Abstract
BACKGROUND
In January 2008, the Centers for Disease Control and Prevention began a nationwide investigation of severe adverse reactions that were first detected in a single hemodialysis facility. Preliminary findings suggested that heparin was a possible cause of the reactions.
METHODS
Information on clinical manifestations and on exposure was collected for patients who had signs and symptoms that were consistent with an allergic-type reaction after November 1, 2007. Twenty-one dialysis facilities that reported reactions and 23 facilities that reported no reactions were included in a case–control study to identify facility-level risk factors. Unopened heparin vials from facilities that reported reactions were tested for contaminants.
RESULTS
A total of 152 adverse reactions associated with heparin were identified in 113 patients from 13 states from November 19, 2007, through January 31, 2008. The use of heparin manufactured by Baxter Healthcare was the factor most strongly associated with reactions (present in 100.0% of case facilities vs. 4.3% of control facilities, P<0.001). Vials of heparin manufactured by Baxter from facilities that reported reactions contained a contaminant identified as oversulfated chondroitin sulfate (OSCS). Adverse reactions to the OSCS-contaminated heparin were often characterized by hypotension, nausea, and shortness of breath occurring within 30 minutes after administration. Of 130 reactions for which information on the heparin lot was available, 128 (98.5%) occurred in a facility that had OSCS-contaminated heparin on the premises. Of 54 reactions for which the lot number of administered heparin was known, 52 (96.3%) occurred after the administration of OSCS-contaminated heparin.
CONCLUSIONS
Heparin contaminated with OSCS was epidemiologically linked to adverse reactions in this nationwide outbreak. The reported clinical features of many of the cases further support the conclusion that contamination of heparin with OSCS was the cause of the outbreak.
Unfractionated heparin is an anti-coagulant medication that is used to prevent or treat thromboembolic disorders. Heparin is also commonly used to prevent clotting of extracorporeal blood during hemodialysis and cardiac surgery, as well as to maintain the patency of intravenous devices. Chemically, heparin is a heterogeneous mixture of sulfated polysaccharides; its main anticoagulant activity is mediated through activation of antithrombin. Commercially available heparin is derived from animal tissues; only porcine-derived heparin is approved for the U.S. market. Although heparin-induced thrombocytopenia is a well-described immune-mediated phenomenon among patients receiving heparin, immediate hypersensitivity reactions (e.g., exanthemas, bronchospasm, angioedema, and anaphylaxis) that are directly attributable to heparin have rarely been reported.1,2
On January 7, 2008, the Missouri Department of Health and Senior Services notified the Centers for Disease Control and Prevention (CDC) about a cluster of allergic-type reactions among patients undergoing hemodialysis at a pediatric hospital.3 Symptoms occurred within minutes after the initiation of a dialysis session, and manifestations included facial edema, tachycardia, hypotension, urticaria, and nausea. The CDC’s national case-finding effort identified, in multiple states, additional clusters of similar reactions among patients undergoing hemodialysis, and subsequently among patients undergoing photopheresis or treatment for cardiac conditions. A common feature that preceded many of the reactions was the receipt of heparin produced by Baxter Healthcare. On January 17, 2008, nine lots of vials of heparin manufactured by Baxter were voluntarily recalled.4 A more extensive recall of heparin products manufactured by Baxter occurred on February 28, 2008.5
In March 2008, the Food and Drug Administration (FDA) announced that a “heparin-like” compound had been identified as a contaminant in the active pharmaceutical ingredient used in heparin manufactured by Baxter. This contaminant was identified as oversulfated chondroitin sulfate (OSCS),6 and the ability of OSCS in the active pharmaceutical ingredient to activate the contact and complement systems was shown.7 In this report, we describe the CDC epidemiologic investigation that was undertaken to establish the cause of the allergic-type reactions among patients undergoing dialysis, provide a clinical description of the reactions that occurred after the administration of heparin, and report on laboratory tests for the presence of OSCS in finished-product heparin vials that were related to these reactions.
METHODS
EPIDEMIOLOGIC INVESTIGATION OF REACTIONS AMONG PATIENTS UNDERGOING DIALYSIS
Case Finding
After being notified about the Missouri cluster, the CDC began active case finding. Inquiries about allergic-type reactions were circulated by various methods, including the use of e-mail distribution lists targeting providers in the field of nephrology and the CDC’s Epidemic Information Exchange. We used a standard form to collect information about the adverse events, as well as information about the demographic and clinical characteristics of the patients who had reactions and exposures of those patients to medication and medical devices. Since the original cluster and the majority of reactions that were reported subsequently occurred among patients undergoing dialysis, our initial case definition was restricted to reactions associated with dialysis. For this aspect of the investigation, we characterized a definite case as the sudden onset of angioedema (i.e., facial edema) or urticaria in a patient within 1 hour after the initiation of a hemodialysis session that occurred after November 1, 2007. A probable case was characterized by the development, within the same period after initiation of hemodialysis, of hypotension, loss of consciousness, or signs and symptoms from at least two of the following categories: sensation of burning, warmth, or flushing; numbness or tingling; difficulty swallowing; shortness of breath, audible wheezing, or chest tightness; tachycardia; and nausea, vomiting, or diarrhea.
