Abstract
Background
While nephron-sparing surgery has been advocated for patients with bilateral renal masses, the long-term functional and oncological outcomes are lacking.
Objective
To determine the outcomes of patients with bilateral renal masses (BRM) and a minimum of 10 years of follow-up.
Design, Setting, and Participants
Patients with BRM evaluated at the National Cancer Institute who underwent their initial surgical intervention at least 10 years ago and had interventions on both renal units were included in our analysis. The data collected included demographics, hereditary diagnosis, number of renal interventions, renal function, and mortality status.
Intervention(s)
Bilateral renal surgery.
Outcome Measurements and Statistical Analysis
Overal and RCC specific survival was assessed. Comparisons of renal function and overall survival between groups containing both renal units and solitary kidneys were performed using the student T-test and Kaplan-Meier analysis.
Results and Limitations
128 patients met our inclusion criteria. The median follow-up of our cohort was 16 years (10-49), mean 17 years. The median number of surgical interventions was 3 (2-10). Eighty-seven patients (68%) required repeat interventions on their ipsilateral renal unit at last follow-up, with a median time between interventions of 6.2 years (0.7-21). Overall and RCC-specific survival of the cohort was 88% and 97%, respectively. Six patients (4.7%) ultimately underwent bilateral nephrectomies.
Although renal function was better preserved in patients with both kidneys (70 vs. 53 mL/min/1.73m2, P=0.0002) there was no difference in overall survival between those with bilateral or solitary kidneys (mean 21.5 vs. 20.8 years, respectively). Limitations of the study are in its retrospective design and inclusion of closely surveilled patients.
Conclusions
At a minimum of 10 years follow-up after initial surgery, nephron-sparing surgery allows for excellent oncologic and functional outcomes. Despite the need for repeat surgical interventions, employing NSS allows for avoidance of dialysis in over 95% of patients.
Keywords: Familial renal cancer (FRC), bilateral renal masses (BRM), nephron-sparing surgery (NSS), partial nephrectomy, outcomes
Introduction
The management of patients with bilateral renal masses (BRM) presents unique challenges. The goal of any surgical renal intervention is two-fold: to perform oncologically sound operations while simultaneously preserving maximal renal function to avoid the need for renal replacement therapy (RRT) and its associated morbidity and mortality. Surgical treatment options upon presentation with BRM include bilateral radical nephrectomy with subsequent RRT as a bridge to renal transplantation, unilateral radical nephrectomy with contralateral partial nephrectomy, or bilateral nephron-sparing surgery (NSS). Pre-operative treatment considerations include tumor characteristics such as size, number, location, and patient factors such as medical comorbidities and baseline renal function.[1]
Recognizing that decreased post-operative renal function is associated with an increased incidence of cardiovascular disease, hospitalization, and overall mortality, NSS has become the treatment of choice for tumors less than 7cm when technically feasible.[2-7] However, evidence demonstrating the long-term renal functional and oncologic outcomes of using NSS for the management of bilateral renal masses is limited.[8-10]
While sporadic BRM are rare and occur in approximately 3-5% of patients with renal cell carcinoma (RCC), the growing incidence of RCC will likely result in a greater number of patients being diagnosed BRM, creating more challenging management questions.[11-13] The National Cancer Institute (NCI) experience in managing these complex patients emerged from the ongoing evaluation and treatment of individuals with bilateral mutifocal renal masses and those with familial renal cancer (FRC) syndromes such as von Hippel-Lindau (VHL), hereditary papillary renal carcinoma (HPRC), hereditary leiomyomatosis and renal cell carcinoma (HLRCC), and Birt-Hogg-Dubé (BHD).[14] Patients with FRC tend to present at a younger age than the sporadic kidney cancer population and have a greater incidence of bilateral multifocal tumors. Despite these differences, however, FRC have similar pathologic features to their sporadic counterparts and make an informative BRM model.[15]
Having a unique opportunity to evaluate the long-term oncologic and functional outcomes of patients with BRM, we describe the NCI experience managing FRC patients who required bilateral renal surgeries and had a minimum of 10 years of post-operative follow-up.
