Abstract
Induction of mild states of hyperketonemia may improve physical and cognitive performance. In this study, we determined the kinetic parameters, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate, a ketone monoester administered in the form of a meal replacement drink to healthy human volunteers. Plasma levels of β-hydroxybutyrate and acetoacetate were elevated following administration of a single dose of the ketone monoester, whether at 140, 357, or 714 mg/kg body weight, while the intact ester was not detected. Maximum plasma levels of ketones were attained within 1–2 h, reaching 3.30 mM and 1.19 mM for β-hydroxybutyrate and acetoacetate, respectively, at the highest dose tested. The elimination half-life ranged from 0.8–3.1 h for β-hydroxybutyrate and 8–14 h for acetoacetate. The ketone monoester was also administered at 140, 357, and 714 mg/kg body weight, three times daily, over 5 days (equivalent to 0.42, 1.07, and 2.14 g/kg/d). The ketone ester was generally well-tolerated, although some gastrointestinal effects were reported, when large volumes of milk-based drink were consumed, at the highest ketone monoester dose. Together, these results suggest ingestion of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate is a safe and simple method to elevate blood ketone levels, compared with the inconvenience of preparing and consuming a ketogenic diet.
Keywords: (R)-3-hydroxybutyl (R)-3-hydroxybutyrate, Ketone, β-Hydroxybutyrate, Acetoacetate, Kinetics, Safety, Tolerability
1. Introduction
Although ketosis has generally been portrayed as an unfavorable pathological state associated with diabetes mellitus and starvation, induction of mild hyperketonemia may have certain therapeutic benefits (Veech, 2004; Veech et al., 2001). For example, a high fat, low carbohydrate, protein-restricted ketogenic diet has been used to treat refractory epilepsy since the early 20th century (Veech, 2004). Glucose oxidation serves as the primary energy source for all living cells; however, under conditions where glucose is limited, such as during caloric deprivation, the body can utilize fats stored as triglycerides in adipose tissue as an energy source (Stanfield and Germann, 2008). During fasting, acetyl-CoA is shunted to the ketogenic pathway in the mitochondria of the liver, resulting in the production of ketone bodies (i.e., D-β-hydroxybutyrate, acetoacetate, and acetone) (Stanfield and Germann, 2008). These ketones are transported to extrahepatic tissues, where they can be converted back to acetyl-CoA and utilized in the citric acid cycle for energy (Manninen, 2004).
The liver of healthy adults is capable of producing up to 185 g of ketones per day (McPherson and McEneny, 2011). Ketones account for 2–6% of an individual’s energy needs following an overnight fast and approximately 40% of energy needs following a 3-day fast (Laffel, 1999). There is evidence to suggest that ketones have a higher metabolic efficiency compared to glucose, providing more energy per unit of oxygen consumed (Cahill and Veech, 2003; Veech, 2004). Early studies suggest β-hydroxybutyrate and acetoacetate increased the motility of sperm, while decreasing oxygen consumption, in contrast to carbohydrates, lipids and other intermediary metabolites (Veech et al., 2001). In an isolated rat heart perfusion model, ketones increased contractility while decreasing oxygen consumption, resulting in 25–28% increase in hydraulic efficiency (Sato et al., 1995; Kashiwaya et al., 1994). These observations were attributed to the fact that D-β-hydroxybutyrate has an inherently greater heat of combustion, releasing approximately 30% more energy per molecule compared to pyruvate (Veech, 2004). The high metabolic efficiency of ketones has important implications for the brain, as ketones can be utilized to meet high energy demands, especially during times of limited glucose availability (Owen, 2005; Owen et al., 1967). It has been proposed that artificially inducing a mild state of ketosis will provide additional acetyl-CoA substrates for the citric acid cycle. This is expected to enhance energy production and thereby improve physical performance and cognitive function, particularly during states of fatigue.
Classic ketogenic diets containing high fat, low carbohydrate and low protein content are difficult to prepare, unpalatable and may present an atherogenic risk as serum levels of cholesterol and triglycerides are often elevated (McPherson and McEneny, 2011). Recently, (R)-3-hydroxybutyl (R)-3-hydroxybutyrate (referred to as ketone monoester hereafter) was synthesized as a method to elevate blood ketone levels without the need to adhere to the strict ketogenic diet. Following ingestion, the ketone monoester was expected to undergo complete hydrolysis into its component parts (i.e., D-β-hydroxybutyrate and R-1,3-butanediol) by carboxylesterases and esterases located throughout the gastrointestinal tract, blood, liver and other tissues (Anders, 1989; Heymann, 1980). R-1,3-butanediol would then be further metabolized to the ketones, D-β-hydroxybutyrate and acetoacetate, in the liver by alcohol and aldehyde dehydrogenase (Desrochers et al., 1995; Tate et al., 1971). Preliminary studies showed the ketone monoester to be hydrolyzed extensively following incubation with human plasma in vitro (unpublished data). Moreover, in studies conducted in rats, oral administration of the ketone monoester readily increased blood levels of D-β-hydroxybutyrate and acetoacetate, whereas the intact ketone monoester was detected only at very low amounts (unpublished data). As such, administration of ketone monoester offers a novel approach to elevate circulating ketone levels.
The levels of β-hydroxybutyrate and acetoacetate in the blood typically range from 0.2–0.5 mM, although levels can increase up to 5–7 mM during periods of limited food intake. Excessively high levels of blood ketones, such as those observed during diabetic ketoacidosis when blood levels of ketones may reach 10–20 mM or higher, are considered pathological. This high level of ketones may overwhelm the body’s buffering capacity, resulting in metabolic acidosis that may potentially result in death if left untreated (Cahill and Veech, 2003). Investigations into the safety profile of the ketone monoester in humans, at doses intended to provide circulating levels of ketones similar to those observed during fasting states (i.e., approximately 5 mM), are warranted given their potential application in athletes and persons undergoing strenuous exercise, as examples. An ascending dose study has been conducted in healthy adults to evaluate the kinetic parameters of orally administered ketone monoester. Furthermore, the safety and tolerability of the ketone monoester were assessed in healthy adults given the ester as part of a meal replacement beverage for five consecutive days.