Facility-Based Case–Control Study
Because a clustering of cases was noted in specific dialysis facilities, and patients within a facility had relatively uniform exposures to medical products, we conducted a case–control study to identify risk factors, using the facility as the unit of analysis. Dialysis facilities that completed a case-report form for at least one definite or probable case (21 facilities in 11 states) were considered to be case facilities and were compared with control facilities that reported no such reactions (23 facilities in 9 states). Control facilities were identified with the use of the Centers for Medicare and Medicaid Services Dialysis Facility Compare Web site.8 These facilities were selected randomly among the pooled dialysis facilities in the 11 states in which case facilities were located. Owing to the pooling of potential controls, two states with case facilities were not represented among the control facilities. A representative of each case and control facility was contacted by telephone and surveyed between January 28, 2008, and February 8, 2008. Oral consent was obtained, and a clinical manager was asked to identify the medical products and supplies that had been used at the facility in the period after December 15, 2007, including heparin products and dialysis equipment (e.g., machines, tubing, and dialyzers), and to describe the facility’s practices with respect to dialyzer reuse and reprocessing. Proportions were compared with the use of Fisher’s exact test and Stata software, version 9.0 (Stata). All reported P values are two-sided.
ASSESSMENT OF REACTIONS, HEPARIN PRODUCTS, AND EXPOSURE TO CONTAMINATED LOTS
Clinical Description of Heparin Reactions
After preliminary findings suggested that heparin was strongly associated with cases and similar reactions were reported among patients who were not undergoing hemodialysis, we expanded our case definition to include reactions in a broader population of patients. For the purposes of describing heparin reactions in this broader population, a probable or definite adverse reaction associated with heparin met the same clinical and temporal criteria as those in the case definition for patients undergoing dialysis but was defined as occurring within 1 hour after the administration of heparin (rather than within 1 hour after the initiation of a hemodialysis session).
Analytic and In Vitro Evaluation of Heparin Products
Unopened finished-product vials of heparin were solicited from health care facilities that reported cases. Heparin vials received by the CDC were categorized by lot number; 10 lots of heparin manufactured by Baxter Healthcare were tested. Samples from each unique lot number and three controls were tested for the presence of OSCS and were assessed for their effect on the amidolytic activity of kallikrein in human plasma as a measure of the activation of the kinin–kallikrein pathway. The quantification of OSCS levels and the measurement of amidolytic activity were performed with the use of previously described methods.6,7
Exposure to Contaminated Heparin Lots
Although the specific lot number of heparin that had been administered in each case patient was requested, most facilities did not routinely record this information. Instead, many facilities reported likely exposures on the basis of the heparin lots that were present in the facility at the time of the reaction. Exposure to heparin lots contaminated with OSCS was described in the following manner. A known exposure to an OSCS-contaminated lot was defined as either documented receipt by a patient of heparin from a lot that tested positive for OSCS or exposure of a patient to one of several heparin lots in the facility, all of which tested positive for OSCS.
RESULTS
DIALYSIS CASES
A total of 131 adverse events met our initial case definition. Of these cases, 128 (97.7%) occurred after the administration of heparin; in 122 of those cases (95.3%), the heparin that was used was manufactured by Baxter Healthcare. The only other factors identified in more than 50% of the cases were the use of acid concentrate manufactured by Minntech (59.5%), the use of dialysis machines (53.8%) and dialyzers (53.5%) manufactured by Gambro, and the practice of reusing hemodialyzers (52.0%).
FACILITY-BASED CASE–CONTROL STUDY
Twenty-one case facilities in 11 states had been identified by late January 2008 and were included in the study. The mean number of cases at case facilities was 4.2 (range, 1 to 11). Fifty-two facilities were contacted to obtain 23 control facilities in nine states. Of the 29 facilities that were contacted but not included as controls, 16 refused to participate, 7 did not respond, 5 reported a possible allergic-type reaction, and 1 was not a dialysis facility.
In univariate analysis, the use of Gambro dialysis machines and the administration of Baxter heparin were significantly associated with the presence of adverse reactions at the facility (Table 1). The factor with the strongest association was the use of Baxter heparin, which was reported by all case facilities and only one control facility (100.0% vs. 4.3%, P<0.001).
Table 1.