Material (Patients) and Methods
All patients were evaluated on a NCI institutional review board-approved research protocol. A prospectively maintained urologic oncology database was queried to identify all patients who underwent renal tumor surgery prior to December 2000 to allow for at least 10 years of follow up (n = 407). This cohort was then limited to those patients requiring bilateral renal surgery for either synchronous or metachronous BRM (n = 132). Ninety-seven percent of patients had complete records available at the NCI (n = 128) and constitute the final cohort used for analyses in this study. Patient demographics, FRC diagnosis, type and number of renal interventions, most recent renal function, presence of metastatic disease, and mortality status were recorded.
Oncologic outcomes were evaluated by overall and renal cell carcinoma-specific survival. Overall survival (OS) was determined by chart review with death from any cause verified using the Social Security Death Index. RCC-specific survival (RCCSS) was attributed to patients with documented evidence of RCC progression as the cause of death. Survival data were obtained on the entire cohort (n=128) and no patient was lost to follow-up. Recurrence-free survival was not assessed because of multifocality of renal masses and inability to differentiate local tumor recurrence from de novo tumor formation in patients with FRC.
Estimated GFR (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) formula using the most recent serum creatinine value available.[16] Comparisons of renal function and overall survival between groups containing both renal units and solitary kidneys were performed using the student T-test and Kaplan-Meier analysis. P value <0.05 was considered significant.
Results
The demographics of the study cohort are summarized in Table 1. Men constituted 57% (n=73) of the sample size. The mean age at initial surgery was 38 years (range 17 to 64) and at last follow-up was 55 years (range 34 to 79). The most common FRC diagnosis seen in our cohort was VHL (n=89, 70%). The median follow-up of our cohort was 16 years (range 10 to 49), mean 17 years.
Table 1.
Patient Characteristics
| Patient number | 128 | |
| Total renal surgeries of the cohort | 437 | |
| Male (%) | 73 | (57) |
| Mean age at first surgery, years (range) | 38 | (17-64) |
| Mean age at last follow-up, years (range) | 55 | (34-79) |
| Median number of total renal surgical interventions per patient (range) |
3 | (2-10) |
| Median number of left renal surgeries | 1 | (1-5) |
| Median number of right renal surgeries | 2 | (1-6) |
| Median time between ipsilateral surgical interventions, years (range) |
6.2 | (0.7-21) |
| Number of patients with solitary kidney at most recent follow-up (%) |
58 | (45.3) |
| Number of patients with bilateral kidneys at most recent follow-up (%) |
64 | (50.0) |
| Number of patients who were anephric at most recent follow-up (%) |
6 | (4.7) |
| Median follow-up of patients with a solitary kidney, years (range) |
17 | (10-48) |
| Median follow-up of patients with bilateral kidneys remaining, years (range) |
14 | (10-37) |
| Median follow-up of anephric patients, years (range) |
17 | (16-20) |
| Median total follow-up, years (range) | 16 | (10-49) |
| Familial cancer diagnosis, number (%)* | ||
| VHL | 89 | (70) |
| BHD | 7 | (5) |
| HPRC - Type 1 | 6 | (5) |
| FRC - Clear | 4 | (3) |
| FRC - Other | 3 | (2) |
| FRO | 2 | (2) |
| RCC - Sporadic | 1 | (1) |
| SDHC | 1 | (1) |
| TS | 1 | (1) |
| BMF with Neg. or Unknown Fam Hx | 14 | (11) |
Abbreviations: VHL - Von Hippel-Lindau, BHD - Birt-Hogg-Dubé, HPRC - Hereditary Papillary Renal Carcinoma, FRC – Familial Renal Cancer, FRO - Familial Renal Oncocytoma, RCC - Renal cell carcinoma, SDHC - Succinate Dehydrogenase C, TS - Tuberous Sclerosis, BMF - Bilateral Multifocal Renal Cancer
Familial cancer diagnosis percentages sum to greater than 100% because of rounding.
The median number of surgical interventions per patient was 3 (range 2 to 10). Eighty-seven patients (68%) required repeat interventions on the same renal unit, with a median time between interventions of 6.2 years (range 0.7 to 21).
