Abstract
Phosphate is absorbed from the diet in the gut, stored as hydroxyapatite in the skeleton, and excreted with the urine. The balance between these compartments determines the circulating phosphate concentration. Fibroblast growth factor 23 (FGF23) has recently been discovered and is part of a previously unrecognised hormonal bone-kidney axis. Phosphate-regulating gene with homologies to endopeptidases on the X chromosome, and dentin matrix protein 1 regulate the expression of FGF23 in osteocytes, which then is O-glycosylated by UDP-N-acetyl-alpha-d-galactosamine: poly-peptide N-acetylgalactosaminyl-transferase 3 and secreted into the circulation. FGF23 binds with high affinity to fibroblast growth factor receptor 1c in the presence of its co-receptor Klotho. It inhibits, either directly or indirectly, reabsorption of phosphate and the synthesis of 1,25-dihydroxy-vita-min-D by the renal proximal tubule and the secretion of parathyroid hormone by the parathyroid glands. Acquired or inborn errors affecting this newly discovered hormonal system can lead to abnormal phosphate homeostasis and/or tissue mineralisation. This chapter will provide an update on the current knowledge of the pathophysiology, the clinical presentation, diagnostic evaluation and therapy of the disorders of phosphate homeostasis and tissue mineralisation.
In contrast to the regulation of calcium homeostasis, which has been extensively studied over the past several decades [1], relatively little is known about the regulation of phosphate homeostasis. Most important, yet often completely unexpected, insights into the regulation of phosphate homeostasis were obtained through the definition of genetic mutations underlying rare inherited disorders in humans. For example, mutations in phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX) provided a molecular genetic explanation for X-linked hypophosphataemia (XLH), the most frequent form of renal phosphate-wasting [2]. Likewise, positional cloning strategies led to the identification of mutations in the chloride channel CLCN5 as the cause of X-linked recessive nephrolithiasis (Dent’s disease) [3], in fibroblast growth factor 23 (FGF23) as the cause of autosomal-dominant hypophosphataemic rickets (ADHR) [4], in dentin matrix protein 1 (DMP1) as a cause of autosomal-recessive hypophosphataemia (ARHP) [5], and in UDP-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyl-transferase 3 (GALNT3) as the cause of a form of tumoral calcinosis [6]. These findings were extended by the generation of a number of animal models, including the Klotho-null mouse, which has led to particularly important insights [7]. This mouse, which has a phenotype resembling hyperphosphataemic familial tumoral calcinosis (HFTC) in humans, lacks the expression of alpha Klotho (KL), which has a single membrane-spanning domain, and was subsequently found to be a co-receptor of FGF23 [8, 9]. Based on the murine phenotype, KL mutations were recently identified by the candidate-gene approach as a cause of hyperphosphataemia in familial tumoral calcinosis type 3 [10] (see below).
The circulating phosphate concentration is determined by the balance between intestinal absorption of phosphate from the diet, storage of phosphate in the skeleton, and reabsorption of phosphate from the urine. It is taken up from the circulation into cells via type II and type III sodium-phosphate co-transporters to facilitate cellular functions such as DNA and membrane lipid synthesis, generation of high-energy phosphate esters, and intracellular signalling. Only 30% of intestinal phosphate absorption occurs in a regulated, 1,25(OH)2D-dependent manner [11]. Consequently, reabsorption of phosphate from the urine in the renal proximal tubules via type II and type III sodium-phosphate co-transporters plays a key role in maintaining serum phosphate homeostasis, while excess phosphate is excreted (for recent reviews, see [12–20]). Renal phosphate reabsorption lies under tight hormonal control by PTH and FGF23 and, to a lesser extent, by insulin, by the hormones of the somatotropic pituitary axis [21], by FGF7 [22] and possibly by matrix extracellular phosphoglyco-protein (MEPE) and secreted frizzled-related protein 4 (sFRP-4) [20].
FGF23 is part of a newly discovered endocrine bone-kidney axis [23, 24]. PHEX and DMP1 regulate the expression of FGF23 in osteocytes, which is secreted into the circulation after undergoing O-glycosylation by GALNT3 [25]. FGF23 binds to FGFR1c and its co-receptor KL [9, 26] and activation of this receptor complex inhibits, either directly or indirectly, the reabsorption of phosphate by reducing the expression of the sodium-phosphate cotransporters NaPi-IIa and NaPi-IIc at the brush border membrane of the proximal renal tubules [27]. FGF23 also decreases the synthesis of 1,25(OH)2D in this portion of the renal tubules [28, 29] and it appears to reduce secretion of PTH by the parathyroid glands [30, 31]. For a more detailed description of phosphate metabolism, see chapter 3.
This chapter will provide an update on the pathophysiology, clinical presentation, diagnostic evaluation and therapy of the disorders associated with these factors. Disorders affecting both calcium and phosphate homeostasis such as parathyroid dysfunction, disorders of vitamin D metabolism and action, and generalised proximal renal tubular dysfunction such as Fanconi syndrome or renal tubular acidosis are important considerations in the differential diagnosis, but will not be discussed here.
Epidemiology
XLH is the most common inherited disorder of phosphate homeostasis affecting 1:20,000 births [32], while the other inherited forms are considered to be rare. Among the acquired disorders of phosphate homeostasis are post-renal transplant hypophosphataemia [33, 34], burn injury-related [35] and post-hepatectomy hypophosphataemia [36], which are increasingly recognised abnormalities. Patients affected by these disorders show an inappropriately high renal phosphate excretion, which appears to go along with elevated FGF23 levels in the case of post-renal transplant hypophosphataemia, while the role of FGF23 in the other two processes remains to be clarified. FGF23 levels are also increased in early chronic kidney disease (stages 2–3) before the development of anaemia, which may help to maintain normophosphataemia, but is likely to suppress 1α-hydroxylase and thus may also support the development of secondary hyperparathyroidism [37, 38]. Tumour-induced osteomalacia, on the other hand, is comparatively rare, with only a few hundred cases described in the literature to date [39, 40].
Clinical Assessment of Phosphate Homeostasis
The clinical assessment of phosphate homeostasis can be challenging: serum phosphate concentrations are influenced by the time of day, relationship to meals, and age of the subject, and none of the methods for determination of tubular reabsorption is entirely satisfactory. To determine the cause of abnormal serum phosphate levels in a patient who has normal parathyroid and renal function, we generally first assess his or her tubular reabsorption for phosphate (%TRP). For this purpose the patient is asked to collect a 3-hour timed urine for phosphate and creatinine, along with the corresponding serum parameters after an eight hour fast. %TRP is then calculated according to the formula depicted in figure 1. A timed post-fasting urine is requested to avoid fluctuations as a result of variable absorption of phosphate and possible effects of insulin or glucose on phosphate handling. The tubular maximum of reabsorption for phosphate (TmP/GFR) is derived from a nomogram, which was devised by Walton and Bijvoet [41] to correct for the non-linear relationships of %TRP and TmP/GFR when TRP is higher than 80%. TmP/GFR reflects the threshold of the serum phosphate concentration above which phosphate is no longer fully reclaimed from the glomerular filtrate in the kidney. While the TmP/GFR derived from the Walton and Bijvoet nomogram is generally sufficient in adults, the nomogram does not accommodate the higher normal range of serum phosphate values in newborns and toddlers and thus calculation of TP/GFR may be more accurate in this paediatric population [42] (fig. 1). Inappropriately high %TRP in the setting of hyperphosphataemia or inappropriately low %TRP in the setting of hypophosphataemia is suggestive of a proximal renal tubular defect as the underlying cause, which can subsequently be further classified by determining the patient’s vitamin D status: concordantly (inappropriately) reduced %TRP and 1,25(OH)2D levels suggest excess FGF23 action, while increased %TRP and 1,25(OH)2D levels suggest diminished activity of this hormone. In contrast, discordantly (appropriately) elevated 1,25(OH)2D levels suggest an FGF23-independent, possibly primary, renal tubular defect leading to abnormal serum phosphate levels (table 1). Excess production of 1,25(OH)2D may lead to increased absorption of calcium in the gut, resulting in hypercalciuria and some suppression of PTH production and may, in the setting of hypophosphataemia, be diagnostic for HHRH [43]. It is important to keep in mind that vitamin D deficiency and secondary hyperparathyroidism may mask these findings and need to be corrected before the above testing [44].
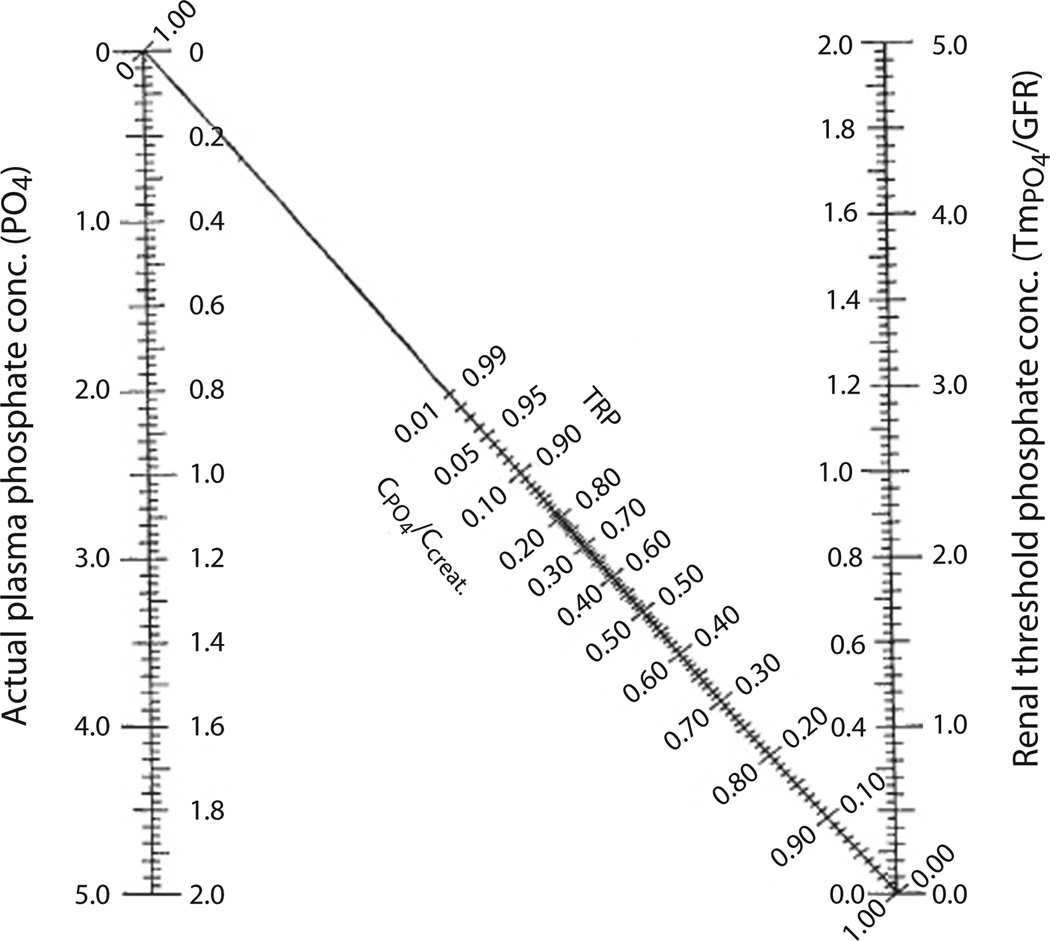
Fig. 1.