Characteristics of Facilities Evaluated in a Facility-Based Case–Control Study.
| Characteristic | Facilities with at Least One Case of Adverse Reaction (N = 21) |
Facilities with No Case of Adverse Reaction (N = 23) |
P Value |
|---|---|---|---|
| no. (%) | |||
| Manufacturer of heparin used | |||
| Baxter Healthcare | 21 (100.0) | 1 (4.3) | <0.001 |
| APP Pharmaceuticals | 2 (9.5) | 20 (87.0) | <0.001 |
| Other | 0 | 2 (8.7) | 0.49 |
| Manufacturer of dialysis machines used | |||
| Gambro | 16 (76.2) | 7 (30.4) | 0.03 |
| Fresenius Medical Care | 9 (42.9) | 13 (56.5) | 0.55 |
| B. Braun | 1 (4.8) | 3 (13.0) | 0.61 |
| Other | 1 (4.8) | 0 | 0.48 |
| Manufacturer of dialyzers used | |||
| Gambro | 10 (47.6) | 8 (34.8) | 0.54 |
| Fresenius Medical Care | 9 (42.9) | 14 (60.9) | 0.37 |
| Other | 7 (33.3) | 6 (26.1) | 0.75 |
| Reused dialyzers | 15 (71.4) | 9 (39.1) | 0.04 |
| Saline priming solution delivered to patient* | 11 (52.4) | 13 (56.5) | 0.76 |
| Patient census >70 patients | 10 (47.6) | 12 (52.2) | 1.00 |
Saline solution is used to clear the tubing and dialyzer of air and residual disinfectant. In some instances, some of the priming solution that has passed through the circuit is delivered to the patient.
ADVERSE REACTIONS ASSOCIATED WITH HEPARIN
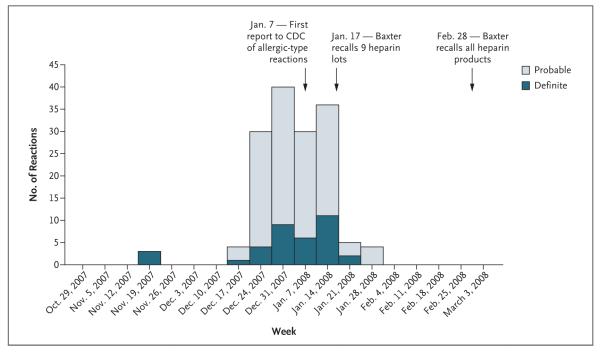
A total of 152 heparin reactions that met our case definition were identified from November 19, 2007, through January 31, 2008 (Fig. 1), among the 194 events that were reported to the CDC. The 152 cases occurred in 113 patients from 13 states and included 130 reactions in 100 patients undergoing hemodialysis, 8 reactions in 6 patients undergoing treatment for cardiac conditions, and 14 reactions in 7 patients undergoing photopheresis. The average age of the 113 case patients was 53 years; 57 (50.4%) of the case patients were women.
Figure 1. Cases of Adverse Reactions Associated with Heparin, According to Week of Onset.
The figure shows a total of 152 cases of adverse reactions associated with heparin that were reported to the Centers for Disease Control and Prevention from November 19, 2007, through January 31, 2008.
Table 2 shows the clinical characteristics of the 152 heparin reactions in 113 case patients. The mean time to a reaction after exposure to heparin was 5.1 minutes among patients undergoing hemodialysis. The most common manifestations were hypotension (50.0%), nausea (48.7%), and shortness of breath (37.5%). Thirty-six reactions (23.7%) involved facial swelling. Although urticaria was reported in several of the cases in the initial cluster, this feature was infrequent (3.3%) among all cases. Fever (0.7%), chills (1.3%), wheezing (0%), and difficulty swallowing (0%) were also rare or absent. In 15.3% of the cases, the reaction required further evaluation in the emergency department, and in 9.0% of the cases, required hospitalization. A total of 149 reactions (98.0%) occurred after intravenous administration of heparin. The other three involved exposure to heparin through other means (e.g., a dialysis circuit primed with heparin). The brand of heparin most commonly used (in 92.8% of the cases) was manufactured by Baxter Healthcare.
Table 2.