2. Material and methods
2.1. Subjects
Healthy male and female subjects (27 males and 27 females) between the age of 18 and 45 years were selected for this study. The enrolled subjects met the following inclusion criteria: a body mass index (BMI) between 18.5 and 29.9 kg/m2; non-pregnant; normal blood ketone (i.e., β-hydroxybutyrate) and glucose levels; normal hemoglobin A1C values; medically healthy with normal or clinically insignificant screening results [laboratory profiles, medical histories, electrocardiograms (ECG), and physical examination]; and negative results for bleeding during rectal examination. Female volunteers were postmenopausal, surgically sterilized, or women of childbearing potential who were non-lactating and using an effective form of birth control during the study and for 30 days after the study period. Individuals were excluded from this study if they had a medical illness, were allergic to milk protein or were lactose intolerant, had a history or presence of alcoholism or drug abuse within the previous 2 years and/or a positive drug or alcohol test at screening or on admission to the study, were taking prescription or over-the-counter medication (other than those taken as nutritional supplements for non-therapeutic indications), used tobacco in excess of five cigarettes per day within 1 month prior to the first dosing, or consumed a restricted diet or were on a high-fat ketogenic diet during 30 days prior to the first dosing. Subjects were not permitted to smoke or perform strenuous exercise, and abstained from any alcohol-containing or caffeine/xanthine-containing products or medications (excluding oral contraceptives) during the study period. The sample size selected was based on the precedent set by other pharmacokinetic studies of similar nature and a power calculation was not conducted.
2.2. Study design
This was a single-center, open-label study that was conducted at dgd Research (now Cetero Research) (San Antonio, Texas, USA). The protocol, including any amendments and consent forms, was approved by the Independent Investigational Review Board Inc. Institutional Review Board, the Department of Defense and the University of Oxford Ethics Committees. This study was conducted in accordance with the guidelines set forth by the International Conference on Harmonisation Guidelines for Good Clinical Practice, the Code of Federal Regulations for Good Clinical Practice, and the Declaration of Helsinki regarding the treatment of human subjects in a study. No randomization was performed as a sequential sample design was used (i.e., first enrolled, first tested). Blinding of the medical staff and subjects was not possible since the ketone monoester test article was administered with a supplemental formula (Ensure®) as necessary to ensure that all subjects received an isocaloric treatment. Compliance was defined as consumption of at least 85% of the ketone monoester meal replacement milkshake beverage and supplemental formula daily within a period of 30 min for each drink.
2.3. Protocol
2.3.1. Test article
The ketone monoester was given to subjects as a constituent of meal replacement milkshake beverages that were manufactured according to Good Manufacturing Practices. Each 100-g serving of the meal replacement milkshake contained approximately 84 kcal and 5.2 g of the ketone ester, with 30% of the calories from the ester. The composition and nutritional information of the meal replacement milkshake drink is summarized in Table 1.
Table 1.
Composition and nutritional information of ketone ester meal replacement milkshake drink.
| Ingredient | Composition (g per 100 g) |
|---|---|
| Composition | |
| Milkshake | 94.6 |
| Cream flavor | 0.2 |
| Ketone ester | 5.2 |
| Total | 100 |
| Macronutrient | Composition (per 100 g)
|
||
|---|---|---|---|
| Grams | Calories | % | |
| Nutritional information and caloric content | |||
| Carbohydrate | 6.7 | 27 | 32 |
| Protein | 3.9 | 16 | 19 |
| Fat | 1.7 | 16 | 19 |
| Ketone ester | 5.2 | 25 | 30 |
| Total | 17.5 | 84 | 100 |
2.3.2. Single dose study
On the morning of the study day following an overnight fast (8–10 h), subjects consumed a meal replacement milkshake drink containing 140, 357, or 714 mg/kg body weight of the ketone ester. So that all subjects received an isocaloric treatment, a supplemental formula (i.e., Ensure®) was consumed as necessary in addition to the ketone monoester meal replacement milkshake (see Table 2 for dosing regimen). The supplemental formula (i.e., Ensure®) was also consumed at 4 and 9 h post-dosing to standardize total daily caloric intake to 34 kcal/kg body weight (bw) (refer to Table 2). Subjects receiving the highest dose (i.e. 714 mg/kg ketone ester) did not receive Ensure® with their ketone ester meal replacement milkshake, as their dose provided exactly one-third of their caloric requirements. Subjects were provided with 8 oz of water at dosing, 4 h post-dose and 9 h post-dose, and were permitted to drink water throughout the day.
Table 2.
Administration schedules for D-β-hydroxybutyrate-R 1,3-butanediol monoester in single- and repeated dose studies.
| Study type | Monoester dose (mg/kg body weight) | Number of doses per day | Number of days | Amount of ketone ester milkshake drink (g/kg)a
|
Amount of supplemental formula (Ensure®) (g/kg)b
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Dosing | 4-h Post-dose | 9-h Post-dose | Dosing | 4-h Post-dose | 9-h Post-dose | ||||
| Single dose study (N = 6 per dose) | 140 | 1 | 1 | 2.69 | 0 | 0 | 8.72 | 10.90 | 10.90 |
| 357 | 1 | 1 | 6.87 | 0 | 0 | 5.35 | 10.90 | 10.90 | |
| 714 | 1 | 1 | 13.73 | 0 | 0 | 0c | 10.90 | 10.90 | |
| Repeated dose studye (N = 12 per dose) | 420 | 3 | 5 | 2.69 | 2.69 | 2.69 | 8.72 | 8.72 | 8.72 |
| 1071 | 3 | 5 | 6.87 | 6.87 | 6.87 | 5.35 | 5.35 | 5.35 | |
| 2142 | 3 | 5 | 13.73 | 13.73 | 13.73 | 0d | 0d | 0d | |
The ketone ester meal replacement milkshake drink contained 84 kcal per 100 g.
The supplemental formula (i.e., Ensure®) contained 104 kcal per 100 g. The amount of supplemental formula required at each meal was calculated based on a daily caloric requirement of 34 kcal/kg body weight, minus the caloric contribution of the ketone ester milkshake drink.
Subjects in the highest dose group (single dose study) had a dose of ketone ester that provided them with one-third of their daily caloric requirements; thus, supplemental formula was not required at dosing.
Subjects in the highest dose group (repeated dose study) had their daily caloric requirements met by the ketone ester milkshake drink; thus, supplemental formula was not required during the 5-day dosing period.
Ketone monoesters were administered as three divided doses of 140 mg/kg bw, 357 mg/kg bw, and 714 mg/kg bw over the course of the day.
For this single dose study, subjects remained in the research center from admission until discharge (approximately 24 h). Subjects returned to the research center 7 days following discharge for a follow-up examination.
2.3.3. Repeated dose study
Subjects consumed a ketone ester meal replacement milkshake drink providing 140, 357, or 714 mg/kg bw of ketone monoester three times daily (at 0, 4, and 9 h) for five consecutive days. Similar to the single dose study, Ensure® was provided as a supplemental formula, so that the total caloric intake per day was standardized to 34 kcal/kg bw (see Table 2 for dosing regimen). Subjects were provided with 8 oz of water at dosing, 4 h post-dose and 9 h post-dose, and were permitted to drink water throughout the day. For this repeated-dose study, subjects remained in the research center from admission until discharge (approximately 5 days). Subjects returned to the research center 7 days following discharge for a follow-up examination.