Calculation of %TRP, TmP/GFR, and TP/GFR. To determine TmP/GRF, first calculate % TRP (% TRP = 1 − (UPO4 × Screat)/(SPO4 × Ucreat) (note that the units for both phosphate and both creatinine measurements must be the same) and then derive TmP/GFR from the Walton and Bijvoet nomogram [41]: The inner axes are mmol/L and the outer axes mg/100 ml. TP/GFR is calculated using the following formula: SPO4 − (UPO4 × Screat/Ucreat), using simultaneous urine and blood creatinine and phosphorus concentrations.
Table 1.
Serum biochemical findings in disorders of phosphate homeostasis and tissue mineralization
| Parameter | Hypophosphataemia |
Hyperphosphataemia |
Normophosphataemia | ||
|---|---|---|---|---|---|
| FGF23- dependent |
FGF23- independent |
FGF23- deficient |
FGF23- resistant |
||
| Acquired | TIO, post-renal transplant | Post hepatectomy | NA | NA | NA |
| Inherited | XLH, ADHR, ARHP, OGD, OSD, FD/MAS, NF1+2 | HHRH | HFTC1, HFTC2 | HFTC3 | pulmonary alveolar microlithiasis, NFTC |
| S-Ca | NL | NL | NL to high | NL to high | NL |
| S-PO4 | low | low | high | high | NL |
| S-PTH | NL to high | NL to low | NL to low | NL to high | NL |
| S-1,25(OH)2D | NL to LOW | HIGH | high | high | NL |
| S-FGF23 | NL to HIGH | low | low | high | NL |
| U-PO4 | HIGH | high | low | low | NL |
| U-Ca | NL to LOW | high | NL to HIGH | NL to HIGH | NL |
| Current treatment | phosphate and alfacalcidol or calcitriol | phosphate replacement only | phosphate binders, acetazolamide, PTH | phosphate binders, acetazolamide, PTH | supportive measures |
Circulating FGF23 levels can be determined from EDTA plasma, which preserves FGF23 relatively well, using several commercially available enzyme-linked immunometric assays [40, 45, 46]. The existing assays permit the diagnosis of FGF23-dependent disorders of phosphate homeostasis, when FGF23 levels are elevated above the normal range [47, 48]. None of the currently available assays, however, is sensitive enough to detect suppressed or inappropriately normal FGF23 levels with sufficient confidence, thus limiting their utility for distinguishing FGF23-independent hypophosphataemic disorders such as HHRH from the FGF23-dependent hypophosphataemic disorders [40, 45]. The C-terminal FGF23 assay (Immutopics, Inc., San Clemente, Calif., USA) uses antibodies directed against two distinct epitopes within the C-terminal region of FGF23 and thus could detect intact FGF23 and C-terminal fragments; this assay appears to be particularly helpful for distinguishing hereditary familial tumoral calcinosis (HFTC) 1 and 2, from HFTC3 (see below for details), if genetic testing is unavailable [10, 49, 50].
Clinical Signs of Hyperphosphataemia
Clinical signs of chronic hyperphosphataemia include ectopic tissue mineralisation of juxta-articular muscular and subcutaneous tissues (fig. 5). Patients with tumoral calcinosis often also show dental pulp stones, which may lead to a complete obliteration of the dental pulp cavities (fig. 3a). Other clinical features, which may constitute the only clinical evidence for tumoral calcinosis, can include eyelid calcifications, vascular calcifications, and/or nephrocalcinosis (fig. 4). There can also be mineralisation of the juxta-articular bone marrow cavities. However, the remaining skeleton often shows low bone mineral density due to a mineralisation defect, which at the moment is only poorly understood [51, 52]. Pulmonary alveolar microlithiasis (PAM) is diagnostic for patients with homozygous loss-of-function mutations of NaPi-IIb (SLC34A2) and may be due to the local accumulation of phosphate (fig. 7) [53]. PAM is initially asymptomatic and may be an incidental finding on radiographic images, but can lead to slow and potentially fatal deterioration of pulmonary function [54].
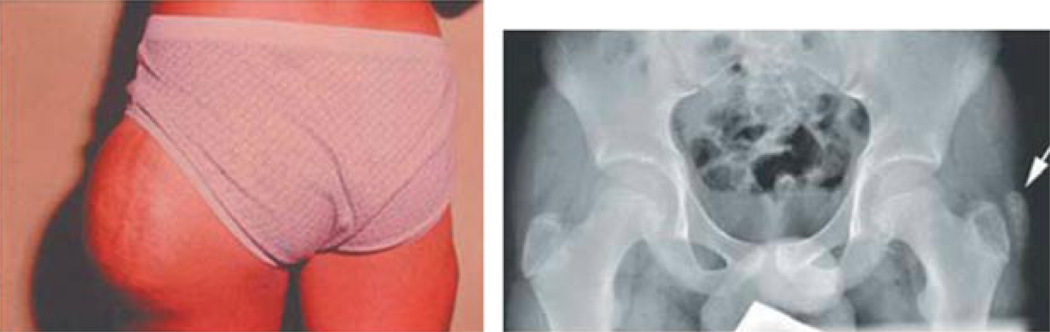
Fig. 5.
Clinical features in HFTC1. The left panel shows a large subcutaneous tumour over the left outer thigh. The right panel shows a periarticular calcified mass over the left acetabulum (fig. 1b from [6], with permission).
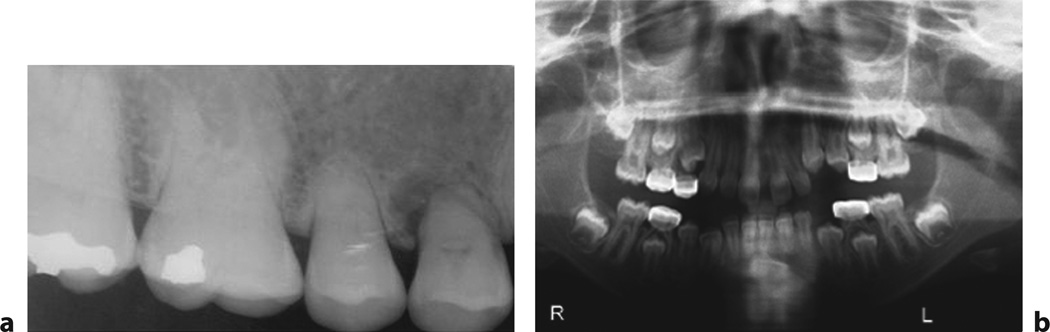
Fig. 3.
a Dental findings. Dental radiograph demonstrating sclerotic teeth with blunt roots and obliterated pulp cavity in a patient with HFTC1 (from fig. 1a in [138], with permission). b Orthopantograph showing deciduous molar teeth with stainless steel crowns and dental cyst at the lower canine teeth (fig. 2 from [139], with permission).
Fig. 4.
Tumoral calcinosis. Clinical features of a patient with HFTC2 showing developmental deformity of the forearm. a Periarticular swelling of the knee. b Eye-lid calcifications. c Renal medullary calcifications. d osteopaenia and osteosclerosis. e and aortic and valvular calcifications of the heart (fig. 1 from [140], with permission).
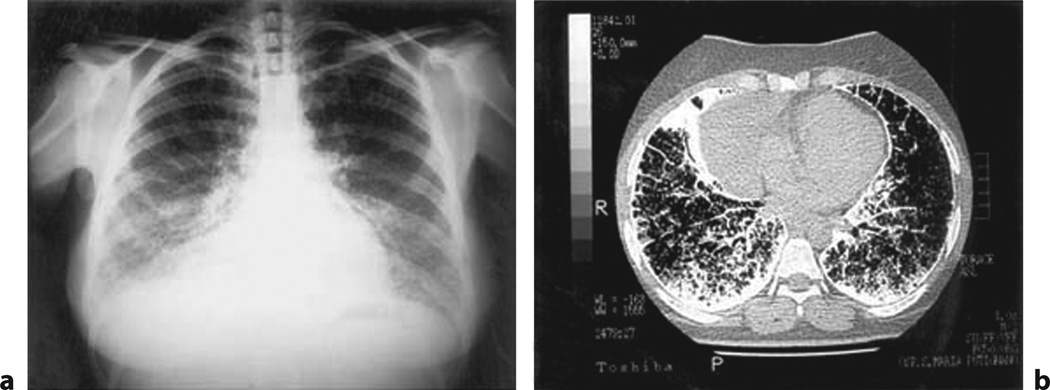
Fig. 7.
Pulmonary alveolar microlithiasis (PAM). a Chest radiograph of a patient affected by PAM, showing fine microliths with a diffuse, uniform spread obscuring the cardiac and diaphragmatic borders (sandstorm lung). b CT scan with diffuse, ground-glass opacities (stony-lung) in all pulmonary fields, and faint calcific densities, sometimes confluent at posterior and inferior subpleural regions (from fig. 1 in [54], with permission).
Clinical Signs of Hypophosphataemia
Bone pain, bowing and waddling gait is the classical diagnostic triad for hypophosphataemic rickets in growing children; osteomalacia is the corresponding finding in adults. The radiological findings of rickets and osteomalacia include undermineralisation of the osteoid, which leads to a blurring of the microtrabecular architecture (fig. 2f, g). The consequences are bone pain and impaired mechanical properties of the affected bones leading to bowing, and stress fractures (looser zones, fig. 2a, b). Lack of chondrocyte apoptosis in the growing skeleton leads to an expansion of the epiphyses, giving rise to swollen wrists and rachitic rosary [55]. Serum biochemical findings suggesting rickets and osteomalacia include elevated bone specific alkaline phosphatase, osteocalcin, procollagen, pyridinoline cross-links and N- and C-telopeptides [56, 57]. When compared to the effects on the skeleton, the mechanism of muscle weakness caused by hypophosphataemia is less well understood and may be related to the role of phosphate in intracellular signal transduction and synthesis of ATP or creatine phosphate [56, 57]. While rickets or osteomalacia are observed to variable degrees with all hypophosphataemic disorders, subtle but important differences can guide the differential diagnostic and therapeutic decisions. Enthesopathies occur in patients with XLH, ADHR and ARHP. The term refers to painful or indolent mineral deposits near the insertion sites of tendons usually at the lower extremities (fig. 2d, e), which can be identified on radiographs [58]. Patients with XLH may also form dental cysts leading to tooth decay (fig. 3b), craniosynostosis, midfacial hypoplasia, and frontal bossing, which may be related to severity of hypophosphataemia, but often cannot be reversed or prevented by therapy with 1,25(OH)2D and phosphate supplements. Thus, local effects of FGF23 excess and activation of canonical FGF receptor (KL-independent) signalling may play a role [58, 59].