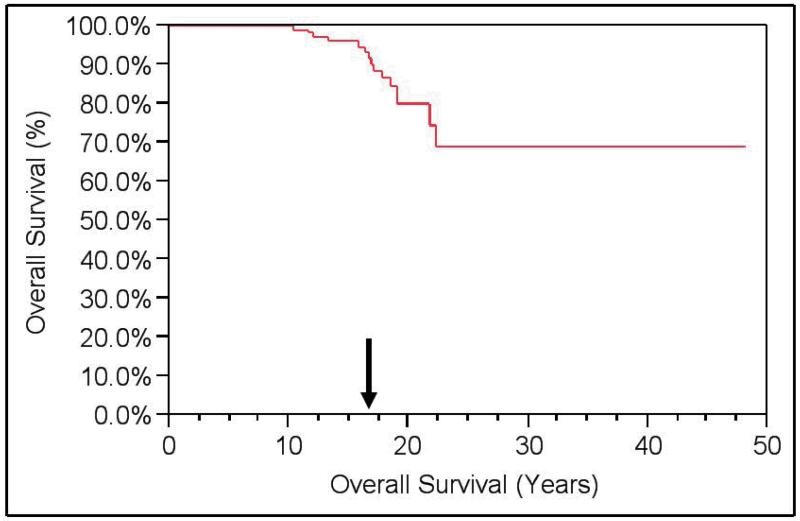
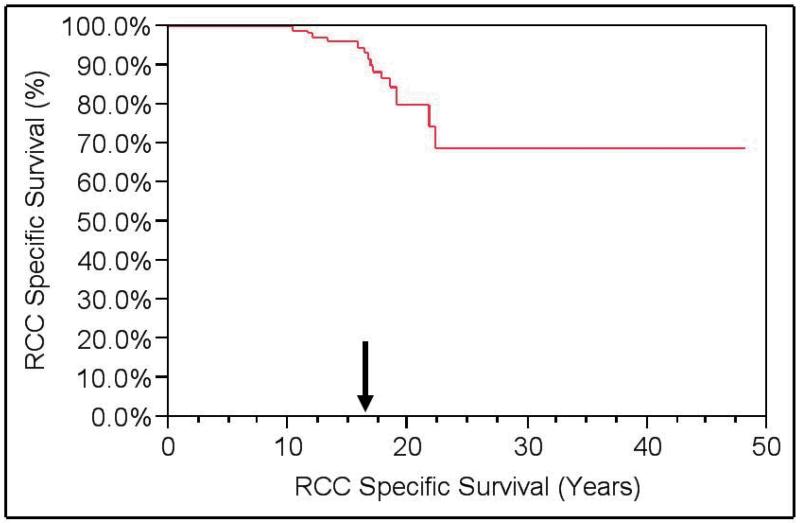
Among the 15 deaths in the cohort, 4 were due to RCC. A total of 11 patients were alive with metastatic disease. OS of the cohort was 88%, but RCCSS survival was 97%. (Figures 1 and 2) Metastasis-free survival was 88%.
Figure 1. Kaplan-Meier Overall Survival (OS).
Overall Survival = 88% at a median follow-up of 16 years.
Figure 2. Kaplan-Meier RCC-Specific Survival (RCCSS).
Renal cell carcinoma-specific survival (RCCSS) = 97% at a median of 16 years of follow-up.