2.4. Sampling and analysis
2.4.1. Single dose study
Blood samples for pharmacokinetic analysis were collected at baseline (prior to dosing), and at 1, 2, 3, 4, 6, 8, 12, and 24 h post-dosing from an indwelling catheter. The levels of D-β-hydroxybutyrate and acetoacetate were determined in all subjects. The levels of R-1,3-butanediol and ketone monoesters were analyzed in samples from three subjects administered the 714 mg/kg bw dose collected during the early time points (up to 4 h post-dosing).
Blood lipids [i.e., total cholesterol, high-density lipoprotein (HDL)-, low-density lipoprotein- and non-HDL-cholesterol, triglycerides and free fatty acids] were measured in samples collected at screening; admission; at 3, 6, and 12 h post-dose; at discharge (24-h post-dose); and at the 7-day follow-up. Blood levels of β-hydroxybutyrate and glucose were assessed using a hand-held device (Abbott Medisense Precision Xtra Advanced Diabetes Monitoring System, Abbott) at screening; on the day of admission (prior to dosing with the test product); at 1.0, 3.0, 6.0, and 12.0 h post-dose; and at discharge. This was to ensure blood levels of β-hydroxybutyrate remained within normal range (0–5.5 mM) and that hypoglycemia (i.e., blood glucose below 65 mg/dL or 2.5 mM) or hyperglycemia (i.e., blood glucose above 400 mg/dL or 22.2 mM) did not develop over the course of the study.
In addition, clinical chemistry, hematology, and urinalysis were conducted at screening, admission (in the morning prior to administration of the test product), discharge (24 h post-dosing) and 7-day follow-up examination. Serum chemistry analysis included albumin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, urea nitrogen, calcium, chloride, cholesterol, creatinine, gamma glutamyl transferase, glucose, iron, lactate dehydrogenase, phosphorus, potassium, sodium, total bilirubin, total protein, triglycerides, and uric acid. Additional serum tests were conducted to measure amylase, creatinine kinase, and magnesium. The hematology parameters evaluated included hematocrit, hemoglobin, mean corpuscular hemoglobin concentration, mean corpuscular volume, platelet count, red cell distribution width, red blood cell count, white blood cell count, and white blood cell differential. Urine was tested for pH, specific gravity, protein, glucose, ketones, bilirubin, occult blood and cells, nitrite, urobilinogen, leukocytes, microscopic examination (performed on abnormal findings unless otherwise specified), and β-human chorionic gonadotropin test (females only).
2.4.2. Repeated dose study
Blood lipids were evaluated in samples collected at screening; admission (prior to initial dosing); at discharge (24-h post-dose); and at 7-day follow-up. Similar to the single-dose study, blood levels of β-hydroxybutyrate and blood glucose were assessed using a hand-held device to ensure hyperketonemia, hypoglycemia and hyperglycemia did not develop. Measurements were taken at screening; admission; three times daily during the 5-day dosing period (i.e., prior to the morning administration of the test product, and 1–2 h following the administration of the second and third test meals); at discharge; and the 7-day follow-up. Clinical chemistry, hematology, and urinalysis parameters were measured at screening; admission (prior to first dose); discharge (morning of day 6); and at the 7-day follow-up.
2.4.3. Detection of ketone monoester and ketones in plasma
Plasma concentrations of acetone, R-1,3-butanediol and the ketone monoester were determined by gas chromatography–mass spectrometry (GC–MS) analyses at the laboratory of Dr. Richard L. Veech at the National Institutes of Health. Acetone concentrations were determined using head-space analysis of the volatile compounds from 20 μL of plasma samples, according to the methods described in Deng et al. (2004). 2H6-acetone was used as an internal standard to quantify plasma acetone concentrations. The ketone monoester and 1,3-butanediol were analyzed as their trimethylsilyl ethers (TMS) in electron impact mode by GC–MS. Twenty μl of neutralized perchloric acid plasma extracts were brought to dryness under a stream of N2 and reacted with 100 μL of the Tri-Sil TBT reagent (Pierce Chemical Co., Rockford, IL, USA) to form the TMS ethers according to standard procedures (Knapp, 1979). Samples were sealed and heated to 60 °C for 5 min. One μL of the derivatized sample extracts was analyzed by capillary column GC–MS using electron impact mode ionization. The 1,3-butanediol was quantified using 1,4-butanediol as an internal standard added to the samples prior to sample extraction. The monoester was quantified using an external standard procedure by spiking plasma with the analyte over a concentration range from 1–100 μM. The lower limit of quantification was 0.05 mM for D-β-hydroxybutyrate and acetoacetate, 0.02 mM for R-1,3-butanediol, and 0.04 mM for the ketone monoester.
2.5. Safety evaluation
The safety assessment of the ketone monoester included regular monitoring for adverse events. Subjects were asked how they felt and were carefully monitored for signs of lethargy, symptoms of dehydration, abdominal pain or cramping, nausea or vomiting, or signs of hypoglycemia. A complete physical examination (including rectal examination) and measurements of vital signs (i.e., ECGs, systolic and diastolic blood pressure, heart rate, respiratory rate, and oral body temperature) were also performed. Laboratory tests were performed as described in Section 2.4.
In the single dose study, adverse event monitoring, physical examination, and vital sign measurements took place at screening; at admission (prior to administration of the test product); at 2, 4, 6, and 12 h post-dosing; at discharge (24 h post-dosing); and at the 7-day follow-up visit. In the repeated-dose study, the same safety evaluations were conducted at screening; on each day of dosing (prior to administration of the test product); at 1–2 h following administration of the third dose; at discharge (24 h after the last dose); and at the 7-day follow-up.
2.6. Statistical analysis
The pharmacokinetic parameters AUC0-t, AUC0-inf, AUCt-inf, Cmax, time to reach maximum peak concentration (Tmax), elimination rate concentration, elimination half life (T1/2), and apparent oral clearance (Cl/F, calculated as Dose/AUC0-inf), were calculated by standard non-compartmental methods using WinNonlin® (Version 5.0.1 or higher). WinNonlin® Model 200 for extravascular input was utilized. The effect of gender on pharmacokinetic parameters was evaluated using an Analyses of Variance model including gender as fixed effects and subjects as a random effect, with differences considered to be statistically significant at p < 0.05. Statistical analyses were completed using SAS® Version 9.1 for Windows.
3. Results
3.1. Participant demographics
Eighteen individuals (8 males and 10 females) were enrolled in the single-dose study, and 36 individuals (18 males and 18 females) were enrolled in the repeated-dose study. A summary of the participant demographics for both the single-dose and repeated- dose study can be found in Table 3. The ages of the participants ranged from 18 to 45, and body weights ranged from 53 to 102 kg. The sample consisted of equal representation by both genders and was predominantly White.