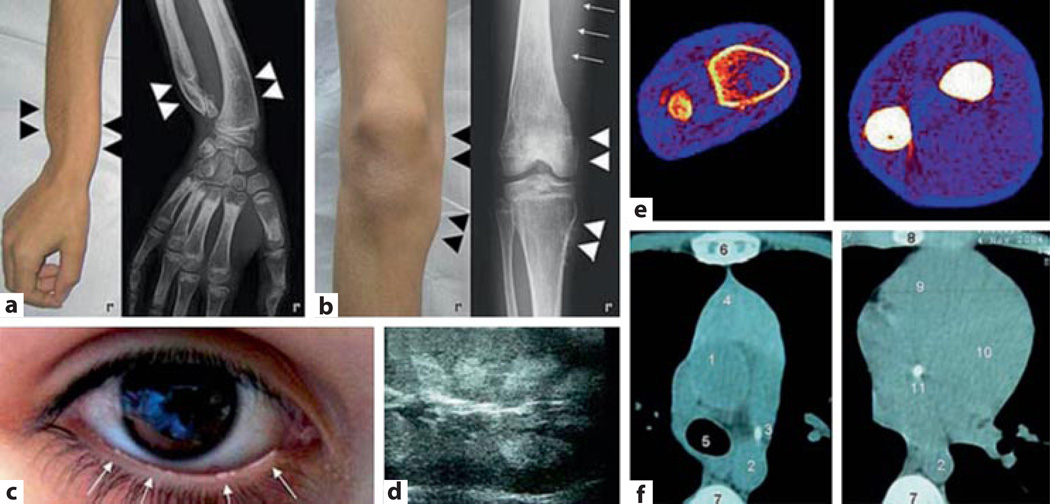
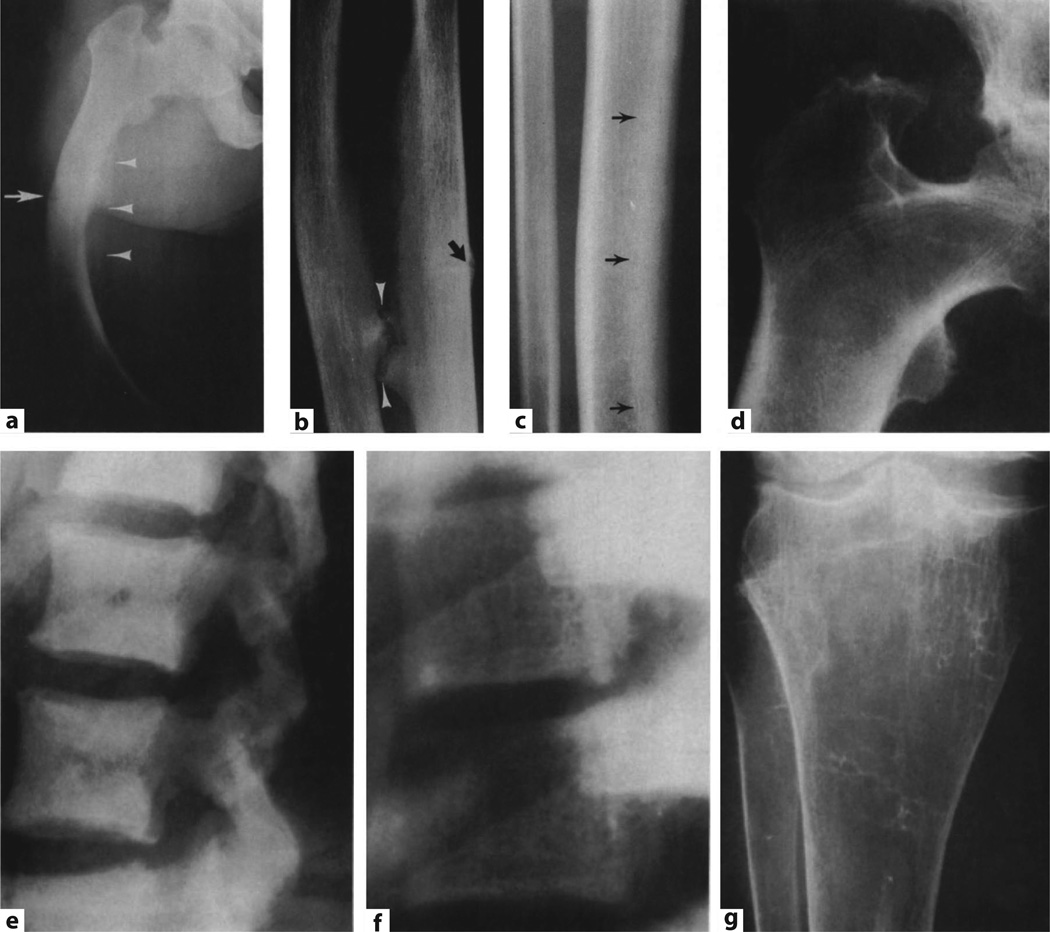
Fig. 2.
Skeletal findings in XLH. a Femoral bowing in XLH with looser zones (arrow) and proliferative changes along the linea aspera (arrowheads). b Periosteal bone proliferation in the forearm along the interosseous membrane with pseudarthrosis formation (arrowheads) and Looser zones (arrow). c Endosteal bone proliferation (arrows). d Enthesopathic changes at both greater and lesser femoral trochanters. e Diffusely increased lateral lumbar bone density. f coarsened vertebral trabecular pattern. g Loss of most metaphyseal trabeculae in the tibia (from [137], with permission).
Genetic Testing
Genetic testing for the inherited disorders discussed is generally done by direct sequence analysis of the affected genes. In the UK testing for genes causing hypophosphataemic rickets is undertaken by the Clinical Genetics Laboratory at the Royal Devon and Exeter Hospital (http://www.rdehospital.nhs.uk/prof/molecular_genetics/tests/clinical_genetics/rickets.html ). In the USA testing is offered by a number of commercial and non-commercial laboratories to which the website of GeneDx provides a helpful directory (http://www.genedx.com/).
Disorders of Renal Phosphate Excretion
Hyperphosphataemic Disorders
Hyperphosphataemic Familial Tumoral Calcinosis, (HFTC) (#211900)
Tumoral calcinosis is a clinically and genetically heterogeneous group of disorders first described by [61] and then by [62]. Tumoral calcinosis is characterised by calcium-phosphate deposits in tissues but, distinct from disorders of hetero-topic ossification, osteogenic cells and matrix formation are absent. For the purpose of this chapter, the hyperphosphataemic forms of familial tumoral calcinosis are classified as types 1–3 (HFTC1–3), all of which follow an autosomal-recessive mode of inheritance and all, furthermore, show inappropriately enhanced renal tubular absorption of phosphate leading to hyperphosphataemia as a common pathophysiological mechanism. The activity of renal 1α-hydroxylase is increased resulting in elevated serum 1,25(OH)2D levels and thus increased intestinal absorption of calcium (and phosphate), suppression of parathyroid hormone production and hypercalciuria. The increased serum calcium-phosphate product then leads to the characteristic tissue mineralisations observed in tumoral calcinosis (fig. 4, 5).
Familial tumoral calcinosis type 1 (HFTC1) is caused by homozygous loss-of-function mutations in the gene encoding GALNT3 [6] (*601756). GALNT3 is responsible for O-glycosylation and proper secretion of intact FGF23 [25]. Patients with HFTC1 characteristically have low or undetectable intact, but increased C-terminal FGF23 levels. HFTC1 is allelic with hyperostosis-hyperphosphataemia syndrome (HSS) (#610233) which, in addition to the serum-biochemical abnormalities, is characterised by recurrent, transient, painful swellings of the long bones associated with the radiographic findings of periosteal reaction and cortical hyperostosis (fig. 6) [63, 64]. HSS can be distinguished from HFTC1 by the presence of bone involvement and the absence of skin involvement.
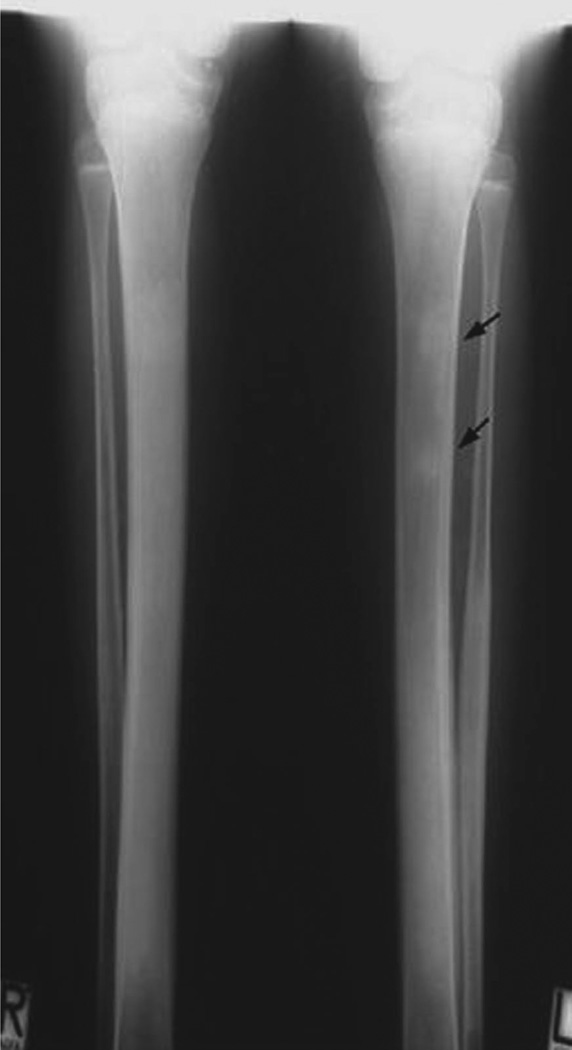
Fig. 6.
Hyperostosis hyperphosphataemia syndrome (HHS). Different from patients with HFTC1 tissue calcifications are missing in HHS. Shown here is the characteristic osteosclerosis and mild metaphyseal dilatation of tibia and fibula. Note the bone islands in the left tibia (arrows) (fig. 2 in [63], with permission).