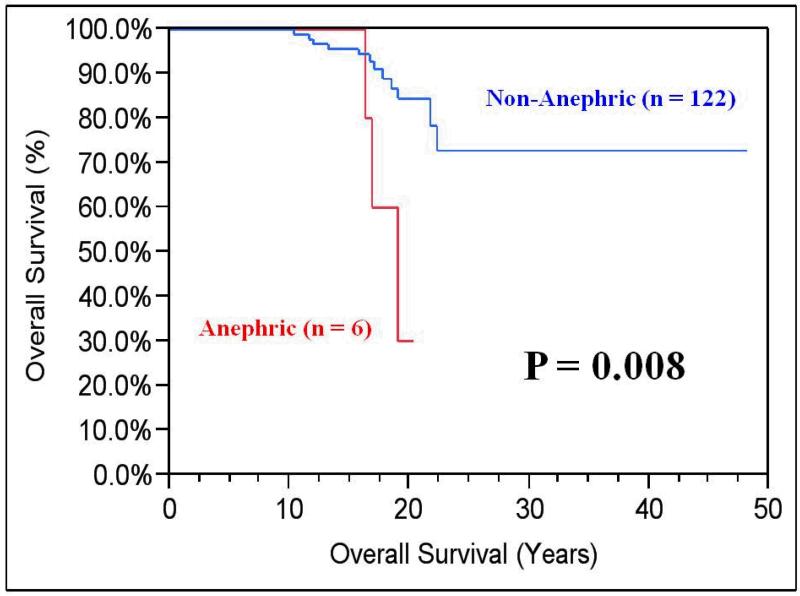
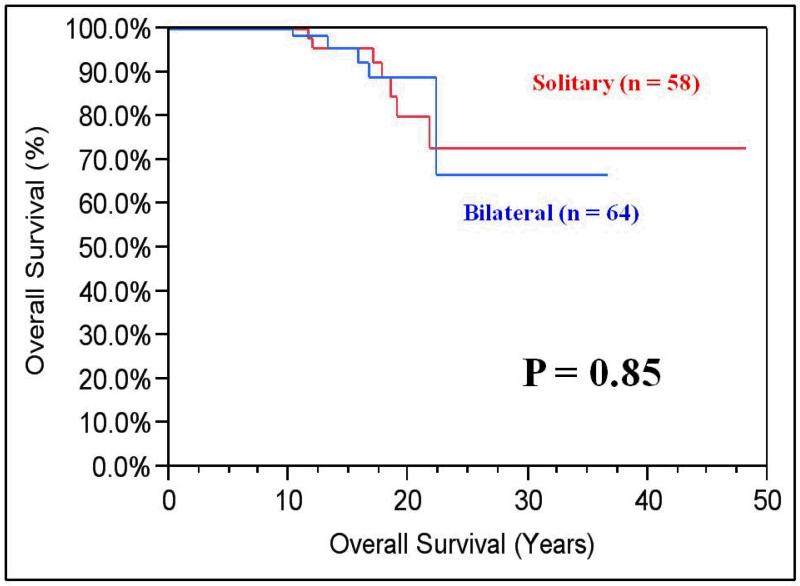
The most recent calculated median eGFR was 57 mL/min/1.73m2 (range 8 to 196). Six patients (4.7%) ultimately underwent bilateral nephrectomies and were noted to have significantly shorter OS compared to patients with one or both renal units (median 19 years vs. median not reached; P=0.008). (Figure 3) The median follow-up for patients with bilateral and solitary renal units was 14 years (range 10-37) vs. 17 years (range 10-48), respectively. Although there was no difference in OS between those with bilateral or solitary kidneys (21.5 vs. 20.8 years, respectively; P=0.85), eGFR was better preserved in patients with both kidneys (70 vs. 53 mL/min/1.73m2, respectively; P=0.0002). (Figure 4).
Figure 3. Kaplan-Maier Overall Survival (OS) of Anephric vs. Non-Anephric Patients.
OS for patients who ultimately became anephric was significantly shorter (median 19 years vs. median not reached; P=0.008) when compared to those who had one or both renal units.
Figure 4. Kaplan-Meier OS Comparing Solitary to Bilateral Renal Units.
There was no difference in overall survival between those with bilateral or solitary kidneys (21.5 vs. 20.8 years, respectively; P=0.85). However, estimated glomerular filtration rate (eGFR) was better preserved in patients with both kidneys (70 vs. 53 mL/min/1.73m2, respectively; P=0.0002).
Discussion
As the number of patients with the diagnosis of RCC increases, the number of patients presenting with BRM will likely increase as well. The old dogma of multifocality as an indication for radical nephrectomy is no longer true with several studies demonstrating oncologic efficacy and acceptable functional outcomes at early or intermediate follow up.[10, 17-20] The present study was undertaken to evaluate long-term outcomes with the follow up of at least 10 years in a cohort with a severe phenotype of BRM requiring bilateral renal surgeries.