Table 3.
Summary of participant demographics.
| Demographic | Statistic | Single dose study (n = 6 per dose)
|
Repeated dose studya (n = 12 per dose)
|
||||
|---|---|---|---|---|---|---|---|
| 140 mg/kg body weight | 357 mg/kg body weight | 714 mg/kg body weight | 420 mg/kg body weight/day | 1071 mg/kg body weight/day | 2142 mg/kg body weight/day | ||
| Age (years) | Mean (SD) | 34.5 (8.02) | 32.3 (9.31) | 32.0 (8.72) | 33.3 (7.20) | 30.7 (6.10) | 35.5 (7.68) |
| Minimum | 23 | 18 | 23 | 19 | 18 | 20 | |
| Maximum | 45 | 43 | 45 | 41 | 38 | 45 | |
| BMI (kg/m2) | Mean (SD) | 26.4 (1.91) | 26.0 (2.40) | 25.4 (2.71) | 26.7 (2.87) | 26.1 (2.19) | 27.0 (2.26) |
| Minimum | 24.0 | 21.6 | 22.2 | 21.3 | 21.9 | 22.2 | |
| Maximum | 29.4 | 27.9 | 28.4 | 29.7 | 29.2 | 29.7 | |
| Height (cm) | Mean (SD) | 167.6 (9.43) | 164.1 (5.97) | 168.1 (8.58) | 168.8 (11.57) | 168.0 (7.23) | 169.3 (8.35) |
| Minimum | 156.6 | 157.1 | 156.5 | 149.7 | 148.2 | 155.4 | |
| Maximum | 180.3 | 174.3 | 182.5 | 188.3 | 174.1 | 188.0 | |
| Weight (kg) | Mean (SD) | 74.7 (12.19) | 70.3 (9.59) | 71.6 (8.96) | 76.1 (10.67) | 73.8 (9.53) | 77.7 (10.82) |
| Minimum | 58.9 | 53.3 | 60.6 | 55.5 | 57.0 | 63.3 | |
| Maximum | 88.7 | 82.8 | 85.3 | 93.2 | 88.2 | 101.8 | |
| Gender | Female | 3 (50.0%) | 4 (66.7%) | 3 (50.0%) | 6 (50.0%) | 6 (50.0%) | 6 (50.0%) |
| Male | 3 (50.0%) | 2 (33.3%) | 3 (50.0%) | 6 (50.0%) | 6 (50.0%) | 6 (50.0%) | |
| Race | Mixedb | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (8.3%) | 0 (0.0%) |
| Black/African American | 2 (33.3%) | 1 (16.7%) | 0 (0.0%) | 6 (50.0%) | 3 (25.0%) | 2 (16.7%) | |
| White | 4 (66.7%) | 5 (83.3%) | 6 (100.0%) | 6 (50.0%) | 8 (66.7%) | 10 (83.3%) | |
BMI = body mass index; SD = standard deviation.
Ketone monoesters were administered as three divided doses of 140 mg/kg bw, 357 mg/kg bw, and 714 mg/kg bw over the course of the day.
Mixed race refers to combination of American Indian/Alaska Native, Black/African America, and White.
3.2. Pharmacokinetic evaluation
Pharmacokinetic analyses were conducted on blood samples collected over 24 h from 17 participants following administration of a single oral dose of ketone monoester. The ketone monoester was not detected in plasma, with the lower limit of quantification being 0.04 mM. R-1,3-butanediol was detected in the plasma of three subjects administered the highest dose of the ketone monoester. However, maximum levels of R-1,3-butanediol were reached at approximately 1.0 mM, and levels returned back to baseline by 4 h following dosing. Since R-1,3-butanediol appears to be readily metabolized, it was not analyzed in the remainder of the subjects and pharmacokinetic analyses focused on D-β-hydroxybutyrate and acetoacetate, the primary metabolites of R-1,3-butanediol.
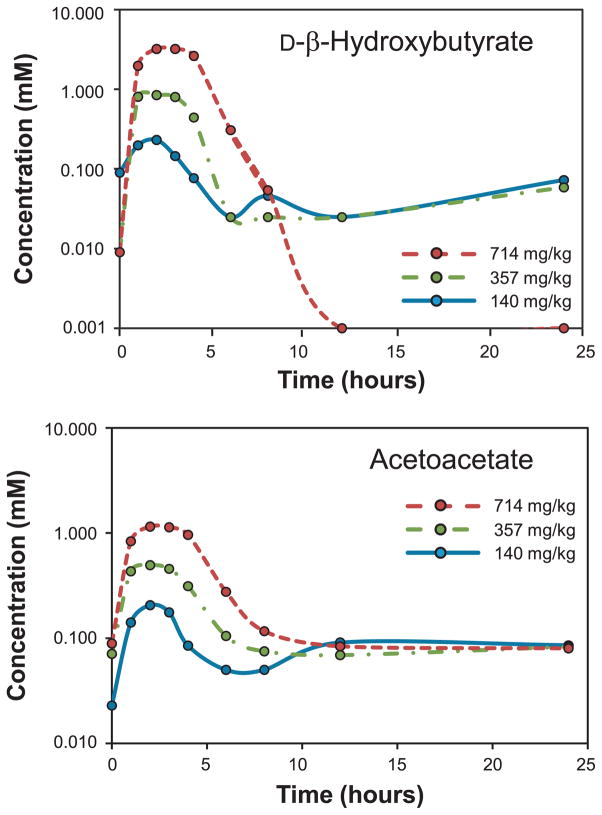
The mean plasma concentration–time profile of D-β-hydroxybutyrate and acetoacetate following administration of three different doses of ketone monoester is presented in Fig. 1. A summary of the pharmacokinetic parameters derived for D-β-hydroxybutyrate and acetoacetate can be found in Table 4. The maximal levels of D-β-hydroxybutyrate attained (Cmax) and area-under-the-curve (AUC; AUC0-t; AUC0-inf) increased proportionately with increasing doses of ketone monoester administered. The Cmax of D-β-hydroxybutyrate was 0.28, 1.00, and 3.30 mM for the 140, 357, and 714 mg/kg bw dose groups, respectively. The time to reach maximum peak concentration of D-β-hydroxybutyrate (Tmax) was comparable for all dose groups, ranging from 1.5 to 2.5 h. The t1/2 of D-β-hydroxybutyrate varied from 0.77 to 3.06 h across the different dose groups. The apparent Cl/F of D-β-hydroxybutyrate was nearly threefold higher for individuals treated with the lowest dose of ketone monoester compared to the highest dose.