Clinical Characteristics of the 152 Adverse Reactions after Administration of Heparin.*
| Characteristic | All Reactions (N = 152) |
Probable Reactions (N = 116) |
Definite Reactions (N = 36) |
|---|---|---|---|
| Time from administration of heparin to reaction — min |
|||
| During hemodialysis | 5.1±9.1 | 5.3±10.0 | 4.5±6.2 |
| During treatment for cardiac conditions | 14±17.2 | 15.9±17.7 | 1±0 |
| During photopheresis† | 30±13.1 | 30±13.1 | — |
| Manifestation — no. (%) | |||
| Facial edema | |||
| Any | 36 (23.7) | 0 | 36 (100) |
| Lips | 23 (15.1) | 0 | 23 (63.9) |
| Eyelids | 17 (11.2) | 0 | 17 (47.2) |
| Throat | 12 (7.9) | 0 | 12 (33.3) |
| Tongue | 11 (7.2) | 0 | 11 (30.6) |
| Mouth | 10 (6.6) | 0 | 10 (27.8) |
| Urticaria | 5 (3.3) | 0 | 5 (13.9) |
| Low blood pressure | 76 (50.0) | 69 (59.5) | 7 (19.4) |
| Systolic pressure <80 mm Hg | 17 (11.2) | 14 (12.1) | 3 (8.3) |
| Nausea | 74 (48.7) | 56 (48.3) | 18 (50.0) |
| Shortness of breath | 57 (37.5) | 38 (32.8) | 19 (52.8) |
| Vomiting | 37 (24.3) | 27 (23.3) | 10 (27.8) |
| Tingling | 36 (23.7) | 28 (24.1) | 8 (22.2) |
| Flushing | 35 (23.0) | 31 (26.7) | 4 (11.1) |
| Tachycardia | 33 (21.7) | 29 (25.0) | 4 (11.1) |
| Diaphoresis | 23 (15.1) | 23 (19.8) | 0 |
| Abdominal pain | 17 (11.2) | 12 (10.3) | 5 (13.9) |
| Diarrhea | 8 (5.3) | 7 (6.0) | 1 (2.8) |
| Loss of consciousness | 6 (3.9) | 6 (5.2) | 0 |
| Chills | 2 (1.3) | 2 (1.7) | 0 |
| Fever | 1 (0.7) | 1 (0.9) | 0 |
| Difficulty swallowing | 0 | 0 | 0 |
| Wheezing | 0 | 0 | 0 |
| Follow-up care — no./total no. (%) | |||
| Blood cultures obtained‡ | 32/144 (22.2) | 26/109 (23.9) | 6/35 (17.1) |
| Evaluation in an emergency department§ | 22/144 (15.3) | 9/109 (8.3) | 13/35 (37.1) |
| Hospitalization§ | 13/144 (9.0) | 5/109 (4.6) | 8/35 (22.9) |
| Use of intravenous heparin — no. (%) | 149 (98.0) | 115 (99.1) | 34 (94.4) |
| Brand of heparin — no. (%) | |||
| Baxter Healthcare | 141 (92.8) | 110 (94.8) | 31 (86.1) |
| APP Pharmaceuticals | 6 (3.9) | 4 (3.4) | 2 (5.6) |
| Baxter or APP | 2 (1.3) | 1 (0.9) | 1 (2.8) |
| Not reported | 3 (2.0) | 1 (0.9) | 2 (5.6) |
Plus–minus values are means ±SD.
Although heparin is administered at the beginning of photophoresis, the patient is not exposed to it until later in the process.
The total numbers exclude eight reactions (one definite and seven probable) owing to missing data.
The total numbers exclude the eight reactions (one definite and seven probable) that occurred in patients undergoing treatment for cardiac conditions because these patients were hospitalized at the time of the adverse reactions.
None of the 113 patients with adverse reactions that met our case definition died immediately after the reaction. Three deaths among patients undergoing treatment for cardiac conditions were reported to the CDC, but the reactions in these patients did not meet our case definition because they occurred between 8 and 11 hours after administration of the heparin.
ANALYTIC AND IN VITRO FINDINGS
The samples tested included 10 different lot numbers of heparin vials collected from facilities and 3 control samples (Fig. 2). Of the lots collected from facilities, Lots A through G and Lot J represent eight of the nine lots of Baxter heparin that were recalled on January 17, 2008. A high level of activation of kallikrein was observed with samples from Lots A through D and F through J at concentrations of 2.5 and 25 μg per milliliter. These concentrations are in the range of a clinically efficacious concentration of heparin of approximately 1 to 5 μg per milliliter, based on a specific activity of approximately 180 U per milligram. There was little activation of kallikrein from heparin samples that did not contain OSCS, including the control samples and Lot E.
Figure 2. Association of Oversulfated Chondroitin Sulfate (OSCS) in Unfractionated Heparin with Induction of Kallikrein Activity.
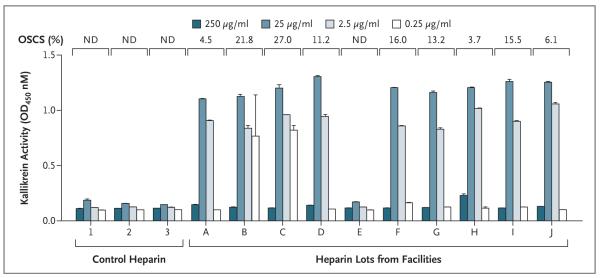
Thirteen samples of heparin, including one sample of non–clinical-grade heparin (control heparin 1), representing both suspect heparin lots and control lots, were analyzed in a blinded fashion for both the presence of OSCS and the ability to activate kallikrein. The presence of OSCS was detected and quantified as described elsewhere.6 The amidolytic activity of kallikrein was assessed at various concentrations of heparin, as indicated. Lots A through D and F through J contained OSCS. Samples from Lot E, as well as controls 1 through 3, contained no detectable OSCS. Lots A through G and Lot J were recalled on January 17, 2008. T bars indicate standard deviations of replicate measurements. ND denotes not detected.
