main findings of increased cortical width and elevations in bone formation on cortical and trabecular surfaces support an anabolic effect of PTH at the bone tissue level, they are based on only two individuals. Therefore, we cannot exclude the possibility that other genetic factors may have contributed to the marked osteosclerosis. For example, activating LRP5 mutations can cause osteosclerosis (30); however, in our patients, there were no physical features (such as torus palatinus) to support further exploration of this or other specific diagnoses. Alternatively, acquired factors such as hepatitis C, heavy metals, and fluoride exposure can influence BMD (6), but these possibilities are also unlikely in our probands based on their history and physical examinations. On the other hand, a recent publication by Long et al. (8) provides further support that high bone mass may be an integral component of the PHP phenotype in some patients. This group showed that total body BMD measurements were significantly greater than normal in 20 children and adults with PHP-Ia; for example, the highest total body BMD Z-score was +4.8 with a PTH value of 253 pg/ml (27.8 pmol/l). Further study of BMD as well as bone histomorphometry in relation to PTH among patients with the 3-kb deletion in STX16 will be helpful in dissecting the relative contribution of elevations in PTH to the osteosclerotic phenotype.
In conclusion, we present novel findings in two patients with PHP-Ib harboring the 3-kb STX16 deletion, including quantitative static and dynamic histomorphometry on trans-iliac specimens. Our bone histomorphometry results suggest that PTH has had an anabolic effect as opposed to a catabolic effect on bone in these cases. In particular, we have shown in these patients that mild elevations in PTH appear to be beneficial for cortical bone structure, although we cannot eliminate the possibility that alterations in other genes may have contributed to the observed skeletal phenotype. Overall, the histomorphometric data provide important insight into the pathogenesis of the bone phenotype at the tissue level in these brothers.
Acknowledgements
Funding
Dr L M Ward is supported by a Canadian Institutes for Health Research New Investigator Award and a Canadian Child Health Clinician Scientist Career Enhancement Award.
The authors thank Guy Charette for technical assistance with biopsy sample processing, Mark Lepik for preparation of the figure, and Isabelle French for assistance with data collection.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Bastepe M. The GNAS locus and pseudohypoparathyroidism. Advances in Experimental Medicine and Biology. 2008;626:27–40. doi: 10.1007/978-0-387-77576-0_3. [DOI] [PubMed] [Google Scholar]
- 2.Linglart A, Bastepe M, Jüppner H. Similar clinical and laboratory findings in patients with symptomatic autosomal dominant and sporadic pseudohypoparathyroidism type Ib despite different epigenetic changes at the GNAS locus. Clinical Endocrinology. 2007;67:822–831. doi: 10.1111/j.1365-2265.2007.02969.x. [DOI] [PubMed] [Google Scholar]
- 3.Kidd GS, Schaaf M, Adler RA, Lassman MN, Wray HL. Skeletal responsiveness in pseudohypoparathyroidism. A spectrum of clinical disease. American Journal of Medicine. 1980;68:772–781. doi: 10.1016/0002-9343(80)90270-3. [DOI] [PubMed] [Google Scholar]
- 4.Murray TM, Rao LG, Wong MM, Waddell JP, McBroom R, Tam CS, Rosen F, Levine MA. Pseudohypoparathyroidism with osteitis fibrosa cystica: direct demonstration of skeletal responsiveness to parathyroid hormone in cells cultured from bone. Journal of Bone and Mineral Research. 1993;8:83–91. doi: 10.1002/jbmr.5650080111. [DOI] [PubMed] [Google Scholar]
- 5.Burnstein MI, Kottamasu SR, Pettifor JM, Sochett E, Ellis BI, Frame B. Metabolic bone disease in pseudohypoparathyroidism: radiologic features. Radiology. 1985;155:351–356. doi: 10.1148/radiology.155.2.3983385. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson HG. Dense bone – too much bone: radiological considerations and differential diagnosis. Part I. Skeletal Radiology. 1985;13:1–20. doi: 10.1007/BF00349088. [DOI] [PubMed] [Google Scholar]
- 7.Balkissoon AR, Hayes CW. Case 14: intramedullary osteosclerosis. Radiology. 1999;212:708–710. doi: 10.1148/radiology.212.3.r99se39708. [DOI] [PubMed] [Google Scholar]
- 8.Long D, Levine M, Germain-Lee E. Bone mineral density in pseudohypoparathyroidism type 1a. Journal of Clinical Endocrinology and Metabolism. 2010;95:4465–4475. doi: 10.1210/jc.2010-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juppner H, Schipani E, Bastepe M, Cole DE, Lawson ML, Mannstadt M, Hendy GN, Plotkin H, Koshiyama H, Koh T, Crawford JD, Olsen BR, Vikkula M. The gene responsible for pseudohypoparathyroidism type Ib is paternally imprinted and maps in four unrelated kindreds to chromosome 20q13.3. PNAS. 1998;95:11798–11803. doi: 10.1073/pnas.95.20.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, Fluoride. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 11.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 13.Glorieux FH, Travers R, Taylor A, Bowen JR, Rauch F, Norman M, Parfitt AM. Normative data for iliac bone histomorphometry in growing children. Bone. 2000;26:103–109. doi: 10.1016/s8756-3282(99)00257-4. [DOI] [PubMed] [Google Scholar]