Fig. 1.
Changes in circulating D-β-hydroxybutyrate and acetoacetate concentrations for 24 h following ingestion of a single dose of the ketone monoester.
Table 4.
| Parameter (units) | β-Hydroxybutyrate
|
Acetoacetate
|
||||
|---|---|---|---|---|---|---|
| 140 mg/kg bw (n = 6c) | 357 mg/kg bw (n = 6d) | 714 mg/kg bw (n = 6) | 140 mg/kg bw (n = 6e) | 357 mg/kg bw (n = 6) | 714 mg/kg bw (n = 6) | |
| AUC0-t (hr·mM) | 0.89 (28.49) | 2.85 (19.45) | 12.75 (25.93) | 1.15 (81.53) | 3.10 (32.28) | 6.66 (22.82) |
| AUC0-inf (hr·mM) | 1.09 (35.79) | 4.00 (18.44) | 13.00 (24.70) | 2.06 (120.20) | 4.19 (33.39) | 7.80 (19.19) |
| AUCt-inf (h mM) | 0.81 (9.88) | 0.77 (14.11) | 0.98 (1.98) | 0.60 (44.20) | 0.74 (6.73) | 0.85 (10.76) |
| Cmax (mM) | 0.28 (45.28) | 1.00 (19.92) | 3.30 (25.89) | 0.23 (36.13) | 0.54 (10.23) | 1.19 (27.02) |
| Tmax (h) | 1.50 (1.00–2.00) | 1.50 (1.00–3.00) | 2.50 (2.00–3.00) | 2.00 (1.00–3.00) | 2.50 (1.00–3.00) | 2.50 (2.00–4.00) |
| Kel (1/h) | 0.5859 (60.91) | 0.3209 (56.88) | 0.9410 (21.02) | 0.2777 (103.20) | 0.1143 (84.38) | 0.0944 (50.41) |
| T1/2 (h) | 1.61 (70.84) | 3.06 (73.25) | 0.77 (21.15) | 14.29 (163.10) | 8.23 (39.42) | 10.47 (74.19) |
| CL/F (mg/h mM) | 11,946.25 (46.51) | 6093.05 (19.94) | 4179.06 (31.91) | 10,338.45 (61.78) | 6554.10 (36.67) | 6745.64 (21.10) |
Abbreviations: AUC = area under the curve; bw = body weight.
Data are presented as arithmetic mean (% coefficient of variation) with the exception of Tmax, which is presented as median (range) for Tmax.
The lower limit of quantitation for both β-hydroxybutyrate and acetoacetate (0.05 mM) was >5% of the Cmax obtained, and on average, less than three half-lives of the pharmacokinetic curve was characterized for individuals treated with the lowest dose of ketone monoester (140 mg/kg bw). As such, the analytical method used to detect the ketones may not have been sufficiently sensitive to measure concentrations at the later time points.
N = 4 for the parameters AUC0-inf, AUCt-inf, Kel, T1/2, CL/F.
N = 3 for the parameters AUC0-inf AUCt-inf, Kel, T1/2, CL/F.
N = 4 for the parameters AUC0-t, AUCt-inf, Kel, T1/2, CL/F.
Similarly, for acetoacetate, Cmax, AUC0-t and AUC0-inf increased proportionately with increasing doses of ketone monoester administered. The Cmax of acetoacetate was 0.23, 0.54, and 1.19 mM for the 140, 357, and 714 mg/kg bw dose groups, respectively. Tmax was generally comparable for all doses, with maximum plasma concentrations of acetoacetate reached at around 2.0–2.5 h. The t1/2 of acetoacetate varied between 8.2 and 14.3 h across the different dose groups. The apparent oral clearance of acetoacetate was greatest in individuals administered the lowest dose of ketone monoester, being approximately twofold higher than the rates observed in the mid- and high dose groups.
The pharmacokinetic parameters of D-β-hydroxybutyrate and acetoacetate did not differ significantly between males and females in any dose group.
3.3. Safety evaluation
3.3.1. Adverse events
3.3.1.1. Single dose
One participant was discontinued from the single dose pharmacokinetic study as a result of non-compliance with the protocol. The only adverse effect reported that was considered to be “possibly” treatment-related was one mild case of increased triglyceride levels in one individual treated with the lowest dose (140 mg/kg bw). This adverse event resolved by the end of the study.
3.3.1.2. Repeated dose
When ketone monoester was administered repeatedly in three divided daily doses over the course of 5 days, adverse events that were deemed to be treatment-related were observed in 4 out of 12 participants in the low dose group (420 mg/kg bw/day), 1 out of 12 participants in the mid-dose group (1071 mg/kg bw/day), and 12 out of 12 participants in the high dose group (2142 mg/kg bw/day). Table 5 lists the frequency of the treatment-related adverse events and their severity. The low and mid- doses of ketone monoester were generally well tolerated, and the few adverse events were all considered to be mild and only “possibly” related to ketone treatment.
Table 5.
Frequency of treatment-related adverse events and their severity.a
| Repeated doseb | 420 mg/kg bw/day
|
1071 mg/kg bw/day
|
2142 mg/kg bw/day
|
|||
|---|---|---|---|---|---|---|
| Adverse event | Male (n = 6) | Female (n = 6) | Male (n = 6) | Female (n = 6) | Male (n = 6) | Female (n = 6) |
| Flatulence | 1 (mild) | – | – | – | 3 (mild) | 2 (mild) |
| Nausea | – | 1 (mild) | – | – | 4 (mild) | 3 (mild) |
| 1 (mod.) | ||||||
| Diarrhea | – | – | 1 (mild) | – | 3 (mild) | 1 (mild) |
| 1 (mod.) | 3 (mod.) | |||||
| Constipation | – | – | – | – | 2 (mild) | 1 (mild) |
| Vomiting | – | – | – | – | 1(mild) | 1 (mild) |
| 2 (mod.) | 2 (mod.) | |||||
| 1 (severe) | ||||||
| Decreased appetite | – | – | 1 (mild) | – | – | – |
| Abdominal distension | – | – | – | – | 6 (mild) | 3 (mild) |
| Abdominal pain | – | – | – | – | 1 (mild) | 1 (mod.) |
| Abdominal pain – lower | – | – | – | – | – | 1 (mild) |
| Abdominal pain – upper | – | – | – | – | 1 (mild) | 2 (mild) |
| 1 (mod.) | ||||||
| Occult blood positive | 1 (mild) | – | – | – | – | – |
| Rhinitis | – | – | – | – | 2 (mild) | – |
| Palpitations | – | – | – | – | 1 (mild) | 1 (mild) |
| Chest pain | – | – | – | – | – | 1 (mod.) |
| Dyspnoea | – | – | – | – | – | 1 (mod.) |
| Dizziness | – | 1 (mild) | – | – | 4 (mild) | 1 (mild) |
| Headache | – | – | – | – | 6 (mild) | 2 (mild) |
| Euphoria | – | 1 (mild) | – | – | – | – |
| Fatigue | – | – | – | – | 2 (mild) | 2 (mild) |
| Lethargy | – | – | – | – | 1 (mild) | – |
| Somnolence | – | – | – | – | – | 1 (mild) |
| Insomnia | – | – | – | – | 1 (mild) | – |
| Anxiety | – | – | – | – | 1 (mild) | – |
| Number of participants reporting at least one AE | 2 | 2 | 1 | 0 | 6 | 6 |
| Total number of AEs reportedc | 3 | 4 | 2 | 0 | 50 | 42 |
AE = adverse event; bw = body weight; mod. = moderate.