Our study highlights several significant findings that may aid in the management of patients with BRM. Despite requiring bilateral renal interventions in all cases the RCCSS was 97% at a median follow-up of 16 years (mean 17 years). Such high RCCSS was observed despite the recurrent nature of the RCC in these patients (with two thirds of the cohort requiring repeat renal surgeries). RCCSS in excess of 90% is consistent with earlier studies from our institution evaluating outcomes of either primary renal surgery, repeat renal surgery, or even salvage procedures (three or more times on the same kidney) after early and intermediate follow up.[18-21]
The present study also provides encouraging long-term renal functional results. The median eGFR of the entire cohort was 57 mL/min/1.73m2. This is approximately the same eGFR seen 1-5 years status post renal transplant.[22] However, unlike transplant patients who have a 30% risk of dying or losing their graft within the 5 years and nearly a 60% risk at 10 years, more than 95% of our patients remained free from renal replacement therapy (RRT) after a median follow-up of 16 years.[23] Even after a median of 3 surgical interventions, we observed the eGFR of 57 mL/min/1.73m2 in our cohort.
Our data suggest that the use of serial NSS to treat BRM provides excellent renal functional outcomes. When the OS of the current study cohort (88% at a median of 16 years) is compared to that of a cohort of VHL patients who were rendered anephric for localized RCC and underwent RRT followed by renal transplantation (65% at 5 years post-transplant), it becomes clear that serial NSS provides a substantial survival benefit.[24] Only six patients (4.7%) from the present study ultimately underwent bilateral nephrectomies and required RRT.
It is important to note that the use of re-operative NSS is challenging and is associated with higher rates of perioperative morbidity when compared to case series of surgically naïve patients.[25] Complications such as increased intra-operative blood loss, longer operative times, urine leak, prolonged hospitalization, loss of the renal unit, rhabdomyolysis, and post-operative death have been encountered.[18-19, 26] Despite these risks, however, the OS of the cohort approaches 90% and is far beyond that which RRT can offer.[24]
The 10 year OS of 88% seen in this cohort exceeds that of 71% from a cohort of 220 patients treated with NSS for synchronous BRM at the Cleveland Clinic.[8] This could be partially attributable to the younger age of our cohort. Most importantly, however, is the fact the 10 year RCCSS specific survival was nearly identical between the two studies, measuring 97% and 96%. Although there was no difference in OS between those with bilateral or solitary kidneys in this study (21.5 vs. 20.8 years, respectively; P=0.85), eGFR rate was better preserved in patients with both kidneys (70 vs. 53 mL/min/1.73m2, respectively; P=0.0002) in the present study. This degree of eGFR preservation is similar to that seen in the patients who had undergone bilateral NSS at the Cleveland Clinic (59 mL/min/1.73m2).[8]
The etiology for the lack of a difference in OS between those patients with bilateral (n=64) and solitary (n=58) renal units is unclear. It may be partially explained by the small number of deaths in this cohort, the good preservation of renal function seen in both groups, and the younger age of our cohort. Additionally, it is possible that while there was a significant difference in eGFR between patients with bilateral kidneys or solitary kidneys, the observed difference in eGFR of less than 20 mL/min/1.73m2 may not be enough to effect the survival of these two groups. Finally, the statistical power to detect a difference in OS may be insufficient in subgroups of this size.
When comparing the findings of this study to the largest single-institution series on sporadic BRM (n=310), the 10 year OS of this cohort is better than that seen in either the synchronous or metachronous Mayo Clinic subgroups (48% vs. 51%, respectively).[9] The 97% 10-year RCCSS from the current study also exceeded both Mayo temporal subgroups (71% vs. 69%, respectively).