HFTC2 is caused by homozygous loss-of-function mutations in FGF23 (*605380), which presumably interfere with normal O-glycosylation and secretion of bioactive intact FGF23. HFTC2 is characterised by low or undetectable circulating intact FGF23 levels [65, 66]. However, C-terminal FGF23 fragments may be secreted and thus also in patients with HFTC2 normal or high levels of FGF23 are often detected in the circulation [50].
Recently, a 13-year-old girl with a disorder resembling HFTC1 and HFTC2 was reported who had extremely high circulating levels of intact FGF23 [10]. Radiographs of the patient showed osteopaenia, patchy sclerosis in the hands, feet, long bones, and calvaria, intracranial calcifications, and calcifications of the dura and carotid arteries. Interestingly, and distinct from the first two forms of HFTC, she had elevated PTH levels due to four-gland parathyroid hyperplasia. Nucleotide sequence analysis of the genes encoding FGF23 and GALNT3 revealed no mutation. Very high circulating levels of intact FGF23 were also observed in mice, in which KL (+604824), the co-receptor for FGF23, had been ablated (see above). The authors therefore next decided to analyse the gene encoding KL, which led to the discovery of a homozygous missense mutation in the second putative beta-glycosidase domain. This mutation presumably inactivates KL, leading to end-organ resistance to FGF23 [10]. We have designated this HFTC3.
Heterozygous carriers of the genetic mutations that cause HFTC1–3 are asymptomatic and do not require specific treatment. Treatment of homozygous individuals currently relies on minimising the intestinal absorption of phosphate through appropriate binders such as aluminium hydroxide, or sevelamer [50], and on inhibiting renal phosphate reabsorption with acetazolamide [67]; in one study, treatment with PTH was attempted [68]. Calcilytic agents that reduce the activity of the calcium-sensing receptor and thus stimulate endogenous PTH secretion [69] or, once available for human use, recombinant FGF23 may become rational treatments of HFTC in the future.
Hypophosphataemic Disorders
Tumour-Induced Osteomalacia (TIO)
Tumour-induced osteomalacia (TIO), also referred to as oncogenic osteomalacia (OOM), is an acquired disorder of FGF23 excess [23], or possibly FGF7 excess [22], which are secreted by usually benign mixed connective tissue tumours. Other factors such as matrix extracellular protein (MEPE) [70] or secreted frizzled related protein 4 (sFRP4) [71] were also isolated from TIO tumours and may contribute to abnormal regulation of renal phosphate handling. Drezner [39] reviewed 120 cases of tumour-induced osteomalacia, and he identified four distinct morphologic patterns:
primitive-appearing, mixed connective tissue tumours,
osteoblastoma-like tumours,
nonossifying fibroma-like tumours, and
ossifying fibroma-like tumours.
Hypophosphataemia was also described in patients with widespread fibrous dysplasia of bone, neurofibromatosis and linear naevus sebaceous syndrome (see further below) and concurrent with breast carcinoma, prostate carcinoma, oat cell carcinoma, small cell carcinoma, multiple myeloma and chronic lymphocytic leukaemia.
Proof of a causal relationship has been that removal of the tumour resulted in appropriate biochemical and radiographic improvements. However, since most cases were reported before the discovery of FGF23, matrix extracellular phosphoglycoprotein (MEPE), or sFRP-4, the phosphaturic factor secreted by these previously reported tumours has not been determined. The tumours are commonly located in the facial skeleton or in the tendons of hands and feet, and may only be a few millimetres large and indolent. They commonly escape detection by physical examination and computed tomography scans, and may require more sensitive techniques for localisation including whole-body octreotide scans [72] or PET-CT scans using [18F]-FDG [73] or [68Ga]-DOTANOC [74] as tracers. Selective vein sampling [75] can permit localisation, particularly in individuals with markedly elevated circulating FGF23 levels [76–78]. Therapy consists of surgical tumour excision, if its location has been revealed, which usually results in normalisation of serum phosphate levels within 24 h.
In those patients, where localisation of the tumour is impossible or if resection of the tumour is incomplete, symptomatic therapy is used as will be described in more detail for the inherited forms of hypophosphataemia below.
Other Acquired Syndromes of Renal Phosphate Wasting
Another increasingly recognised acquired syndrome of renal phosphate wasting is post-renal transplant hypophosphataemia, which often cannot be attributed to tertiary hyperparathyroidism alone [79, 80]. Bhan et al. [33] and Pande et al. [34] recently showed that post-transplant hypophosphataemia correlated inversely with serum FGF23 levels and coined the term ‘tertiary hyperphosphatonism’ due to persistent production of FGF23, which is longer than would be expected from the half-life of the hormone [33, 81]. Hypophosphataemia in the setting of inappropriate renal phosphate excretion has also been recognised with severe burn injuries [82, 83], and after partial hepatectomy [36], although it may be independent of FGF23 in these cases [35].
X-Linked Dominant Hypophosphataemia (XLH) (#307800)
XLH, the most common form of hypophosphataemia, was first recognised by Albright et al. [84] in 1937. Lack of male-to-male transmission was observed by Winters et al. [85] in 1958 and suggested X-linked inheritance. Using a positional cloning approach, the genetic defect was ultimately identified in 1995 [2] and a large number of different loss-of-function mutations in PHEX, phosphate-regulating gene with homologies to endopeptidases on the X chromosome (*300550), have since been reported [86]. Details of these mutations are available on a dedicated database (http://www.phexdb.mcgill.ca/). It remains uncertain why males and females are equally affected. Deletion of the Phex gene in hyp mice results in increased FGF23 gene transcription in osteocytes resulting in increased circulating levels of FGF23 and thus renal phosphate wasting [87], which is similar to findings in human XLH patients [58, 59]. It was therefore concluded that PHEX may be involved in the feedback regulation of FGF23 secretion [14]. XLH can be severe, often leading to stunted growth despite treatment with phosphate and active vitamin D analogues, although some patients have normal growth [88]. Additional clinical features include craniosynostosis, frontal bossing and mid-facial hypoplasia as described above [58, 59] (see chapter 15, case 23).
Treatment consists of oral phosphate supplementation and active vitamin D analogues, which provides symptomatic relief and improves the bone abnormalities, but is usually unable to normalise serum phosphate levels. Treatment is furthermore complicated by the development of secondary hyperparathyroidism, hypercalciuria and nephrocalcinosis [58, 59]. Thus, treatment needs to be monitored carefully for these complications. We prefer potassium-containing over sodium-containing phosphate supplements since the former seem to induce less sodium-related phosphaturia, although formal studies to support this practice are still missing. Parathyroidectomy may be required to control tertiary hyperparathyroidism and is generally associated with a reduced phosphate requirement and improved control. It is conceivable that the development of this complication may be slowed in the future by the use of calcium-sensing receptor agonists such as cinacalcet, which has been successfully used to normalise parathyroid hormone secretion and to reduce the magnitude of phosphaturia in XLH patients [89, 90]. Thiazide diuretics may be helpful in slowing the progression of nephrocalcinosis [91]. Replacement of the membrane-anchored PHEX with a soluble form of PHEX did not prove effective to reverse hypophosphataemia in hyp mice, while treatment with anti-FGF23 antibodies has been successful in these animals and holds promise to become a therapeutic option for humans with XLH [92]. Growth hormone therapy has been reported to improve linear growth in some patients, although it remains unclear whether the observed improvements have been in part attributable to an increase in tubular reabsorption of phosphate during growth hormone treatment [93].
Autosomal-Dominant Hypophosphataemic Rickets (ADHR) (#193100)
ADHR follows an autosomal-dominant mode of inheritance and is caused by heterozygous ‘gain-of-function’ mutations in FGF23 (*605380) that prevent cleavage at the RXXR site [4]. The two amino acid residues of FGF23, R176 or R179 that are mutated in patients affected by ADHR constitute a site for cleavage by subtilisin/furin-like endopeptidases. Just like O-linked glycosylation of T178 in wild-type FGF23 [25], mutation of either residue protects FGF23 from proteolytic cleavage and degradation [94] resulting in persistent FGF23 activity, with an incompletely understood feedback mechanism that escapes normal regulation by phosphate and 1,25(OH)2D [45]. The clinical course of ADHR is comparable to mild forms of XLH. As a result, phosphate and 1,25(OH)2D supplementation are often only required during skeletal growth in childhood.
A sporadic case resembling autosomal-dominant hypophosphataemic rickets (%612089) was recently reported by Brownstein et al. [95], who described a 13-month-old girl, who initially presented with hyperparathyroidism. Her hypophosphataemia persisted after surgical cure of her hyperparathyroidism, and mutations in the known candidates including PHEX, FGF23, FGF-receptor 1 (FGFR1) and DMP1 were excluded. Cytogenetic analysis revealed a de novo chromosomal translocation with breakpoint adjacent to the gene encoding for alpha-KL. Indeed, her plasma alpha KL levels and beta-glucuronidase activity were increased and her FGF23 levels were elevated, which may explain increased proximal tubular action of FGF23 leading to renal phosphate wasting. This condition would appear to be the converse of HFTC3.
Autosomal-Recessive Hypophosphataemia (ARHP) (#241520)
ARHP is caused by homozygous, presumably loss-of-function mutations in DMP1 [5] (*600980). Intact DMP1 is cleaved into a 35- and a 57-kDa fragment, possibly by bone morphogenic protein 1 (BMP1) [96] which, in turn, is activated by a complex consisting of the endopeptidase SPC2 and the co-activator 7B2 [97]. Transgenic overexpression of 57 kDa DMP1 is both necessary and sufficient to rescue the bone phenotype (and probably the hypophosphataemia resulting from increased FGF23 secretion) of DMP1 null mice [98]. The 57-kDa C-terminal fragment of DMP1 appears to have nuclear effects which, when lost, leads to excess secretion of FGF23 resulting in renal phosphate wasting and hypophosphataemia [98]. Thus, the 57-kDa fragment may be required for suppression and/or feedback regulation of gene transcription and/or secretion of FGF23 [5].
Treatment of ARHP is symptomatic and relies, like XLH and ADHR, on oral phosphate supplementation and repletion of 1,25(OH)2D with either alfacalcidol or calcitriol to suppress development of hyperparathyroidism.
Hereditary Hypophosphataemic Rickets with Hypercalciuria (HHRH) (#241530)
HHRH is a rare disorder of autosomal-recessive inheritance that was first described in 1985 in a large consanguineous Bedouin kindred [43]. Unlike patients with XLH, individuals affected by HHRH do not develop dental abscesses or craniofacial abnormalities (i.e. frontal bossing, scaphocephaly, Chiari I malformation) [99, 100] and, different from patients affected by XLH, ADHR, and ARHR, FGF23 levels of HHRH patients appear to be suppressed [101], contributing to a compensatory increase in the plasma level of 1,25(OH)2D. This appropriate rise in the biologically active form of vitamin D results in absorptive hypercalciuria, the cardinal feature that distinguishes HHRH from most other Mendelian hypophosphataemic disorders. The measurement of 1,25(OH)2D and urinary calcium excretion is thus essential for establishing the diagnosis of HHRH, although both may be normal if vitamin D deficiency is present [44].