The number of individuals reporting the symptoms listed is provided.
Ketone monoesters were administered as three divided doses of 140 mg/kg bw, 357 mg/kg bw, and 714 mg/kg bw over the course of the day.
Includes repeat occurrences of the same adverse event in the same individual.
At the highest dose of ketone monoester administered (2142 mg/kg bw/day), two participants were discontinued from the study as a result of adverse events. These included severe vomiting in one individual, and nausea, diarrhea, chest pain, abdominal distension, and upper abdominal pain in the other. A number of gastrointestinal symptoms were also reported by participants in the high dose group who completed the study. These include flatulence, nausea, diarrhea, constipation, vomiting, abdominal distension, and abdominal pain ranging from mild to moderate in severity. Some of these events were deemed to be “highly probable” in their relation to ketone drink. Headaches, dizziness, lethargy, and somnolence were also reported in some participants, although these were considered to be mild in severity and were deemed to be “probable” in relation to the ketone treatment. All other adverse events reported were considered mild in severity with “probable” or “possible” relation to ketone treatment. It must be noted that, at this dose, the subjects were consuming ~1.1 liters of the milk-based drink within 30 min (i.e., 3.3 liters of drink per day).
The adverse events reported at all doses of ketone monoester resolved by the end of the study, with the exception of the positive fecal occult test observed in one individual in the lowest dose group (420 mg/kg bw/day).
3.3.2. Clinical laboratory parameters, physical examination, and vital signs
No abnormal changes in the levels of blood lipids, as well as hematology, clinical biochemistry and urinalysis parameters were observed following any dose of ketone monoester in either the single or repeated dose study. Moreover, blood ketone levels and glucose levels did not deviate from ranges deemed to be safe, with blood D-β-hydroxybutyrate not exceeding the maximum acceptable levels of 5.5 mM, and glucose levels remaining above 65 mg/dL (2.5 mM) and below 400 mg/dL (22.2 mM). Vital signs were also stable throughout the course of the study, and no treatment-related abnormalities were reported upon physical examination for any participant.
4. Discussion
In this study, the pharmacokinetic parameters of a novel synthetic ketone monoester were determined in healthy volunteers. The ketone monoester was not detected in systemic circulation following ingestion of a single dose at up to 714 mg/kg bw, though plasma ketone levels were found to readily increase. Maximum plasma levels of ketones were achieved within 1.5–2.5 h, reaching 3.30 mM and 1.19 mM for β-hydroxybutyrate and acetoacetate, respectively, at the highest dose of the ketone monoester tested. Similar findings have been reported in a study where the ketone monoester was provided in the diet of rats for up to 66 days. Plasma levels of the ketone monoester were below the limit of detection (< 1 μM), while the levels of β-hydroxybutyrate were nearly twice as high as the levels found in animals fed a control diet (unpublished data). Moreover, in a pharmacokinetic study conducted in rats, the ketone monoester was detected only at very low levels in the blood (0.05–0.11 mM) when the compound was administered by oral gavage at 5 mg/kg bw. Maximum blood levels of the ketone monoester were reached within 15 min, and its short half-life (6.5 min in females and 14 min in males) suggests the occurrence of rapid hydrolysis in systemic circulation. Accordingly, blood ketone levels were readily elevated, with D-β-hydroxybutyrate reaching maximum levels of 9–12 mM and acetoacetate reaching 6 mM within 30 min. Furthermore, R-1,3-butanediol was found to reach maximal levels of 1.3–2.9 mM by 60 min (unpublished data). Carboxylesterases and esterases have been identified throughout the gastrointestinal tract, blood, liver and other tissues (Anders, 1989; Heymann, 1980), and preliminary studies have shown the ketone monoester to be hydrolyzed extensively following incubation with human plasma in vitro (unpublished data). Together, these data provide evidence that ketone monoester is primarily hydrolyzed to its component parts (i.e., D-β-hydroxybutyrate and R-1,3-butanediol), thereby substantially elevating the levels of ketones in systemic circulation.
Following its release from the monoester, R-1,3-butanediol is converted to ketone bodies in the liver, specifically D-β-hydroxybutyrate and acetoacetate, by alcohol and aldehyde dehydrogenase (Desrochers et al., 1995; Tate et al., 1971). These ketone bodies have relatively short half-lives, ranging between 0.8–3.1 h for D-β-hydroxybutyrate and 8.2–14.3 h for acetoacetate. These compounds are rapidly metabolized by the body and accumulation is not expected to occur following repeated consumption. Once formed, ketones can be utilized as an energy source in organs such as the brain, heart, kidney cortex, and skeletal muscles, particularly during times of limited glucose availability or when glucose cannot be utilized effectively. During ketolysis, D-β-hydroxybutyrate is oxidized to acetoacetate, which is metabolized to acetoacetyl- CoA and subsequently to acetyl-CoA for utilization in the citric acid cycle (Laffel, 1999; McPherson and McEneny, 2011). Ketones that are not utilized are filtered by the kidneys, and the fraction that is not re-absorbed is excreted in the urine (Laffel, 1999). It has been demonstrated in rats that nearly all of the filtered β-hydroxybutyrate and acetoacetate were reabsorbed in the kidney under physiological conditions where plasma ketones levels were <2 mM (Barac-Nieto, 1985; Ferrier et al., 1992). However, re-absorption decreases as plasma levels of ketones increase, with approximately 70–80% reabsorbed at plasma levels of ketones between 2 and 5 mM, whereas 40–50% are reabsorbed at plasma levels in excess of 10 mM (Barac-Nieto, 1985; Ferrier et al., 1992). The increased excretion of ketones in the urine may reflect saturation of reabsorption processes, and sodium-dependent transporters of ketones have been identified in the kidneys of rats and rabbits (Ferrier et al., 1992). Ketonuria is observed during certain pathological states, such as extensive starvation or diabetic ketoacidosis where very high levels of ketones are reached. Ketones were not detected in the urine of any of the participants at all doses of the ketone monoester tested in both the single dose and repeated dose study (data not shown), suggesting urinary excretion is unlikely to contribute substantially to its elimination.