EXPOSURE TO CONTAMINATED HEPARIN LOTS
Information on the lot of Baxter heparin was reported for 130 of the 152 heparin reactions. In 128 of the 130 (98.5%), OSCS-contaminated heparin was present in the facility; in 106 of the 130 (81.5%), facility records indicated that only OSCS-contaminated heparin could have been received by the patient (either because all heparin lots present in the facility tested positive for OSCS or because the single lot received by the patient was known and tested positive). Furthermore, of 54 heparin reactions for which the specific lot number of administered heparin was known, 52 (96.3%) resulted from an OSCS-contaminated lot.
The 106 reactions for which there was known exposure to OSCS-contaminated heparin occurred in 77 case patients. Of these case patients, 40 (51.9%) had a reported allergy to medication, most commonly to antibiotics. Six patients (7.8%) had a reported food allergy. An angiotensin-converting–enzyme (ACE) inhibitor was prescribed for 20 of these case patients (26.0%) at the time of the reaction.
Table 3 shows the clinical characteristics of the heparin reactions in case patients who were known to have received OSCS-contaminated heparin (106 reactions) and heparin that contained more than 20% OSCS (18 reactions). Low blood pressure was documented in 58.5% of the reactions; a systolic pressure lower than 80 mm Hg, however, was reported for only 9.4% of the reactions. Other common symptoms in case patients included nausea (46.2% of reactions), vomiting (28.3%), shortness of breath (25.5%), flushing (25.5%), tingling (24.5%), and tachycardia (24.5%). Facial swelling was associated with 17 reactions (16.0%). The 18 reactions involving heparin contaminated with more than 20% OSCS showed similar manifestations, although nausea was more frequent in this subgroup of cases (occurring in 72.2% of reactions), as was shortness of breath (38.9%).
Table 3.
Clinical Characteristics of the 106 Adverse Reactions in Patients Confirmed to Have Received Heparin Contaminated with OSCS and with >20% OSCS.*
| Characteristic | Reaction with OSCS- Contaminated Heparin (N = 106) |
Reaction with Heparin Contaminated with >20% OSCS (N = 18) |
|---|---|---|
| Case status — no. (%) | ||
| Probable | 89 (84.0) | 16 (88.9) |
| Definite | 17 (16.0) | 2 (11.1) |
| Patient population — no. (%) | ||
| Dialysis | 85 (80.2) | 18 (100) |
| Cardiac | 7 (6.6) | 0 |
| Photopheresis† | 14 (13.2) | 0 |
| Time from administration of heparin to reaction — min | ||
| During dialysis | 4.8±9.0 | 3.7±1.7 |
| During treatment for cardiac conditions | 15.9±17.7 | — |
| During photopheresis | 30±13.2 | — |
| Manifestations — no. (%) | ||
| Facial edema | ||
| Any | 17 (16.0) | 2 (11.1) |
| Eyelids | 11 (10.4) | 0 |
| 11 (10.4) | 1 (5.6) | |
| Tongue | 5 (4.7) | 1 (5.6) |
| Mouth | 5 (4.7) | 2 (11.1) |
| Throat | 3 (2.8) | 1 (5.6) |
| Urticaria | 2 (1.9) | 0 |
| Low blood pressure | 62 (58.5) | 10 (55.6) |
| Systolic pressure <80 mm Hg | 10 (9.4) | 0 |
| Nausea | 49 (46.2) | 12 (66.7) |
| Vomiting | 30 (28.3) | 4 (22.2) |
| Shortness of breath | 27 (25.5) | 7 (38.9) |
| Flushing | 27 (25.5) | 4 (22.2) |
| Tachycardia | 26 (24.5) | 0 |
| Tingling | 26 (24.5) | 7 (38.9) |
| Diaphoresis | 17 (16.0) | 5 (27.8) |
| Loss of consciousness | 4 (3.8) | 0 |
| Difficulty swallowing | 0 | 0 |
Forty-six reactions are not included: 2 were in patients who did not receive OSCS-contaminated heparin, and 22 were in patients who may have received OSCS-contaminated heparin but for whom receipt could not be confirmed; for the remaining 22 reactions, we did not receive lot information and were thus unable to make a determination. Plus–minus values are means ±SD. OSCS denotes oversulfated chondroitin sulfate.
Although heparin is administered at the beginning of photophoresis, the patient is not exposed to it until later in the process.
DISCUSSION
We describe findings from a national investigation of adverse reactions among patients who received heparin and characterize 152 cases that occurred between November 19, 2007, and January 31, 2008. Our initial evaluation of reactions among patients undergoing hemodialysis suggested that heparin was a potential cause, and a facility-based case–control study confirmed a strong epidemiologic association between the receipt of heparin produced by Baxter Healthcare and reactions. Reports to the CDC of heparin reactions declined after the initial Baxter recall of nine lots of heparin vials on January 17, 2008 (Fig. 1). By February 28, 2008, when all Baxter heparin products had been recalled, the CDC was no longer receiving reports of cases. Our findings confirm the presence of OSCS in finished-product vials of heparin manufactured by Baxter Healthcare that were used by facilities that reported cases, as well as the ability of these vials to induce kallikrein activation, and provide evidence that the vast majority of the patients with reactions had been exposed to heparin vials contaminated with OSCS.