It is likely that a high RCCSS observed in the present study is explained by the fact that the majority of patients in our cohort were managed with active surveillance until the largest tumor reached 3 cm.[21, 27-28] Nevertheless, the patients in this study had their original surgery before the year 2000, and the first publication of the “3cm rule” was in 1999, suggesting that some patients were not treated for small tumors exclusively. Notably, the “3cm rule” was utilized long before the data was initially published in 1999. Unfortunately, due to the large number of resected tumors and many historic records of our cohort, we were unable to determine the number and size of the tumors treated at the time of prior (older) surgeries. We, therefore, are unable to reliably comment on the size of the tumors resected in this cohort. Considering the data from our recent publications describing outcomes on similar patient cohorts we can point out that some surgeries were likely performed for as many as several dozen tumors.[10, 29]
We recognize that the limitations of this study are its retrospective nature, the inclusion of many patients who were closely surveilled, and that it comes from a single institution with a special interest in the management of BRM. However, the strengths of this project are its large sample size, the completeness of the medical records (97% of patients treated for BRM were eligible for inclusion), recent follow-up data (median time from last visit was 6 months), and, to our knowledge, the longest follow-up of patients with BRM managed with surgical intervention (median of 16 years). This study provides a compelling argument for the use of NSS to manage patients with BRM.
Conclusions
At a minimum of 10 years after initial surgery, NSS allows for excellent oncologic and functional outcomes. RCCSS of 97% and RRT-free survival of more than 95% further support the role of aggressive surgical intervention in the management of patients presenting with BRM.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Nguyen CT, Campbell SC, Novick AC. Choice of operation for clinically localized renal tumor. Urol Clin North Am. 2008;35(4):645–55. vii. doi: 10.1016/j.ucl.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182(4):1271–9. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 3.McKiernan J, Simmons R, Katz J, Russo P. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002;59(6):816–20. doi: 10.1016/s0090-4295(02)01501-7. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179(2):468–71. doi: 10.1016/j.juro.2007.09.077. discussion 472-3. [DOI] [PubMed] [Google Scholar]
- 5.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7(9):735–40. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 7.Ljungberg B, Hanbury DC, Kuczyk MA, Merseburger AS, Mulders PF, Patard JJ, et al. Renal cell carcinoma guideline. Eur Urol. 2007;51(6):1502–10. doi: 10.1016/j.eururo.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Simmons MN, Brandina R, Hernandez AV, Gill IS. Surgical management of bilateral synchronous kidney tumors: functional and oncological outcomes. J Urol. 2010;184(3):865–72. doi: 10.1016/j.juro.2010.05.042. quiz 1235. [DOI] [PubMed] [Google Scholar]
- 9.Boorjian SA, Crispen PL, Lohse CM, Leibovich BC, Blute ML. The impact of temporal presentation on clinical and pathological outcomes for patients with sporadic bilateral renal masses. Eur Urol. 2008;54(4):855–63. doi: 10.1016/j.eururo.2008.04.079. [DOI] [PubMed] [Google Scholar]
- 10.Gupta GN, Peterson J, Thakore KN, Pinto PA, Linehan WM, Bratslavsky G. Oncological outcomes of partial nephrectomy for multifocal renal cell carcinoma greater than 4 cm. J Urol. 2010;184(1):59–63. doi: 10.1016/j.juro.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall FF, Stewart AK, Menck HR. The National Cancer Data Base: report on kidney cancers. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1997;80(11):2167–74. [PubMed] [Google Scholar]
- 12.Grimaldi G, Reuter V, Russo P. Bilateral non-familial renal cell carcinoma. Ann Surg Oncol. 1998;5(6):548–52. doi: 10.1007/BF02303649. [DOI] [PubMed] [Google Scholar]