HHRH is caused by homozygous or compound heterozygous loss-of-function mutations in NaPi-IIc/SLC34A3 [101, 102] (*609826). Heterozygous mutations can lead to increased urinary calcium excretion, and occasionally some of the above biochemical features of HHRH, while bone changes are missing. Individuals with two mutated SLC34A3 alleles can initially present with renal stones alone even if clinical symptoms of rickets or osteomalacia are missing [44, 103].
In contrast to patients with XLH, ADHR or ARHP, who are usually treated with high doses of alfacalcidol or calcitriol and multiple daily doses of oral phosphate, the effective therapy of individuals affected by HHRH consists of oral phosphate supplementation alone. The prescription of biologically active vitamin D analogues is contraindicated and may lead to hypercalcaemia, hypercalciuria, nephrocalcinosis, and possibly renal insufficiency [44, 103].
Hypophosphataemia with Osteoporosis and Nephrolithiasis Type I (#612286) and Type II (#612287)
Prie et al. [104] investigated a heterogeneous group of patients with idiopathic hypercalciuria, osteoporosis and renal stones. Using a candidate gene approach they found 2/20 individuals heterozygous for non-synonymous SNPs in NaPi-IIa/SLC34A1 [104] (*182309) and 7/94 individuals heterozygous for non-synonymous SNPs in NHERF-1/SLC9A3R1 [105] (*604990). Although the authors present experimental in vitro evidence for dominant negative effects of the NaPi-IIa/SLC34A1 alterations on proximal renal tubular phosphate reabsorption, their findings have been challenged by others [106]. Likewise, some of the identified NHERF-1 alterations are listed in the NCBI dbSNP database as low frequency polymorphisms [107]. Further study is thus required to prove that mutations in NaPi-IIa or NHERF-1 are indeed responsible in the described clinical syndromes.
Other Inherited Forms of Renal Phosphate Wasting
Osteoglophonic dysplasia (OGD) (#166250) is an autosomal-dominant disorder caused by activating missense mutations in the gene encoding fibroblast growth factor receptor-1 (FGFR1) [108, 109] (*136350). Affected individuals have clinical features of the syndromes caused by all three fibroblast growth factor receptors, including craniosynostosis, midfacial hypoplasia, prognathism and rhizomelic chondrodysplasia. In addition, they have symmetrical radiolucent metaphyseal defects, which appear to produce FGF23. Consequently, most affected individuals develop hypophosphataemia due to renal phosphate wasting while their 1,25(OH)2D levels remain inappropriately normal [110, 111].
Opsismodysplasia (OSD) (%258480) [112] is an autosomal-recessive skeletal dysplasia that is characterised by a delay in epiphyseal ossification, platyspondyly and metaphyseal cupping, resulting in brachydactyly with short metacarpals and phalanges. The genetic defect is unknown. Like OGD, opsismodysplasia can go along with FGF23 excess leading to renal phosphate wasting [113].
The Schimmelpenning-Feuerstein-Mims syndrome (%163200) encompasses linear naevus sebaceous syndrome (LNSS), epidermal naevus syndrome (ENS) (see chapter 15, case 24) and phakomatosis pigmentokeratotica. It is characterised by sebaceous naevi, often in the face, abnormalities of the central nervous system, ocular anomalies, including coloboma, and skeletal defects [114–117]. Most patients with LNSS or ENS carry mosaic FGFR3 mutations [118]. LNSS/ENS may go along with hypophosphataemic rickets [119, 120], and some of the naevi were shown to secrete FGF23 thus providing an explanation for the underlying renal phosphate wasting [119–124]. However, it is unknown whether the FGFR3 mutations alone or additional unknown somatic mutations lead to renal phosphate wasting.
Fibrous dysplasia (FD)/McCune-Albright syndrome (MAS) (#174800) is caused by somatic activating missense mutations in the alpha subunit of the stimulatory G-protein (encoded by GNAS) [125, 126] (+139320). The classical triad of MAS includes polyostotic FD, café-au-lait spots, typically large and with a ragged ‘coast of Maine’ appearance, and non-central precocious puberty, particularly in girls. However, a number of other endocrine disorders such as thyrotoxicosis, pituitary gigantism, and Cushing syndrome are often present as well [125]. The non-mineralising bone lesions of FD/MAS may secrete FGF23, which can lead to hypophosphataemic rickets or osteomalacia [127–129]. Phosphate levels should always be checked and, if necessary, corrected before the bone lesions of MAS are treated with bisphosphonates.
FGF23-mediated hypophosphataemia can also be observed in Jansen’s metaphyseal chondrodysplasia (#156400), which is caused by heterozygous activating PTH/PTHrP receptor mutations (*168468) and may be, as in FD/MAS, a consequence of agonist-independent Gs-alpha activation [130].
Finally, hypophosphataemia leading to osteomalacia has been described in some individuals with neurofibromatosis 1 (+162200) and 2 (#101000) [131, 132], although the mechanism remains to be clarified.
Disorders of Tissue Mineralisation
Normophosphataemic Tumoral Calcinosis (NFTC) (#610455)
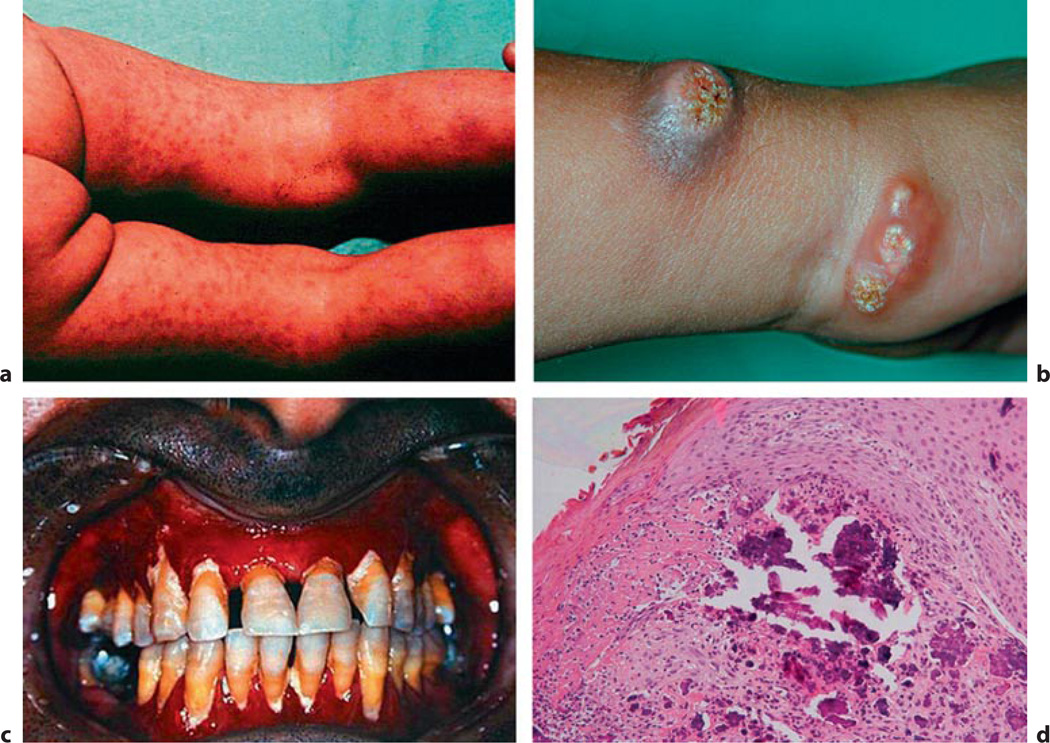
NFTC is an autosomal-recessive disorder caused by homozygous loss-of function mutations in sterile alpha motif domain-containing protein 9 (SAMD9) (*610456), a factor involved in the physiologic responses to tissue injury [133–135]. The genetic defect was identified by homozygosity mapping in five families of Jewish Yemenite origin. Distinct from HFTC, serum biochemical parameters including calcium, phosphate, vitamin D3 metabolites, and PTH levels are normal in this disorder, which resembles the dystrophic calcinosis seen at sites of (chronic) tissue injury. Affected individuals develop reddish-to-hyperpigmented skin lesions over the extremities during the first year of life, which later become calcified. In addition, severe conjunctivitis and gingivitis were observed in most of the affected individuals (fig. 8). Treatment consists of local supportive measures.
Fig. 8.
Normocalcaemic tumoral calcinosis. Clinical features of NFTC include erythematous papular eruption (a), these papules later become calcified ulcerating tumours (b), severe gingivitis (c), and calcified material in the upper dermis (d) (fig. 2 in [135], with permission).
Pulmonary Alveolar Microlithiasis (#265100)
Pulmonary alveolar microlithiasis is a rare autosomal-recessive disorder characterised by the deposition of calcium phosphate microliths throughout the lungs [53]. The diagnosis is often made incidentally since most patients are asymptomatic for several years or even for decades. A ‘sandstorm-appearing’ chest X-ray is a typical diagnostic finding. The onset of this potentially lethal disease varies from the neonatal period to old age, and the disease follows a long-term progressive course, resulting in a slow deterioration of lung function.
Genetic linkage studies led to the discovery of homozygous loss-of-function mutations in the sodium-phosphate co-transporter NaPi-IIb/SLC34A2 (*604217) [53, 136]. This co-transporter is closely related to NaPi-IIa and NaPi-IIc, but unlike these transporters, it is mainly expressed in the intestine, the lung and testicles. Therapy consists in supportive measures.
Conclusions
In the past few years there have been considerable advances in our understanding of the regulation of phosphate homeostasis, in which the newly discovered hormone, FGF23, plays a particularly important role. Although much remains to be learned about this novel hormonal system, our present understanding has already provided better tools for the diagnosis of a number of genetic disorders of phosphate metabolism, both those associated with hyper- and with hypophosphataemia, and provided a rationale for their treatment. In the future, it may also allow newer drugs to be developed that are more effective in treating these conditions.
Table 2.