Kishimoto et al.7 previously demonstrated kallikrein activation from OSCS in the active pharmaceutical ingredient of contaminated heparin. Since finished-product vials may contain heparin from more than one lot of the active pharmaceutical ingredient, the vials tested in this investigation best represent the heparin product that case patients received. We found similar biologic activity among multiple vials of heparin that were known to result in adverse reactions, and the clinical picture described among the outbreak cases nationally is consistent with the biologic mediators previously identified in response to OSCS.
The adverse reactions that were reported to the CDC encompassed a constellation of signs and symptoms, initially among patients undergoing hemodialysis. Some manifestations were overt (e.g., facial edema), others were more subtle (e.g., flushing), and many were not uncommon for patients undergoing hemodialysis or treatment for cardiac conditions (e.g., hypotension and dyspnea). Our initial case definition was intentionally broad to accommodate the many presentations and potential sources of allergic-type reactions in the absence of a clear cause or mechanism.
Similar adverse reactions have been documented among patients undergoing dialysis and have, in the past, been attributed to many causes, including dialyzer membranes, water impurities, residual disinfectants, and medications such as ACE inhibitors.9-11 A systemic inflammatory response has also been described in the setting of cardiopulmonary bypass and has been attributed to activation of the contact system resulting from interaction of blood with the artificial surfaces of the bypass circuit and other mechanisms that activate the kinin–kallikrein pathway, complement system, and other systems.12
Heparin alone rarely causes the symptoms, such as angioedema, that were observed in this investigation.13 Heparin has antiinflammatory properties, and the use of heparin-coated devices is thought to decrease the risk of an inflammatory response.14-17 Although there have been occasional adverse reactions associated with heparin that have been attributed to animal proteins or allergens,2 most adverse events reported in association with heparin products have been the result of either intrinsic or extrinsic microbial contamination.18-20
Various clinical manifestations were observed in patients with adverse reactions who received OSCS-contaminated heparin. Although hypotension was the most common, a large proportion of case patients had nausea, shortness of breath, vomiting, tingling, flushing, and diaphoresis. Urticaria was not a prominent feature among the case patients. This finding is consistent with reactions that are not mediated by mast cells and supports the role of bradykinin and other mediators instead.21 In addition, there was no substantial difference between reactions that occurred as a result of heparin contaminated with OSCS and those that occurred as a result of heparin contaminated with a high concentration of OSCS (>20%). This finding may be consistent with data showing that at clinical concentrations of heparin (2.5 and 25 μg per milliliter), the level of kallikrein activation is similar, regardless of the concentration of OSCS. Furthermore, the relatively infrequent need for case patients to be evaluated in the emergency department or hospitalized supports the notion that the clinical manifestations of these adverse reactions were mostly transient.
Twenty-six percent of case patients were taking an ACE inhibitor when they received OSCS-contaminated heparin and had the reaction. ACE inhibitors cause an accumulation of bradykinin and, thus, might be expected to predispose patients to a reaction or to worsen the reaction. The prevalence of ACE-inhibitor use among patients who received OSCS-contaminated heparin at these facilities but did not have reactions is unknown. In two studies of patients undergoing hemodialysis, the prevalence of ACE-inhibitor use was 24% and 51%,22,23 suggesting that the prevalence among case patients in this investigation was not greater than expected.
On the basis of available evidence, an allergic mechanism seems to be an unlikely cause of these reactions. Thus, it is interesting that a preexisting allergy to medication was identified among 51.9% of case patients, although it is possible that these reports of a history of intolerance to medication did not truly indicate allergic phenomena, and the prevalence of preexisting allergies among patients who did not have a reaction is unknown.
It should be recognized that the cases described in this report do not represent the entire outbreak. As of May 31, 2008, the FDA Adverse Event Reporting System had received reports of more deaths after heparin administration (238 deaths) than we had reports of cases.24 No determination of a causal association between these deaths and heparin administration has been reported, and many deaths occurred among patients with severe underlying or life-threatening conditions.
We could not calculate attack rates because we did not know the total number of patients in the United States who received heparin during this period. We suspect that OSCS-contaminated heparin was used more broadly than in only those facilities that reported cases. According to national sales distribution data from the IMS National Sales Perspectives (IMS Health), approximately 7,163,700 single-dose and 3,339,400 multidose vials of Baxter heparin were sold by the manufacturer to distributors in the United States during the period from November 2007 through January 2008 (data abstracted by the FDA from IMS Health: IMS National Sales Perspectives: Retail and Non-Retail. Nov ’07–Jan. ’08. Extracted September 4, 2008). These data do not provide a direct estimate of use, but they do provide a national estimate of the number of vials sold by the manufacturer into retail and nonretail channels of distribution. The lack of reports from other facilities may represent underreporting or underrecognition of reactions, evidence of intermittent contamination, patterns of distribution and use, or other factors.