- 13.Klatte T, Wunderlich H, Patard JJ, Kleid MD, Lam JS, Junker K, et al. Clinicopathological features and prognosis of synchronous bilateral renal cell carcinoma: an international multicentre experience. BJU Int. 2007;100(1):21–5. doi: 10.1111/j.1464-410X.2007.06877.x. [DOI] [PubMed] [Google Scholar]
- 14.Singer EA, Bratslavsky G, Middelton L, Srinivasan R, Linehan WM. Impact of genetics on the diagnosis and treatment of renal cancer. Curr Urol Rep. 2011;12(1):47–55. doi: 10.1007/s11934-010-0156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosner I, Bratslavsky G, Pinto PA, Linehan WM. The clinical implications of the genetics of renal cell carcinoma. Urol Oncol. 2009;27(2):131–6. doi: 10.1016/j.urolonc.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, Knight EL, Hogan ML, Singh AK. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol. 2003;14(10):2573–80. doi: 10.1097/01.asn.0000088721.98173.4b. [DOI] [PubMed] [Google Scholar]
- 17.Liu NW, Khurana K, Sudarshan S, Pinto PA, Linehan WM, Bratslavsky G. Repeat partial nephrectomy on the solitary kidney: surgical, functional and oncological outcomes. J Urol. 2010;183(5):1719–24. doi: 10.1016/j.juro.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson A, Sudarshan S, Liu J, Linehan WM, Pinto PA, Bratslavsky G. Feasibility and outcomes of repeat partial nephrectomy. J Urol. 2008;180(1):89–93. doi: 10.1016/j.juro.2008.03.030. discussion 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bratslavsky G, Liu JJ, Johnson AD, Sudarshan S, Choyke PL, Linehan WM, et al. Salvage partial nephrectomy for hereditary renal cancer: feasibility and outcomes. J Urol. 2008;179(1):67–70. doi: 10.1016/j.juro.2007.08.150. [DOI] [PubMed] [Google Scholar]
- 20.Herring JC, Enquist EG, Chernoff A, Linehan WM, Choyke PL, Walther MM. Parenchymal sparing surgery in patients with hereditary renal cell carcinoma: 10-year experience. J Urol. 2001;165(3):777–81. [PubMed] [Google Scholar]
- 21.Bratslavsky G, Linehan WM. Long-term management of bilateral, multifocal, recurrent renal carcinoma. Nat Rev Urol. 2010;7(5):267–75. doi: 10.1038/nrurol.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.USRDS 2010 USRDS Annual Report. 2010 [cited 2012 February 3]; Volume 2:[Chapter 7 - Transplantation]. Available from: http://www.usrds.org/2010/pdf/v2_07.pdf.
- 23.USRDS 2011 USRDS Annual Report. 2011 [cited 2012 February 3]; Volume 2:[Chapter 7 - Transplantation]. Available from: http://www.usrds.org/2011/pdf/v2_ch07_11.pdf.
- 24.Goldfarb DA, Neumann HP, Penn I, Novick AC. Results of renal transplantation in patients with renal cell carcinoma and von Hippel-Lindau disease. Transplantation. 1997;64(12):1726–9. doi: 10.1097/00007890-199712270-00017. [DOI] [PubMed] [Google Scholar]
- 25.Singer EA, Bratslavsky G. Management of locally recurrent kidney cancer. Curr Urol Rep. 2010;11(1):15–21. doi: 10.1007/s11934-009-0085-9. [DOI] [PubMed] [Google Scholar]
- 26.Kowalczyk KJ, Hooper HB, Linehan WM, Pinto PA, Wood BJ, Bratslavsky G. Partial nephrectomy after previous radio frequency ablation: the National Cancer Institute experience. J Urol. 2009;182(5):2158–63. doi: 10.1016/j.juro.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walther MM, Choyke PL, Glenn G, Lyne JC, Rayford W, Venzon D, et al. Renal cancer in families with hereditary renal cancer: prospective analysis of a tumor size threshold for renal parenchymal sparing surgery. J Urol. 1999;161(5):1475–9. doi: 10.1016/s0022-5347(05)68930-6. [DOI] [PubMed] [Google Scholar]
- 28.Duffey BG, Choyke PL, Glenn G, Grubb RL, Venzon D, Linehan WM, et al. The relationship between renal tumor size and metastases in patients with von Hippel-Lindau disease. J Urol. 2004;172(1):63–5. doi: 10.1097/01.ju.0000132127.79974.3f. [DOI] [PubMed] [Google Scholar]
- 29.Fadahunsi AT, Sanford T, Linehan WM, Pinto PA, Bratslavsky G. Feasibility and outcomes of partial nephrectomy for resection of at least 20 tumors in a single renal unit. J Urol. 2011;185(1):49–53. doi: 10.1016/j.juro.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]