Table of genetically determined hyperphosphataemic and hypophosphataemic disorders with their OMIM numbers, the genes involved together with their OMIM numbers and the chromosomal locations of the genes
| Disorder | Abbrevia- tion |
OMIM | Inherit- ance |
Gene | OMIM | Gene location | |
|---|---|---|---|---|---|---|---|
| Hyperphosphataemic disorders | |||||||
| Hyperphosphataemic familial tumoral calcinosis | HFTC | ||||||
| Hyperphosphataemic familial tumoral calcinosis type 1 | HFTC1 | #211900 | AR | UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyl-transferease 3 | GALNT3 | *601756 | 2q24-q31 |
| Hyperostosis-Hyperphosphataemia Syndrome | HSS | #610233 | AR | ||||
| Hyperphosphataemic familial tumoral calcinosis type 2 | HFTC2 | #211900 | AR | fibroblast growth factor 23 | FGF23 | *605380 | 12p13.3 |
| Hyperphosphataemic familial tumoral calcinosis type 3 | HFTC3 | #211900 | AR | Klotho | KL | +604824 | 13q12 |
| Hypophosphataemic disorders | |||||||
| X-linked dominant hypophosphataemic Rickets | XLHR | #307800 | XLD | phosphate-regulating gene with homologies to endopeptidases on the X chromosome | PHEX | *300550 | Xp22.2-p22.1 |
| Autosomal-dominant Hypophosphataemic rickets | ADHR1 | #193100 | AD | fibroblast growth factor 23 | FGF23 | *605380 | 12p13.3 |
| ADHR2 | %612089 | AD | KL | KL | +604824 | 13q12 | |
| Autosomal-recessive hypophosphataemia | ARHP | #241520 | AR | dentin matrix acidic phosphoprotein 1 | DMP1 | *600980 | 4q21 |
| Hereditary hypophosphataemic rickets with hypercalciuria | HHRH | #241530 | AR | solute carrier family 34 (sodium/phosphate cotransporter), member 3 | SLC34A3 | *609826 | 9q34 |
| Hypophosphataemia with osteoporosis and nephrolithiasis type I | NPHLOP1 | #612286 | AD | solute carrier family 34 (sodium/phosphate cotransporter), member 1 (see text p. 148) | SLC34A1? | *182309 | 5q35 |
| Hypophosphataemia with osteoporosis and nephrolithiasis type II | NPHLOP2 | #612287 | AD | solute carrier family 9, isoform A3, regulatory factor 1 (see text p. 148) | SLC9A3R1? | *604990 | 17q25.1 |
| Osteoglophonic dysplasia | OGD | #166250 | AD | fibroblast growth factor receptor 1 | FGFR1 | *136350 | 8p11.2-p11.1 |
| Opsismodysplasia | OSD | %258480 | AR | not known | |||
| Schimmelpenning-Feuerstein-Mims syndrome | %163200 | sporadic somatic mutation | fibroblast growth factor receptor 33 | FGFR3 | 4p16.3 | ||
| McCune-Albright fibrous dysplasia | MAS/FD | #174800 | sporadic somatic mutation | GNAS complex locus | GNAS | +139320 | 20q13.2 |
| Jansen′s metaphyseal chondrodysplasia | #156400 | AR | PTH/PTHrP receptor 1 | PTHR1 | *168468 | 3p22-p21.1 | |
| Neurofibromatosis type I | NF1 | +162200 | AD | neurofibronim | NF1 | 17q11.2 | |
| Neurofibromatosis type II | NF2 | #101000 | AD | neurofibronim 2 (merlin) | NF2 | *607379 | 22q12.2 |
| Tissue mineralisation disorders | |||||||
| Normophosphataemic tumoral calcinosis | NFTC | #610455 | AR | sterile alpha motif domain-containing protein 9 | SAMD9 | *610456 | 7q21 |
| Pulmonary alveolar microlithiasis | #265100 | AR | solute carrier family 34 (sodium/phosphate cotransporter), member 2 | SLC34A2 | *604217 | 4p15.31- p15.2 | |
References
- 1.Potts JT., Jr Hyperparathyroidism and other hyper-calcemic disorders. Adv Intern Med. 1996;41:165–212. [PubMed] [Google Scholar]
- 2.The HYP Consortium: A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd SE, Pearce SH, Fisher SE, et al. A common molecular basis for three inherited kidney stone diseases. Nature. 1996;379:445–449. doi: 10.1038/379445a0. [DOI] [PubMed] [Google Scholar]
- 4.White KE, Evans WE, O’Riordan JLH, et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topaz O, Shurman DL, Bergman R, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 7.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 8.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 10.Ichikawa S, Imel EA, Kreiter ML, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilz DR, Gray RW, Dominguez JH, Lemann J., Jr Plasma 1,25-(OH)2-vitamin D concentrations and net intestinal calcium, phosphate, and magnesium absorption in humans. Am J Clin Nutr. 1979;32:2052–2060. doi: 10.1093/ajcn/32.10.2052. [DOI] [PubMed] [Google Scholar]
- 12.Forster IC, Hernando N, Biber J, Murer H. Proximal tubular handling of phosphate: A molecular perspective. Kidney Int. 2006;70:1548–1559. doi: 10.1038/sj.ki.5001813. [DOI] [PubMed] [Google Scholar]
- 13.Kuro-o M. Endocrine FGFs and Klothos emerging concepts. Trends Endocrinol Metab. 2008;19:239–245. doi: 10.1016/j.tem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto K, Ito M, Tatsumi S, Kuwahata M, Segawa H. New aspect of renal phosphate reabsorption the type IIc sodium-dependent phosphate transporter. Am J Nephrol. 2007;27:503–515. doi: 10.1159/000107069. [DOI] [PubMed] [Google Scholar]
- 16.Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflügers Arch. 2004;447:763–767. doi: 10.1007/s00424-003-1072-5. [DOI] [PubMed] [Google Scholar]
- 17.Prie D, Beck L, Silve C, Friedlander G. Hypophosphatemia and calcium nephrolithiasis. Nephron Exp Nephrol. 2004;98:e50–e54. doi: 10.1159/000080256. [DOI] [PubMed] [Google Scholar]
- 18.Shaikh A, Berndt T, Kumar R. Regulation of phosphate homeostasis by the phosphatonins and other novel mediators. Pediatr Nephrol. 2008;23:1203–1210. doi: 10.1007/s00467-008-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strom TM, Juppner H. PHEX, FGF23, DMP1 and beyond. Curr Opin Nephrol Hypertens. 2008;17:357–362. doi: 10.1097/MNH.0b013e3282fd6e5b. [DOI] [PubMed] [Google Scholar]
- 20.White KE, Larsson TE, Econs MJ. The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis frizzled related protein-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23. Endocr Rev. 2006;27:221–241. doi: 10.1210/er.2005-0019. [DOI] [PubMed] [Google Scholar]
- 21.Bringhurst FR, Leder BZ. Regulation of calcium and phosphate homeostasis. In: De Groot LJ, Jameson JL, editors. Endocrinology. Philadelphia: Saunders; 2006. pp. 805–843. [Google Scholar]
- 22.Carpenter TO, Ellis BK, Insogna KL, Philbrick WM, Sterpka J, Shimkets R. Fibroblast growth factor 7:an inhibitor of phosphate transport derived from oncogenic osteomalacia-causing tumors. J Clin Endocrinol Metab. 2005;90:1012–1020. doi: 10.1210/jc.2004-0357. [DOI] [PubMed] [Google Scholar]
- 23.Shimada T, Mizutani S, Muto T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 25.Kato K, Jeanneau C, Tarp MA, et al. Polypeptide GalNAc-transferase T3 familial tumoral calcinosis secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281:18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 26.Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15:437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- 27.Yan X, Yokote H, Jing X, et al. Fibroblast growth factor 23 reduces expression of type IIa Na+/Pi co-transporter by signaling through a receptor functionally distinct from the known FGFRs in opossum kidney cells. Genes Cells. 2005;10:489–502. doi: 10.1111/j.1365-2443.2005.00853.x. [DOI] [PubMed] [Google Scholar]
- 28.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 29.Razzaque MS: FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player? Am J Physiol Renal Physiol. 2008 doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krajisnik T, Bjorklund P, Marsell R, et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 32.Burnett SA, Dent CE, Harper C, Warland BJ. Vitamin D-resistant rickets analysis of twenty-four pedigrees with hereditary and sporadic cases. Am J Med. 1964;36:222–232. doi: 10.1016/0002-9343(64)90085-3. [DOI] [PubMed] [Google Scholar]
- 33.Bhan I, Shah A, Holmes J, et al. Post-transplant hypophosphatemia tertiary ‘hyper-phosphatoninism’? Kidney Int. 2006;70:1486–1494. doi: 10.1038/sj.ki.5001788. [DOI] [PubMed] [Google Scholar]
- 34.Pande S, Ritter CS, Rothstein M, et al. FGF-23 and sFRP-4 in chronic kidney disease and post-renal transplantation. Nephron Physiol. 2006;104:23–32. doi: 10.1159/000093277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salem RR, Tray K. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2005;241:343–348. doi: 10.1097/01.sla.0000152093.43468.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nafidi O, Lepage R, Lapointe RW, D’Amour P. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2007;245:1000–1002. doi: 10.1097/SLA.0b013e31805d0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 38.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 39.Drezner MK. Tumor-induced osteomalacia. Rev Endocr Metab Disord. 2001;2:175–186. doi: 10.1023/a:1010006811394. [DOI] [PubMed] [Google Scholar]
- 40.Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 41.Walton RJ, Bijvoet OL. Nomogram for derivation of renal threshold phosphate concentration. Lancet. 1975;ii:309–310. doi: 10.1016/s0140-6736(75)92736-1. [DOI] [PubMed] [Google Scholar]
- 42.Alon U, Hellerstein S. Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr Nephrol. 1994;8:250–251. doi: 10.1007/BF00865491. [DOI] [PubMed] [Google Scholar]
- 43.Tieder M, Modai D, Samuel R, et al. Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med. 1985;312:611–617. doi: 10.1056/NEJM198503073121003. [DOI] [PubMed] [Google Scholar]
- 44.Kremke B, Bergwitz C, Ahrens W, et al. Hypophosphatemic rickets with hypercalciuria due to mutation in SLC34A3/NaPi-IIc can be masked by vitamin D deficiency and can be associated with renal calcifications. Exp Clin Endocrinol Diabetes. s2008 doi: 10.1055/s-2008-1076716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imel EA, Hui SL, Econs MJ. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res. 2007;22:520–526. doi: 10.1359/jbmr.070107. [DOI] [PubMed] [Google Scholar]