There were several challenges to this investigation. First, health care facilities rarely recorded lot numbers of the heparin that was administered to patients. In many cases, we could determine only the heparin lots that a patient might have received. Second, our case descriptions are limited by the accuracy of information provided by health care personnel at reporting facilities. However, the fact that health care personnel reported reactions with the use of standard case-report forms probably increased the overall quality of the clinical information we obtained, as compared with information obtained by other methods. Finally, it was difficult to build precise case definitions owing to inherent uncertainties in this investigation. We attempted to reduce misclassification by establishing limits with respect to the time of onset and the symptoms that were required to be classified as a case, but some true cases may have been misclassified as noncases.
This report of a nationwide outbreak attributed to a newly discovered contaminant in heparin products contributes to our understanding of the epidemiology and biology of the adverse reactions that occurred. It also underscores the importance of a public health mechanism to address serious noninfectious adverse events in health care settings, the pivotal role of clinicians who recognize and report clusters of unusual events to public health authorities, and the need for ongoing collaboration among public health agencies, clinicians, basic-science researchers, and industry to prevent future safety threats associated with medications.
Acknowledgments
The CDC investigation had no external funding. The laboratory analysis was supported in part by grants from the National Institutes of Health (GM57073 and HL080279, to Dr. Sasisekharan).
Dr. Venkataraman reports being an employee of Momenta Pharmaceuticals and holding equity in the company, which performs the analysis and characterization of complex mixtures, including heparin, and being an inventor on patent applications for Momenta Pharmaceuticals; Dr. Kishimoto, being an employee of, holding equity in, and being an inventor on patent applications for Momenta Pharmaceuticals; Dr. Langer, receiving consulting fees from Momenta Pharmaceuticals and Scientific Protein, having equity in Momenta Pharmaceuticals, and serving on a paid board of Momenta Pharmaceuticals; Dr. Shriver, being an employee of Momenta Pharmaceuticals and holding equity in the company; Dr. Sasisekharan, holding equity in Momenta Pharmaceuticals and receiving consulting fees or serving on a paid board of Momenta Pharmaceuticals and Scientific Protein Labs; Dr. Austen, receiving consulting fees from Momenta Pharmaceuticals; Dr. Elward, receiving consulting fees or serving on a paid advisory board for Trinity and receiving grant support from SAGE Products; and Dr. Schillie, holding equity in Pfizer, Lilly, Monsanto, Walgreens, General Electric, and Abbott.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
We thank the many health care professionals at facilities at which adverse events occurred for reporting to public health authorities and for sending heparin vials for laboratory testing; the state epidemiologists and representatives of the End Stage Renal Disease Network for their assistance in facilitating contact between facilities with adverse events and the CDC; Laura Governale (IMS Health), Gary Warns (Gambro), and Bill Singley (Minntech) for the timely information they provided; and representatives of the FDA for their close collaboration, especially in the early stages of this outbreak investigation, in particular, Karen Deasy and Tarun Mallick, who served as important links between the FDA and the CDC and furthered our collective efforts.
Footnotes
No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Bircher AJ, Harr T, Hohenstein L, Tsakiris DA. Hypersensitivity reactions to anticoagulant drugs: diagnosis and management options. Allergy. 2006;61:1432–40. doi: 10.1111/j.1398-9995.2006.01227.x. [DOI] [PubMed] [Google Scholar]
- 2.Bottio T, Pittarello G, Bonato R, Fagiolo U, Gerosa G. Life-threatening anaphylactic shock caused by porcine heparin intravenous infusion during mitral valve repair. J Thorac Cardiovasc Surg. 2003;126:1194–5. doi: 10.1016/s0022-5223(03)00813-4. [DOI] [PubMed] [Google Scholar]
- 3.Acute allergic-type reactions among patients undergoing hemodialysis — multiple states, 2007–2008. MMWR Morb Mortal Wkly Rep. 2008;57:124–5. [PubMed] [Google Scholar]
- 4.Recall — firm press release: Baxter issues urgent nationwide voluntary recall of heparin 1,000 units/ml 10 and 30ml multidose vials. Food and Drug Administration; Rockville, MD: [Accessed November 24, 2008]. 2008. at http://www.fda.gov/oc/po/firmrecalls/baxter01_08.html. [Google Scholar]