A number of studies have been conducted to determine the kinetics of acetoacetate and β-hydroxybutyrate metabolism (Hall et al., 1984; Wastney et al., 1984). There is evidence to suggest that utilization of ketone bodies may be a saturable process, specifically for the uptake of ketones by skeletal muscle (Balasse and Féry, 1989). Higher ketone levels have been associated with slower rates of clearance, even when the hyperketonemia was artificially induced through infusion of exogenous ketones (Balasse and Féry, 1989; Hall et al., 1984; Wastney et al., 1984). Accordingly, individuals administered the high dose of ketone monoester in this study were found to have the slower rates of clearance of both β-hydroxybutyrate and acetoacetate.
The safety and tolerability of the ketone monoester was also examined in this study. Ingestion of the ketone monoester had no adverse effects in participants administered a single-dose at up to 714 mg/kg bw in the pharmacokinetic study. Furthermore, the ketone monoester was generally well tolerated when administered repeatedly at doses up to 714 mg/kg bw taken three times daily (corresponding to 2142 mg/kg bw/day) for five consecutive days. Although many of the participants administered the highest dose in the repeated-dose study (i.e., 2142 mg/kg bw/day) developed adverse effects that were primarily gastrointestinal in nature, these were considered mild to moderate in severity and resolved spontaneously by the end of the study. The gastrointestinal side effects with the high level of ketone monoester were likely attributable to the consumption of large volumes (~1.1 l) of a milk-based drink within a short time-frame, rather than adverse effects produced from elevated ketone levels. No clinically significant changes in vital signs, blood lipid levels, hematology, clinical biochemistry, and urinalysis parameters were observed following consumption of the ketone monoester at any of the doses tested. Blood ketone and glucose levels were strictly monitored over the course of the study, and levels were not altered beyond the range deemed to be of safety concern.
An excess of circulating ketones, which are strong organic acids that dissociate fully at physiological pH and overload the buffering capacity of serum and tissues, may result in metabolic acidosis, a potentially life-threatening condition. Moreover, increased ketone levels may affect the permeability of the brain microvascular endothelium, and cerebral edema has been associated with diabetic ketoacidosis (Edge et al., 2001; Isales et al., 1999; Wootton-Gorges et al., 2005). However, such effects are observed only when ketones reach supra-physiological levels (10–20 mM or higher) during pathological states (Cahill and Veech, 2003; Laffel, 1999). Ketones are normally present in the blood of healthy individual in small amounts following an overnight fast or prolonged exercise, with plasma levels reported in the ranges of 0.2–0.5 mM (Laffel, 1999). Circulating ketone levels can increase up to 50-fold during periods of caloric deprivation, with β-hydroxybutyrate levels reported to be 4–5 mM in the blood following a 5- to 8-day fast (Cahill, 2006; Mensink et al., 1992; Owen et al., 1967; VanItallie and Nufert, 2003). From the pharmacokinetic analyses, the highest dose of ketone monoester tested (714 mg/kg bw) produced mean maximal concentrations of β-hydroxybutyrate and acetoacetate pf 3.30 mM and 1.19 mM, respectively. Moreover, plasma levels of β-hydroxybutyrate did not exceed 5.5 mM following ingestion of the ketone monoester beverage in the repeated dose study at the highest dose tested (2142 mg/kg bw/day in three divided doses of 714 mg/kg bw each). Thus, the mild ketosis induced by consumption of the ketone monoester can be considered similar to the physiological response observed during fasting states in humans.
The ability of ketones to serve as an alternative energy source is of particular importance in the brain as they supply nearly two-thirds of the energy required during periods of prolonged fasting and starvation (Laffel, 1999). The ketone monoester was found to enhance cognitive function following exercise to exhaustion in rats, as measured by performance in a maze task (unpublished data). Similarly, administration of a ketogenic diet improved cognitive function, as measured by performance in the T-maze and object recognition tests, in aged rats under both normoxic and hypoxic conditions (Xu et al., 2010). The higher metabolic efficiency of ketones compared to glucose has also been shown to result in increased hydraulic efficiency in an isolated, perfused rat heart (Kashiwaya et al., 1994; Sato et al., 1995). So the ketone monoester may be used to enhance cognitive function and physical performance, particularly in athletes performing strenuous exercise. Induction of a mild hyperketonemic state has been proposed to be beneficial in a number of diseases, particularly those involving insulin resistance or insufficiency of metabolic substrates, as well as those resulting from free radical damage or hypoxia (reviewed in Veech (2004)). Elevations of ketone levels can be achieved through administration of the classic ketogenic diet containing high fat and low carbohydrate. However, such diets are unpalatable, so have poor compliance, and may increase blood cholesterol and triglycerides, which are well documented atherogenic risk factors (McPherson and McEneny, 2011). The ketone monoester may provide an alternative method of elevating ketone levels without the adverse effects of ketogenic diets.
In summary, the pharmacokinetic properties and safety of a synthetic ketone monoester was evaluated in healthy adult humans. The ketone monoester was completely hydrolyzed to its components (D-β-hydroxybutyrate and R-1,3-butanediol), resulting in increased plasma levels of the ketones, D-β-hydroxybutyrate and acetoacetate. Ingestion of the ketone monoester over a period of 5 days was generally well tolerated. Some gastrointestinal disturbances were observed in individuals who consumed the highest dose (2142 mg/kg bw/day taken in three divided doses of 714 mg/kg bw/day daily), though these were considered to be related to the large volumes (>1 l) of a milk-based drink consumed in a short period, rather than effects caused by the ketone monoester.
Acknowledgments
The authors thank Dr. Joseph Bielitsky, the late Dr. Catherine Golden, Dr. Brett Giroir and Dr. Kerrie DeMarco (Defense Advanced Research Projects Agency (DARPA) of the United States), whose constant support and advice made this study possible.
Funding sources statement: The authors thank the Defense Advanced Research Projects Agency (DARPA) of the United States for funding this work.