- 46.Yamazaki Y, Okazaki R, Shibata M, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 47.Endo I, Fukumoto S, Ozono K, et al. Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients proposal of diagnostic criteria using FGF23 measurement. Bone. 2008;42:1235–1239. doi: 10.1016/j.bone.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 48.Imel EA, Peacock M, Pitukcheewanont P, et al. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2006;91:2055–2061. doi: 10.1210/jc.2005-2105. [DOI] [PubMed] [Google Scholar]
- 49.Ichikawa S, Guigonis V, Imel EA, et al. Novel GALNT3 mutations causing hyperostosis-hyperphosphatemia syndrome result in low intact fibro-blast growth factor 23 concentrations. J Clin Endocrinol Metab. 2007;92:1943–1947. doi: 10.1210/jc.2006-1825. [DOI] [PubMed] [Google Scholar]
- 50.Larsson T, Yu X, Davis SI, et al. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab. 2005;90:2424–2427. doi: 10.1210/jc.2004-2238. [DOI] [PubMed] [Google Scholar]
- 51.Martin A, David V, Laurence JS, et al. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins) ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology. 2008;149:1757–1772. doi: 10.1210/en.2007-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sitara D, Kim S, Razzaque MS, et al. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 2008;4:e1000154. doi: 10.1371/journal.pgen.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corut A, Senyigit A, Ugur SA, et al. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular micro-lithiasis. Am J Hum Genet. 2006;79:650–656. doi: 10.1086/508263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castellana G, Gentile M, Castellana R, Fiorente P, Lamorgese V. Pulmonary alveolar microlithiasis clinical features, evolution of the phenotype, and review of the literature. Am J Med Genet. 2002;111:220–224. doi: 10.1002/ajmg.10530. [DOI] [PubMed] [Google Scholar]
- 55.Donohue MM, Demay MB. Rickets in VDR null mice is secondary to decreased apoptosis of hypertrophic chondrocytes. Endocrinology. 2002;143:3691–3694. doi: 10.1210/en.2002-220454. [DOI] [PubMed] [Google Scholar]
- 56.Francis RM, Selby PL. Osteomalacia. Baillières Clin Endocrinol Metab. 1997;11:145–163. doi: 10.1016/s0950-351x(97)80569-1. [DOI] [PubMed] [Google Scholar]
- 57.Narchi H, El Jamil M, Kulaylat N. Symptomatic rickets in adolescence. Arch Dis Child. 2001;84:501–503. doi: 10.1136/adc.84.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Econs MJ, Samsa GP, Monger M, Drezner MK, Feussner JR. X-Linked hypophosphatemic rickets a disease often unknown to affected patients. Bone Miner. 1994;24:17–24. doi: 10.1016/s0169-6009(08)80127-4. [DOI] [PubMed] [Google Scholar]
- 59.DiMeglio LA, Econs MJ. Hypophosphatemic rickets. Rev Endocr Metab Disord. 2001;2:165–173. doi: 10.1023/a:1010054727323. [DOI] [PubMed] [Google Scholar]
- 60.McKusick V. Online Mendelian Inheritance in Man, OMIM. McKusick-Nathans Institute of Genetic Medicine, Baltimore, Johns Hopkins University and Bethesda, National Center for Biotechnology Information, National Library of Medicine. http://www.ncbi.nlm.nih.gov/omim/
- 61.Giard JM. Sur la calcification hibernale. CR Séances Soc Biol Soc. 1898:1013–1015. [Google Scholar]
- 62.Duret MH. Tumeurs multiples et singulaires des bourses. Bull Mem Soc Ant Paris. 1899;74:725–731. [Google Scholar]
- 63.Frishberg Y, Ito N, Rinat C, et al. Hyperostosis-hyperphosphatemia syndrome a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res. 2007;22:235–242. doi: 10.1359/jbmr.061105. [DOI] [PubMed] [Google Scholar]
- 64.Frishberg Y, Topaz O, Bergman R, et al. Identification of a recurrent mutation in GALNT3 demonstrates that hyperostosis-hyperphosphatemia syndrome and familial tumoral calcinosis are allelic disorders. J Mol Med. 2005;83:33–38. doi: 10.1007/s00109-004-0610-8. [DOI] [PubMed] [Google Scholar]
- 65.Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 66.Ichikawa S, Lyles KW, Econs MJ. A novel GALNT3 mutation in a pseudoautosomal dominant form of tumoral calcinosisevidence that the disorder is autosomal recessive. J Clin Endocrinol Metab. 2005;90:2420–2423. doi: 10.1210/jc.2004-2302. [DOI] [PubMed] [Google Scholar]
- 67.Yamaguchi T, Sugimoto T, Imai Y, Fukase M, Fujita T, Chihara K. Successful treatment of hyperphosphatemic tumoral calcinosis with long-term acetazolamide. Bone. 1995;16:247S–250S. doi: 10.1016/8756-3282(95)00019-a. [DOI] [PubMed] [Google Scholar]
- 68.Lufkin EG, Wilson DM, Smith LH, et al. Phosphorus excretion in tumoral calcinosis response to parathyroid hormone and acetazolamide. J Clin Endocrinol Metab. 1980;50:648–653. doi: 10.1210/jcem-50-4-648. [DOI] [PubMed] [Google Scholar]
- 69.Gowen M, Stroup GB, Dodds RA, et al. Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J Clin Invest. 2000;105:1595–1604. doi: 10.1172/JCI9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rowe PS, de Zoysa PA, Dong R, et al. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000;67:54–68. doi: 10.1006/geno.2000.6235. [DOI] [PubMed] [Google Scholar]
- 71.Berndt T, Craig TA, Bowe AE, et al. Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J Clin Invest. 2003;112:785–794. doi: 10.1172/JCI18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nguyen BD, Wang EA. Indium-111 pentetreotide scintigraphy of mesenchymal tumor with oncogenic osteomalacia. Clin Nucl Med. 1999;24:130–131. doi: 10.1097/00003072-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 73.Dupond JL, Magy N, Mahammedi M, et al. Oncogenic osteomalacia: the role of the phosphatonins diagnostic usefulness of the Fibroblast Growth Factor 23 measurement in one patient. Rev Med Interne. 2005;26:238–241. doi: 10.1016/j.revmed.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Hesse E, Moessinger E, Rosenthal H, et al. Oncogenic osteomalacia exact tumor localization by co-registration of positron emission and computed tomography. J Bone Miner Res. 2007;22:158–162. doi: 10.1359/jbmr.060909. [DOI] [PubMed] [Google Scholar]
- 75.Takeuchi Y, Suzuki H, Ogura S, et al. Venous sampling for fibroblast growth factor-23 confirms pre-operative diagnosis of tumor-induced osteomalacia. J Clin Endocrinol Metab. 2004;89:3979–3982. doi: 10.1210/jc.2004-0406. [DOI] [PubMed] [Google Scholar]
- 76.Nasu T, Kurisu S, Matsuno S, et al. Tumor-induced hypophosphatemic osteomalacia diagnosed by the combinatory procedures of magnetic resonance imaging and venous sampling for FGF23. Intern Med. 2008;47:957–961. doi: 10.2169/internalmedicine.47.0745. [DOI] [PubMed] [Google Scholar]
- 77.van Boekel G, Ruinemans-Koerts J, Joosten F, Dijkhuizen P, van Sorge A, de Boer H. Tumor producing fibroblast growth factor 23 localized by two-staged venous sampling. Eur J Endocrinol. 2008;158:431–437. doi: 10.1530/EJE-07-0779. [DOI] [PubMed] [Google Scholar]
- 78.Westerberg PA, Olauson H, Toss G, et al. Preoperative tumor localization by means of venous sampling for fibroblast growth factor-23 in a patient with tumor-induced osteomalacia. Endocr Pract. 2008;14:362–367. doi: 10.4158/EP.14.3.362. [DOI] [PubMed] [Google Scholar]
- 79.Levi M. Post-transplant hypophosphatemia. Kidney Int. 2001;59:2377–2387. doi: 10.1046/j.1523-1755.2001.00755.x. [DOI] [PubMed] [Google Scholar]
- 80.Parfitt AM, Kleerekoper M, Cruz C. Reduced phosphate reabsorption unrelated to parathyroid hormone after renal transplantation implications for the pathogenesis of hyperparathyroidism in chronic renal failure. Miner Electrolyte Metab. 1986;12:356–362. [PubMed] [Google Scholar]
- 81.Khosravi A, Cutler CM, Kelly MH, et al. Determination of the elimination half-life of fibro-blast growth factor-23. J Clin Endocrinol Metab. 2007;92:2374–2377. doi: 10.1210/jc.2006-2865. [DOI] [PubMed] [Google Scholar]
- 82.Dickerson RN, Gervasio JM, Sherman JJ, Kudsk KA, Hickerson WL, Brown RO. A comparison of renal phosphorus regulation in thermally injured and multiple trauma patients receiving specialized nutrition support. J Parenter Enteral Nutr. 2001;25:152–159. doi: 10.1177/0148607101025003152. [DOI] [PubMed] [Google Scholar]
- 83.Nordstrom H, Lennquist S, Lindell B, Sjoberg HE. Hypophosphataemia in severe burns. Acta Chir Scand. 1977;143:395–399. [PubMed] [Google Scholar]
- 84.Albright F, Butler AM, Bloomberg E. Rickets resistant to vitamin D therapy. Am J Dis Child. 1937;54:529–547. [Google Scholar]
- 85.Winters RW, Graham JB, Williams TF, McFalls VW, Burnett CH. A genetic study of familial hypophosphatemia and vitamin D resistant rickets with a review of the literature. 1958. Medicine (Baltimore) 1991;70:215–217. [PubMed] [Google Scholar]
- 86.Sabbagh Y, Jones AO, Tenenhouse HS. PHEXdb, a locus-specific database for mutations causing X-linked hypophosphatemia. Hum Mutat. 2000;16:1–6. doi: 10.1002/1098-1004(200007)16:1<1::AID-HUMU1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 87.Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 88.Makras P, Hamdy NA, Kant SG, Papapoulos SE. Normal growth and muscle dysfunction in X-linked hypophosphatemic rickets associated with a novel mutation in the PHEX gene. J Clin Endocrinol Metab. 2008;93:1386–1389. doi: 10.1210/jc.2007-1296. [DOI] [PubMed] [Google Scholar]
- 89.Alon US, Levy-Olomucki R, Moore WV, Stubbs J, Liu S, Quarles LD. Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin J Am Soc Nephrol. 2008;3:658–664. doi: 10.2215/CJN.04981107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Geller JL, Khosravi A, Kelly MH, Riminucci M, Adams JS, Collins MT. Cinacalcet in the management of tumor-induced osteomalacia. J Bone Miner Res. 2007;22:931–937. doi: 10.1359/jbmr.070304. [DOI] [PubMed] [Google Scholar]
- 91.Seikaly MG, Baum M. Thiazide diuretics arrest the progression of nephrocalcinosis in children with X-linked hypophosphatemia. Pediatrics. 2001;108:E6. doi: 10.1542/peds.108.1.e6. [DOI] [PubMed] [Google Scholar]
- 92.Yamazaki Y, Tamada T, Kasai N, et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res. 2008;23:1509–1518. doi: 10.1359/jbmr.080417. [DOI] [PubMed] [Google Scholar]
- 93.Seikaly MG, Brown R, Baum M. The effect of recombinant human growth hormone in children with X-linked hypophosphatemia. Pediatrics. 1997;100:879–884. doi: 10.1542/peds.100.5.879. [DOI] [PubMed] [Google Scholar]