- 5.Recall — firm press release: Baxter to proceed with recall of remaining heparin sodium vial products. Food and Drug Administration; Rockville, MD: [Accessed November 24, 2008]. 2008. at http://www.fda.gov/oc/po/firmrecalls/baxter02_08.html. [Google Scholar]
- 6.Guerrini M, Beccati D, Shriver Z, et al. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat Biotechnol. 2008;26:669–75. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kishimoto TK, Viswanathan K, Ganguly T, et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med. 2008;358:2457–67. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Information on Dialysis Facility Compare. Centers for Medicare & Medicaid Services; Baltimore: [Accessed November 24, 2008]. 2008. at http://www.medicare.gov/Dialysis/Include/DataSection/Questions/SearchCriteria.asp?version=default&browser=IE%7C6%7CWinXP&language=English&defaultstatus=0&pagelist=Home. [Google Scholar]
- 9.Arduino MJ. CDC investigations of noninfectious outbreaks of adverse events in hemodialysis facilities, 1979-1999. Semin Dial. 2000;13:86–91. doi: 10.1046/j.1525-139x.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- 10.Ebo DG, Bosmans JL, Couttenye MM, Stevens WJ. Haemodialysis-associated anaphylactic and anaphylactoid reactions. Allergy. 2006;61:211–20. doi: 10.1111/j.1398-9995.2006.00982.x. [DOI] [PubMed] [Google Scholar]
- 11.Pegues DA, Beck-Sague CM, Woollen SW, et al. Anaphylactoid reactions associated with reuse of hollow-fiber hemodialyzers and ACE inhibitors. Kidney Int. 1992;42:1232–7. doi: 10.1038/ki.1992.409. [DOI] [PubMed] [Google Scholar]
- 12.Zakkar M, Taylor K, Hornick PI. Immune system and inflammatory responses to cardiopulmonary bypass. In: Gravlee GP, Davis RF, Stammers AH, Ungerleider RM, editors. Cardiopulmonary bypass: principles and practice. 3rd ed Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 321–7. [Google Scholar]
- 13.Berkun Y, Haviv YS, Schwartz LB, Shalit M. Heparin-induced recurrent anaphylaxis. Clin Exp Allergy. 2004;34:1916–8. doi: 10.1111/j.1365-2222.2004.02129.x. [DOI] [PubMed] [Google Scholar]
- 14.Walenga JM. Non-anticoagulant effects of unfractionated and low-molecular weight heparins. Clin Adv Hematol Oncol. 2007;5:759–60. [PubMed] [Google Scholar]
- 15.Ludwig RJ, Alban S, Boehncke WH. Structural requirements of heparin and related molecules to exert a multitude of anti-inflammatory activities. Mini Rev Med Chem. 2006;6:1009–23. doi: 10.2174/138955706778195180. [DOI] [PubMed] [Google Scholar]
- 16.Maharaj C, Laffey JG. New strategies to control the inflammatory response in cardiac surgery. Curr Opin Anaesthesiol. 2004;17:35–48. doi: 10.1097/00001503-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Wan S, LeClerc JL, Antoine M, DeSmet JM, Yim AP, Vincent JL. Heparin-coated circuits reduce myocardial injury in heart or heart-lung transplantation: a prospective, randomized study. Ann Thorac Surg. 1999;68:1230–5. doi: 10.1016/s0003-4975(99)00701-8. [DOI] [PubMed] [Google Scholar]
- 18.Pseudomonas bloodstream infections associated with a heparin/saline flush — Missouri, New York, Texas, and Michigan, 2004–2005. MMWR Morb Mortal Wkly Rep. 2005;54:269–72. [PubMed] [Google Scholar]
- 19.Souza Dias MB, Habert AB, Borrasca V, et al. Salvage of long-term central venous catheters during an outbreak of Pseudomonas putida and Stenotrophomonas maltophilia infections associated with contaminated heparin catheter-lock solution. Infect Control Hosp Epidemiol. 2008;29:125–30. doi: 10.1086/526440. [DOI] [PubMed] [Google Scholar]
- 20.Vonberg RP, Gastmeier P. Hospital-acquired infections related to contaminated substances. J Hosp Infect. 2007;65:15–23. doi: 10.1016/j.jhin.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz LB. Heparin comes clean. N Engl J Med. 2008;358:2505–9. doi: 10.1056/NEJMe0803599. [DOI] [PubMed] [Google Scholar]
- 22.Bishu K, Gricz KM, Chewaka S, Agar-wal R. Appropriateness of antihypertensive drug therapy in hemodialysis patients. Clin J Am Soc Nephrol. 2006;1:820–4. doi: 10.2215/CJN.00060106. [DOI] [PubMed] [Google Scholar]
- 23.Leypoldt JK, Cheung AK, Delmez JA, et al. Relationship between volume status and blood pressure during chronic hemodialysis. Kidney Int. 2002;61:266–75. doi: 10.1046/j.1523-1755.2002.00099.x. [DOI] [PubMed] [Google Scholar]
- 24.Information on adverse event reports and heparin. Food and Drug Administration; Rockville, MD: [Accessed November 24, 2008]. 2008. at http://www.fda.gov/cder/drug/infopage/heparin/adverse_events.htm. [Google Scholar]