Abbreviations
- AUC
area-under-the-curve
- Ketone monoester
(R)-3-hydroxybutyl (R)-3-hydroxybutyrate
- BMI
body mass index
- bw
body weight
- Cmax
maximum peak concentration
- Cl/F
oral clearance calculated as Dose/AUC0-inf
- ECG
electrocardiograms
- GC–MS
gas chromatography-mass spectrometry
- T1/2
elimination half life
- Tmax
time to reach maximum peak concentration
- TMS
trimethylsilyl ethers
Footnotes
Conflict of interest statement
The intellectual property covering the uses of ketone bodies and ketone esters are owned by BTG Ltd., the University of Oxford and the National Institutes of Health. Should royalties ever accrue from these patents, Dr. Richard L. Veech, Professor Kieran Clarke, and Mr. Todd King, as inventors, will receive a share of the royalties under the terms prescribed by each institution. Professor Kieran Clarke is a non-executive director of TdeltaS Ltd., a company spun out of the University of Oxford to develop products based on the science of ketone bodies in human nutrition. Drs Kathy Musa- Veloso, Manki Ho, and Ashley Roberts received financial support from DARPA and TdeltaS Limited for consulting services and manuscript preparation.
References
- Anders MW. Biotransformation and bioactivation of xenobiotics by the kidney. In: Hutson D, Caldwell J, Paulson GD, editors. Intermediary Xenobiotic Metabolism in Animals: Methodology, Mechanisms and Significance. Taylor and Francis; New York: 1989. pp. 81–97. [Google Scholar]
- Balasse EO, Féry F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev. 1989;5:247–270. doi: 10.1002/dmr.5610050304. (abstract only) [DOI] [PubMed] [Google Scholar]
- Barac-Nieto M. Renal hydroxybutyrate and acetoacetate reabsorption and utilization in the rat. Am J Physiol. 1985;249:F40–F48. doi: 10.1152/ajprenal.1985.249.1.F40. [DOI] [PubMed] [Google Scholar]
- Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- Cahill GF, Jr, Veech RL. Ketoacids? Good medicine? Trans Am Clin Climatol Assoc. 2003;114:149–161. (discussion 162–163) [PMC free article] [PubMed] [Google Scholar]
- Deng C, et al. Rapid determination of acetone in human plasma by gas chromatography–mass spectrometry and solid-phase microextraction with on-fiber derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;805:235–240. doi: 10.1016/j.jchromb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Desrochers S, et al. Metabolism of (R, S)-1,3-butanediol acetoacetate esters, potential parenteral and enteral nutrients in conscious pigs. Am J Physiol. 1995;268:E660–E667. doi: 10.1152/ajpendo.1995.268.4.E660. [DOI] [PubMed] [Google Scholar]
- Edge JA, et al. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch Dis Child. 2001;85:16–22. doi: 10.1136/adc.85.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier B, et al. Transport of beta-hydroxybutyrate and acetoacetate along rat nephrons: a micropuncture study. Am J Physiol. 1992;262:F762–F769. doi: 10.1152/ajprenal.1992.262.5.F762. [DOI] [PubMed] [Google Scholar]
- Hall SE, et al. Ketone body kinetics in humans: the effects of insulin-dependent diabetes, obesity, and starvation. J Lipid Res. 1984;25:1184–1194. [PubMed] [Google Scholar]
- Heymann E. Carboxylesterases and amidases. In: Jakoby W, editor. Enzymatic Basis of Detoxification. Biochemical Pharmacology and Toxicology. Vol. 2. Academic Press; New York: 1980. pp. 291–323. [Google Scholar]
- Isales CM, Min L, Hoffman WH. Acetoacetate and β-hydroxybutyrate differentially regulate endothelin-1 and vascular endothelial growth factor in mouse brain microvascular endothelial cells. J Diabet Compl. 1999;13:91–97. doi: 10.1016/s1056-8727(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Kashiwaya Y, et al. Control of glucose utilization in working perfused rat heart. J Biol Chem. 1994;269:25502–25514. [PubMed] [Google Scholar]
- Knapp DR. Handbook of Analytical Derivatization Reactions. Wiley-Interscience Publication; New York: 1979. Derivatization of particular compound types: hydroxyl, sulfhydryl, and epoxy compounds. 1.1 Derivatives of alcohols; pp. 30–39. [Google Scholar]
- Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diab Metab Res Rev. 1999;15:412–426. doi: 10.1002/(sici)1520-7560(199911/12)15:6<412::aid-dmrr72>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Manninen AH. Metabolic effects of the very-low-carbohydrate diets: misunderstood “villains” of human metabolism. J Int Soc Sports Nutr. 2004;1:7–11. doi: 10.1186/1550-2783-1-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson PA, McEneny J. The biochemistry of ketogenesis and its role in weight management, neurological disease and oxidative stress. J Physiol Biochem (Advance, Publication – October, 8, 2011) 2011 doi: 10.1007/s13105-011-0112-4. [DOI] [PubMed] [Google Scholar]
- Mensink RP, et al. Effects of dietary saturated, cis-and trans-monounsaturated and polyunsaturated fatty acids on fasting blood ketone levels in man. In: Sinclair A, Gibson R, editors. Essential Fatty Acids and Eicosanoids. Invited Papers from the Third International Congress; Adelaide, Australia. Champaign, IL: American Oil Chemists’ Society (ACS); 1992. pp. 274–278. [Google Scholar]
- Owen OE. Ketone bodies as a fuel for the brain during starvation. Biochem Mol Biol Educ. 2005;33:246–251. [Google Scholar]
- Owen OE, et al. Brain metabolism during fasting. J Clin Invest. 1967;46:1589– 1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, et al. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- Stanfield CL, Germann WJ. Principles of Human Physiology. 3. Pearson Benjamin Cummings; San Francisco: 2008. Cell metabolism; pp. 58–93. [Google Scholar]
- Tate RL, Mehlman MA, Tobin RB. Metabolic fate of 1,3-butanediol in the rat: conversion to hydroxybutyrate. J Nutr. 1971;101:1719–1726. doi: 10.1093/jn/101.12.1719. [DOI] [PubMed] [Google Scholar]
- VanItallie TB, Nufert TH. Ketones: metabolism’s ugly duckling. Nutr Rev. 2003;61:327–341. doi: 10.1301/nr.2003.oct.327-341. [DOI] [PubMed] [Google Scholar]
- Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Veech RL, et al. Ketone bodies, potential therapeutic uses. IUBMB Life. 2001;51:241–247. doi: 10.1080/152165401753311780. [DOI] [PubMed] [Google Scholar]
- Wastney ME, Hall SE, Berman M. Ketone body kinetics in humans: a mathematical model. J Lipid Res. 1984;25:160–174. [PubMed] [Google Scholar]
- Wootton-Gorges SL, et al. Detection of cerebral β-hydroxy butyrate, acetoacetate, and lactate on proton MR spectroscopy in children with diabetic ketoacidosis. AJNR Am J Neuroradiol. 2005;26:1286–1291. [PMC free article] [PubMed] [Google Scholar]
- Xu K, et al. Diet-induced ketosis improves cognitive performance in aged rats. Adv Exp Med Biol. 2010;662:71–75. doi: 10.1007/978-1-4419-1241-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]