- 94.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 95.Brownstein CA, Adler F, Nelson-Williams C, et al. A translocation causing increased alpha-Klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.von Marschall Z, Fisher LW. Dentin matrix pro-tein-1 isoforms promote differential cell attachment and migration. J Biol Chem. 2008;283:32730–32740. doi: 10.1074/jbc.M804283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuan B, Meudt J. 7B2 protein mediated inhibition of DMP1 cleavage in osteoblasts enhances FGF-23 production in hyp-mice. JBMR. 2008;23:s16. (abstract 1053). [Google Scholar]
- 98.Lu Y, Qin C, Xie Y, Bonewald LF, Feng JQ. Studies of the DMP1 57-kDa functional domain both in vivo and in vitro. Cells Tissues Organs. 2008;189:175–185. doi: 10.1159/000151727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones A, Tzenova J, Frappier D, et al. Hereditary hypophosphatemic rickets with hypercalciuria is not caused by mutations in the Na/Pi cotransporter NPT2 gene. J Am Soc Nephrol. 2001;12:507–514. doi: 10.1681/ASN.V123507. [DOI] [PubMed] [Google Scholar]
- 100.Tenenhouse HS, Econs MJ. Mendelian hypophosphatemias. In: Scriver CR, Beaud AL, Valleet D, et al., editors. The Metabolic Basis of Inherited Diseases. New York: McGraw-Hill; 2001. pp. 5039–5067. [Google Scholar]
- 101.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, et al. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet. 2006;78:193–201. doi: 10.1086/499410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bergwitz C, Roslin NM, Tieder M, et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006;78:179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jaureguiberry G, Carpenter TO, Forman S, Juppner H, Bergwitz C. A novel missense mutation in SLC34A3 that causes hereditary hypophosphatemic rickets with hypercalciuria in humans identifies threonine 137 as an important determinant of sodium-phosphate cotransport in NaPi-IIc. Am J Physiol Renal Physiol. 2008;295:F371–F379. doi: 10.1152/ajprenal.00090.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Prie D, Huart V, Bakouh N, et al. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med. 2002;347:983–991. doi: 10.1056/NEJMoa020028. [DOI] [PubMed] [Google Scholar]
- 105.Karim Z, Gerard B, Bakouh N, et al. NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med. 2008;359:1128–1135. doi: 10.1056/NEJMoa0802836. [DOI] [PubMed] [Google Scholar]
- 106.Virkki LV, Forster IC, Hernando N, Biber J, Murer H. Functional characterization of two naturally occurring mutations in the human sodium-phosphate cotransporter type IIa. J Bone Miner Res. 2003;18:2135–2141. doi: 10.1359/jbmr.2003.18.12.2135. [DOI] [PubMed] [Google Scholar]
- 107.Bergwitz C, Bastepe M. NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med. 2008;359:2615–2616. doi: 10.1056/NEJMc086284. [DOI] [PubMed] [Google Scholar]
- 108.Beighton P, Cremin BJ, Kozlowski K. Osteoglophonic dwarfism. Pediatr Radiol. 1980;10:46–50. doi: 10.1007/BF01644343. [DOI] [PubMed] [Google Scholar]
- 109.Sklower BS, Kassner G, Qazi Q, Keogh MJ, Gorlin RJ. Osteoglophonic dysplasia review and further delineation of the syndrome. Am J Med Genet. 1996;66:154–162. doi: 10.1002/(SICI)1096-8628(19961211)66:2<154::AID-AJMG6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 110.Farrow EG, Davis SI, Mooney SD, et al. Extended mutational analyses of FGFR1 in osteoglophonic dysplasia. Am J Med Genet [A] 2006;140:537–539. doi: 10.1002/ajmg.a.31106. [DOI] [PubMed] [Google Scholar]
- 111.White KE, Cabral JM, Davis SI, et al. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet. 2005;76:361–367. doi: 10.1086/427956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maroteaux P, Stanescu V, Stanescu R, Le Marec B, Moraine C, Lejarraga H. Opsismodysplasia a new type of chondrodysplasia with predominant involvement of the bones of the hand and the vertebrae. Am J Med Genet. 1984;19:171–182. doi: 10.1002/ajmg.1320190117. [DOI] [PubMed] [Google Scholar]
- 113.Zeger MD, Adkins D, Fordham LA, et al. Hypophosphatemic rickets in opsismodysplasia. J Pediatr Endocrinol Metab. 2007;20:79–86. doi: 10.1515/jpem.2007.20.1.79. [DOI] [PubMed] [Google Scholar]
- 114.Gellis SS, Feingold M. Linear nevus sebaceous syndrome. Am J Dis Child. 1970;120:139–140. [PubMed] [Google Scholar]
- 115.Menascu S, Donner EJ. Linear nevus sebaceous syndromecase reports and review of the literature. Pediatr Neurol. 2008;38:207–210. doi: 10.1016/j.pediatrneurol.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 116.Rogers M. Epidermal nevi the epidermal nevus syndromes a review of 233 cases. Pediatr Dermatol. 1992;9:342–344. doi: 10.1111/j.1525-1470.1992.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 117.Solomon LM, Fretzin DF, Dewald RL. The epidermal nevus syndrome. Arch Dermatol. 1968;97:273–285. [PubMed] [Google Scholar]
- 118.Hafner C, van Oers JM, Vogt T, et al. Mosaicism of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116:2201–2207. doi: 10.1172/JCI28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Skovby F, Svejgaard E, Moller J. Hypophosphatemic rickets in linear sebaceous nevus sequence. J Pediatr. 1987;111:855–857. doi: 10.1016/s0022-3476(87)80204-4. [DOI] [PubMed] [Google Scholar]
- 120.Zutt M, Strutz F, Happle R, et al. Schimmelpenning-Feuerstein-Mims syndrome with hypophosphatemic rickets. Dermatology. 2003;207:72–76. doi: 10.1159/000070948. [DOI] [PubMed] [Google Scholar]
- 121.Chou YY, Chao SC, Shiue CN, Tsai WH, Lin SJ. Hypophosphatemic rickets associated with epidermal nevus syndrome and giant hairy nevus. J Pediatr Endocrinol Metab. 2005;18:93–95. doi: 10.1515/jpem.2005.18.1.93. [DOI] [PubMed] [Google Scholar]
- 122.Hoffman WH, Jain A, Chen H, Fedarko NS. Matrix extracellular phosphoglycoprotein (MEPE) correlates with serum phosphorus prior to and during octreotide treatment and following excisional surgery in hypophosphatemic linear sebaceous nevus syndrome. Am J Med Genet [A] 2008;146A:2164–2168. doi: 10.1002/ajmg.a.32395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hoffman WH, Jueppner HW, Deyoung BR, O’dorisio MS, Given KS. Elevated fibroblast growth factor-23 in hypophosphatemic linear nevus sebaceous syndrome. Am J Med Genet A. 2005;134:233–236. doi: 10.1002/ajmg.a.30599. [DOI] [PubMed] [Google Scholar]
- 124.John M, Shah NS. Hypophosphatemic rickets with epidermal nevus syndrome. Indian Pediatr. 2005;42:611–612. [PubMed] [Google Scholar]
- 125.Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein alpha-sub-unit mutations and the role of genomic imprinting. Endocr Rev. 2001;22:675–705. doi: 10.1210/edrv.22.5.0439. [DOI] [PubMed] [Google Scholar]
- 126.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 127.Collins MT, Chebli C, Jones J, et al. Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J Bone Miner Res. 2001;16:806–813. doi: 10.1359/jbmr.2001.16.5.806. [DOI] [PubMed] [Google Scholar]
- 128.Riminucci M, Collins MT, Fedarko NS, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamamoto T, Miyamoto KI, Ozono K, et al. Hypophosphatemic rickets accompanying McCune-Albright syndrome: evidence that a humoral factor causes hypophosphatemia. J Bone Miner Metab. doi: 10.1007/s007740170012. [DOI] [PubMed] [Google Scholar]
- 130.Brown WW, Juppner H, Langman CB, et al. Hypophosphatemia with elevations in serum FGF23 in a child with Jansen’s metaphyseal chondrodysplasia (FGF23 in Jansen’s syndrome) J Clin Endocrinol Metab. 2009;94:17–20. doi: 10.1210/jc.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Haviv YS, Silver J. Late onset oncogenic osteomalacia-associated with neurofibromatosis type II. Clin Nephrol. 2000;54:429–430. [PubMed] [Google Scholar]
- 132.Konishi K, Nakamura M, Yamakawa H, et al. Hypophosphatemic osteomalacia in von Reck-linghausen neurofibromatosis. Am J Med Sci. 1991;301:322–328. doi: 10.1097/00000441-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 133.Chefetz I, Ben Amitai D, Browning S, et al. Normophosphatemic familial tumoral calcinosis is caused by deleterious mutations in SAMD9, encoding a TNF-alpha responsive protein. J Invest Dermatol. 2008;128:1423–1429. doi: 10.1038/sj.jid.5701203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li CF, MacDonald JR, Wei RY, et al. Human sterile alpha motif domain 9, a novel gene identified as down-regulated in aggressive fibromatosis, is absent in the mouse. BMC Genomics. 2007;8:92. doi: 10.1186/1471-2164-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Topaz O, Indelman M, Chefetz I, et al. A deleterious mutation in SAMD9 causes normophosphatemic familial tumoral calcinosis. Am J Hum Genet. 2006;79:759–764. doi: 10.1086/508069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huqun Izumi S, Miyazawa H, et al. Mutations in the SLC34A2 gene are associated with pulmonary alveolar microlithiasis. Am J Respir Crit Care Med. 2007;175:263–268. doi: 10.1164/rccm.200609-1274OC. [DOI] [PubMed] [Google Scholar]
- 137.Hardy DC, Murphy WA, Siegel BA, Reid IR, Whyte MP. X-linked hypophosphatemia in adults prevalence of skeletal radiographic and scintigraphic features. Radiology. 1989;171:403–414. doi: 10.1148/radiology.171.2.2539609. [DOI] [PubMed] [Google Scholar]
- 138.Specktor P, Cooper JG, Indelman M, Sprecher E. Hyperphosphatemic familial tumoral calcinosis caused by a mutation in GALNT3 in a European kindred. J Hum Genet. 2006;51:487–490. doi: 10.1007/s10038-006-0377-6. [DOI] [PubMed] [Google Scholar]
- 139.Batra P, Tejani Z, Mars M. X-linked hypophosphatemia dental and histologic findings. J Can Dent Assoc. 2006;72:69–72. [PubMed] [Google Scholar]
- 140.Chefetz I, Heller R, Galli-Tsinopoulou A, et al. A novel homozygous missense mutation in FGF23 causes familial tumoral calcinosis associated with disseminated visceral calcification. Hum Genet. 2005;118:261–266. doi: 10.1007/s00439-005-0026-8. [DOI] [PubMed] [Google Scholar]