- 14.Rauch F, Travers R, Glorieux FH. Intracortical remodeling during human bone development – a histomorphometric study. Bone. 2007;40:274–280. doi: 10.1016/j.bone.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Bastepe M, Fröhlich LF, Hendy GN, Indridason OS, Josse RG, Koshiyama H, Korkko J, Nakamoto JM, Rosenbloom AL, Slyper AH, Sugimoto T, Tsatsoulis A, Crawford JD, Jüppner H. Autosomal dominant pseudohypoparathyroidism type Ib is associated with a heterozygous microdeletion that likely disrupts a putative imprinting control element of GNAS. Journal of Clinical Investigation. 2003;112:1255–1263. doi: 10.1172/JCI19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantovani G, de Sanctis L, Barbieri AM, Elli FM, Bollati V, Vaira V, Labarile P, Bondioni S, Peverelli E, Lania AG, Beck-Peccoz P, Spada A. Pseudohypoparathyroidism and GNAS epigenetic defects: clinical evaluation of albright hereditary osteodystrophy and molecular analysis in 40 patients. Journal of Clinical Endocrinology and Metabolism. 2010;95:651–658. doi: 10.1210/jc.2009-0176. [DOI] [PubMed] [Google Scholar]
- 17.Parfitt AM. Renal bone disease: a new conceptual framework for the interpretation of bone histomorphometry. Current Opinion in Nephrology and Hypertension. 2003;12:387–403. doi: 10.1097/00041552-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Rauch F. Watching bone cells at work: what we can see from bone biopsies. Pediatric Nephrology. 2006;21:457–462. doi: 10.1007/s00467-006-0025-6. [DOI] [PubMed] [Google Scholar]
- 19.Duquesnoy B, Wemeau JL, Hardouin P, Delfosse JM, Grimbert I, Truquet B, Hubaud P, Decoulx M, Delcambre B. Histomorphometric data in 5 cases of pseudohypoparathyroidism: discussion on bone sensitivity to parathyroid hormone. Revue du Rhumatisme et des Maladies Ostéo-Articulaires. 1986;53:243–248. [PubMed] [Google Scholar]
- 20.Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. Journal of Bone and Mineral Research. 2003;18:1932–1941. doi: 10.1359/jbmr.2003.18.11.1932. [DOI] [PubMed] [Google Scholar]
- 21.Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetic K, Muller R, Bilezikian J, Lindsay R. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. Journal of Bone and Mineral Research. 2001;16:1846–1853. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 22.Tollin SR, Perlmutter S, Aloia JF. Serial changes in bone mineral density and bone turnover after correction of secondary hyperparathyroidism in a patient with pseudohypoparathyroidism type Ib. Journal of Bone and Mineral Research. 2000;15:1412–1416. doi: 10.1359/jbmr.2000.15.7.1412. [DOI] [PubMed] [Google Scholar]
- 23.Laspa E, Bastepe M, Juppner H, Tsatsoulis A. Phenotypic and molecular genetic aspects of pseudohypoparathyroidism type Ib in a Greek kindred: evidence for enhanced uric acid excretion due to parathyroid hormone resistance. Journal of Clinical Endocrinology and Metabolism. 2004;89:5942–5947. doi: 10.1210/jc.2004-0249. [DOI] [PubMed] [Google Scholar]
- 24.Parfitt AM. Parathyroid hormone and periosteal bone expansion. Journal of Bone and Mineral Research. 2002;17:1741–1743. doi: 10.1359/jbmr.2002.17.10.1741. [DOI] [PubMed] [Google Scholar]
- 25.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. New England Journal of Medicine. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 26.Jilka RL, O’Brien CA, Ali AA, Roberson PK, Weinstein RS, Manolagas SC. Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone. 2009;44:275–286. doi: 10.1016/j.bone.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1–34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. Journal of Bone and Mineral Research. 2007;22:495–502. doi: 10.1359/jbmr.070104. [DOI] [PubMed] [Google Scholar]
- 28.Balena R, Shih MS, Parfitt AM. Bone resorption and formation on the periosteal envelope of the ilium: a histomorphometric study in healthy women. Journal of Bone and Mineral Research. 1992;7:1475–1482. doi: 10.1002/jbmr.5650071216. [DOI] [PubMed] [Google Scholar]
- 29.Epker BN, Frost HM. Periosteal appositional bone growth from age two to age seventy in man. A tetracycline evaluation. Anatomical Record. 1966;154:573–577. doi: 10.1002/ar.1091540307. [DOI] [PubMed] [Google Scholar]
- 30.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. Journal of Clinical Investigation. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]